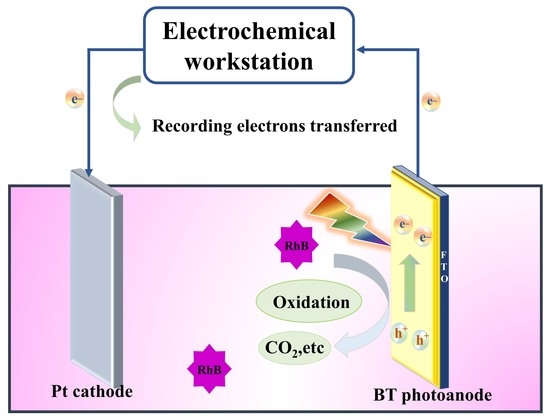
Efficient Dye Contaminant Elimination and Simultaneously Electricity Production via a Bi-Doped TiO2 Photocatalytic Fuel Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.2. Characterization
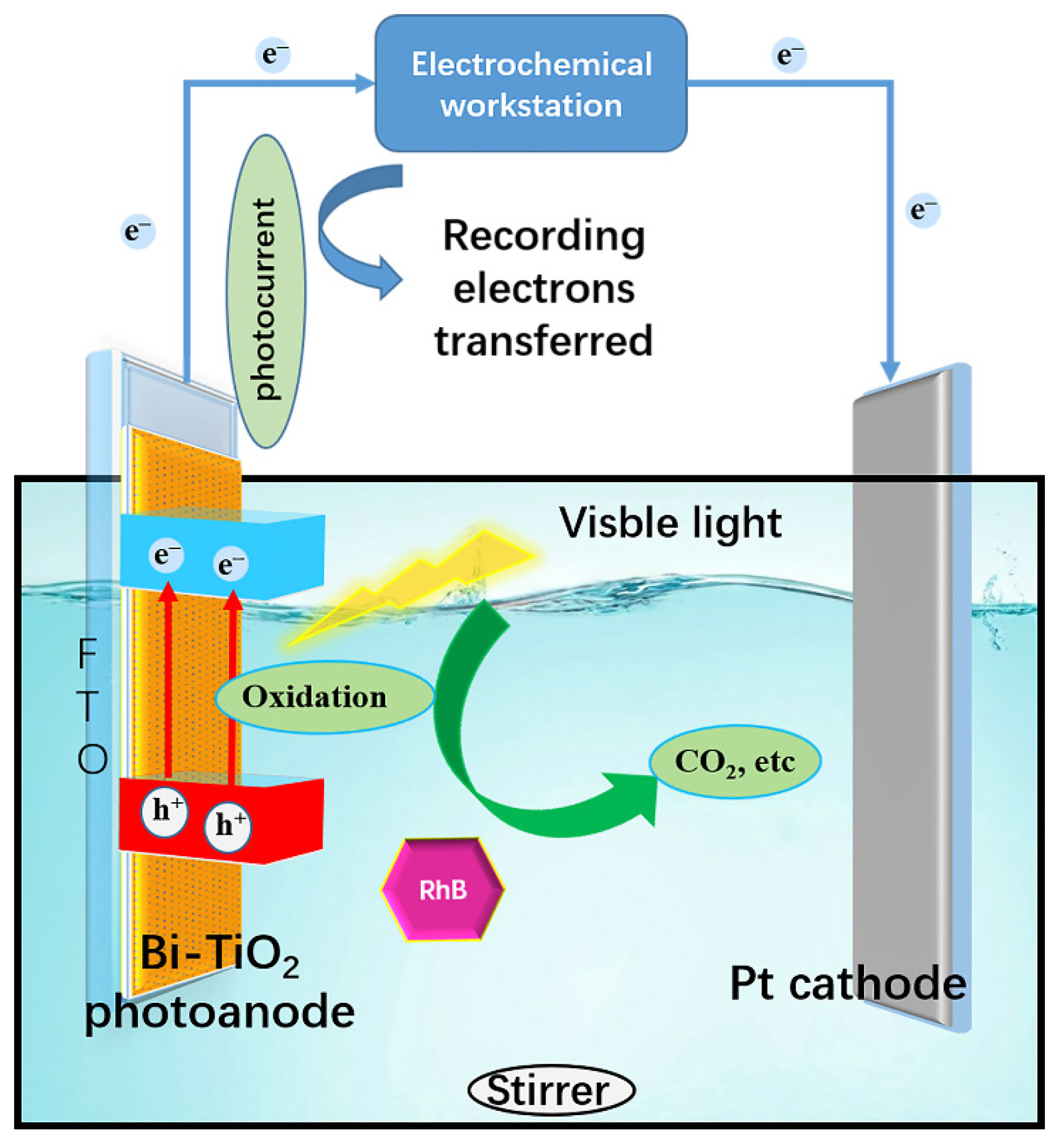
2.3. PFC Performance
3. Results
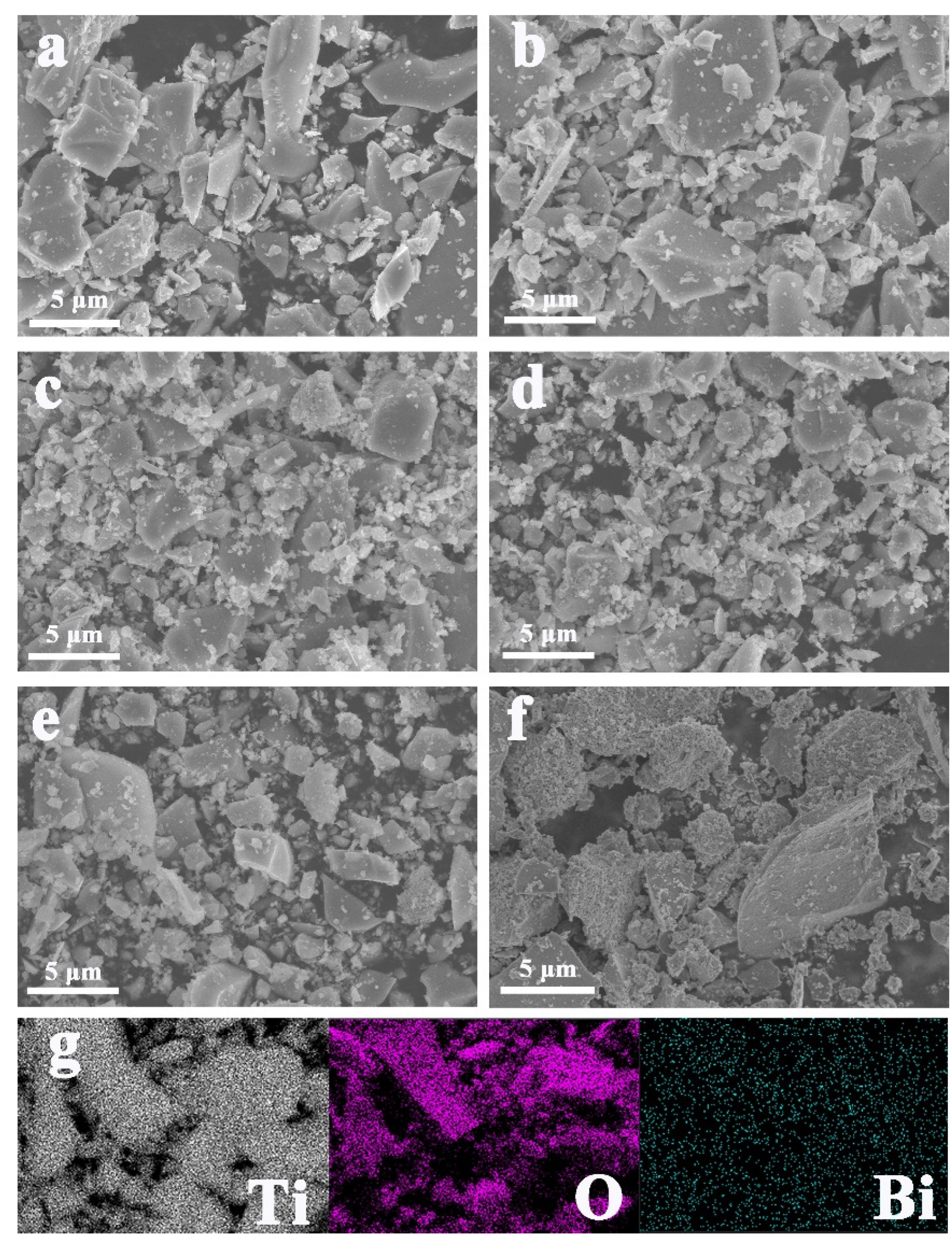
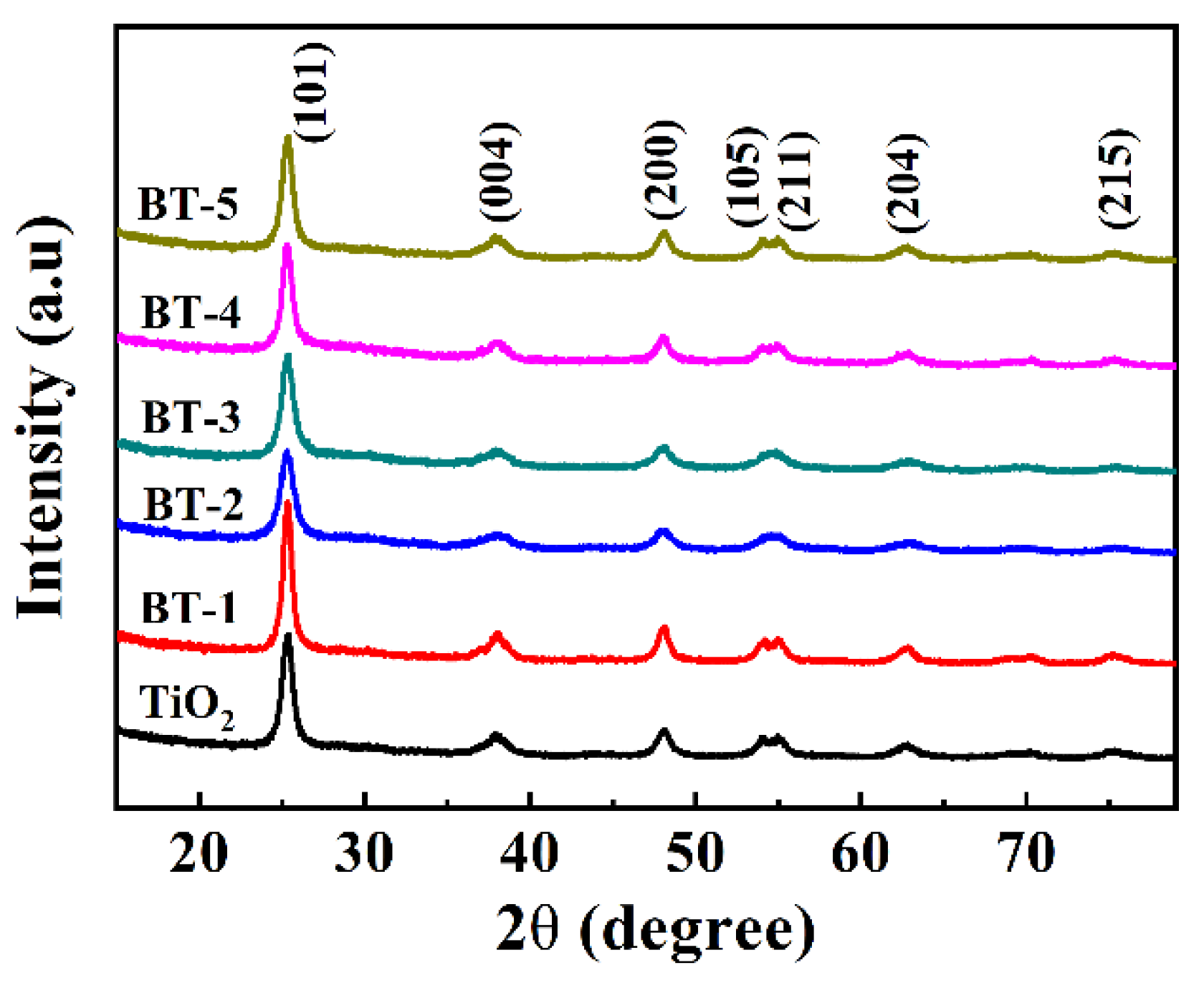
3.1. Structural and Morphological Characteristics
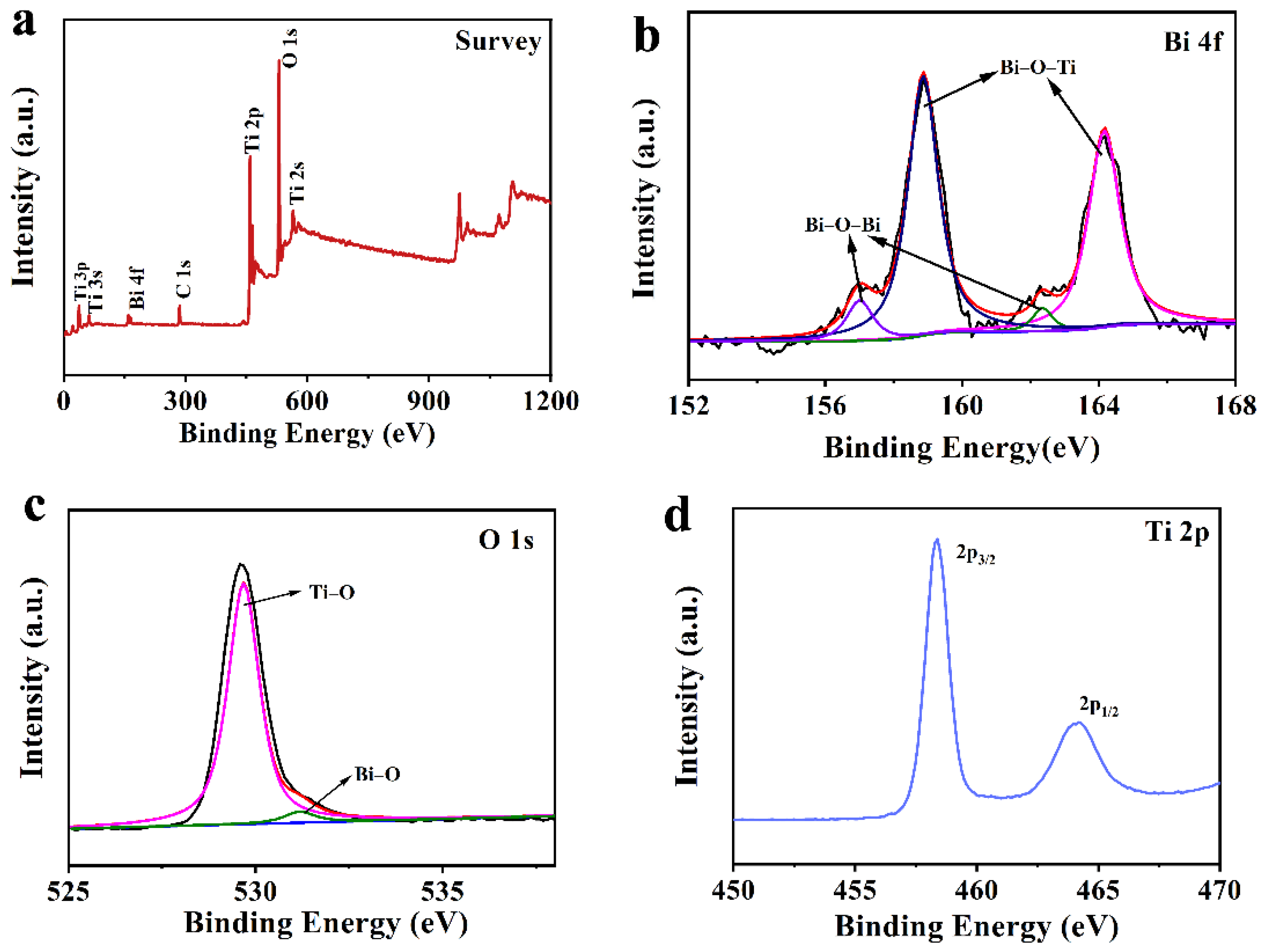
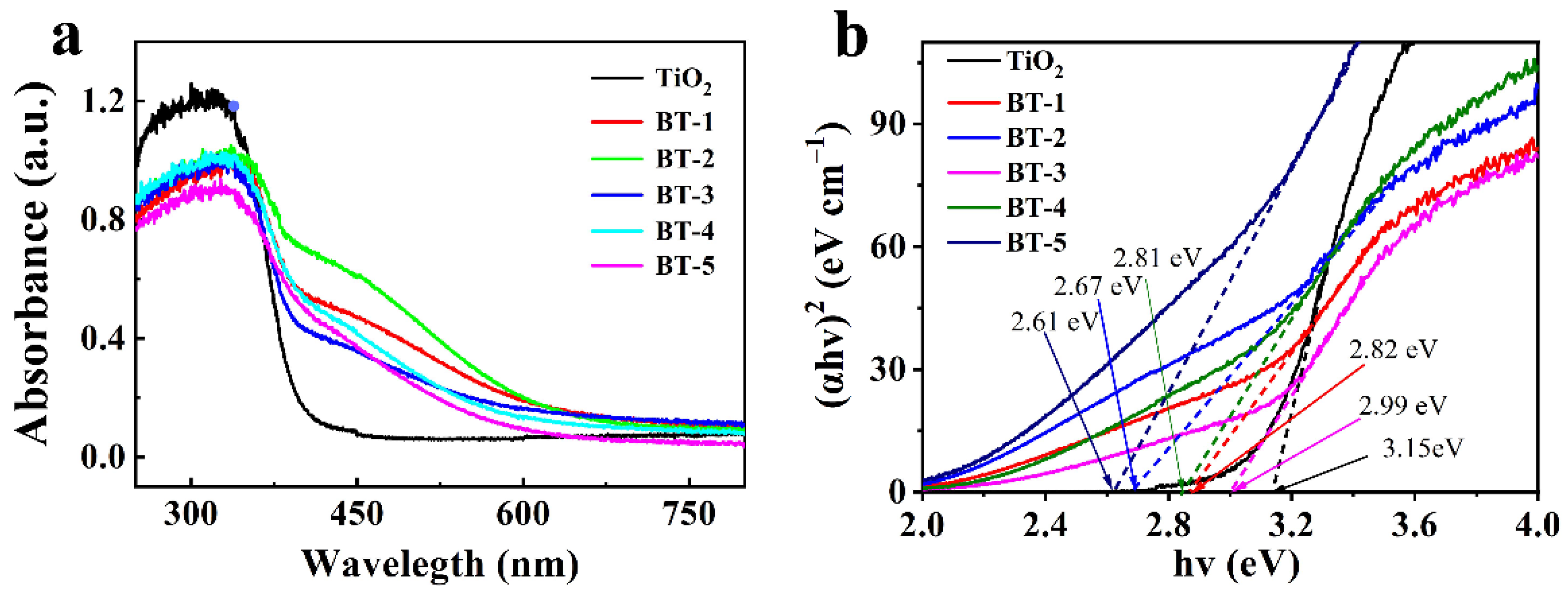
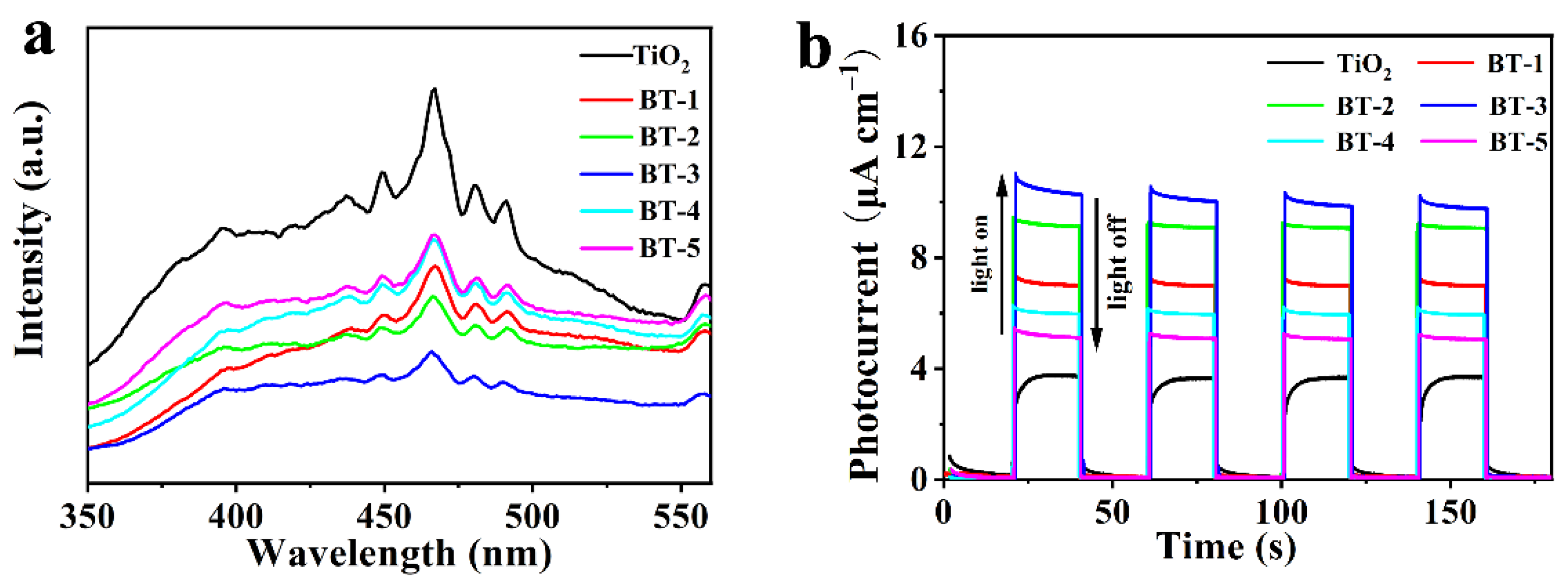
3.2. Spectral and Photoelectric Properties
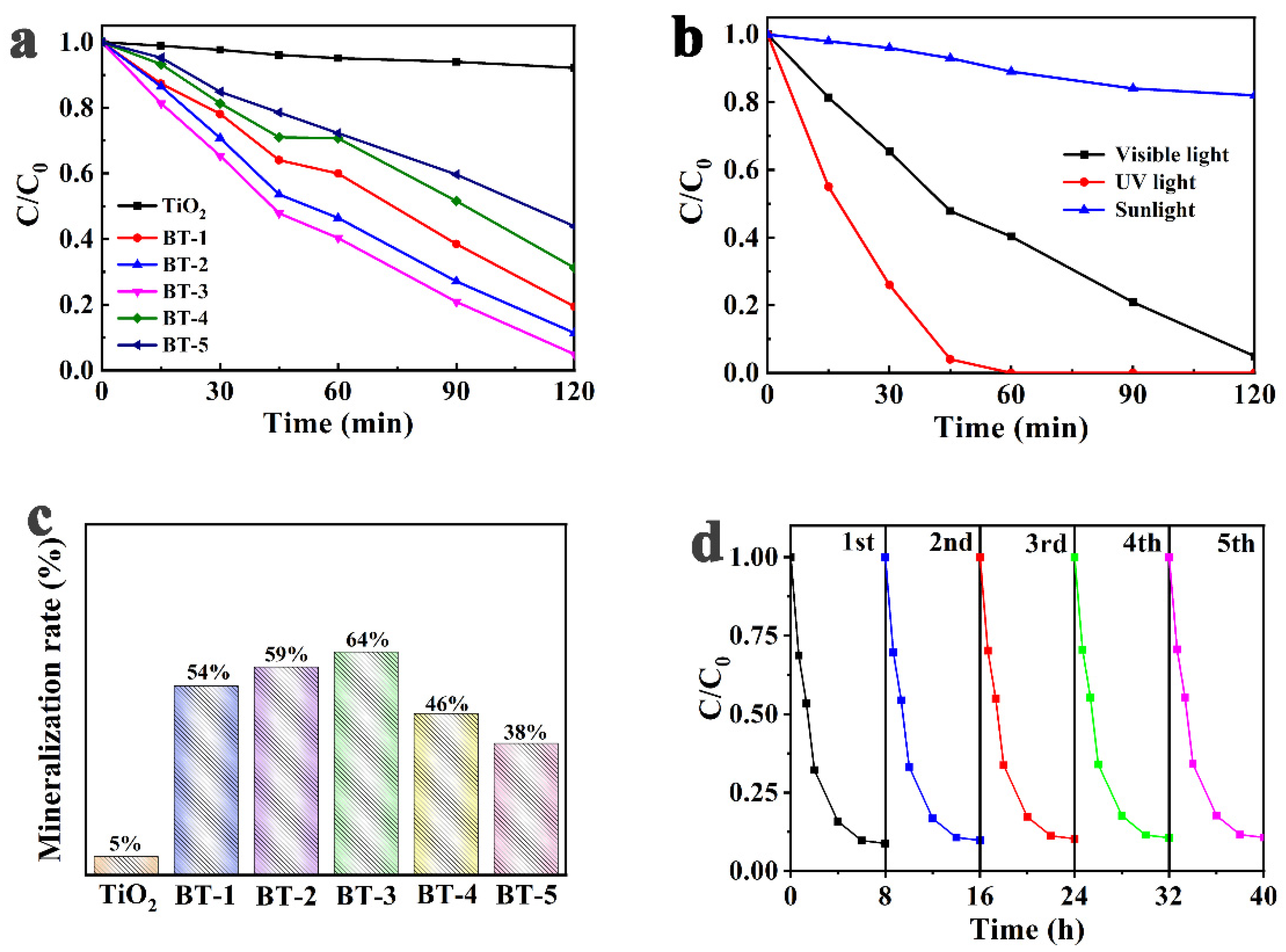
3.3. Effect of Bi Content on the PFC Performance
3.4. Photocurrent Properties for Determination of COD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaushik, J.; Kumar, V.; Garg, A.K.; Dubey, P.; Tripathi, K.M.; Sonkar, S.K. Bio-mass derived functionalized graphene aerogel: A sustainable approach for the removal of multiple organic dyes and their mixtures. New J. Chem. 2021, 45, 9073–9083. [Google Scholar] [CrossRef]
- Kaushik, J.; Himanshi, K.V.; Tripathi, K.M.; Sonkar, S.K. Sunlight-promoted photodegradation of Congo red by cadmium-sulfide decorated graphene aerogel. Chemosphere 2022, 287, 132225. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Yang, J.; Zhou, H.; Murugananthan, M.; Zhang, Y. Electrochemically Self-doped TiO2 nanotube arrays for efficient visible light photoelectrocatalytic degradation of contaminants. Electrochim. Acta 2014, 136, 310–317. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Si, M.; Yu, Y.; Zhang, H. (Yb3+, Er3+) co-doped TiO2/Ag3PO4 hybrid photocatalyst with enhanced activity for photodegradation of phenol. Appl. Surf. Sci. 2019, 463, 159–168. [Google Scholar] [CrossRef]
