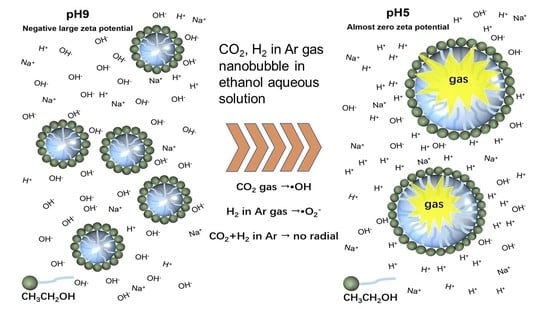
Stability and Free Radical Production for CO2 and H2 in Air Nanobubbles in Ethanol Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Nanobubble Preparation and Measurements
2.2.2. Radical Preparation and Measurement by ESR
3. Results and Discussion
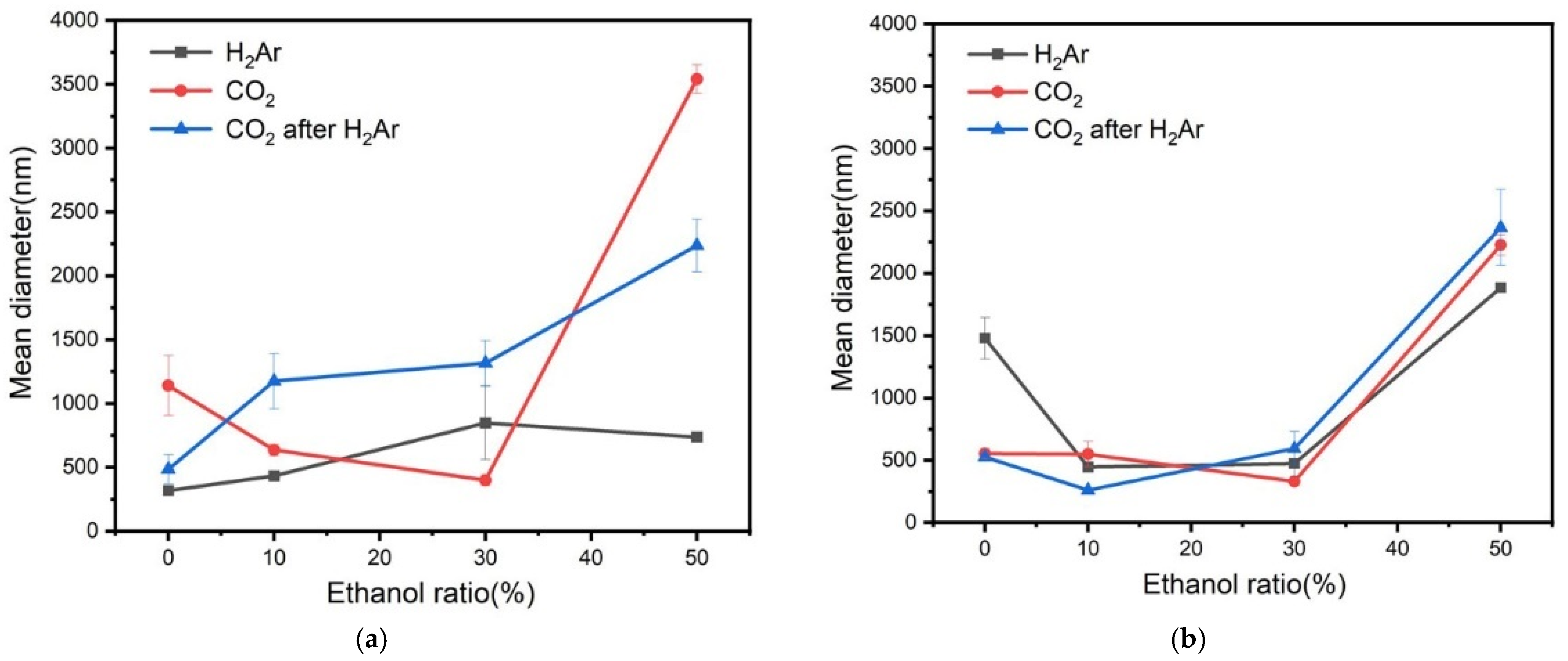
3.1. Determination of Diameter of Nanobubbles
