Βio-Based Epoxy/Amine Reinforced with Reduced Graphene Oxide (rGO) or GLYMO-rGO: Study of Curing Kinetics, Mechanical Properties, Lamination and Bonding Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GRMs
2.2.1. Preparation of GO
2.2.2. Preparation of rGO
2.2.3. Preparation of GLYMO-rGO
2.3. Characterisation of GRM Flakes
2.4. Preparation, Characterization and Testing of GRM/Polymer Nanocomposites
2.4.1. DSC Characterisation
2.4.2. Thermogravimetric Analysis
2.4.3. Tensile Testing
2.4.4. SEM Characterisation
2.4.5. Fabrication of CFRP Laminates, Lap Shear Joints and Lap Shear Testing
3. Results and Discussion
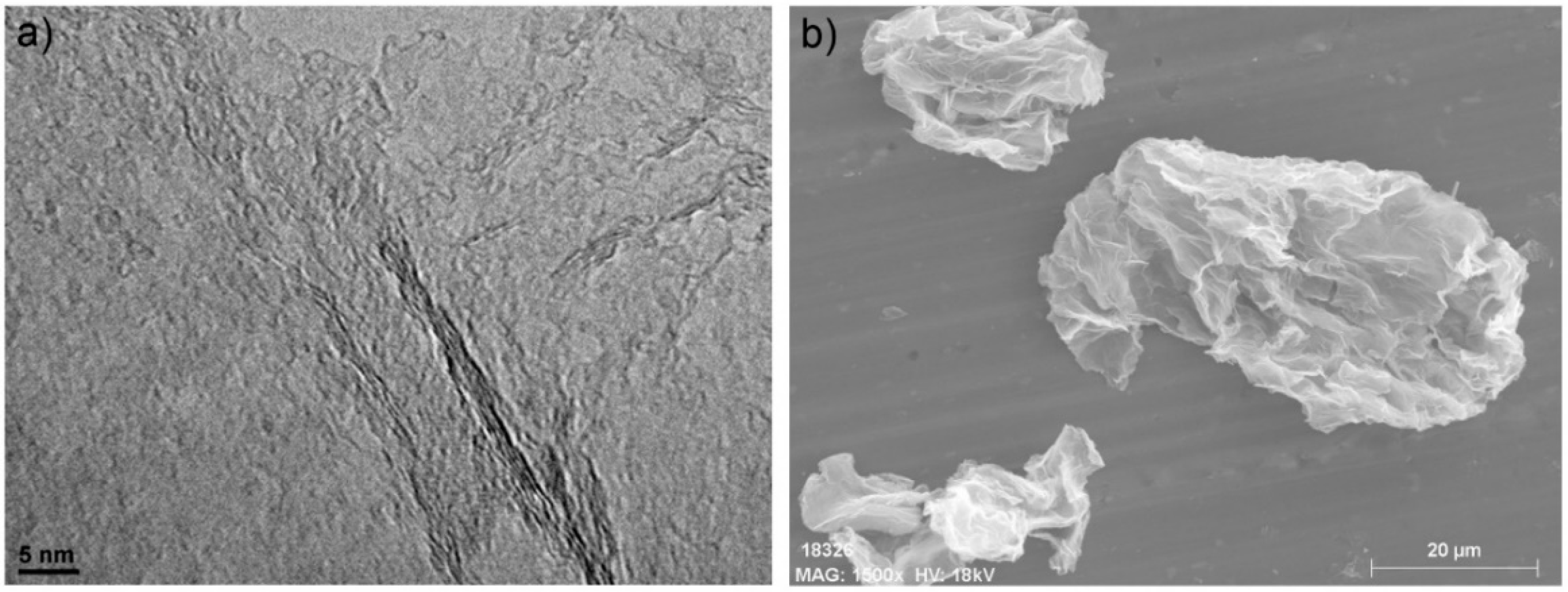
3.1. Characterisation of GRMs
3.2. Curing Study of GRM/Polymer Nanocomposites by DSC
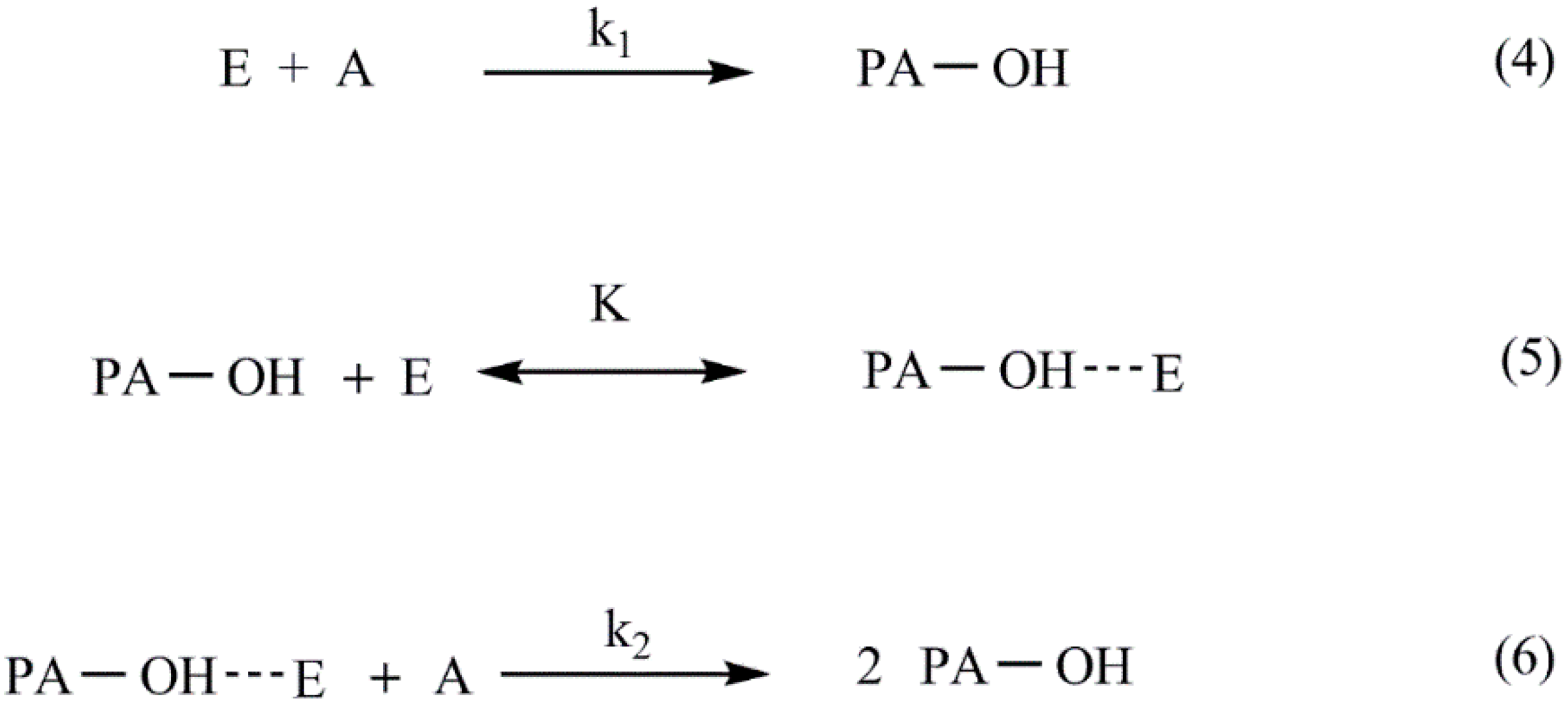
3.2.1. Curing Mechanism and Kinetics
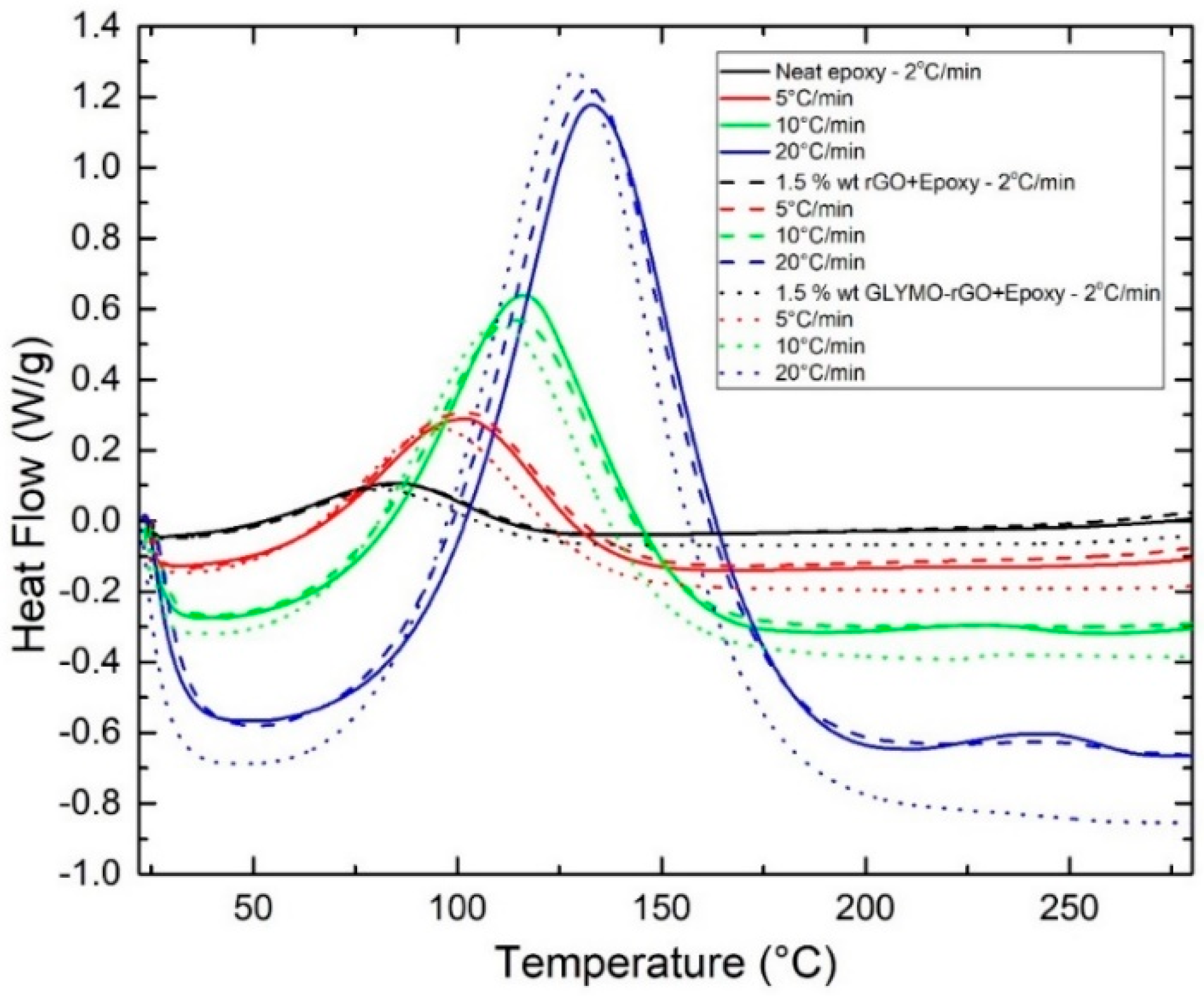
3.2.2. Non-Isothermal Curing Scanning Method
3.2.3. Isothermal Scanning Method
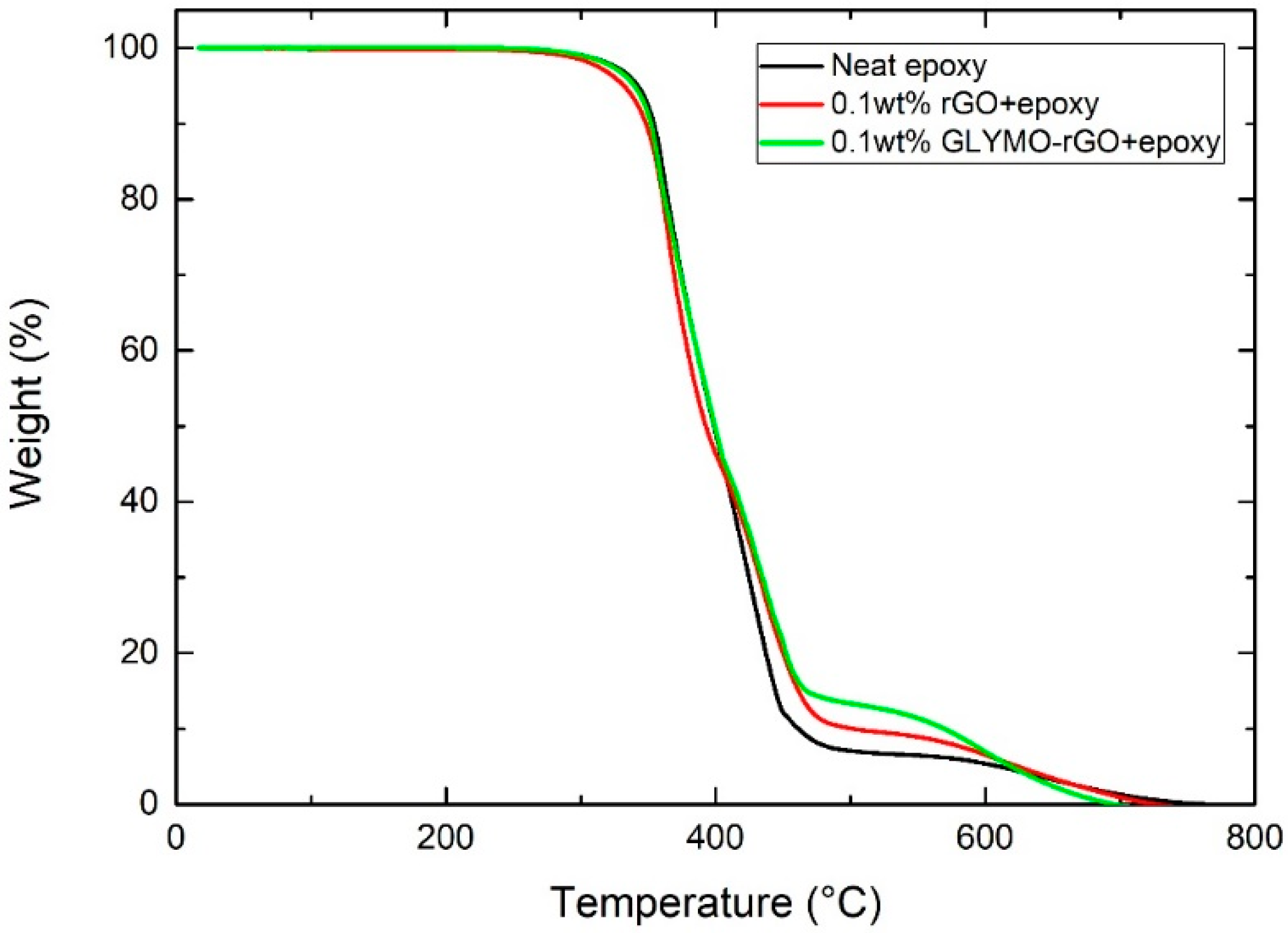
3.3. Thermogravimetric Analysis
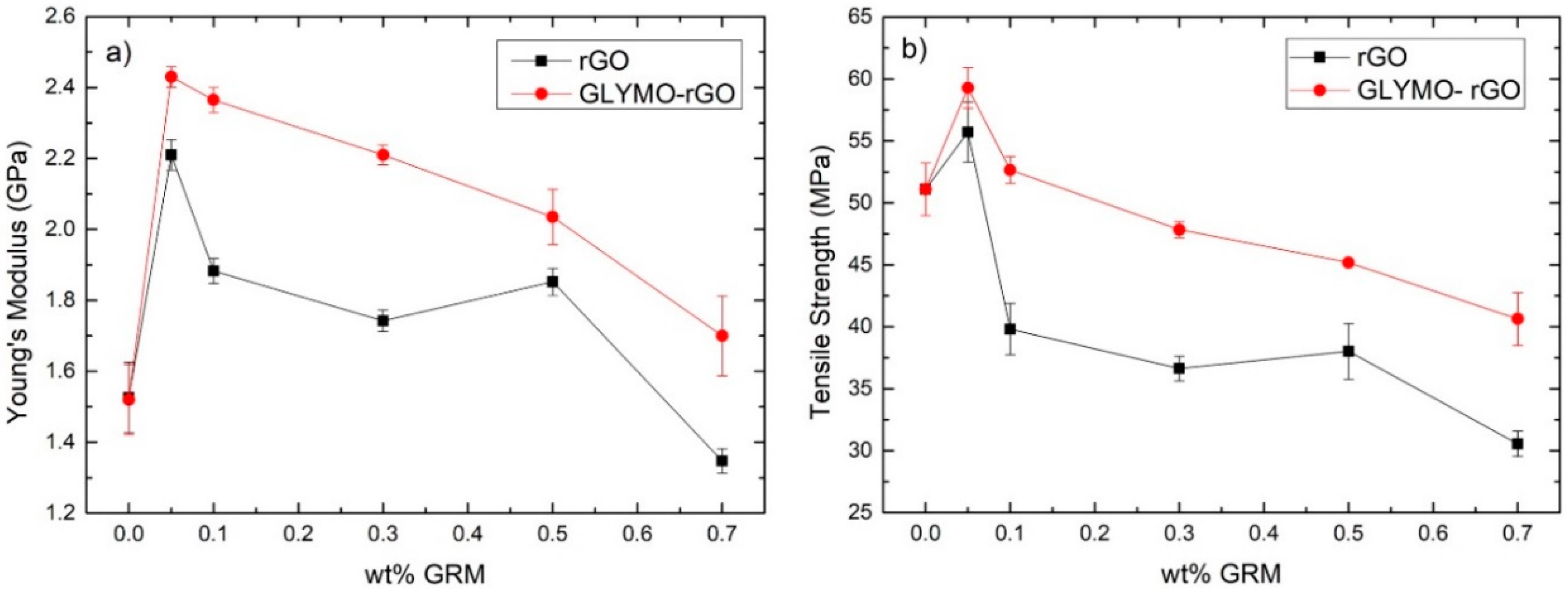
3.4. Mechanical Properties
3.4.1. Tensile Testing GRM/Polymer Nanocomposites
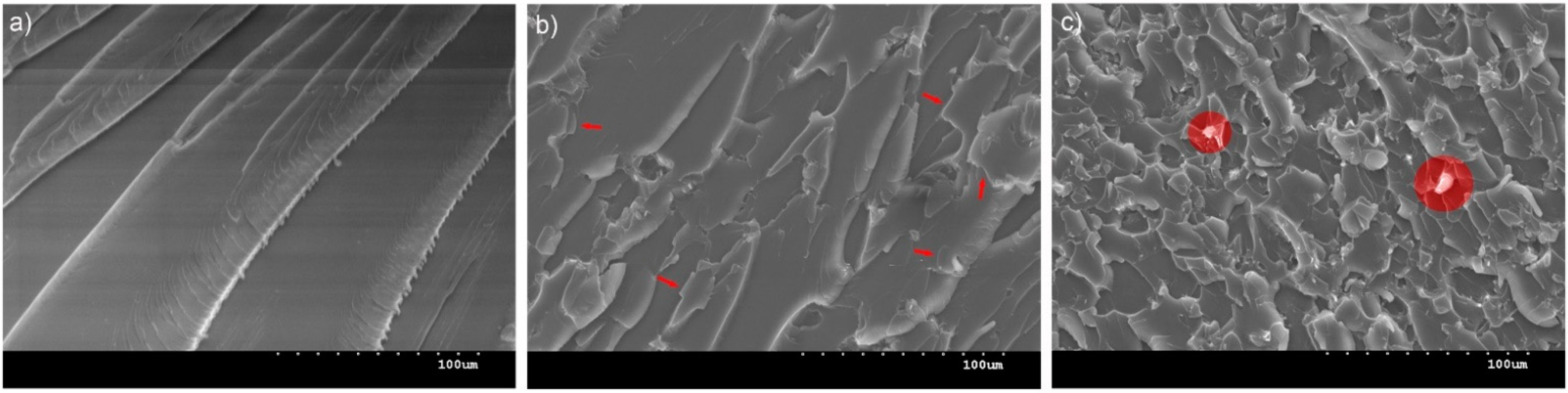
3.4.2. Microscopical Investigation
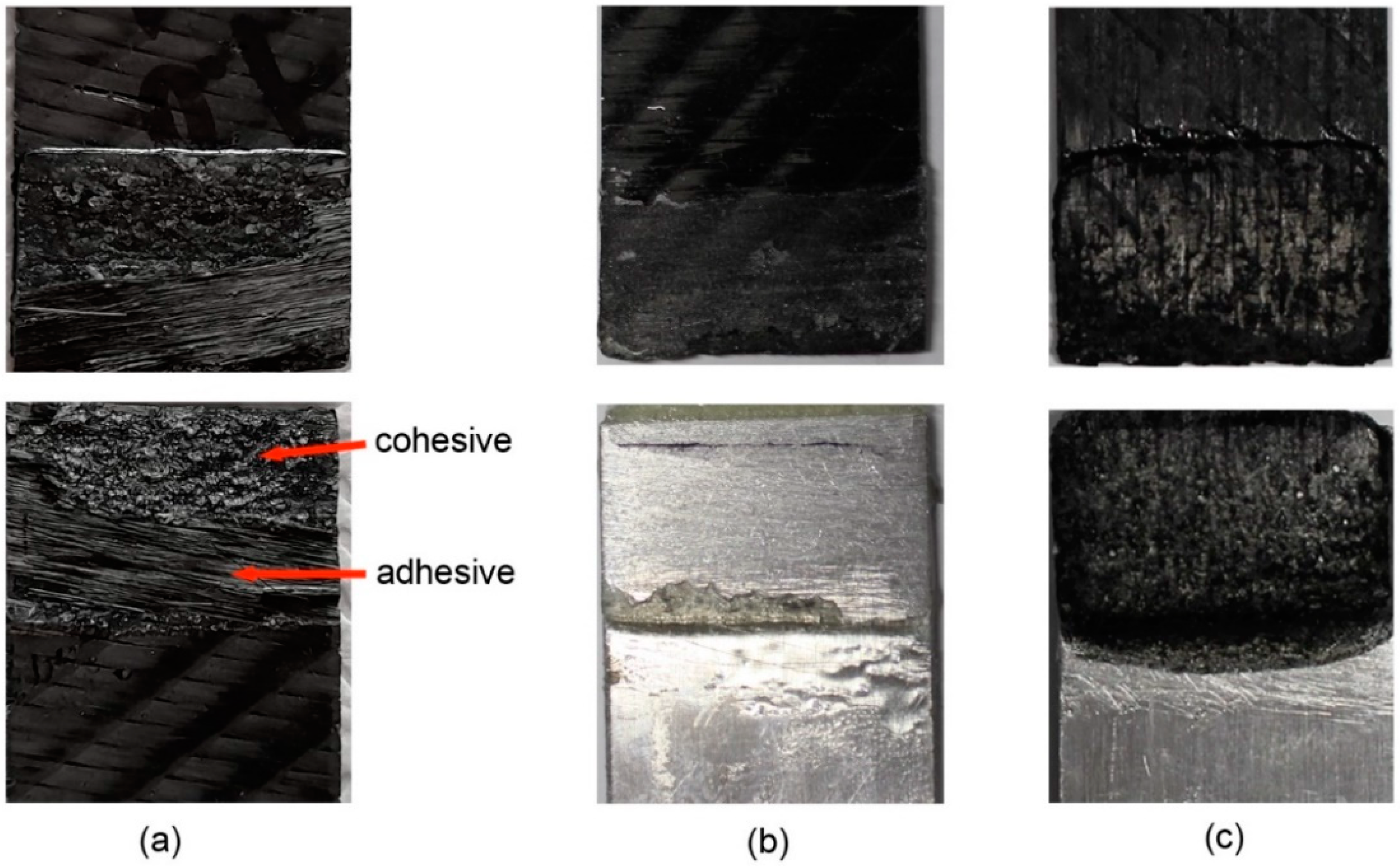
3.4.3. Lap Shear Joints with CFRP/CFRP or CFRP/Aluminium Laminate Adherents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taub, A.; De Moor, E.; Luo, A.; Matlock, D.K.; Speer, J.G.; Vaidya, U. Materials for Automotive Lightweighting. Annu. Rev. Mater. Res. 2019, 49, 327–359. [Google Scholar] [CrossRef]
- Koffler, C. Life cycle assessment of automotive lightweighting through polymers under US boundary conditions. Int. J. Life Cycle Assess. 2014, 19, 538–545. [Google Scholar] [CrossRef]
- Bandivadekar, A.E.A. On the Road in 2035—Reducing Transportation’s Petroleum Consumption and GHG Emissions; Massachusetts Institute of Technology: Cambridge, MA, USA, 2008. [Google Scholar]