- Xia, P.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 2D/2D g-C3N4/MnO2 nanocomposite as a direct Z-scheme photocatalyst for enhanced photocatalytic activity. ACS Sustain. Chem. Eng. 2017, 6, 965–973. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase: A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef] [Green Version]
- Subhas, C.; Naidu, K.R.; Reddy, Y.V. Preparation, characterization, and activity evaluation of CuO/F-TiO2 photocatalyst. Mater. Chem. Phy. 2012, 134, 951–957. [Google Scholar]
- Janus, M.; Kusiak, E.; Morawski, A.W. Carbon modified TiO2 photocatalyst with enhanced adsorptivity for dyes from water. Catal. Lett. 2009, 131, 506–511. [Google Scholar] [CrossRef]
- Zhai, C.; Zhu, M.; Lu, Y.; Ren, F.; Wang, C.; Du, Y.; Yang, P. Reduced graphene oxide modified highly ordered TiO2 nanotube arrays photoelectrode with enhanced photoelectrocatalytic performance under visible-light irradiation. Phys. Chem. Chem. Phys. 2014, 16, 14800–14807. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, S.; Weckhuysen, B.M.; Pettignano, A.; Pignataro, B. Multi-doped brookite-prevalent TiO2 photocatalyst with enhanced activity in the visible light. Catal. Lett. 2018, 148, 1–13. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Gao, R.; Zhao, Q.; Yang, Z.; Gao, X.; Wang, Z.; Zhang, F.; Wu, W. Enhanced visible light photoelectrocatalytic degradation of tetracycline hydrochloride by I and P co-doped TiO2 photoelectrode. J. Hazard. Mater. 2021, 406, 124309. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.K.; Bhavani, K.; Srinivas, B.; Kumar, S.N.; Sudhakar, M.; Naresh, G.; Venugopal, A. Nano structured bismuth and nitrogen co-doped TiO2 as an efficient light harvesting photocatalyst under natural sunlight for the production of H2 by H2O splitting. Appl. Catal. A: Gen. 2016, 515, 91–100. [Google Scholar] [CrossRef]
- Cho, Y.; Choi, W.; Lee, C.H.; Hyeon, T.; Lee, H.I. Visible light-induced degradation of carbon tetrachloride on dye-sensitized TiO2. Environ. Sci. Technol. 2001, 35, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tian, Y.; An, Y.; Yang, Q.; Xiong, S.; Feng, J.; Qian, Y. Robust and flexible polymer/MXene-derived two dimensional TiO2 hybrid gel electrolyte for dendrite-free solid-state zinc-ion batteries. Chem. Eng. J. 2022, 430, 132748. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Marami, M.B.; Farahmandjou, M.; Khoshnevisan, B. Sol-gel synthesis of Fe-doped TiO2 nanocrystals. J. Electron. Mater. 2018, 47, 3741–3748. [Google Scholar] [CrossRef]
- Jo, W.K.; Kumar, S.; Isaacs, M.A.; Lee, A.F.; Karthikeyan, S. Cobalt promoted TiO2/GO for the photocatalytic degradation of oxytetracycline and Congo Red. Appl. Catal. B: Environ. 2017, 201, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Wang, Y.; Liang, H.; Liang, A.; Zhang, J. Mechanical properties and tribological behavior of fullerene-like hydrogenated carbon films prepared by changing the flow rates of argon gas. Appl. Surf. Sci. 2016, 364, 288–293. [Google Scholar] [CrossRef]
