Challenges and Strategies in Developing an Enzymatic Wearable Sweat Glucose Biosensor as a Practical Point-Of-Care Monitoring Tool for Type II Diabetes
Abstract
:1. Introduction
1.1. Wearable Sweat Biosensors Based on Colorimetric
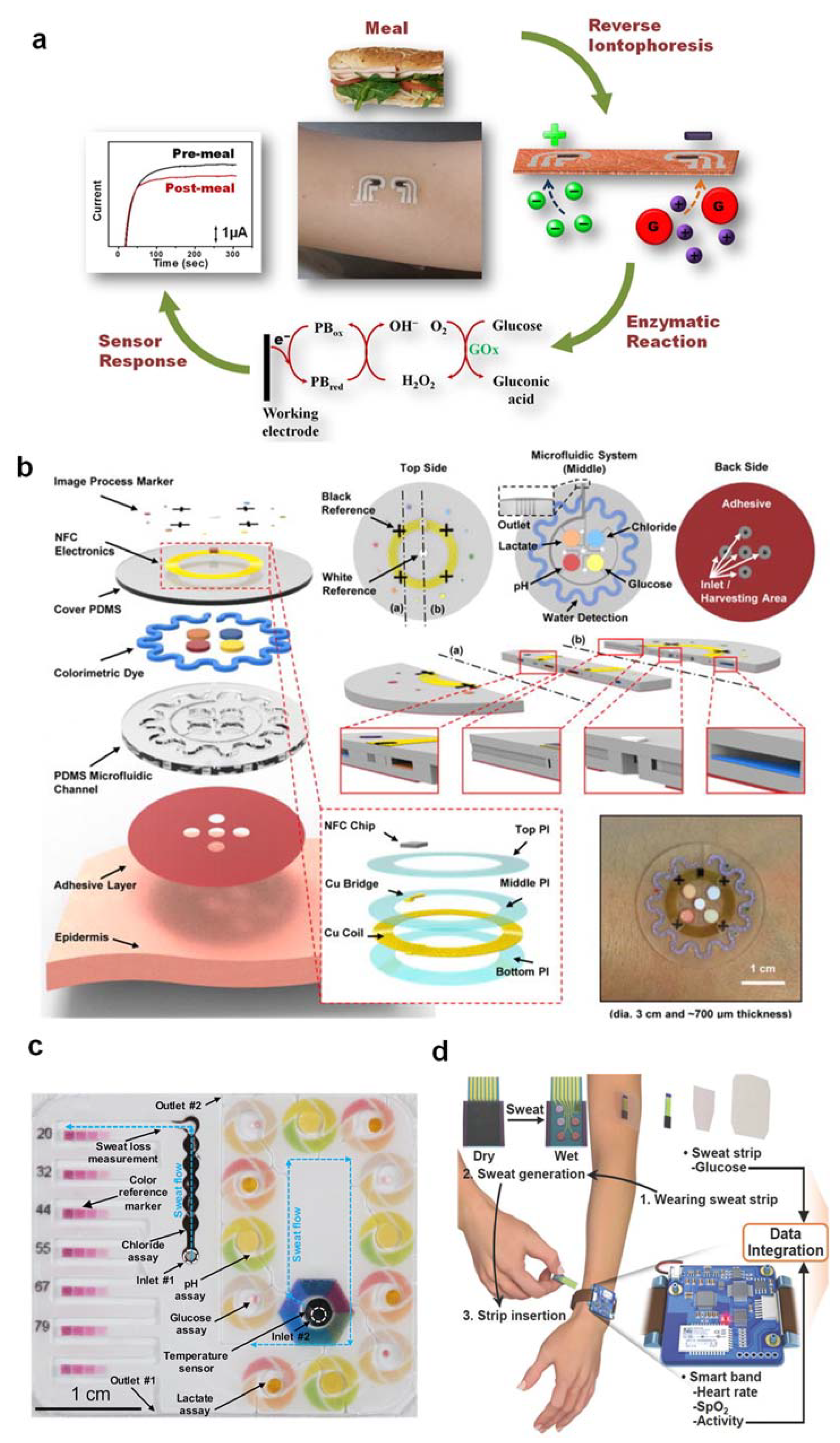
1.2. Wearable Sweat Biosensors Based on Electrochemical
2. Wearable Biosensors for Non-Invasive Control of Sweat Glucose
