Nanostructured MoS2 and WS2 Photoresponses under Gas Stimuli
Abstract
1. Introduction
2. Materials and Fabrication Methods
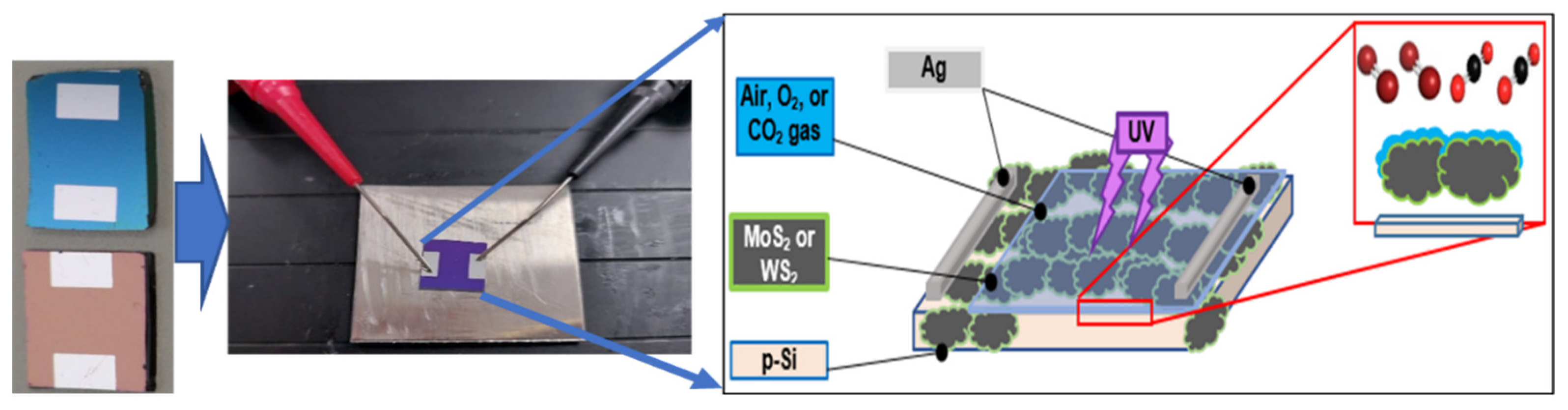
2.1. Fabrication of Thin Films
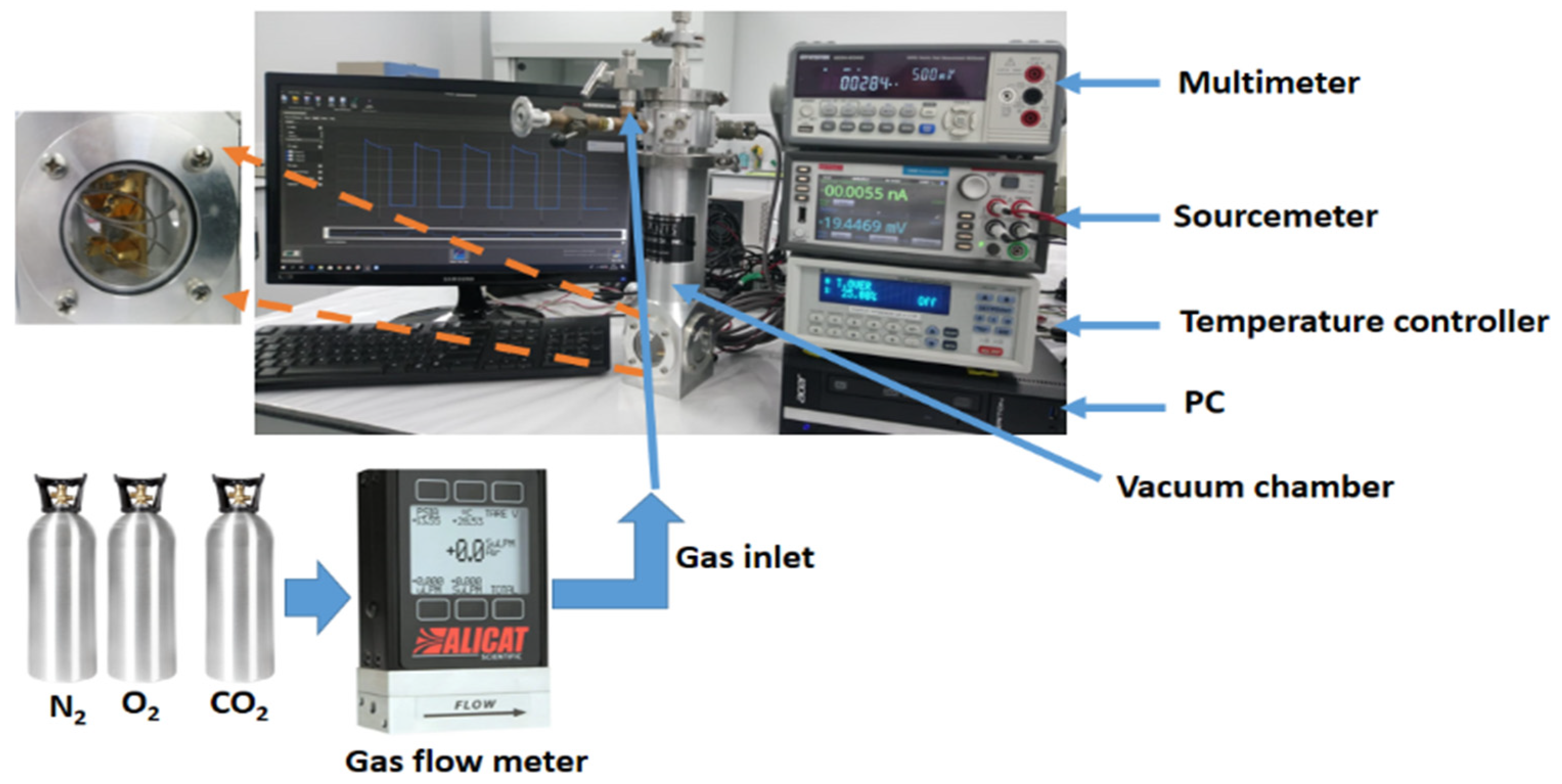
2.2. Characterization Techniques
3. Results and Discussion
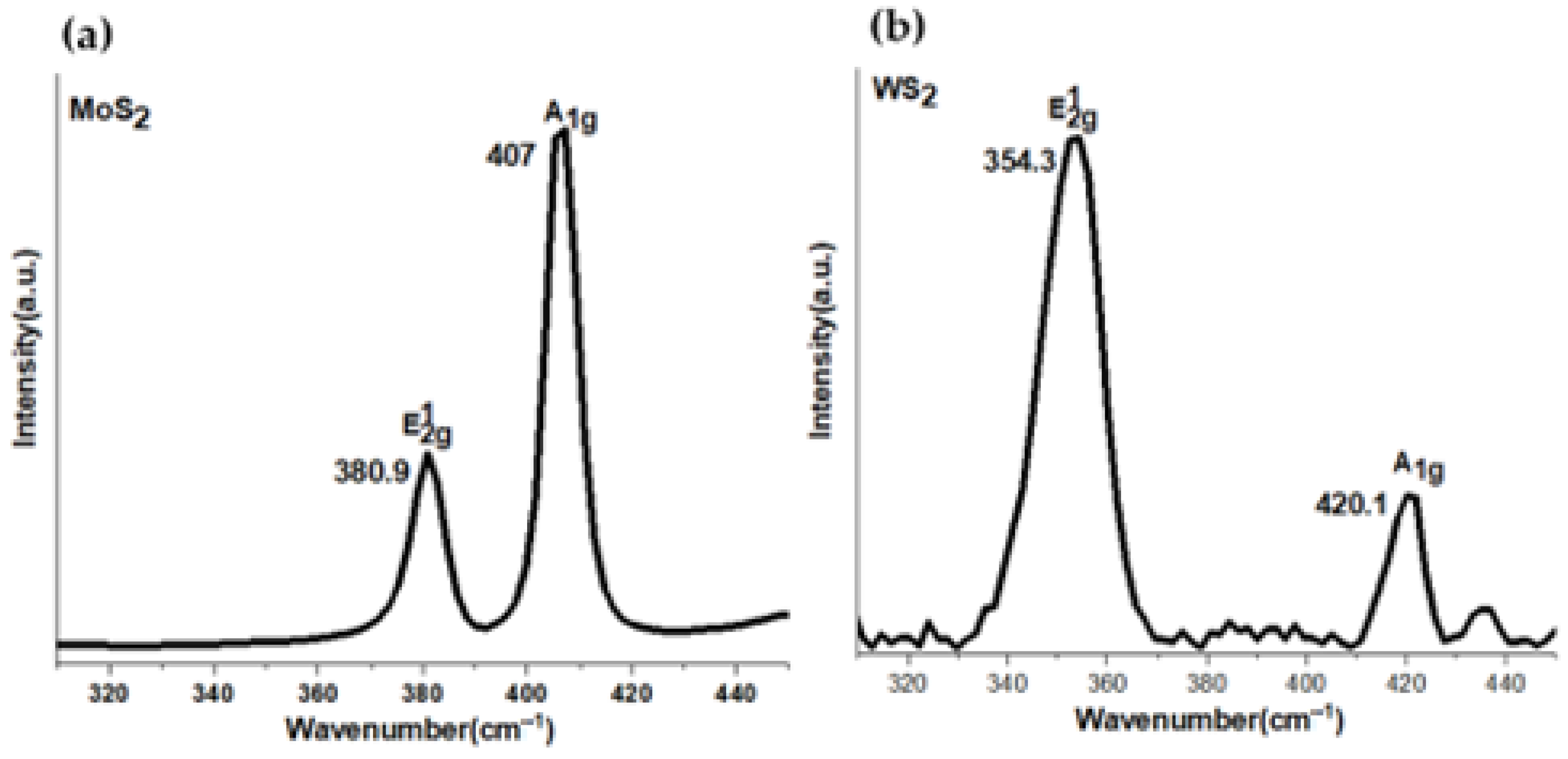
3.1. Raman and PL Spectra
3.2. Surface Morphology and Topography
3.3. XPS and Oxidation States
3.4. Sheet Resistance and Hall Coefficient
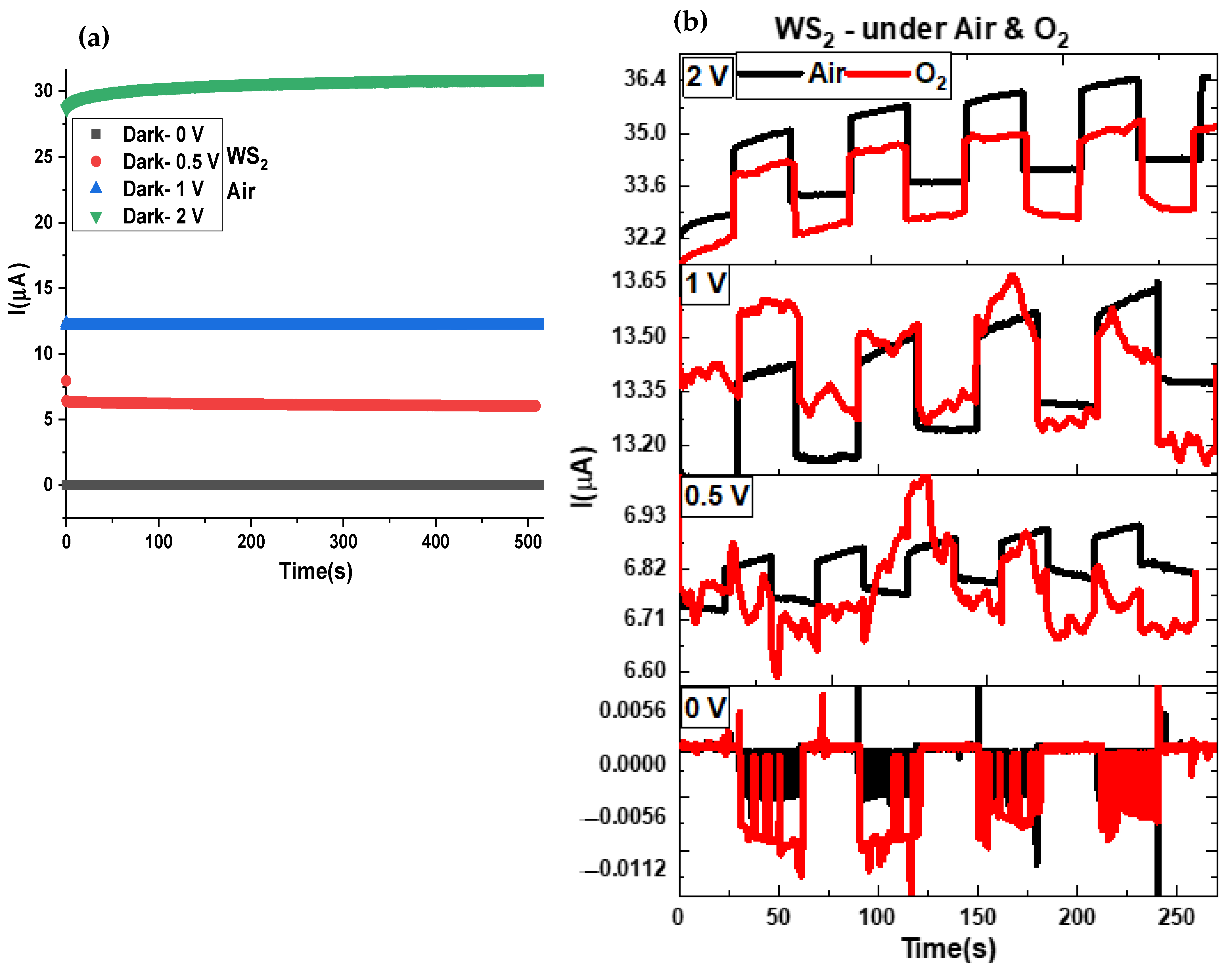
3.5. Electric and Optoelectronic Characteristics
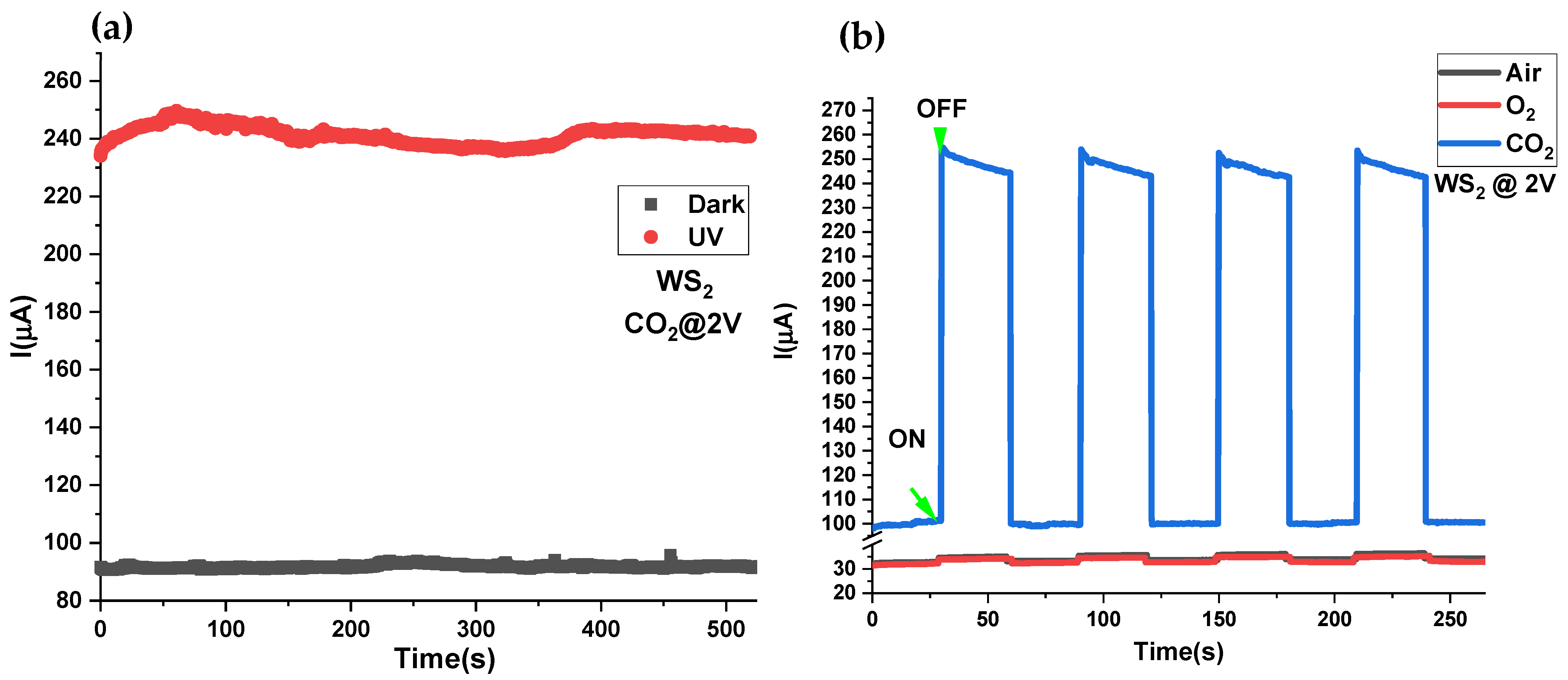
3.6. Transient Response
3.7. Photocurrent Gain (Pg), Photoresponsivity (), External Quantum Efficiency (EQE) and Detectivity ()
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzaalez-Cuevas, J.A.; Refaat, T.F.; Abedin, M.N.; Elsayed-Ali, H.E. Modeling of the Temperature-Dependent Spectral Response of In_{1−x}Ga_{x}Sb Infrared Photodetectors. Opt. Eng. 2006, 45, 44001. [Google Scholar]
- Basyooni, M.A.; Shaban, M.; El Sayed, A.M. Enhanced Gas Sensing Properties of Spin-coated Na-doped ZnO Nanostructured Films. Sci. Rep. 2017, 7, 41716. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.E.; Basyooni, M.A. Ultra-sensitive gas sensor based fano resonance modes in periodic and fibonacci quasi-periodic Pt/PtS2 structures. Sci. Rep. 2022, 12, 9759. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.E.; Basyooni, M.A.; Shaban, M.; Rabia, M.; Eker, Y.R.; Attia, G.F.; Yilmaz, M.; Ahmed, A.M. Role of oxygen vacancies in vanadium oxide and oxygen functional groups in graphene oxide for room temperature CO2 gas sensors. Sens. Actuators A Phys. 2019, 294, 17–24. [Google Scholar] [CrossRef]
- Owen, T. The Composition and Early History of the Atmosphere of Mars; Mars Publication: Hong Kong, China, 2018; pp. 818–834. [Google Scholar]