- Gibson, G. Chapter 27—Epoxy Resins. In Gilbert MBT-BPM; Eighth, E., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 773–797. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization. John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent Development of Biobased Epoxy Resins: A Review. Polym. Plast. Technol. Eng. 2018, 57, 133–155. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Kumar Yadav, S.; Palmese, G.R.; Stanzione, J.F., III. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 45. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/Polymer Nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Vo, T.; Inam, F. Epoxy/graphene nanocomposites—Processing and properties: A review. RSC Adv. 2015, 5, 73510–73524. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; Paul, K.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, P.G.; Hodge, S.A.; Lombardi, L.; Tomarchio, F.; Decorde, N.; Milana, S.; Goykhman, I.; Su, Y.; Mesite, S.V.; Johnstone, D.N.; et al. Microfluidization of Graphite and Formulation of Graphene-Based Conductive Inks. ACS Nano 2017, 11, 2742–2755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Eigler, S.; Grimm, S.; Enzelberger-Heim, M.; Müller, P.; Hirsch, A. Graphene oxide: Efficiency of reducing agents. Chem. Commun. 2013, 49, 7391–7393. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Abdelkader, A.M.; Alonso, C.; Andrieux-Ledier, A.; Arenal, R.; Azpeitia, J.; Balakrishnan, N.; Banszerus, L.; Barjon, J.; Bartali, R. Production and processing of graphene and related materials. 2D Materials 2020, 7, 22001. [Google Scholar] [CrossRef]