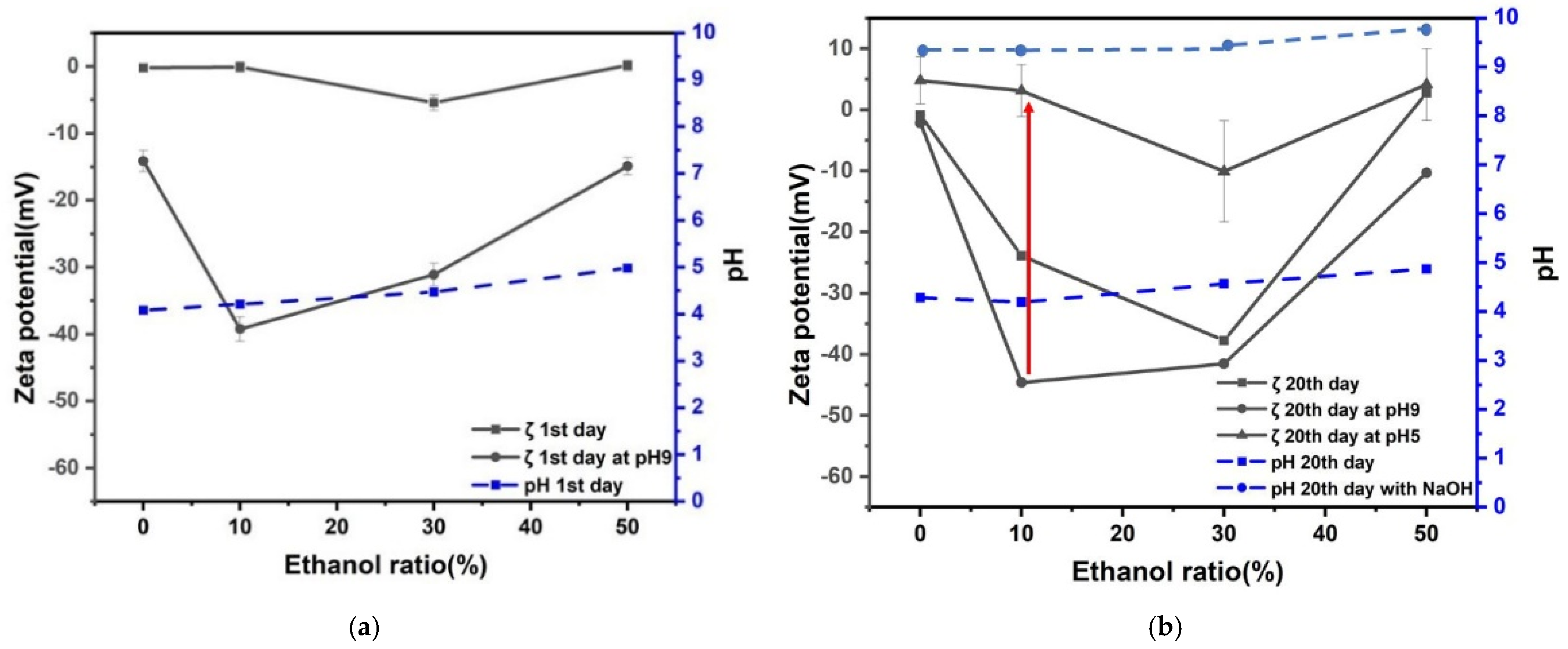
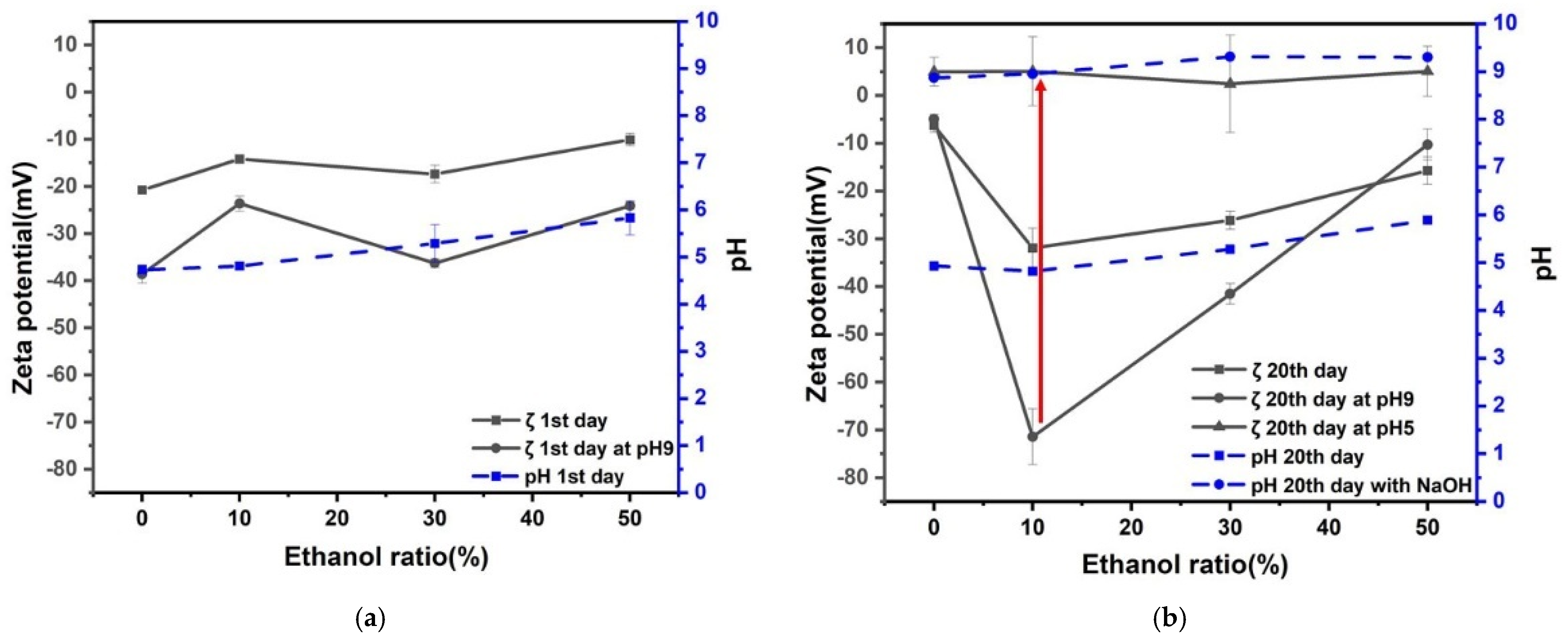
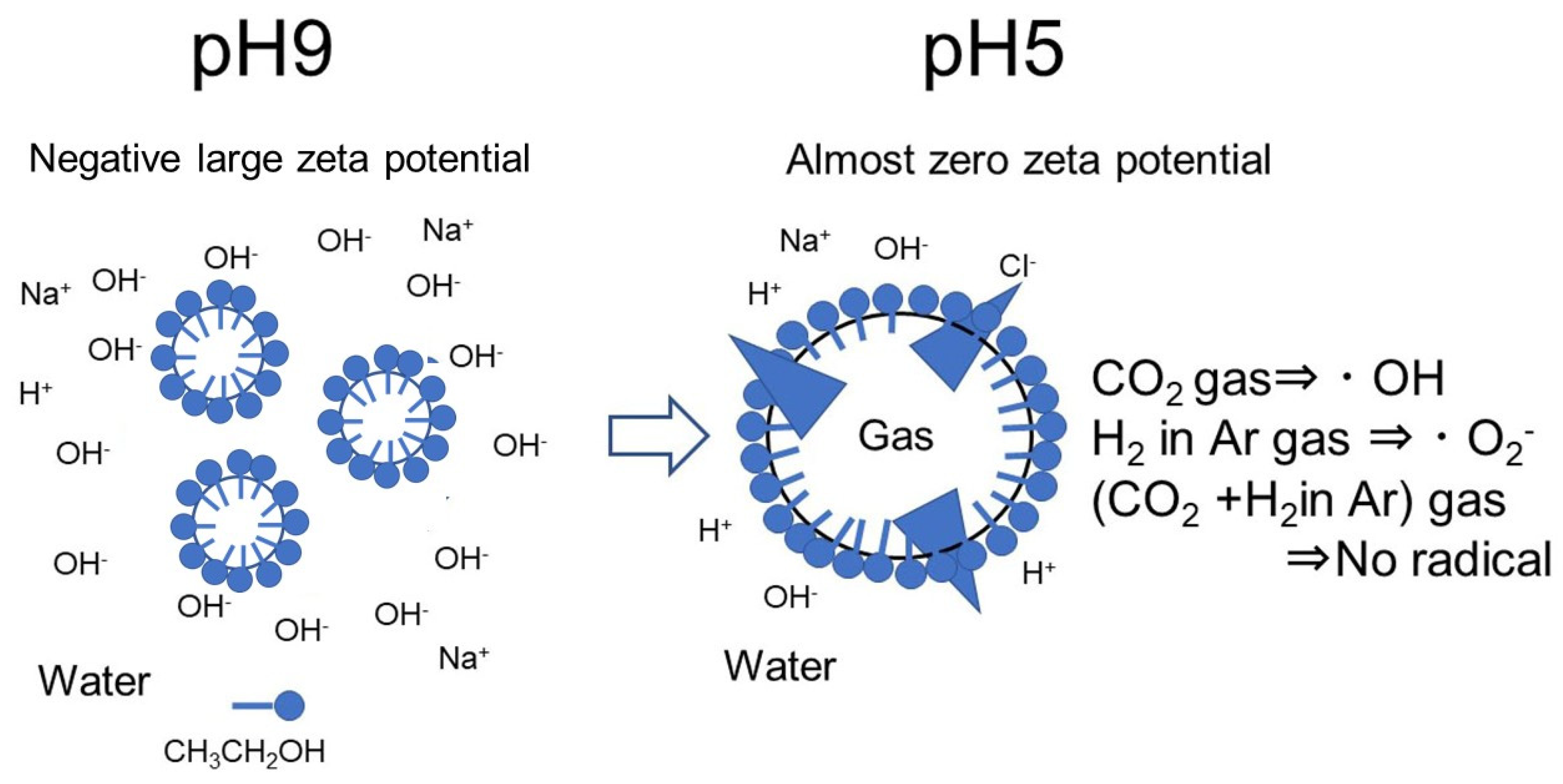
3.2. Effect of Ethanol Ratio in Zeta Potential and pH of Nanobubbles