2.1. Enzymatic Biosensors
2.2. Non-Enzymatic Sensors
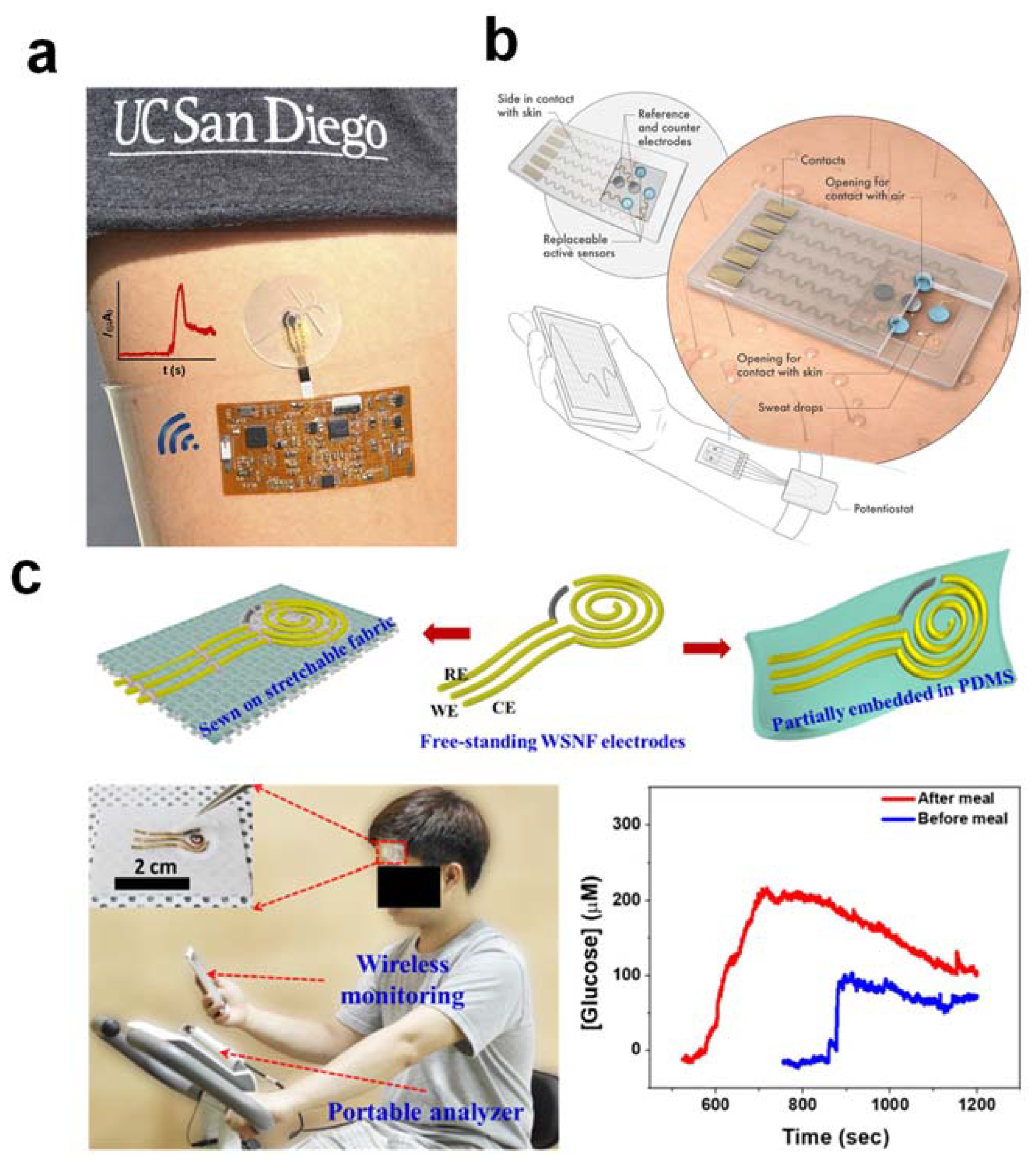
3. Ten Specific Requirements for the Development of a Desired Wearable Sweat-Based Glucose Biosensor for Successful Type II Diabetes Management: Challenges and Solutions
3.1. A Fully Integrated and Autonomous Platform
3.2. A Wearable Material That Is Soft, Flexible, and Stretchable
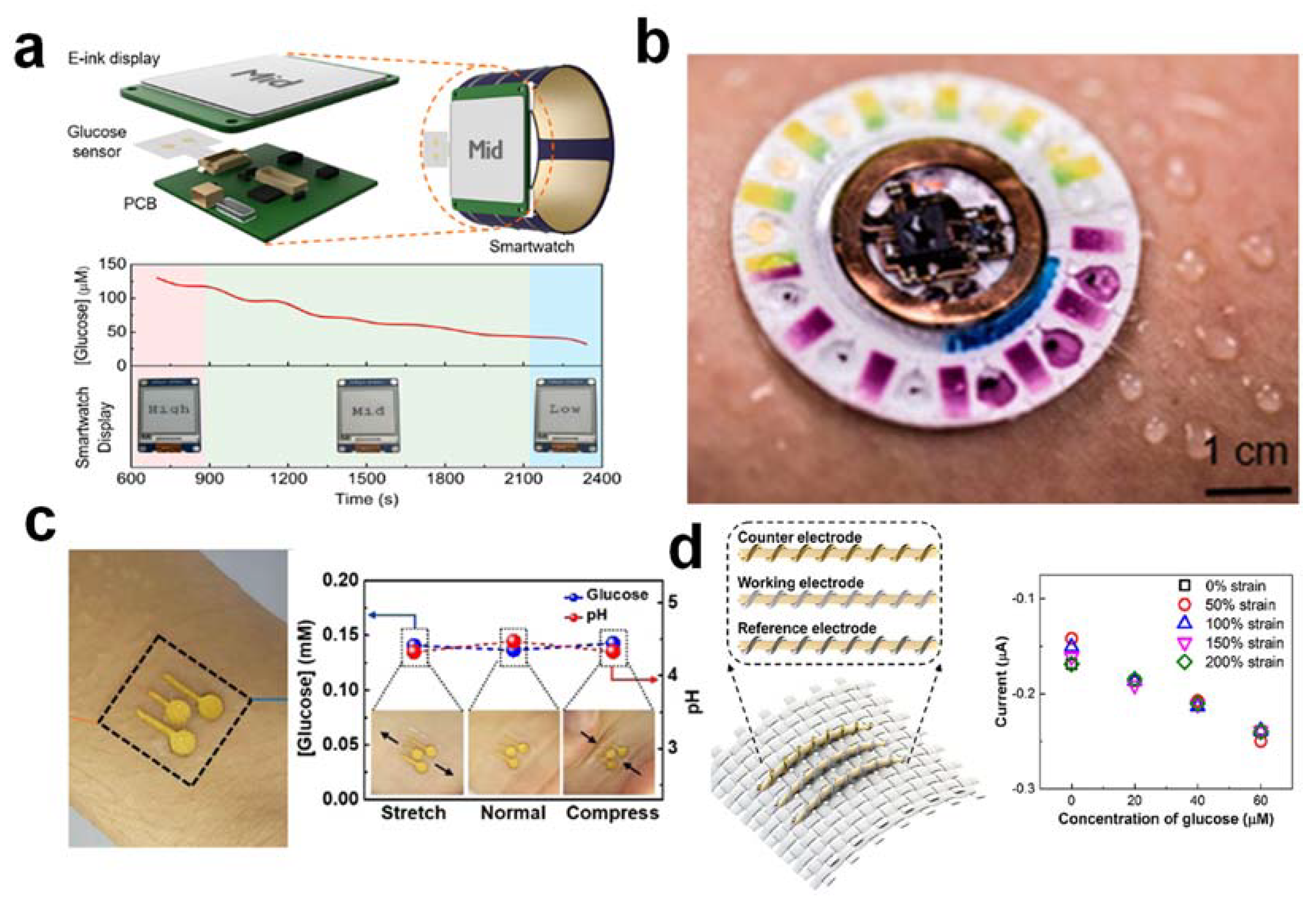
