Converting non-Mesogenic to Mesogenic Stacking of Amino-s-Triazine-Based Dendrons with p-CN Phenyl Unit by Eliminating Peripheral Dipole
Abstract
:1. Introduction
2. Experimental Section
2.1. General Methods
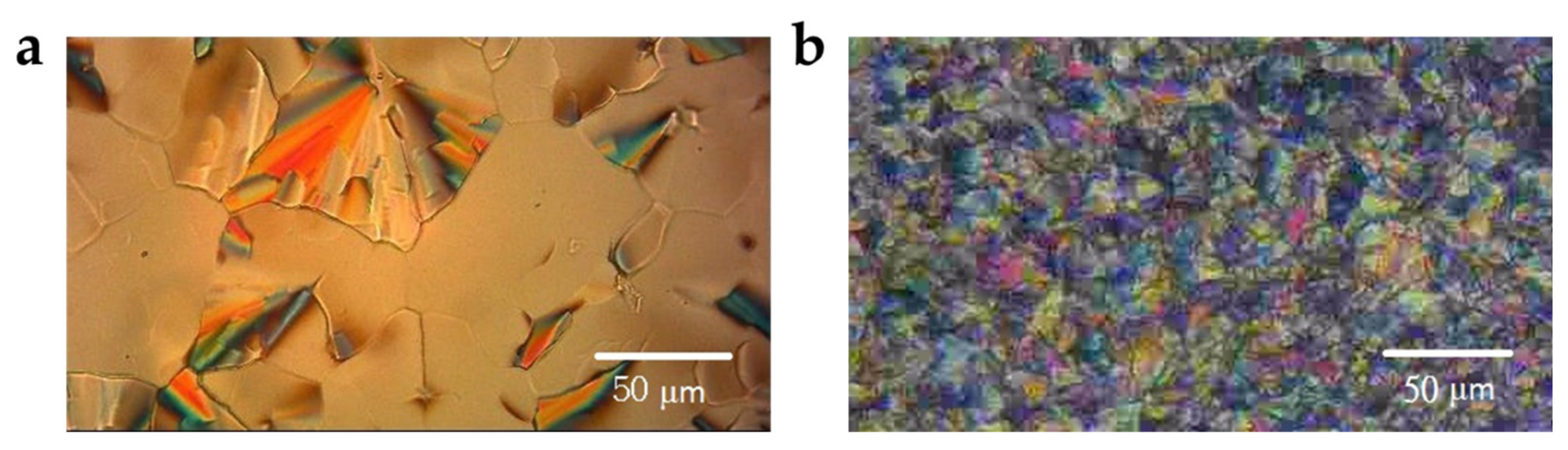
2.2. Sample Characterization by POM
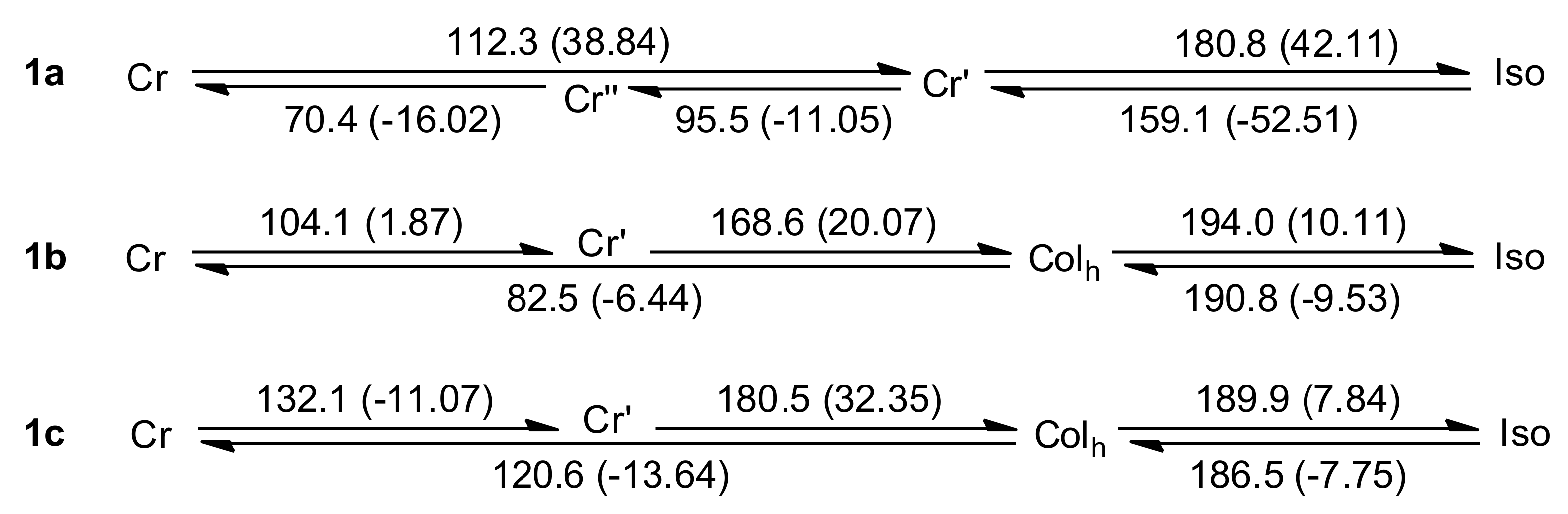