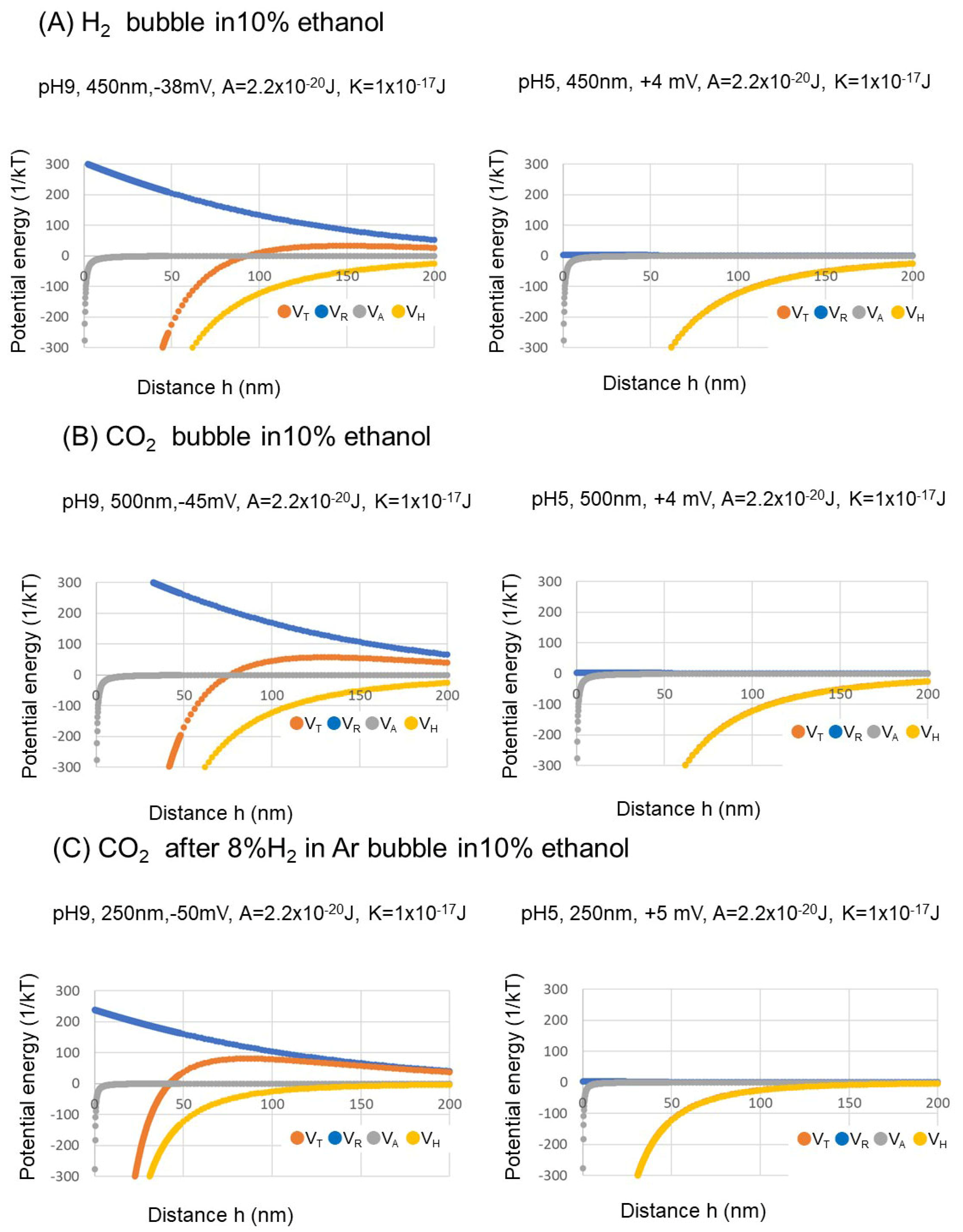
3.3. Nanobubble Stability Evaluation Using Extended DLVO Theory
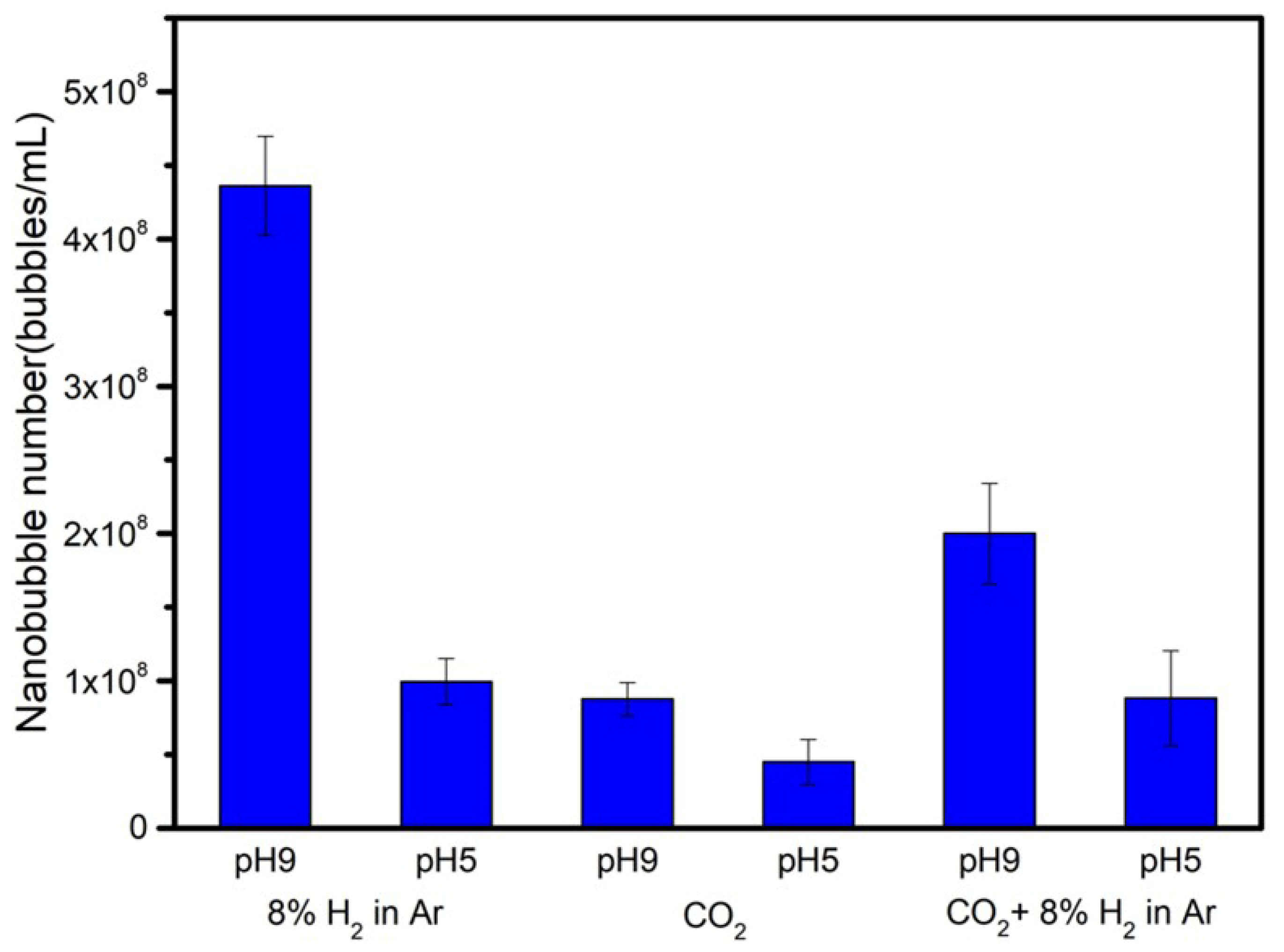
3.4. Number of Nanobubbles