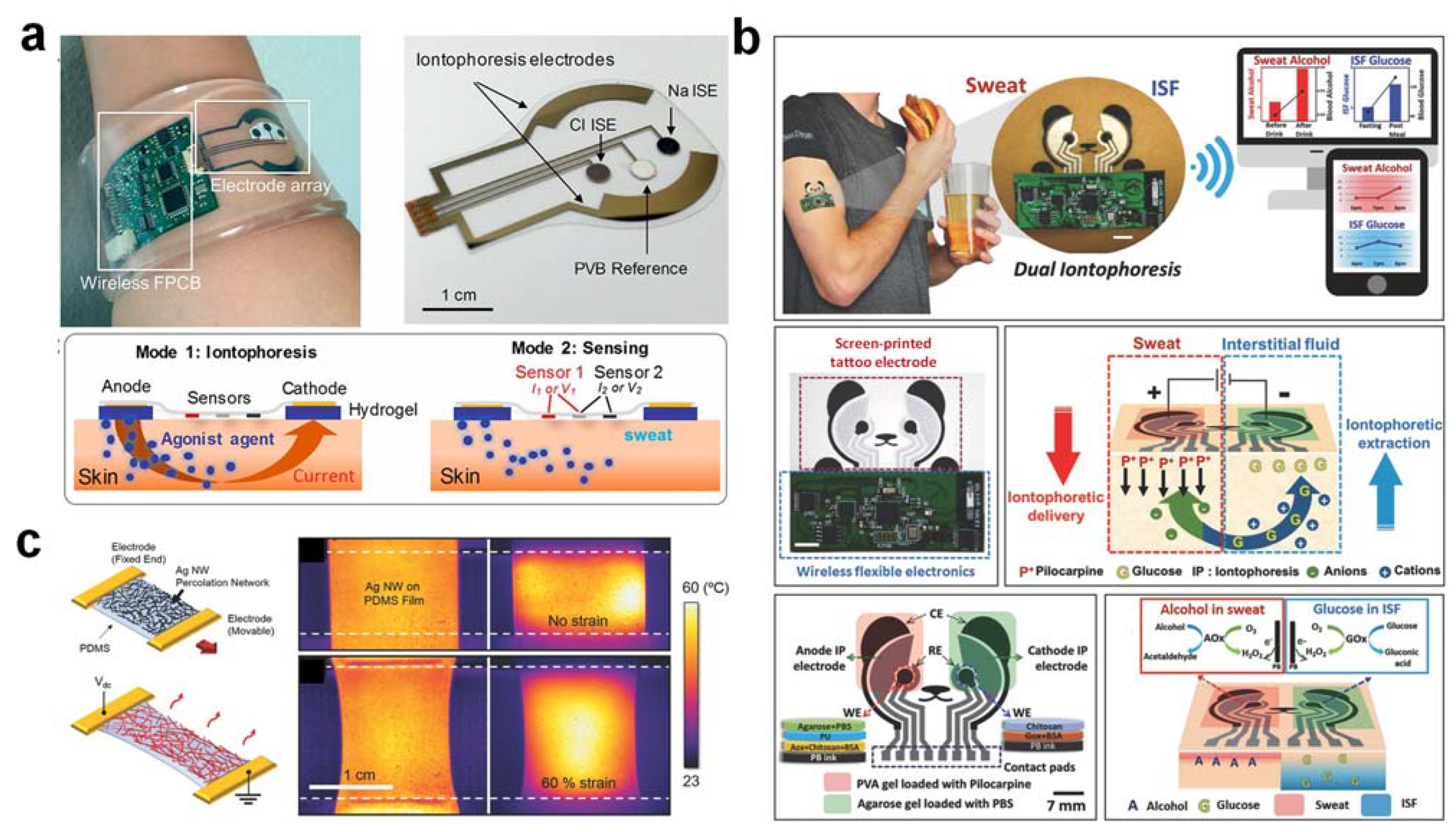
3.3. Real-Time Sweat Stimulation and Extraction
3.3.1. Physical Exercise
3.3.2. Iontophoresis
3.3.3. Wearable Stretchable Heater for Non-Invasive Continuous Sweat Extraction
3.3.4. The Collection and Detection of Sweat Components at Rest via Hydrogel
3.4. Wearable Self-Powered System
3.4.1. A Wearable Solar Cell and Biofuel Cell