2.3. Sample Characterization by DSC
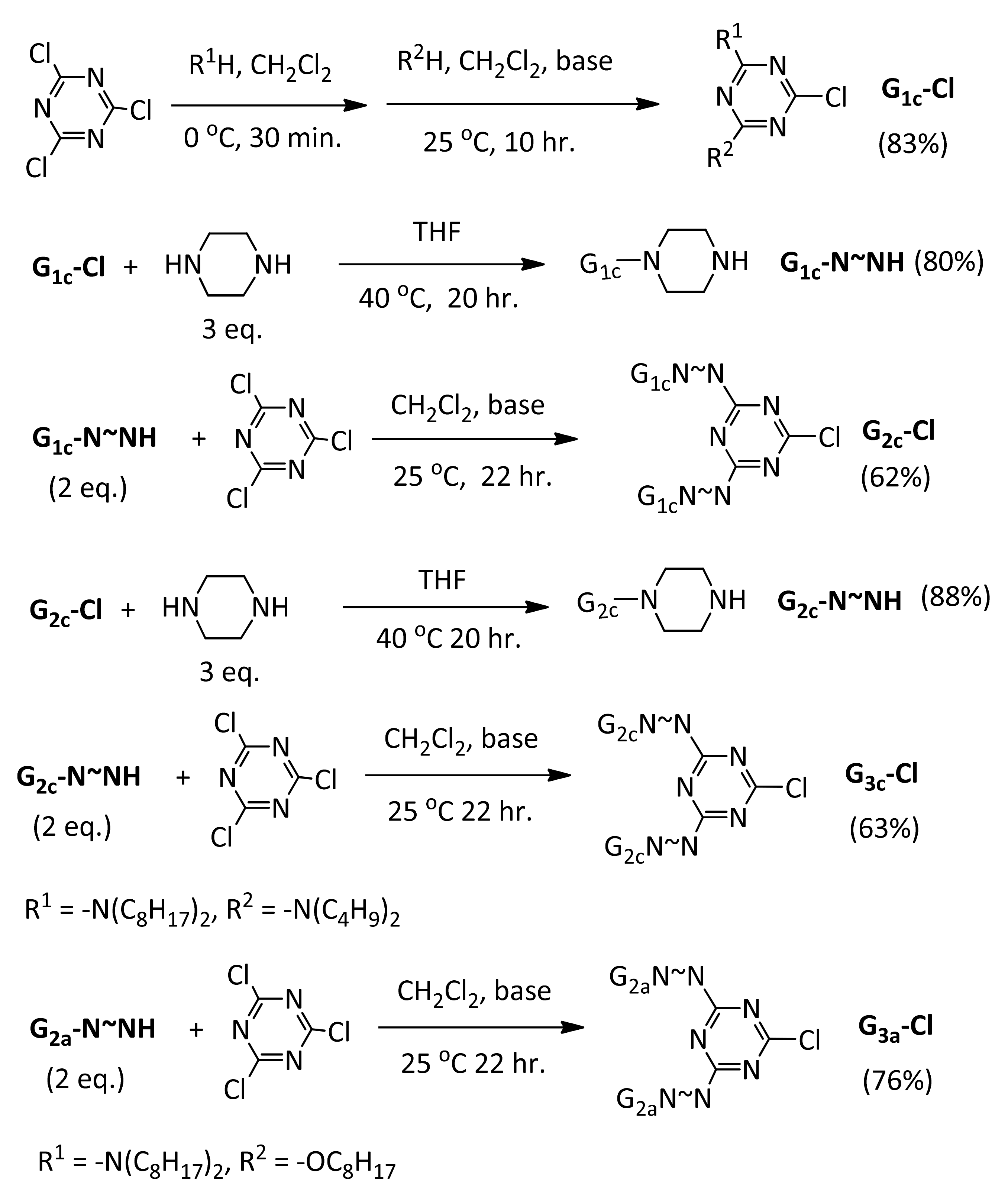
2.4. Prepartion of Chloro-Dendrons G3a-Cl and G3c-Cl