- Ling, X.; Zhang, J.; Ye, C.; Tian, B.; Feng, C.; Anpo, M. Synergistic effects of doped Fe3+ and deposited Au on improving the photocatalytic activity of TiO2. Catal. Lett. 2006, 111, 207–211. [Google Scholar]
- Liu, D.; Zhou, J.; Wang, J.; Tian, R.; Li, X.; Nie, E.; Piao, X.; Sun, Z. Enhanced visible light photoelectrocatalytic degradation of organic contaminants by F and Sn co-doped TiO2 photoelectrode. Chem. Eng. J. 2018, 344, 332–341. [Google Scholar] [CrossRef]
- Hasan, M.R.; Abd Hamid, S.B.; Basirun, W.J.; Chowdhury, Z.Z.; Kandjani, A.E.; Bhargava, S.K. Ga doped RGO-TiO2 composite on an ITO surface electrode for investigation of photoelectrocatalytic activity under visible light irradiation. New J. Chem. 2015, 39, 369–376. [Google Scholar] [CrossRef]
- Bhowmick, G.D.; Noori, M.T.; Das, I.; Neethu, B.; Ghangrekar, M.M.; Mitra, A. Bismuth doped TiO2 as an excellent photocathode catalyst to enhance the performance of microbial fuel cell. Int. J. Hydrogen Energ. 2018, 43, 7501–7510. [Google Scholar] [CrossRef]
- Nischk, M.; Mazierski, P.; Wei, Z.; Siuzdak, K.; Kouame, N.A.; Kowalska, E.; Remita, H.; Zaleska-Medynska, A. Enhanced photocatalytic, electrochemical and photoelectrochemical properties of TiO2 nanotubes arrays modified with Cu, AgCu and Bi nanoparticles obtained via radiolytic reduction. Appl. Surf. Sci. 2016, 387, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Zhang, W.; Lv, X.; Sun, Y.; Dong, F.; Zhang, Y. Noble metal-free Bi nanoparticles supported on TiO2 with plasmon-enhanced visible light photocatalytic air purification. Environ. Sci. Nano 2016, 3, 1306–1317. [Google Scholar] [CrossRef]