3.4.2. A Wireless Power Transmitter
3.4.3. A Small Flexible/Wearable Aqueous Rechargeable Battery
3.5. Flexible, Microfluidic Sweat-Sampling System
3.6. A Porous Interface to Enhance Sweat Glucose Biosensing Sensitivity

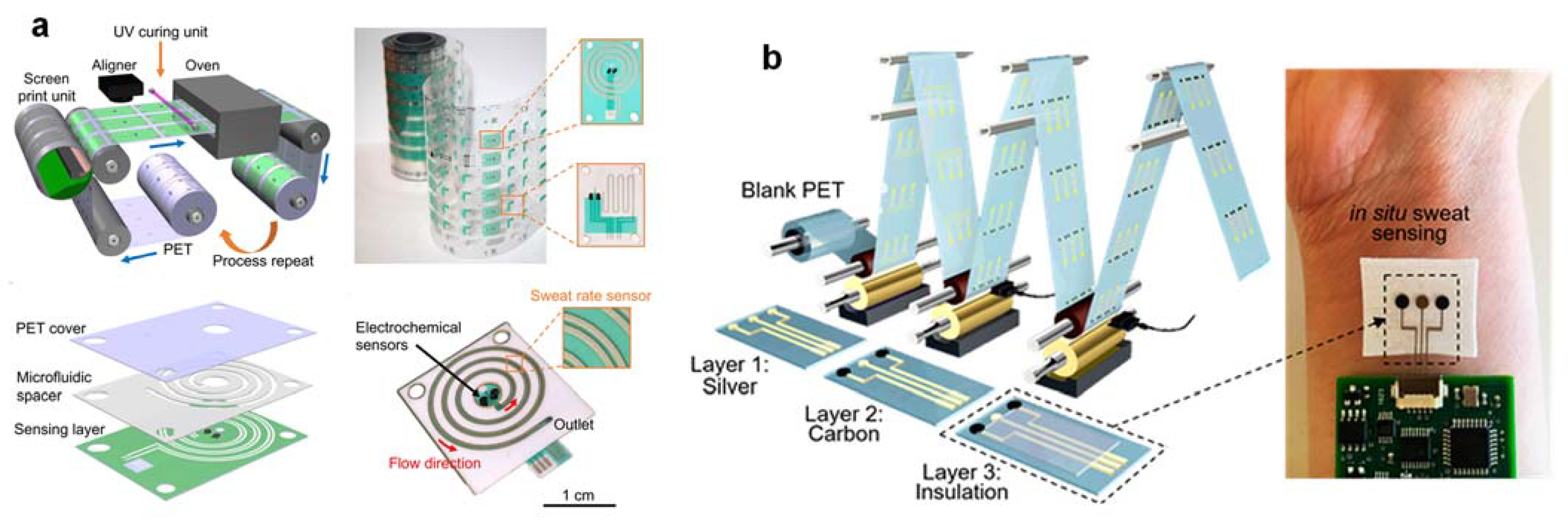
3.7. High-Throughput Roll-To-Roll (R2R) Device Fabrication Technique for Large Population Studies
3.8. Correlation with Blood Glucose