- Maddalena, L.; Gomez, J.; Fina, A.; Carosio, F. Effects of Graphite Oxide Nanoparticle Size on the Functional Properties of Layer-by-Layer Coated Flexible Foams. Nanomaterials 2021, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Aradhana, R.; Mohanty, S.; Nayak, S.K. Comparison of mechanical, electrical and thermal properties in graphene oxide and reduced graphene oxide filled epoxy nanocomposite adhesives. Polymer 2018, 141, 109–123. [Google Scholar] [CrossRef]
- Olowojoba, G.B.; Kopsidas, S.; Eslava, S.; Gutierrez, E.S.; Kinloch, A.J.; Mattevi, C.; Victoria, G.R.; Taylor, A.C. A facile way to produce epoxy nanocomposites having excellent thermal conductivity with low contents of reduced graphene oxide. J. Mater. Sci. 2017, 52, 7323–7344. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Chen, G.; Cao, J.; Yang, W.; Xie, B.; Yang, M. Functionalized graphene oxide with ethylenediamine and 1,6-hexanediamine. New Carbon Mater. 2012, 27, 370–376. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- He, H.; Gao, C. General Approach to Individually Dispersed, Highly Soluble, and Conductive Graphene Nanosheets Functionalized by Nitrene Chemistry. Chem. Mater. 2010, 22, 5054–5064. [Google Scholar] [CrossRef]
- Lei, L.; Xia, Z.; Zhang, L.; Zhang, Y.; Zhong, L. Preparation and properties of amino-functional reduced graphene oxide/waterborne polyurethane hybrid emulsions. Prog. Org. Coat. 2016, 97, 19–27. [Google Scholar] [CrossRef]
- Liu, Z.; Duan, X.; Qian, G.; Zhou, X.; Yuan, W. Eco-friendly one-pot synthesis of highly dispersible functionalized graphene nanosheets with free amino groups. Nanotechnology 2013, 24, 45609. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.-J.; Gong, L.-X.; Tang, L.-C.; Wu, L.-B.; Jiang, J.-X. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide. Compos. Part A Appl. Sci. Manuf. 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Song, L.; Yu, B.; Hu, Y.; Yeoh, G.H. Preparation of UV-curable functionalized graphene/polyurethane acrylate nanocomposite with enhanced thermal and mechanical behaviors. React. Funct. Polym. 2013, 73, 854–858. [Google Scholar] [CrossRef]