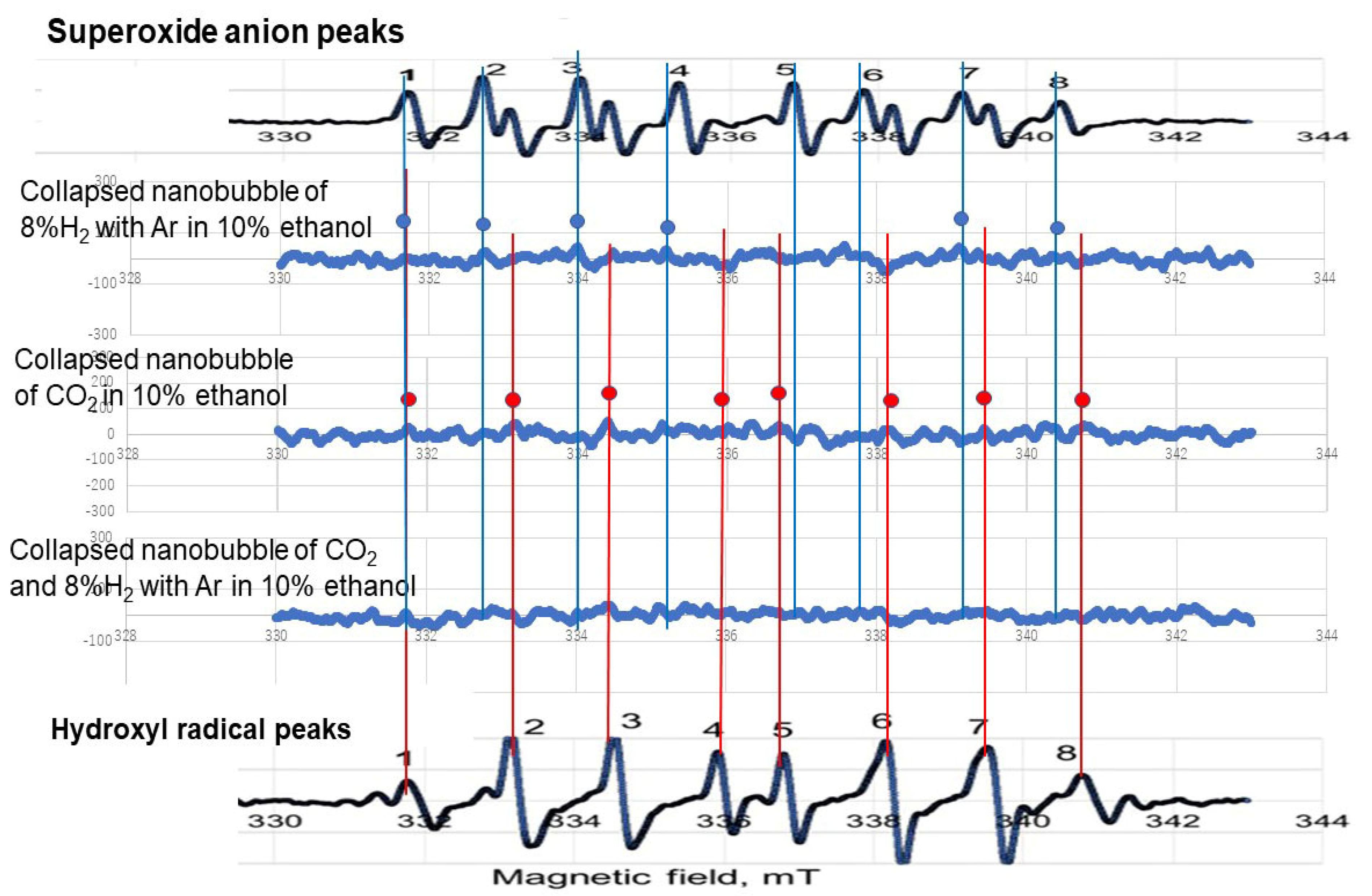
3.5. Radical Observation by Changing the pH
4. Conclusions
- The prepared nanobubbles were stable for 20 days owing to a high negative zeta potential at alkaline pH 9.