- Nishikawa, M.; Yuto, S.; Nakajima, T.; Tsuchiya, T.; Saito, N. Effect of lattice distortion on photocatalytic performance of TiO2. Catal. Lett. 2017, 147, 292–300. [Google Scholar] [CrossRef]
- Wu, M.C.; Chih, J.S.; Huang, W.K. Bismuth doping effect on TiO2 nanofibres for morphological change and photocatalytic performance. CrystEngComm 2014, 16, 10692–10699. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, G.; Li, S. The doping effect of Bi on TiO2 for photocatalytic hydrogen generation and photodecolorization of rhodamine B. J. Phys. Chem. C 2009, 113, 9950–9955. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, T.; Liu, E.; Ma, X.; Ge, Q.; Gong, J. Understanding electronic and optical properties of anatase TiO2 photocatalysts co-doped with nitrogen and transition metals. Phys. Chem. Chem. Phys. 2013, 15, 9549–9561. [Google Scholar] [CrossRef]
- Solís, M.; Rincón, M.E.; Calva, J.C.; Alvarado, G. Bismuth sulfide sensitized TiO2 arrays for photovoltaic applications. Electrochim. Acta 2013, 112, 159–163. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, F.; Wang, F.; Luo, S.; Yin, X. Synthesis, characterization, and activities of visible light-driven Bi2O3-TiO2 composite photocatalysts. J. Alloy. Compd. 2010, 498, 179–184. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Shang, M.; Gao, E.; Zhang, Z.; Ren, J. Electrospun nanofibers of Bi-doped TiO2 with high photocatalytic activity under visible light irradiation. J. Hazard. Mater. 2011, 196, 426–430. [Google Scholar] [CrossRef]