- Bhowmik, K.; Pramanik, S.; Medda, S.K.; De, G. Covalently functionalized reduced graphene oxide by organically modified silica: A facile synthesis of electrically conducting black coatings on glass. J. Mater. Chem. 2012, 22, 24690–24697. [Google Scholar] [CrossRef]
- Marami, G.; Nazari, S.A.; Faghidian, S.A.; Vakili-Tahami, F.; Etemadi, S. Improving the mechanical behavior of the adhesively bonded joints using RGO additive. Int. J. Adhes. Adhes. 2016, 70, 277–286. [Google Scholar] [CrossRef]
- Xue, G.; Zhang, B.; Sun, M.; Zhang, X.; Li, J.; Wang, L.; Song, C. Morphology, thermal and mechanical properties of epoxy adhesives containing well-dispersed graphene oxide. Int. J. Adhes. Adhes. 2019, 88, 11–18. [Google Scholar] [CrossRef]
- Jojibabu, P.; Jagannatham, M.; Haridoss, P.; Janaki Ram, G.D.; Deshpande, A.P.; Bakshi, S.R. Effect of different carbon nano-fillers on rheological properties and lap shear strength of epoxy adhesive joints. Compos. Part A Appl. Sci. Manuf. 2016, 82, 53–64. [Google Scholar] [CrossRef]
- Moriche, R.; Prolongo, S.G.; Sánchez, M.; Jiménez-Suárez, A.; Chamizo, F.J.; Ureña, A. Thermal conductivity and lap shear strength of GNP/epoxy nanocomposites adhesives. Int. J. Adhes. Adhes. 2016, 68, 407–410. [Google Scholar] [CrossRef]
- Salom, C.; Prolongo, M.G.; Toribio, A.; Martínez-Martínez, A.J.; De Cárcer, I.A.; Prolongo, S.G. Mechanical properties and adhesive behavior of epoxy-graphene nanocomposites. Int. J. Adhes. Adhes. 2018, 84, 119–125. [Google Scholar] [CrossRef]
- Han, S.; Meng, Q.; Araby, S.; Liu, T.; Demiral, M. Mechanical and electrical properties of graphene and carbon nanotube reinforced epoxy adhesives: Experimental and numerical analysis. Compos. Part A Appl. Sci. Manuf. 2019, 120, 116–126. [Google Scholar] [CrossRef]