- Elías, A.L.; Perea-López, N.; Castro-Beltrán, A.; Berkdemir, A.; Lv, R.; Feng, S.; Long, A.D.; Hayashi, T.; Kim, Y.A.; Endo, M.; et al. Controlled synthesis and transfer of large-area WS2 sheets: From single layer to few layers. ACS Nano 2013, 7, 5235–5242. [Google Scholar] [CrossRef]
- Huo, N.; Yang, S.; Wei, Z.; Li, S.S.; Xia, J.B.; Li, J. Photoresponsive and Gas Sensing Field-Effect Transistors based on Multilayer WS2 Nanoflakes. Sci. Rep. 2014, 4, 5209. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, T.; Jalil, R.; Belle, B.D.; Britnell, L.; Gorbachev, R.V.; Morozov, S.V.; Kim, Y.J.; Gholinia, A.; Haigh, S.J.; Makarovsky, O.; et al. Vertical field-effect transistor based on graphene-WS2 heterostructures for flexible and transparent electronics. Nat. Nanotechnol. 2013, 8, 100–103. [Google Scholar] [CrossRef]
- Bernardi, M.; Palummo, M.; Grossman, J.C. Extraordinary sunlight absorption and one nanometer thick photovoltaics using two-dimensional monolayer materials. Nano Lett. 2013, 13, 3664–3670. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.S.; Cha, E.; Kim, J.; Choi, W. Synthesis of uniform single layer WS2 for tunable photoluminescence. Sci. Rep. 2017, 7, 16121. [Google Scholar] [CrossRef]
- Das, U.; Bhattacharjee, S.; Mahato, B.; Prajapat, M.; Sarkar, P.; Roy, A. Uniform, large-scale growth of WS2 nanodomains via CVD technique for stable non-volatile RRAM application. Mater. Sci. Semicond. Process. 2020, 107, 104837. [Google Scholar] [CrossRef]
- Lin, Y.; Adilbekova, B.; Firdaus, Y.; Yengel, E.; Faber, H.; Sajjad, M.; Zheng, X.; Yarali, E.; Seitkhan, A.; Bakr, O.M.; et al. 17% Efficient Organic Solar Cells Based on Liquid Exfoliated WS2 as a Replacement for PEDOT:PSS. Adv. Mater. 2019, 31, 1902965. [Google Scholar] [CrossRef]
- Sharma, D.; Motayed, A.; Shah, P.B.; Amani, M.; Georgieva, M.; Glen Birdwell, A.; Dubey, M.; Li, Q.; Davydov, A. V Transfer characteristics and low-frequency noise in single- and multi-layer MoS2 field-effect transistors. Appl. Phys. Lett. 2015, 107, 162102. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef]