- Ali, I.; Kim, S.R.; Kim, S.P.; Kim, J.O. Anodization of bismuth doped TiO2 nanotubes composite for photocatalytic degradation of phenol in visible light. Catal. Today 2017, 282, 31–37. [Google Scholar] [CrossRef]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of interfaces in two-dimensional photocatalyst for water splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Yi, M.; Xiuli, W.; Yushuai, J.; Xiaobo, C.; Hongxian, H.; Can, L. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar]
- Liao, Q.; Li, L.; Chen, R.; Zhu, X.; Wang, H.; Ye, D.; Cheng, X.; Zhang, M.; Zhou, Y. Respective electrode potential characteristics of photocatalytic fuel cell with visible-light responsive photoanode and air-breathing cathode. Int. J. Hydrogen Energ. 2015, 40, 16547–16555. [Google Scholar] [CrossRef]
- Lee, S.L.; Ho, L.N.; Ong, S.A.; Wong, Y.S.; Voon, C.H.; Khalik, W.F.; Yusoff, N.A.; Nordin, N. Enhanced electricity generation and degradation of the azo dye Reactive Green 19 in a photocatalytic fuel cell using ZnO/Zn as the photoanode. J. Clean. Prod. 2016, 127, 579–584. [Google Scholar] [CrossRef]
- Xie, S.; Ouyang, K. Degradation of refractory organic compounds by photocatalytic fuel cell with solar responsive WO3/FTO photoanode and air-breathing cathode. J. Colloid. Interface. Sci. 2017, 500, 220–227. [Google Scholar] [CrossRef]
- Ye, Y.; Bruning, H.; Li, X.; Yntema, D.; Rijnaarts, H.H.M. Significant enhancement of micropollutant photocatalytic degradation using a TiO2 nanotube array photoanode based photocatalytic fuel cell. Chem. Eng. J. 2018, 354, 553–562. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, X.; Yang, X.; Li, J.; Zhang, X.; Zhao, J. Energy storage ability and anti-corrosion properties of Bi-doped TiO2 nanotube arrays. Electrochim. Acta 2015, 169, 227–232. [Google Scholar]