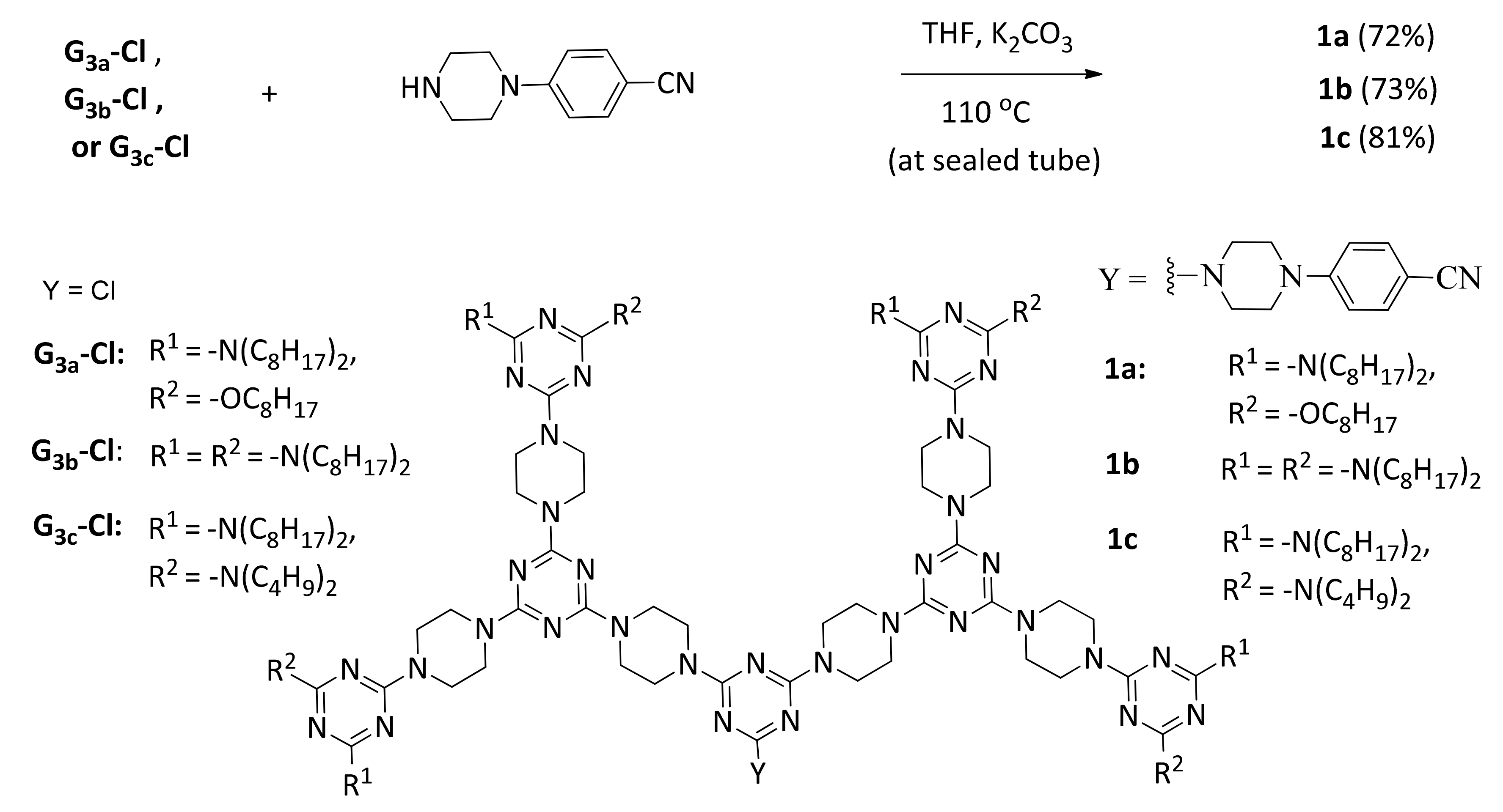
2.5. The Typical Procedure for Preparing Dendrons 1a–1c