- Coleman, J.N. ChemInform Abstract: Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. ChemInform 2011, 42, 568–571. [Google Scholar] [CrossRef]
- Yu, X.; Prévot, M.S.; Sivula, K. Multiflake thin film electronic devices of solution processed 2D MoS2 enabled by sonopolymer assisted exfoliation and surface modification. Chem. Mater. 2014, 26, 5892–5899. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef]
- Varrla, E.; Backes, C.; Paton, K.R.; Harvey, A.; Gholamvand, Z.; McCauley, J.; Coleman, J.N. Large-scale production of size-controlled MoS2 nanosheets by shear exfoliation. Chem. Mater. 2015, 27, 1129–1139. [Google Scholar] [CrossRef]
- Knirsch, K.C.; Berner, N.C.; Nerl, H.C.; Cucinotta, C.S.; Gholamvand, Z.; McEvoy, N.; Wang, Z.; Abramovic, I.; Vecera, P.; Halik, M.; et al. Basal-Plane Functionalization of Chemically Exfoliated Molybdenum Disulfide by Diazonium Salts. ACS Nano 2015, 9, 6018–6030. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y. Photoenergy and Thin Film Materials; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Giannazzo, F.; Lara Avila, S.; Eriksson, J.; Sonde, S. Integration of 2D Materials for Electronics Applications. Crystals 2019, 7, 162. [Google Scholar]
- Ali, M.; Mohamed, B. VO2 Ince Film Içeren Iki Boyutlu Ve Çok Katmanli Yapilardaki Arayüzey Gerilmelerinin Termokromik Ve Fotodetektör Performanslara Etkisi. Ph.D. Thesis, Necmettin Erbakan University, Konya, Turkey, 2020. [Google Scholar]
- Basyooni, M.A.; Zaki, S.E.; Shaban, M.; Eker, Y.R.; Yilmaz, M. Efficient MoWO3/VO2/MoS2/Si UV Schottky photodetectors; MoS2 optimization and monoclinic VO2 surface modifications. Sci. Rep. 2020, 10, 15926. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, J.; Zhang, W.; Chang, H. Synthesis of high quality two-dimensional materials via chemical vapor deposition. Chem. Sci. 2015, 6, 6705–6716. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, L.; Lee, Y.H.; Fang, W.; Hsu, A.; Herring, P.; Chin, M.; Dubey, M.; Li, L.J.; Kong, J.; et al. Large-scale 2D electronics based on single-layer MoS2 grown by chemical vapor deposition. In Proceedings of the Technical Digest—International Electron Devices Meeting, IEDM, San Francisco, CA, USA, 10–13 December 2012. [Google Scholar]
- Browning, R.; Padigi, P.; Solanki, R.; Tweet, D.J.; Schuele, P.; Evans, D. Atomic layer deposition of MoS2 thin films. Mater. Res. Express 2015, 2, 025006. [Google Scholar] [CrossRef]
- Loh, T.A.J.; Chua, D.H.C. Growth mechanism of pulsed laser fabricated few-layer MoS2 on metal substrates. ACS Appl. Mater. Interfaces 2014, 6, 15966–15971. [Google Scholar] [CrossRef] [PubMed]
- Serrao, C.R.; Diamond, A.M.; Hsu, S.L.; You, L.; Gadgil, S.; Clarkson, J.; Carraro, C.; Maboudian, R.; Hu, C.; Salahuddin, S. Highly crystalline MoS2 thin films grown by pulsed laser deposition. Appl. Phys. Lett. 2015, 106, 052101. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Al-Dossari, M.; Zaki, S.E.; Eker, Y.R.; Yilmaz, M.; Shaban, M. Tuning the Metal–Insulator Transition Properties of VO2 Thin Films with the Synergetic Combination of Oxygen Vacancies, Strain Engineering, and Tungsten Doping. Nanomaterials 2022, 12, 1470. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shi, M. Thermal evaporation deposition of few-layer MoS2 films. Nano-Micro Lett. 2013, 5, 135–139. [Google Scholar] [CrossRef]
- Wu, S.; Huang, C.; Aivazian, G.; Ross, J.S.; Cobden, D.H.; Xu, X. Vapor-solid growth of high optical quality MoS2 monolayers with near-unity valley polarization. ACS Nano 2013, 7, 2768–2772. [Google Scholar] [CrossRef] [PubMed]