- Jee, S.M.; Ahn, C.-H.; Park, J.H.; Kim, T.A.; Park, M. Solvent-free encapsulation of curing agents for high performing one-component epoxy adhesives. Compos. Part B Eng. 2020, 202, 108438. [Google Scholar] [CrossRef]
- Nascimento, H.; Dos Reis, M.O.; Monteiro, E.C.; Ávila, A.F. An investigation on industrial adhesive nano-modified by graphene nanoplatelets under extreme environmental conditions. Int. J. Adhes. Adhes. 2021, 111, 102982. [Google Scholar] [CrossRef]
- Carosio, F.; Maddalena, L.; Gomez, J.; Saracco, G.; Fina, A. Graphene Oxide Exoskeleton to Produce Self-Extinguishing, Nonignitable, and Flame Resistant Flexible Foams: A Mechanically Tough Alternative to Inorganic Aerogels. Adv. Mater. Interfaces 2018, 5, 1801288. [Google Scholar] [CrossRef]
- Gómez, J.; Villaro, E.; Navas, A.; Recio, I. Testing the influence of the temperature, RH and filler type and content on the universal power law for new reduced graphene oxide TPU composites. Mater. Res. Express 2017, 4, 105020. [Google Scholar] [CrossRef] [Green Version]
- King, A.A.K.; Davies, B.R.; Noorbehesht, N.; Newman, P.; Church, T.L.; Harris, A.T.; Razal, J.M.; Minett, A.I. A New Raman Metric for the Characterisation of Graphene oxide and its Derivatives. Sci. Rep. 2016, 6, 19491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, F.; Creighton, M.; Chen, Y.; Hurt, R.; Külaots, I. Porous structures in stacked, crumpled and pillared graphene-based 3D materials. Carbon 2014, 66, 476–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prime, R.B. Thermosets. In Thermal Characterization of Polymeric Materials, 2nd ed.; Turi, E.A., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 1379–1766. [Google Scholar]
- Smith, I.T. The mechanism of the crosslinking of epoxide resins by amines. Polymer 1961, 2, 95–108. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Mechanism and kinetics of epoxy-amine cure studied by differential scanning calorimetry. Macromolecules 1996, 29, 1867–1873. [Google Scholar] [CrossRef]