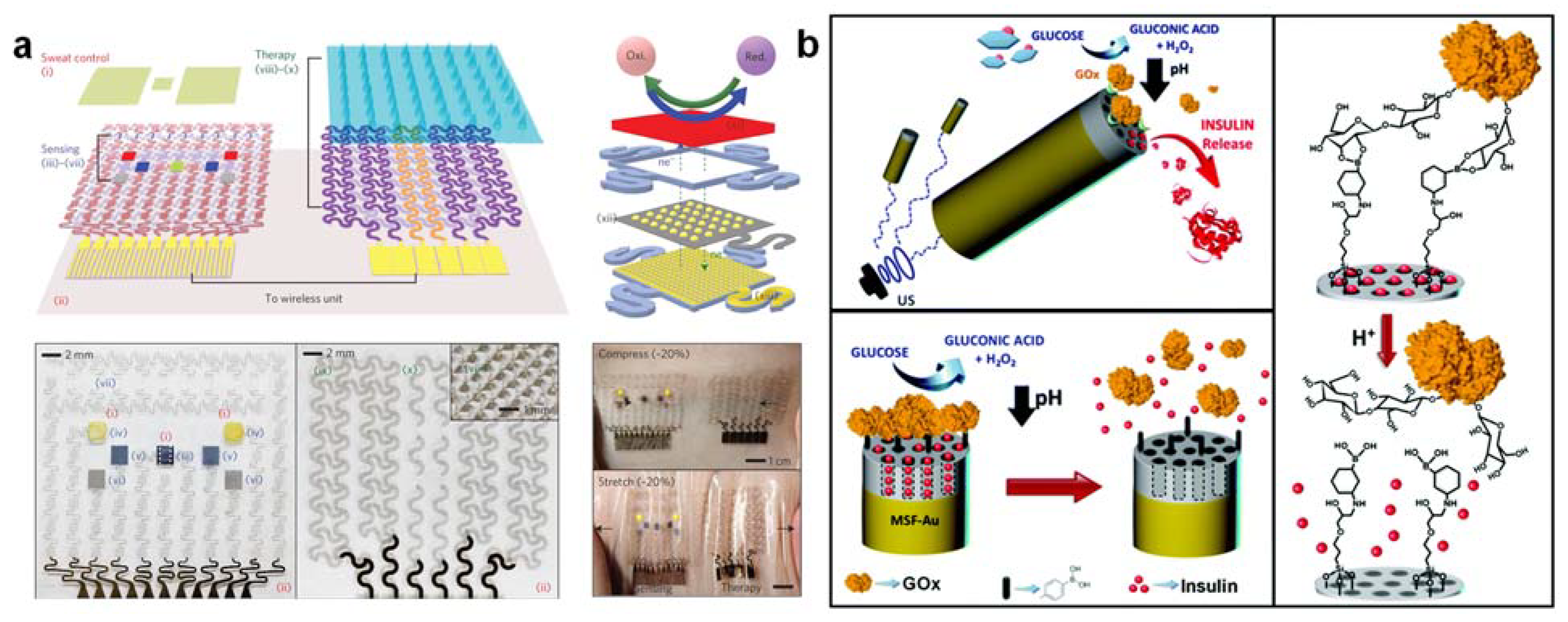
3.9. Glucose-Triggered Insulin and Therapeutic Drug Delivery Close-Loop System for Precision Theranostics
3.10. Security and Privacy Issues for Personalized Medicine in Wireless Wearable Biosensor Networks
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Diabetes. Available online: http://www.who.int/mediacentre/factsheets/fs312/en/ (accessed on 10 November 2021).