- Pacley, S.; Hu, J.; Jespersen, M.; Hilton, A.; Waite, A.; Brausch, J.; Beck-Millerton, E.; Voevodin, A.A. Impact of reduced graphene oxide on MoS2 grown by sulfurization of sputtered MoO3 and Mo precursor films. J. Vac. Sci. Technol. A Vac. Surf. Film. 2016, 34, 041505. [Google Scholar] [CrossRef]
- Hussain, S.; Shehzad, M.A.; Vikraman, D.; Khan, M.F.; Singh, J.; Choi, D.C.; Seo, Y.; Eom, J.; Lee, W.G.; Jung, J. Synthesis and characterization of large-area and continuous MoS2 atomic layers by RF magnetron sputtering. Nanoscale 2016, 8, 4340–4347. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Ohashi, T.; Muneta, I.; Ishihara, S.; Kakushima, K.; Tsutsui, K.; Ogura, A.; Wakabayashi, H. Low-Carrier-Density Sputtered MoS2 Film by Vapor-Phase Sulfurization. J. Electron. Mater. 2018, 47, 3497–3501. [Google Scholar] [CrossRef]
- Furchi, M.M.; Polyushkin, D.K.; Pospischil, A.; Mueller, T. Mechanisms of photoconductivity in atomically thin MoS2. Nano Lett. 2014, 14, 6165–6170. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Adler, E.R.; Liu, Y.; St Marie, L.; El Fatimy, A.; Melis, S.; van Keuren, E.; Barbara, P. Ambient effects on photogating in MoS2 photodetectors. Nanotechnology 2019, 30, 284004. [Google Scholar] [CrossRef]
- Pospischil, A.; Mueller, T. Optoelectronic devices based on atomically thin transition metal dichalcogenides. Appl. Sci. 2016, 6, 78. [Google Scholar] [CrossRef]
- Klement, P.; Steinke, C.; Chatterjee, S.; Wehling, T.O.; Eickhoff, M. Effects of the Fermi level energy on the adsorption of O2 to monolayer MoS2. 2D Mater. 2018, 5, 045025. [Google Scholar] [CrossRef]
- Kumar, R.; Zheng, W.; Liu, X.; Zhang, J.; Kumar, M. MoS2-Based Nanomaterials for Room-Temperature Gas Sensors. Adv. Mater. Technol. 2020, 5, 1901062. [Google Scholar] [CrossRef]
- Khan, M.A.; Kumawat, K.L.; Nanda, K.K.; Krupanidhi, S.B. Reduced graphene oxide-based broad band photodetector and temperature sensor: Effect of gas adsorption on optoelectrical properties. J. Nanoparticle Res. 2018, 20, 293. [Google Scholar] [CrossRef]