- When the pH of ethanol alkaline aqueous solution with nanobubbles was adjusted to acidic at around pH 5, the zeta potential of nanobubbles was almost zero. The numbers of nanobubble decreased at almost zero charge (pH 5) were identified by measuring their numbers using the particle trajectory method (Nano site).
- The extended Derjaguin, Landau, Verwey, and Overbeek (DLVO) theory was used to evaluate the nanobubble stability (repulsion between bubbles) in alkaline conditions, and its instability (attraction between bubbles) in acidic conditions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asenbaum, A.; Pruner, C.; Wilhelm, E.; Mijakovic, M.; Zoranic, L.; Sokolic, F.; Kezic, B.; Perera, A. Structural changes in ethanol–water mixtures: Ultrasonics, Brillouin scattering and molecular dynamics studies. Vib. Spectrosc. 2012, 60, 102–106. [Google Scholar] [CrossRef]
- Gereben, O.; Pusztai, L. Hydrogen bond connectivities in water–ethanol mixtures: On the influence of the H-bond definition. J. Mol. Liq. 2016, 220, 836–841. [Google Scholar] [CrossRef]
- Gereben, O. Ring structure analysis of ethanol–water mixtures. J. Mol. Liq. 2015, 211, 812–820. [Google Scholar] [CrossRef]
- Dolenko, T.A.; Burikov, S.A.; Dolenko, S.A.; Efitorov, A.O.; Plastinin, I.V.; Yuzhakov, V.I.; Patsaeva, S.V. Raman Spectroscopy of Water–Ethanol Solutions: The Estimation of Hydrogen Bonding Energy and the Appearance of Clathrate-like Structures in Solutions. J. Phys. Chem. A 2015, 119, 10806–10815. [Google Scholar] [CrossRef]
- Rak, D.; Sedlák, M. Comment on “Bulk Nanobubbles or Not Nanobubbles: That is the Question”. Langmuir 2020, 36, 15618–15621. [Google Scholar] [CrossRef] [PubMed]
- Alheshibri, M.; Craig, V.S. Generation of nanoparticles upon mixing ethanol and water; Nanobubbles or Not? J. Colloid Interface Sci. 2019, 542, 136–143. [Google Scholar] [CrossRef]
- Alheshibri, M.; Al Baroot, A.; Shui, L.; Zhang, M. Nanobubbles and nanoparticles. Curr. Opin. Colloid Interface Sci. 2021, 55, 101470. [Google Scholar] [CrossRef]
- Chan, C.U.; Ohl, C.-D. Total-Internal-Reflection-Fluorescence Microscopy for the Study of Nanobubble Dynamics. Phys. Rev. Lett. 2012, 109, 174501. [Google Scholar] [CrossRef]
- Häbich, A.; Ducker, W.; Dunstan, D.; Zhang, X. Do Stable Nanobubbles Exist in Mixtures of Organic Solvents and Water? J. Phys. Chem. B 2010, 114, 6962–6967. [Google Scholar] [CrossRef] [PubMed]
- Rak, D.; Ovadová, M.; Sedlak, M. (Non)Existence of Bulk Nanobubbles: The Role of Ultrasonic Cavitation and Organic Solutes in Water. J. Phys. Chem. Lett. 2019, 10, 4215–4221. [Google Scholar] [CrossRef]
- Jadhav, A.J.; Barigou, M. Bulk Nanobubbles or Not Nanobubbles: That is the Question. Langmuir 2020, 36, 1699–1708. [Google Scholar] [CrossRef] [Green Version]
- Yano, Y.F. Correlation between surface and bulk structures of alcohol–water mixtures. J. Colloid Interface Sci. 2005, 284, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Millare, J.C.; Basilia, B.A. Dispersion and electrokinetics of scattered objects in ethanol-water mixtures. Fluid Phase Equilibria 2019, 481, 44–54. [Google Scholar] [CrossRef]
- Nirmalkar, N.; Pacek, A.W.; Barigou, M. On the Existence and Stability of Bulk Nanobubbles. Langmuir 2018, 34, 10964–10973. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; An, H.; Ohl, C.-D. Stability of surface and bulk nanobubbles. Curr. Opin. Colloid Interface Sci. 2021, 53, 101428. [Google Scholar] [CrossRef]
- Chen, N.; Wen, Z.; Li, X.; Ye, Z.; Ren, D.; Xu, J.; Chen, Q.; Ma, S. Controllable preparation and formation mechanism of mono-dispersed bulk nanobubbles in dilute ethanol-water solutions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126372. [Google Scholar] [CrossRef]
- Qiu, J.; Zou, Z.; Wang, S.; Wang, X.; Wang, L.; Dong, Y.; Zhao, H.; Zhang, L.; Hu, J. Formation and Stability of Bulk Nanobubbles Generated by Ethanol-Water Exchange. ChemPhysChem 2017, 18, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, H.Z.; Zhang, D.X.; Li, G.; Hu, J. Nanobubbles at the interface of hopg and ethanol solution. Int. J. Nanosci. 2011, 4, 399–407. [Google Scholar] [CrossRef]