- Olarte, O.; Chilo, J.; Pelegri-Sebastia, J.; Barbé, K.; Moer, W.V. Glucose detection in human sweat using an electronic nose. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013. [Google Scholar]
- Bandodkar, A.J.; Jia, W.Z.; Yardimci, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.J.; Lee, H.; Kim, J.; Lee, M.; Choi, H.J.; Hyeon, T.; Kim, D.H. Multifunctional wearable system that integrates sweat-based sensing and vital-sign monitoring to estimate pre-/post-exercise glucose levels. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Xu, T.; Gu, Z.; Gao, W.; Xu, L.-P.; Pan, T.; Zhang, X. Flexible and superwettable bands as a platform toward sweat sampling and sensing. Anal. Chem. 2019, 91, 4296–4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, G.; He, J.; Chen, X.; Qiao, Y.; Wang, F.; Xia, Q.; Yu, L.; Lu, Z. A wearable, cotton thread/paper-based microfluidic device coupled with smartphone for sweat glucose sensing. Cellulose 2019, 26, 4553–4562. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeang, W.J.; Ghaffari, R.; Rogers, J.A. Wearable sensors for biochemical sweat analysis. Annu. Rev. Anal. Chem. 2019, 12, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Bandodkar, A.J.; Reeder, J.T.; Ray, T.R.; Turnquist, A.; Kim, S.B.; Nyberg, N.; Hourlier-Fargette, A.; Model, J.B.; Aranyosi, A.J.; et al. Soft, skin-integrated multifunctional microfluidic systems for accurate colorimetric analysis of sweat biomarkers and temperature. ACS Sens. 2019, 4, 379–388. [Google Scholar] [CrossRef]
- Zhu, X.F.; Ju, Y.H.; Chen, J.; Liu, D.Y.; Liu, H. Nonenzymatic wearable sensor for electrochemical analysis of perspiration glucose. ACS Sens. 2018, 3, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, T.; Zhang, J.J.; Lu, Y. Transforming the blood glucose meter into a general healthcare meter for in vitro diagnostics in mobile health. Biotechnol. Adv. 2016, 34, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Toghill, K.E.; Compton, R.G. Electrochemical non-enzymatic glucose sensors: A perspective and an evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301. [Google Scholar]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized enzymes in biosensor applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.D.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.F.; et al. Epidermal microfluidic electrochemical detection system: Enhanced sweat sampling and metabolite detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef]
- Lei, Y.J.; Zhao, E.N.; Zhang, Y.Z.; Jiang, Q.; He, J.H.; Baeumner, A.; Wolfbeis, O.S.; Wang, Z.L.; Salama, K.N.; Aishareef, H.N. A MXene-based wearable biosensor system for high-performance in vitro perspiration analysis. Small 2019, 15, 1901190. [Google Scholar] [CrossRef] [Green Version]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Toi, P.T.; Trung, T.Q.; Dang, T.M.L.; Bae, C.W.; Lee, N.E. Highly electrocatalytic, durable, and stretchable nanohybrid fiber for on-body sweat glucose detection. ACS Appl. Mater. Interfaces 2019, 11, 10707–10717. [Google Scholar] [CrossRef]
- Olejnik, A.; Siuzdak, K.; Karczewski, J.; Grochowska, K. A flexible nafion coated enzyme-free glucose sensor based on Au-dimpled Ti structures. Electroanalysis 2019, 32, 323–332. [Google Scholar] [CrossRef]
- Park, S.; Park, S.; Jeong, R.A.; Boo, H.; Park, J.; Kim, H.C.; Chung, T.D. Nonenzymatic continuous glucose monitoring in human whole blood using electrified nanoporous Pt. Biosens. Bioelectron. 2012, 31, 284–291. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.V.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.Q.; Lin, Y.J.; Wu, J.B.; Nyein, H.Y.Y.; Bariya, M.; Tai, L.C.; Chao, M.H.; Ji, W.B.; Zhang, G.; Fan, Z.Y.; et al. A fully integrated and self-powered smartwatch for continuous sweat glucose monitoring. ACS Sens. 2019, 4, 1925–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.H. Enzyme-based glucose sensor: From invasive to wearable device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi, Z.; Johansen, M.D.; Christiansen, J.S.; Hejlesen, O. Comparison between one-point calibration and two-point calibration approaches in a continuous glucose monitoring algorithm. J. Diabetes Sci. Technol. 2014, 8, 709–719. [Google Scholar] [CrossRef] [Green Version]