- Yan, H.J.; Xu, B.; Shi, S.Q.; Ouyang, C.Y. First-principles study of the oxygen adsorption and dissociation on graphene and nitrogen doped graphene for Li-air batteries. J. Appl. Phys. 2012, 112, 112602. [Google Scholar] [CrossRef]
- Guo, R.; Han, D.; Chen, W.; Dai, L.; Ji, K.; Xiong, Q.; Li, S.; Reb, L.K.; Scheel, M.A.; Pratap, S.; et al. Degradation mechanisms of perovskite solar cells under vacuum and one atmosphere of nitrogen. Nat. Energy 2021, 6, 977–986. [Google Scholar] [CrossRef]
- Karataş, A.; Yılmaz, M. Molybdenum disulfide thin films fabrication from multi-phase molybdenum oxide using magnetron sputtering and CVD systems together. Superlattices Microstruct. 2020, 143, 106555. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Eker, Y.R.; Yilmaz, M. Van-der Waals 2-Dimensional Nanostructured Molybdenum and Tungsten Disulfide-Si Heterostructure Photodetector under Different Environment Stimuli for High Yield Optoelectronics. Mühendislik Bilimleri 2020, 2. [Google Scholar]
- Shanmugam, M.; Durcan, C.A.; Yu, B. Layered semiconductor molybdenum disulfide nanomembrane based Schottky-barrier solar cells. Nanoscale 2012, 4, 7399–7405. [Google Scholar] [CrossRef] [PubMed]
- Basyooni, M.A.; Zaki, S.E.; Tihtih, M.; Eker, Y.R.; Ateş, Ş. Photonic bandgap engineering in (VO2)n/(WSe2)nphotonic superlattice for versatile near- and mid-infrared phase transition applications. J. Phys. Condens. Matter 2022, 34, 325901. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Cheng, Y.C.; Schwingenschlögl, U. Giant spin-orbit-induced spin splitting in two-dimensional transition-metal dichalcogenide semiconductors. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 84, 153402. [Google Scholar] [CrossRef]