- Parker, J.L.; Claesson, P.M.; Attard, P. Bubbles, cavities, and the long-ranged attraction between hydrophobic surfaces. J. Phys. Chem. 1994, 98, 8468–8480. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, F.; Guo, X.; Qiao, Z.; Zhu, Y.; Jin, N.; Cui, Y.; Yang, W. Research progress on bulk nanobubbles. Particuology 2021, 60, 99–106. [Google Scholar] [CrossRef]
- Yu, G.-H.; Kuzyakov, Y. Fenton chemistry and reactive oxygen species in soil: Abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling. Earth-Sci. Rev. 2021, 214, 103525. [Google Scholar] [CrossRef]
- Takahashi, M.; Chiba, K.; Li, P. Free-Radical Generation from Collapsing Microbubbles in the Absence of a Dynamic Stimulus. J. Phys. Chem. B 2007, 111, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Takahashi, M.; Chiba, K. Degradation of phenol by the collapse of microbubbles. Chemosphere 2009, 75, 1371–1375. [Google Scholar] [CrossRef]
- Takahashi, M.; Ishikawa, H.; Asano, T.; Horibe, H. Effect of Microbubbles on Ozonized Water for Photoresist Removal. J. Phys. Chem. C 2012, 116, 12578–12583. [Google Scholar] [CrossRef]
- Takahashi, M.; Shirai, Y.; Sugawa, S. Free-Radical Generation from Bulk Nanobubbles in Aqueous Electrolyte Solutions: ESR Spin-Trap Observation of Microbubble-Treated Water. Langmuir 2021, 37, 5005–5011. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Oshita, S.; Kawabata, S.; Makino, Y.; Yoshimoto, T. Identification of ROS Produced by Nanobubbles and Their Positive and Negative Effects on Vegetable Seed Germination. Langmuir 2016, 32, 11295–11302. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, M.; Zhang, J.; Lyu, T.; Cooper, M.; Pan, G. Reducing arsenic toxicity using the interfacial oxygen nanobubble technology for sediment remediation. Water Res. 2021, 205, 117657. [Google Scholar] [CrossRef] [PubMed]
- Tada, K.; Maeda, M.; Nishiuchi, Y.; Nagahara, J.; Hata, T.; Zhuowei, Z.; Yoshida, Y.; Watanabe, S.; Ohmori, M. ESR Measurement of Hydroxyl Radicals in Micro-nanobubble Water. Chem. Lett. 2014, 43, 1907–1908. [Google Scholar] [CrossRef]
- Ahmed, A.K.A.; Shi, X.; Hua, L.; Manzueta, L.; Qing, W.; Marhaba, T.; Zhang, W. Influences of Air, Oxygen, Nitrogen, and Carbon Dioxide Nanobubbles on Seed Germination and Plant Growth. J. Agric. Food Chem. 2018, 66, 5117–5124. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Kurokawa, H.; Han, Z.; Zhou, Y.; Matsui, H.; Ponou, J.; Dodbiba, G.; He, C.; Wei, Y. Free radical degradation in aqueous solution by blowing hydrogen and carbon dioxide nanobubbles. Sci. Rep. 2021, 11, 3068. [Google Scholar] [CrossRef] [PubMed]
- Safamirzaei, M.; Modarress, H.; Mohsen-Nia, M. Modeling the hydrogen solubility in methanol, ethanol, 1-propanol and 1-butanol. Fluid Phase Equilibria 2010, 289, 32–39. [Google Scholar] [CrossRef]
- Dalmolin, I.; Skovroinski, E.; Biasi, A.; Corazza, M.L.; Dariva, C.; Oliveira, J.V. Solubility of carbon dioxide in binary and ternary mixtures with ethanol and water. Fluid Phase Equilibria 2006, 245, 193–200. [Google Scholar] [CrossRef]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, H. A review of bulk nanobubbles and their roles in flotation of fine particles. Powder Technol. 2021, 395, 618–633. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, Z.; He, C.; Feng, Q.; Wang, K.; Wang, Y.; Luo, N.; Dodbiba, G.; Wei, Y.; Otsuki, A.; et al. Long-Term Stability of Different Kinds of Gas Nanobubbles in Deionized and Salt Water. Materials 2021, 14, 1808. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Yamashita, S.; Midorikawa, M.; Saiki, S.; Muroya, Y.; Kamibayashi, M.; Yamashita, M.; Anzai, K.; Katsumura, Y. Spin-Trapping Reactions of a Novel Gauchetype Radical Trapper G-CYPMPO. Anal. Chem. 2011, 83, 9600–9604. [Google Scholar] [CrossRef]