- Mao, H.; Jin, Z.; Zhang, F.; He, H.; Chen, J.; Qian, Y. A high efficiency photocatalyst based on porous Bi-doped TiO2 composites. Ceram. Int. 2018, 44, 17535–17538. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Liu, X.; Yu, C.; Yan, D.; Sun, Z.; Pan, L. Sn doped TiO2 nanotube with oxygen vacancy for highly efficient visible light photocatalysis. J. Alloy. Compd. 2016, 679, 454–462. [Google Scholar] [CrossRef]
- Liu, D.; Tian, R.; Wang, J.; Nie, E.; Piao, X.; Li, X.; Sun, Z. Photoelectrocatalytic degradation of methylene blue using F doped TiO2 photoelectrode under visible light irradiation. Chemosphere 2017, 185, 574–581. [Google Scholar] [CrossRef]
- Sajjad, S.; Leghari, S.A.; Chen, F.; Zhang, J. Bismuth-doped ordered mesoporous TiO2: Visible-light catalyst for simultaneous degradation of phenol and chromium. Chemistry 2010, 16, 795–804. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, D.; Zhang, S.; Catterall, K.P.; John, R. Development of a direct photoelectrochemical method for determination of chemical oxygen demand. Anal. Chem. 2015, 76, 155–160. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Xian, Y.; Ying, X.; Liu, M.; Jin, L. Preparation and application of TiO2 photocatalytic sensor for chemical oxygen demand determination in water research. Water Res. 2005, 39, 1340–1346. [Google Scholar] [CrossRef]
- Heng, W.; Zhang, W.; Zhang, Q.; Wang, H.; Li, Y. Photoelectrocatalytic microfluidic reactors utilizing hierarchical TiO2 nanotubes for determination of chemical oxygen demand. RSC Adv. 2016, 6, 49824–49830. [Google Scholar] [CrossRef]