- Zhao, W.; Ghorannevis, Z.; Chu, L.; Toh, M.; Kloc, C.; Tan, P.H.; Eda, G. Evolution of electronic structure in atomically thin sheets of ws2 and wse2. ACS Nano 2013, 7, 791–797. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, L. Exciton dynamics and annihilation in WS2 2D semiconductors. Nanoscale 2015, 7, 7402–7408. [Google Scholar] [CrossRef]
- Hwang, J.D.; Chang, W.T.; Chen, Y.H.; Kung, C.Y.; Hu, C.H.; Chen, P.S. Suppressing the dark current of metal-semiconductor-metal SiGe/Si heterojunction photodetector by using asymmetric structure. Thin Solid Films 2007, 515, 3837–3839. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Q.; Shen, W.; Wang, D. Self-cleaning antireflective coating with a hierarchical texture for light trapping in micromorph solar cells. J. Mater. Chem. C 2017, 5, 103–109. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W.; Ma, L.; Li, H.; Li, H.; Huang, F.; Xu, Z.; Zhang, Q.; Lee, J.Y. Graphene-like MoS2/amorphous carbon composites with high capacity and excellent stability as anode materials for lithium ion batteries. J. Mater. Chem. 2011, 21, 6251–6257. [Google Scholar] [CrossRef]
- He, H.Y. Assembly of 1T-WSe2: Sn nanosheets/graphene by a modified hydrothermal process for water splitting. J. Sol-Gel Sci. Technol. 2020, 93, 554–562. [Google Scholar] [CrossRef]
- Xiong, M.; Qian, J.; Yang, K.; Chen, Z.; Mei, T.; Wang, J.; Li, J.; Yu, L.; Wang, X. Efficient polysulfide anchor: Brain coral-like WS2 nanosheets. J. Mater. Sci. 2020, 55, 12031–12040. [Google Scholar] [CrossRef]
- Ko, T.S.; Huang, C.C.; Lin, D.Y. Optical and transport properties of Ni-MoS2. Appl. Sci. 2016, 6, 227. [Google Scholar] [CrossRef]
- Method of Making an Integrated Photodetector in Which a Silicon Nitride Layer Forms an Anti-Reflective Film and Part of Multi-Layer Insulator within Transistor Structures. U.S. Patents US6803249B2, 12 October 2014. Available online: https://patents.google.com/patent/US6803249B2/en (accessed on 2 July 2020).