- Kamibayashi, M.; Oowada, S.; Kameda, H.; Okada, T.; Inanami, O.; Ohta, S.; Ozawa, T.; Makino, K.; Kotake, Y. Synthesis and characterization of a practically better DEPMPO-type spin trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrrolineN-oxide (CYPMPO). Free Radic. Res. 2006, 40, 1166–1172. [Google Scholar] [CrossRef]
- Sueishi, Y.; Kamogawa, E.; Nakamura, H.; Ukai, M.; Kunieda, M.; Okada, T.; Shimmei, M.; Kotake, Y. Kinetic Evaluation of Spin Trapping Rate Constants of New CYPMPO-type Spin Traps for Superoxide and Other Free Radicals. Z. Phys. Chem. 2015, 229, 317–326. [Google Scholar] [CrossRef]
- Ma, X.; Li, M.; Pfeiffer, P.; Eisener, J.; Ohl, C.-D.; Sun, C. Ion adsorption stabilizes bulk nanobubbles. J. Colloid Interface Sci. 2021, 606, 1380–1394. [Google Scholar] [CrossRef]
- European Commission. Drinking Water Directive, Drinking Water Legislation—Environment—European Commission; European Commission: Brussels, Belgium, 2021; Available online: https://european-union.europa.eu/select-language?destination=/node/1 (accessed on 18 November 2021).
- Cerrón-Calle, G.A.; Magdaleno, A.L.; Graf, J.C.; Apul, O.G.; Garcia-Segura, S. Elucidating CO2 nanobubble interfacial reactivity and impacts on water chemistry. J. Colloid Interface Sci. 2021, 607, 720–728. [Google Scholar] [CrossRef]
- Zheng, X.; Jang, J. Hydraulic Properties of Porous Media Saturated with Nanoparticle-Stabilized Air-Water Foam. Sustainability 2016, 8, 1317. [Google Scholar] [CrossRef] [Green Version]
- Yoon, R.-H.; Aksoy, B. Hydrophobic Forces in Thin Water Films Stabilized by Dodecylammonium Chloride. J. Colloid Interface Sci. 1999, 211, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yoon, R.-H. Hydrophobic Forces in the Foam Films Stabilized by Sodium Dodecyl Sulfate: Effect of Electrolyte. Langmuir 2004, 20, 11457–11464. [Google Scholar] [CrossRef] [PubMed]
- Prieve, D.C. Intermolecular and surface forces with applications to colloidal and biological systems. J. Colloid Interface Sci. 1987, 116, 300. [Google Scholar] [CrossRef]
- Wiącek, A.; Chibowski, E. Application of an extended DLVO theory for the calculation of the interactions between emulsified oil droplets in alcohol solutions. Colloids Surf. B Biointerfaces 1999, 14, 19–26. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces with Application to Colloidal and Biological Systems; Academic Press: Cambridge, MA, USA, 1985; p. 17. [Google Scholar]
- Tuinier, R.; Rieger, J.; de Kruif, C. Depletion-induced phase separation in colloid–polymer mixtures. Adv. Colloid Interface Sci. 2003, 103, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Otsuki, A.; Bryant, G. Characterization of the interactions within fine particle mixtures in highly concentrated suspensions for advanced particle processing. Adv. Colloid Interface Sci. 2015, 226, 37–43. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Kurokawa, H.; Matsui, H.; He, C.; Wang, K.; Wei, Y.; Dodbiba, G.; Otsuki, A.; Fujita, T. Stability and Free Radical Production for CO2 and H2 in Air Nanobubbles in Ethanol Aqueous Solution. Nanomaterials 2022, 12, 237. https://doi.org/10.3390/nano12020237
Han Z, Kurokawa H, Matsui H, He C, Wang K, Wei Y, Dodbiba G, Otsuki A, Fujita T. Stability and Free Radical Production for CO2 and H2 in Air Nanobubbles in Ethanol Aqueous Solution. Nanomaterials. 2022; 12(2):237. https://doi.org/10.3390/nano12020237
Chicago/Turabian StyleHan, Zhenyao, Hiromi Kurokawa, Hirofumi Matsui, Chunlin He, Kaituo Wang, Yuezou Wei, Gjergj Dodbiba, Akira Otsuki, and Toyohisa Fujita. 2022. "Stability and Free Radical Production for CO2 and H2 in Air Nanobubbles in Ethanol Aqueous Solution" Nanomaterials 12, no. 2: 237. https://doi.org/10.3390/nano12020237
APA StyleHan, Z., Kurokawa, H., Matsui, H., He, C., Wang, K., Wei, Y., Dodbiba, G., Otsuki, A., & Fujita, T. (2022). Stability and Free Radical Production for CO2 and H2 in Air Nanobubbles in Ethanol Aqueous Solution. Nanomaterials, 12(2), 237. https://doi.org/10.3390/nano12020237