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.; Sekine, Y.; Reeder, J.T.; Jeang, W.J.; Aranyosi, A.J.; Lee, S.P.; Model, J.B.; et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 2019, 5, eaav3294. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.Y.; Hong, S.Y.; Jeong, Y.R.; Yun, J.; Park, H.; Jin, S.W.; Lee, G.; Oh, J.H.; Lee, H.; Lee, S.S.; et al. Skin-attachable, stretchable electrochemical sweat sensor for glucose and pH detection. ACS Appl. Mater. Interfaces 2018, 10, 13729–13740. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Hong, S.; Cho, H.M.; Lee, J.; Suh, Y.D.; Ham, J.; Ko, S.H. Highly sensitive and stretchable multidimensional strain sensor with prestrained anisotropic metal nanowire percolation networks. Nano Lett. 2015, 15, 5240–5247. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhai, Q.; Dong, D.; An, T.; Gong, S.; Shi, Q.; Cheng, W. Highly stretchable and strain-insensitive fiber-based wearable electrochemical biosensor to monitor glucose in the sweat. Anal. Chem. 2019, 91, 6569–6576. [Google Scholar] [CrossRef] [PubMed]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Kim, J.; Sempionatto, J.R.; Imani, S.; Hartel, M.C.; Barfidokht, A.; Tang, G.D.; Campbell, A.S.; Mercier, P.P.; Wang, J. Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv. Sci. 2018, 5, 1800880. [Google Scholar] [CrossRef] [Green Version]
- Won, P.; Park, J.J.; Lee, T.; Ha, I.; Han, S.; Choi, M.; Lee, J.; Hong, S.; Cho, K.J.; Ko, S.H. Stretchable and Transparent Kirigami Conductor of Nanowire Percolation Network for Electronic Skin Applications. Nano Lett. 2019, 19, 6087–6096. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Lee, H.; Lee, J.; Kwon, J.; Han, S.; Suh, Y.D.; Cho, H.; Shin, J.; Yeo, J.; Ko, S.H. Highly stretchable and transparent metal nanowire heater for wearable electronics applications. Adv. Mater. 2015, 27, 4744–4751. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.J.; Kimpinski, K. Test-retest reliability of quantitative sudomotor axon reflex testing. J. Clin. Neurophysiol. 2013, 30, 308–312. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Bariya, M.; Tran, B.; Ahn, C.H.; Brown, B.J.; Ji, W.; Davis, N.; Javey, A. A wearable patch for continuous analysis of thermoregulatory sweat at rest. Nat. Commun. 2021, 12, 1823. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Moon, J.-M.; Wang, J. Touch-Based Fingertip Blood-Free Reliable Glucose Monitoring: Personalized Data Processing for Predicting Blood Glucose Concentrations. ACS Sens. 2021, 6, 1875–1883. [Google Scholar] [CrossRef]
- Nagamine, K.; Mano, T.; Nomura, A.; Ichimura, Y.; Izawa, R.; Furusawa, H.; Matsui, H.; Kumaki, D.; Tokito, S. Noninvasive Sweat-Lactate Biosensor Emplsoying a Hydrogel-Based Touch Pad. Sci. Rep. 2019, 9, 10102. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Wang, B.; Zhao, Y.; Shih, R.; Cheng, X.; Yu, W.; Hojaiji, H.; Lin, H.; Hoffman, C.; Ly, D.; et al. Natural Perspiration Sampling and in Situ Electrochemical Analysis with Hydrogel Micropatches for User-Identifiable and Wireless Chemo/Biosensing. ACS Sens. 2020, 5, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, R.C.; Mahbub, I. Wearable self-powered biosensors. Curr. Opin. Electrochem. 2019, 19, 55–62. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Sempionatto, J.R.; Barfidokht, A.; Mishra, R.K.; Campbell, A.S.; Hubble, L.J.; Wang, J. Wearable bioelectronics: Enzyme-based body-worn electronic devices. Acc. Chem. Res. 2018, 51, 2820–2828. [Google Scholar] [CrossRef]
- Grattieri, M.; Minteer, S.D. Self-powered biosensors. ACS Sens. 2018, 3, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Hackwoth, S.A.; Liu, X.; Li, C.; Sun, M. Wireless power delivery for wearable sensors and implants in body sensor networks. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 692–695. [Google Scholar]
- Song, W.J.; Lee, S.; Song, G.; Park, S. Stretchable aqueous batteries: Progress and prospects. ACS Energy Lett. 2019, 4, 177–186. [Google Scholar] [CrossRef]
- Li, S.; Ma, Z.; Cao, Z.L.; Pan, L.J.; Shi, Y. Advanced wearable microfluidic sensors for healthcare monitoring. Small 2019, 16, 1903822. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.L.; Ouyang, Y.W.; Duarte, G.R.M.; Carrilho, E.; Krauss, S.T.; Landers, J.P. Inexpensive, rapid prototyping of microfluidic devices using overhead transparencies and a laser print, cut and laminate fabrication method. Nat. Protoc. 2015, 10, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, Y.; Su, L.; Zhao, D.; Zhao, L.; Zhang, X. Microfluidic Chip-Based Wearable Colorimetric Sensor for Simple and Facile Detection of Sweat Glucose. Anal. Chem. 2019, 91, 14803–14807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.J.; Bariya, M.; Nyein, H.Y.Y.; Kivimaki, L.; Uusitalo, S.; Jonsson, E.; Ji, W.B.; Yuan, Z.; Happonen, T.; Liedert, C.; et al. Porous enzymatic membrane for nanotextured glucose sweat sensors with high stability toward reliable noninvasive health monitoring. Adv. Funct. Mater. 2019, 29, 1902521. [Google Scholar] [CrossRef]