- Oliva, N.; Casu, E.A.; Yan, C.; Krammer, A.; Rosca, T.; Magrez, A.; Stolichnov, I.; Schueler, A.; Martin, O.J.F.; Ionescu, A.M. van der Waals MoS2/VO2 heterostructure junction with tunable rectifier behavior and efficient photoresponse. Sci. Rep. 2017, 7, 14250. [Google Scholar] [CrossRef] [PubMed]
- Basyooni, M.A.; Zaki, S.E.; Ertugrul, S.; Yilmaz, M.; Eker, Y.R. Fast response of CO2 room temperature gas sensor based on Mixed-Valence Phases in Molybdenum and Tungsten Oxide nanostructured thin films. Ceram. Int. 2020, 46, 9839–9853. [Google Scholar] [CrossRef]
- Tongay, S.; Zhou, J.; Ataca, C.; Liu, J.; Kang, J.S.; Matthews, T.S.; You, L.; Li, J.; Grossman, J.C.; Wu, J. Broad-range modulation of light emission in two-dimensional semiconductors by molecular physisorption gating. Nano Lett. 2013, 13, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, X.; Guo, T.; Tian, W.; Hao, J.; Guo, Q. The competitive adsorption mechanism of CO2, H2O and O2 on a solid amine adsorbent. Chem. Eng. J. 2021, 416, 129007. [Google Scholar] [CrossRef]
- Ko, P.J.; Abderrahmane, A.; Kim, N.H.; Sandhu, A. High-performance near-infrared photodetector based on nano-layered MoSe2. Semicond. Sci. Technol. 2017, 32, 065015. [Google Scholar] [CrossRef]
- Wu, J.M.; Chang, W.E. Ultrahigh responsivity and external quantum efficiency of an ultraviolet-light photodetector based on a single VO2 microwire. ACS Appl. Mater. Interfaces 2014, 6, 14286–14292. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Weng, W.-J.; Wei, D.-H. Enhanced UV photodetector response and recovery times using a nonpolar ZnO sensing layer. J. Vac. Sci. Technol. A Vac. Surf. Films 2016, 34, 02D106. [Google Scholar] [CrossRef]
- Liu, X.; Gu, L.; Zhang, Q.; Wu, J.; Long, Y.; Fan, Z. All-printable band-edge modulated ZnO nanowire photodetectors with ultra-high detectivity. Nat. Commun. 2014, 5, 4007. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, A.; Hall, S.; Ragsdale, S.W.; Armstrong, F.A. Selective visible-light-driven CO2 reduction on a p-type dye-sensitized nio photocathode. J. Am. Chem. Soc. 2014, 136, 13518–13521. [Google Scholar] [CrossRef] [PubMed]












| Sample | Region | Min (nm) | Max (nm) | Mid (nm) | Rq (nm) | Ra (nm) | Rz (nm) | Rsk | Rku |
|---|---|---|---|---|---|---|---|---|---|
| MoS2 | Line | −24.584 | 22.080 | −1.252 | 46.664 | 10.689 | 9.200 | 45.329 | 0.439 |
| WS2 | Line | −4.964 | 6.682 | 0.859 | 11.646 | 2.761 | 2.099 | 11.374 | −0.645 |
| Sample | Rs (Ω/sq) | Rho (/Ω-cm) | VH (V) | RH (m3/C) | Type | Ns/Ps (/cm2) | N/P (/cm3) | Mobility (cm2/V) |
|---|---|---|---|---|---|---|---|---|
| MoS2 | 1890 | 0.00284 | −0.0129 | −0.00000268 | N | −3,500,000,000,000 | −2,330,000,000,000,000,000 | 946 |
| WS2 | 38,200 | 0.0573 | 0.00819 | 0.00000171 | P | 5,500,000,000,000 | 3,670,000,000,000,000,000 | 2980 |
| Sample | Gas | Photocurrent (A) | Photocurrent Gain (a.u.) | Responsivity (µA/mW) | EQE | |
|---|---|---|---|---|---|---|
| MoS2 | Air | 0.0000131 | 0.0270 | 6.5745 | 13,900,000 | 372.05 |
| CO2 | 0.00000635 | 0.0265 | 3.1765 | 6,740,000 | 256.34 | |
| WS2 | Air | 0.00000207 | 0.0631 | 1.035 | 2,200,000 | 225.76 |
| CO2 | 0.000153 | 1.51 | 76.50 | 162,000,000 | 9509.10 | |
| O2 | 0.00000183 | 0.0568 | 0.915 | 1,940,000 | 201.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basyooni, M.A.; Zaki, S.E.; Alfryyan, N.; Tihtih, M.; Eker, Y.R.; Attia, G.F.; Yılmaz, M.; Ateş, Ş.; Shaban, M. Nanostructured MoS2 and WS2 Photoresponses under Gas Stimuli. Nanomaterials 2022, 12, 3585. https://doi.org/10.3390/nano12203585
Basyooni MA, Zaki SE, Alfryyan N, Tihtih M, Eker YR, Attia GF, Yılmaz M, Ateş Ş, Shaban M. Nanostructured MoS2 and WS2 Photoresponses under Gas Stimuli. Nanomaterials. 2022; 12(20):3585. https://doi.org/10.3390/nano12203585
Chicago/Turabian StyleBasyooni, Mohamed A., Shrouk E. Zaki, Nada Alfryyan, Mohammed Tihtih, Yasin Ramazan Eker, Gamal F. Attia, Mücahit Yılmaz, Şule Ateş, and Mohamed Shaban. 2022. "Nanostructured MoS2 and WS2 Photoresponses under Gas Stimuli" Nanomaterials 12, no. 20: 3585. https://doi.org/10.3390/nano12203585
APA StyleBasyooni, M. A., Zaki, S. E., Alfryyan, N., Tihtih, M., Eker, Y. R., Attia, G. F., Yılmaz, M., Ateş, Ş., & Shaban, M. (2022). Nanostructured MoS2 and WS2 Photoresponses under Gas Stimuli. Nanomaterials, 12(20), 3585. https://doi.org/10.3390/nano12203585