- Flammersheim, H.J. Kinetics and mechanism of the epoxide–amine polyaddition1Presented at the Twelfth Ulm-Freiberg Conference, Freiberg, Germany, 19–21 March 19971. Thermochim. Acta 1998, 310, 153–159. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Prolongo, M.G.; Salom, C.; Arribas, C.; Sánchez-Cabezudo, M.; Masegosa, R.M.; Prolongo, S.G. Influence of graphene nanoplatelets on curing and mechanical properties of graphene/epoxy nanocomposites. J. Therm. Anal. Calorim. 2016, 125, 629–636. [Google Scholar] [CrossRef] [Green Version]
- Verchère, D.; Sautereau, H.; Pascault, J.P.; Riccardi, C.C.; Moschiar, S.M.; Williams, R.J.J. Buildup of epoxycycloaliphatic amine networks. Kinetics, vitrification, and gelation. Macromolecules 1990, 23, 725–731. [Google Scholar] [CrossRef]
- Rehman, S.; Akram, S.; Kanellopoulos, A.; Elmarakbi, A.; Karagiannidis, P.G. Development of new graphene/epoxy nanocomposites and study of cure kinetics, thermal and mechanical properties. Thermochim. Acta 2020, 694, 178785. [Google Scholar] [CrossRef]
- De Bakker, C.J.; St John, N.A.; George, G.A. Simultaneous differential scanning calorimetry and near-infra-red analysis of the curing of tetraglycidyldiaminodiphenylmethane with diaminodiphenylsulphone. Polymer 1993, 34, 716–725. [Google Scholar] [CrossRef]
- Ramos-Galicia, L.; Mendez, L.N.; Martínez-Hernández, A.L.; Espindola-Gonzalez, A.; Galindo-Esquivel, I.R.; Fuentes-Ramirez, R.; Velasco-Santos, C. Improved Performance of an Epoxy Matrix as a Result of Combining Graphene Oxide and Reduced Graphene. Int. J. Polym. Sci. 2013, 2013, 493147. [Google Scholar] [CrossRef]