- Bae, C.W.; Toi, P.T.; Kim, B.Y.; Lee, W.I.; Lee, H.B.; Hanif, A.; Lee, E.H.; Lee, N.E. Fully stretchable capillary microfluidics-integrated nanoporous gold electrochemical sensor for wearable continuous glucose monitoring. ACS Appl. Mater. Interfaces 2019, 11, 14567–14575. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Bariya, M.; Kivimaki, L.; Uusitalo, S.; Liaw, T.S.; Jansson, E.; Ahn, C.H.; Hangasky, J.A.; Zhao, J.Q.; Lin, Y.J.; et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 2019, 5, eaaw9906. [Google Scholar] [CrossRef] [Green Version]
- Bariya, M.; Shahpar, Z.; Park, H.; Sun, J.F.; Jung, Y.; Gao, W.; Nyein, H.Y.Y.; Liaw, T.S.; Tai, L.C.; Ngo, Q.P.; et al. Roll-to-roll gravure printed electrochemical sensors for wearable and medical devices. ACS Nano 2018, 12, 6978–6987. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Avila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Clarke, W.L.; Gonder-Frederick, L.; Cox, D.; Kovatchev, B. A critical appraisal of the continuous glucose-error grid analysis: Response to Wentholt et al. Diabetes Care 2007, 30, 449–450, author reply 450–441. [Google Scholar] [CrossRef] [Green Version]
- Contreras, I.; Vehi, J. Artificial intelligence for diabetes management and decision support: Literature review. J. Med. Internet Res. 2018, 20, e10775. [Google Scholar] [CrossRef] [PubMed]
- Diez, P.; de Avila, B.E.F.; Ramirez-Herrera, D.E.; Villalonga, R.; Wang, J. Biomedical nanomotors: Efficient glucose-mediated insulin release. Nanoscale 2017, 9, 14307–14311. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.S.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Mo, R.; Di, J.; Subramanian, V.; Gu, X.; Buse, J.B.; Gu, Z. Bio-inspired synthetic nanovesicles for glucose-responsive release of insulin. Biomacromolecules 2014, 15, 3495–3502. [Google Scholar] [CrossRef]
- Di, J.; Yu, J.C.; Ye, Y.Q.; Ranson, D.; Jindal, A.; Gu, Z. Engineering synthetic insulin-secreting cells using hyaluronic acid microgels integrated with glucose-responsive nanoparticles. Cell. Mol. Bioeng. 2015, 8, 445–454. [Google Scholar] [CrossRef]
- Karimi, M.; Eslami, M.; Sahandi-Zangabad, P.; Mirab, F.; Farajisafiloo, N.; Shafaei, Z.; Ghosh, D.; Bozorgomid, M.; Dashkhaneh, F.; Hamblin, M.R. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2016, 8, 696–716. [Google Scholar] [CrossRef] [Green Version]
- Ke, W.C.; Singh, M.M. Wearable technology devices security and privacy vulnerability analysis. Int. J. Netw. Secur. Its Appl. 2016, 8, 19–30. [Google Scholar]
- Kirby, A.M. Detection of insulin in saliva for prediction of blood insulin level after meals and development of hyperinsulinemia. Patent WO2019036802, 28 February 2019. [Google Scholar]
- Rocha, E.M.; Cunha, D.A.; Carneiro, E.M.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A. Identification of insulin in the tear film and insulin receptor and IGF-I receptor on the human ocular surface. Investig. Ophthalmol. Vis. Sci. 2002, 43, 963–967. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khor, S.M.; Choi, J.; Won, P.; Ko, S.H. Challenges and Strategies in Developing an Enzymatic Wearable Sweat Glucose Biosensor as a Practical Point-Of-Care Monitoring Tool for Type II Diabetes. Nanomaterials 2022, 12, 221. https://doi.org/10.3390/nano12020221
Khor SM, Choi J, Won P, Ko SH. Challenges and Strategies in Developing an Enzymatic Wearable Sweat Glucose Biosensor as a Practical Point-Of-Care Monitoring Tool for Type II Diabetes. Nanomaterials. 2022; 12(2):221. https://doi.org/10.3390/nano12020221
Chicago/Turabian StyleKhor, Sook Mei, Joonhwa Choi, Phillip Won, and Seung Hwan Ko. 2022. "Challenges and Strategies in Developing an Enzymatic Wearable Sweat Glucose Biosensor as a Practical Point-Of-Care Monitoring Tool for Type II Diabetes" Nanomaterials 12, no. 2: 221. https://doi.org/10.3390/nano12020221
APA StyleKhor, S. M., Choi, J., Won, P., & Ko, S. H. (2022). Challenges and Strategies in Developing an Enzymatic Wearable Sweat Glucose Biosensor as a Practical Point-Of-Care Monitoring Tool for Type II Diabetes. Nanomaterials, 12(2), 221. https://doi.org/10.3390/nano12020221