- Domini, C.E.; Hidalgo, M.; Marken, F.; Canals, A. Comparison of three optimized digestion methods for rapid determination of chemical oxygen demand: Closed microwaves, open microwaves and ultrasound irradiation. Anal. Chim. Acta 2006, 569, 275–276. [Google Scholar] [CrossRef]
- Ying, D.; Cao, R.; Li, C.; Tang, T.; Li, K.; Wang, H.; Wang, Y.; Jia, J. Study of the photocurrent in a photocatalytic fuel cell for wastewater treatment and the effects of TiO2 surface morphology to the apportionment of the photocurrent. Electrochim. Acta 2016, 192, 319–327. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Li, C.; Zhao, C.; Nie, E.; Wang, J.; Zhou, J.; Zhao, Q. Efficient Dye Contaminant Elimination and Simultaneously Electricity Production via a Bi-Doped TiO2 Photocatalytic Fuel Cell. Nanomaterials 2022, 12, 210. https://doi.org/10.3390/nano12020210
Liu D, Li C, Zhao C, Nie E, Wang J, Zhou J, Zhao Q. Efficient Dye Contaminant Elimination and Simultaneously Electricity Production via a Bi-Doped TiO2 Photocatalytic Fuel Cell. Nanomaterials. 2022; 12(2):210. https://doi.org/10.3390/nano12020210
Chicago/Turabian StyleLiu, Dong, Chunling Li, Congyue Zhao, Er Nie, Jianqiao Wang, Jun Zhou, and Qian Zhao. 2022. "Efficient Dye Contaminant Elimination and Simultaneously Electricity Production via a Bi-Doped TiO2 Photocatalytic Fuel Cell" Nanomaterials 12, no. 2: 210. https://doi.org/10.3390/nano12020210
APA StyleLiu, D., Li, C., Zhao, C., Nie, E., Wang, J., Zhou, J., & Zhao, Q. (2022). Efficient Dye Contaminant Elimination and Simultaneously Electricity Production via a Bi-Doped TiO2 Photocatalytic Fuel Cell. Nanomaterials, 12(2), 210. https://doi.org/10.3390/nano12020210