- Peng, M.; Tang, X.; Zhou, Y. Fast phase transfer of graphene oxide from water to triglycidyl para-aminophenol for epoxy composites with superior nanosheet dispersion. Polymer 2016, 93, 1–8. [Google Scholar] [CrossRef]
- Olowojoba, G.B.; Eslava, S.; Gutierrez, E.S.; Kinloch, A.J.; Mattevi, C.; Rocha, V. In situ thermally reduced graphene oxide/epoxy composites: Thermal and mechanical properties. Appl. Nanosci. 2016, 6, 1015–1022. [Google Scholar] [CrossRef] [PubMed]







| GRM | D (cm−1) | Gapp (cm−1) | ID/IGapp | FWHMD | FWHMG | I2D/ID | IDD’/I2D | D’inf | D’inf − Gapp |
|---|---|---|---|---|---|---|---|---|---|
| rGO | 1363.9 | 1581.5 | 0.68 | 180.3 | 101.8 | 0.015 | 2.29 | 1588.7 | 3.89 |
| GLYMO-rGO | 1359.6 | 1588.0 | 0.74 | 146.6 | 92.8 | 0.010 | 2.44 | 1595.8 | 7.80 |
| GRM | C 1s (%) | O 1s (%) | Si 2p (%) | C/O |
|---|---|---|---|---|
| rGO | 88.7 | 11.0 | - | 8.1 |
| GLYMO-rGO | 92.5 | 7.2 | 0.45 | 12.8 |
| GRM (wt%) | φ (°C/min) | Tinit (°C) | Tfinal (°C) | Tp (°C) | ΔHult a (J/g) | ΔHult b (kJ/mol) | α (%) |
|---|---|---|---|---|---|---|---|
| 0 (neat epoxy) | 2 | 28.72 | 276.52 | 83.10 | 173.35 | 59.38 | 55.5 |
| 5 | 30.12 | 283.06 | 100.56 | 243.62 | 83.46 | 78.0 | |
| 10 | 33.98 | 281.96 | 114.53 | 275.57 | 94.40 | 88.2 | |
| 20 | 42.025 | 258.16 | 132.83 | 275.80 | 94.48 | 88.3 | |
| 1.5 rGO | 2 | 27.77 | 278.22 | 84.71 | 193.98 | 66.45 | 62.1 |
| 5 | 32.46 | 277.66 | 99.49 | 243.72 | 83.49 | 78.0 | |
| 10 | 36.74 | 284.59 | 112.78 | 268.47 | 91.97 | 86.0 | |
| 20 | 44.75 | 283.19 | 130.84 | 287.36 | 98.44 | 92.0 | |
| 1.5 GLYMO-rGO | 2 | 28.29 | 276.02 | 79.07 | 212.13 | 72.67 | 68.0 |
| 5 | 30.15 | 284.91 | 93.99 | 276.61 | 94.76 | 88.6 | |
| 10 | 33.98 | 283.92 | 109.12 | 290.26 | 99.44 | 92.9 | |
| 20 | 37.74 | 287.78 | 127.35 | 312.32 | 106.99 | 100 |
| GRM (wt%) | T (°C) | ΔHso (J/g) | ΔHiso (kJ/mol) | ΔHyn (J/g) | ΔHdyn (kJ/mol) | α1 (%) | α2 (%) | Tg (°C) |
|---|---|---|---|---|---|---|---|---|
| 0 (neat epoxy) | 50 | 209.16 | 71.65 | 35.22 | 12.06 | 85.6 | 66.7 | 53.1 |
| 70 | 214.37 | 73.44 | 0.120 | 0.041 | 99.8 | 68.6 | 73.3 | |
| 90 | 261.21 | 89.48 | 0 | 0 | 100 | 83.6 | 83.6 | |
| 1.5 rGO | 50 | 242.63 | 83.12 | 26.09 | 8.92 | 96.5 | 77.7 | 64.5 |
| 70 | 255.86 | 87.65 | 0.24 | 0.082 | 99.9 | 81.9 | 84.2 | |
| 90 | 268.94 | 92.13 | 0 | 0 | 100 | 86.1 | 94.2 | |
| 1.5 GLYMO-rGO | 50 | 243.30 | 83.35 | 24.35 | 8.33 | 96.7 | 77.9 | 55.9 |
| 70 | 260.96 | 89.40 | 0.318 | 0.108 | 99.9 | 83.6 | 83.4 | |
| 90 | 291.66 | 99.92 | 0 | 0 | 100 | 93.4 | 97.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, S.; Gomez, J.; Villaro, E.; Cossey, D.; Karagiannidis, P.G. Βio-Based Epoxy/Amine Reinforced with Reduced Graphene Oxide (rGO) or GLYMO-rGO: Study of Curing Kinetics, Mechanical Properties, Lamination and Bonding Performance. Nanomaterials 2022, 12, 222. https://doi.org/10.3390/nano12020222
Rehman S, Gomez J, Villaro E, Cossey D, Karagiannidis PG. Βio-Based Epoxy/Amine Reinforced with Reduced Graphene Oxide (rGO) or GLYMO-rGO: Study of Curing Kinetics, Mechanical Properties, Lamination and Bonding Performance. Nanomaterials. 2022; 12(2):222. https://doi.org/10.3390/nano12020222
Chicago/Turabian StyleRehman, Sheikh, Julio Gomez, Elvira Villaro, Dwane Cossey, and Panagiotis G. Karagiannidis. 2022. "Βio-Based Epoxy/Amine Reinforced with Reduced Graphene Oxide (rGO) or GLYMO-rGO: Study of Curing Kinetics, Mechanical Properties, Lamination and Bonding Performance" Nanomaterials 12, no. 2: 222. https://doi.org/10.3390/nano12020222
APA StyleRehman, S., Gomez, J., Villaro, E., Cossey, D., & Karagiannidis, P. G. (2022). Βio-Based Epoxy/Amine Reinforced with Reduced Graphene Oxide (rGO) or GLYMO-rGO: Study of Curing Kinetics, Mechanical Properties, Lamination and Bonding Performance. Nanomaterials, 12(2), 222. https://doi.org/10.3390/nano12020222