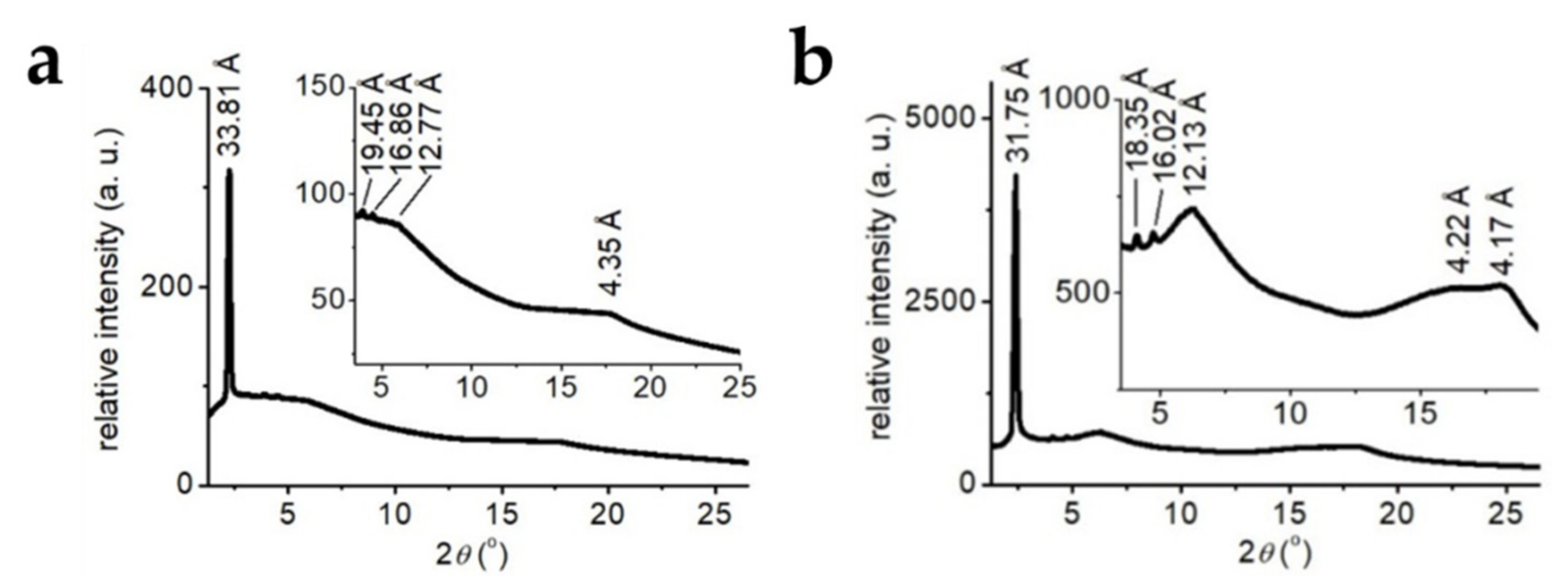
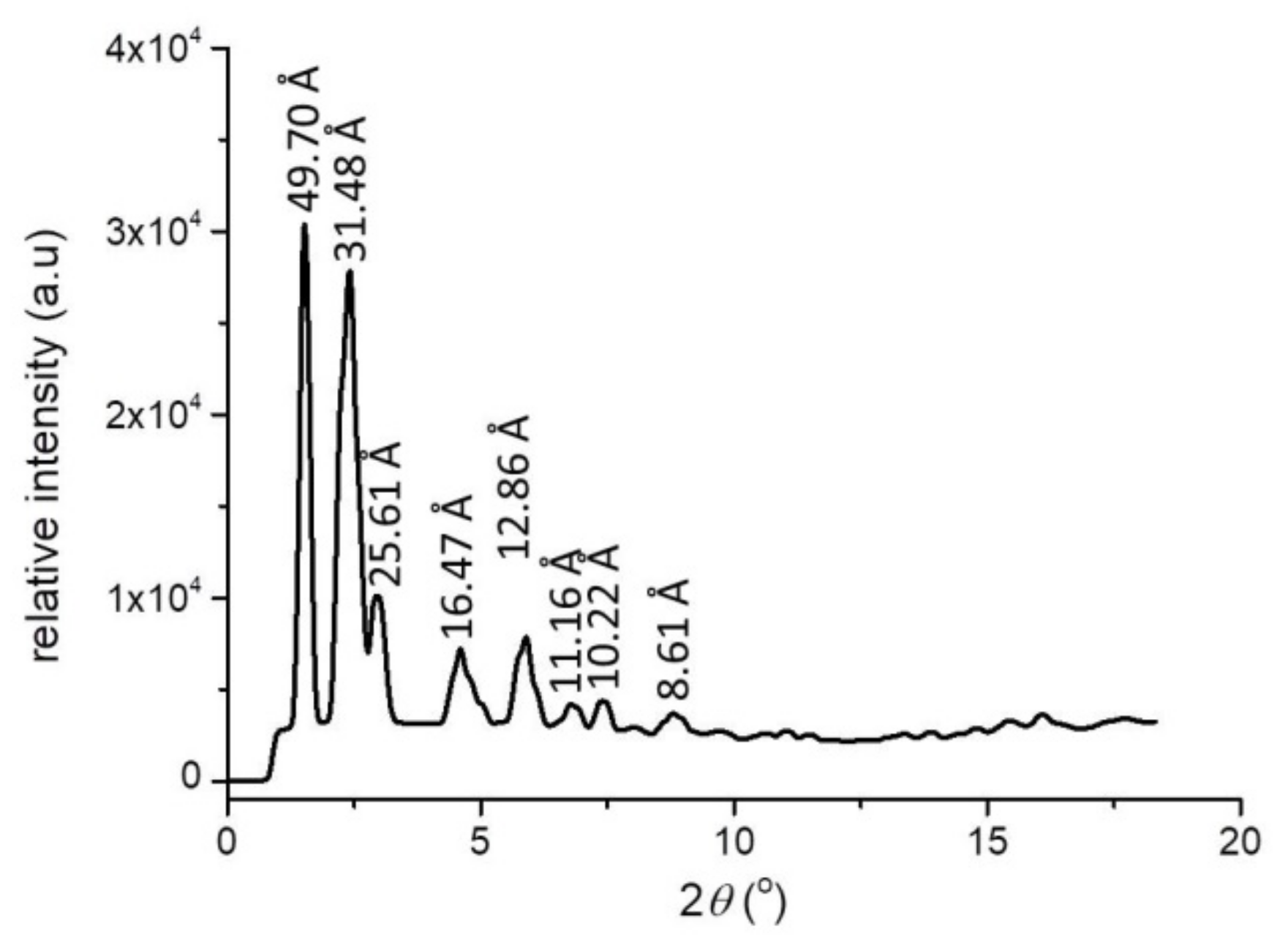
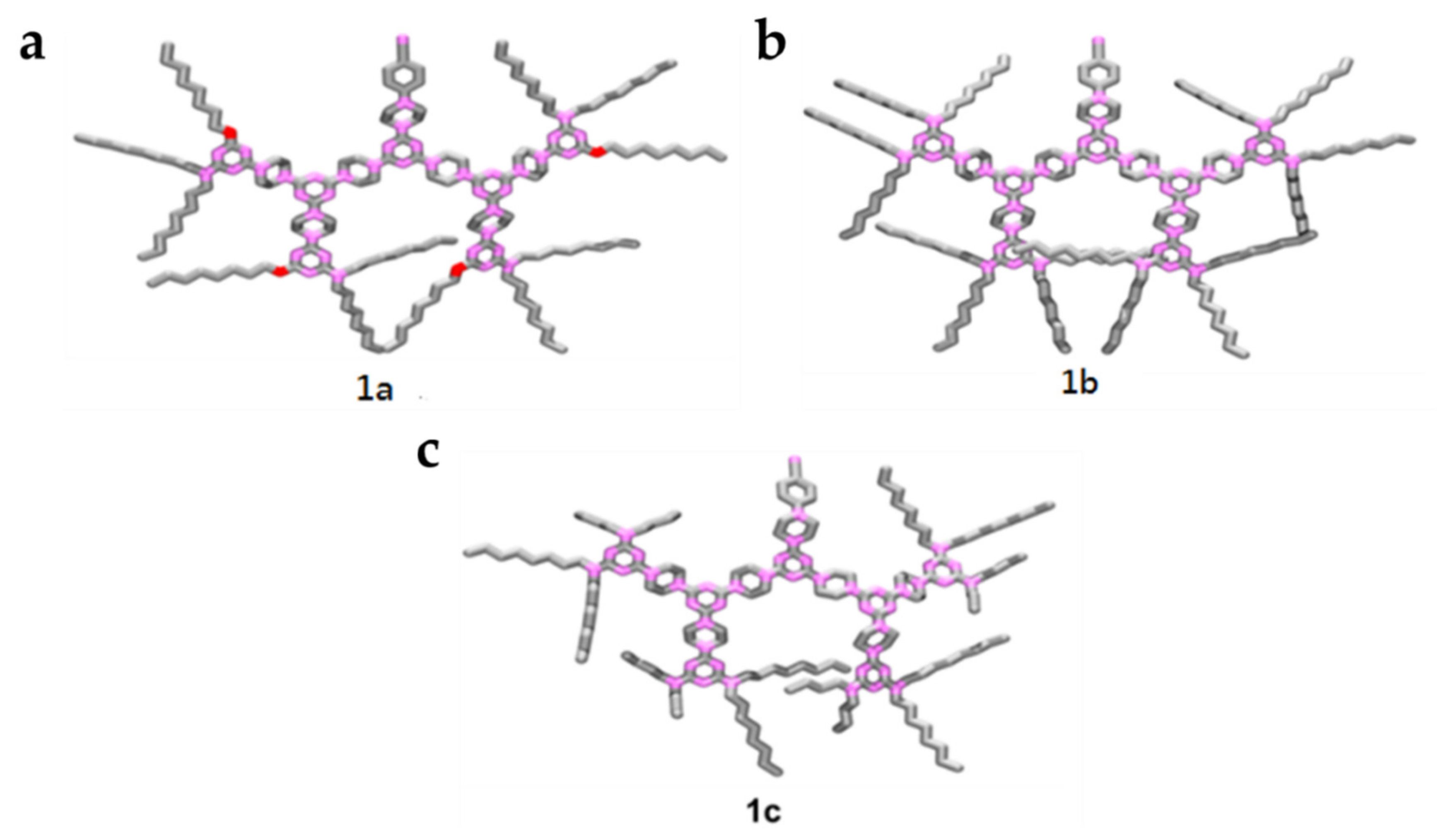
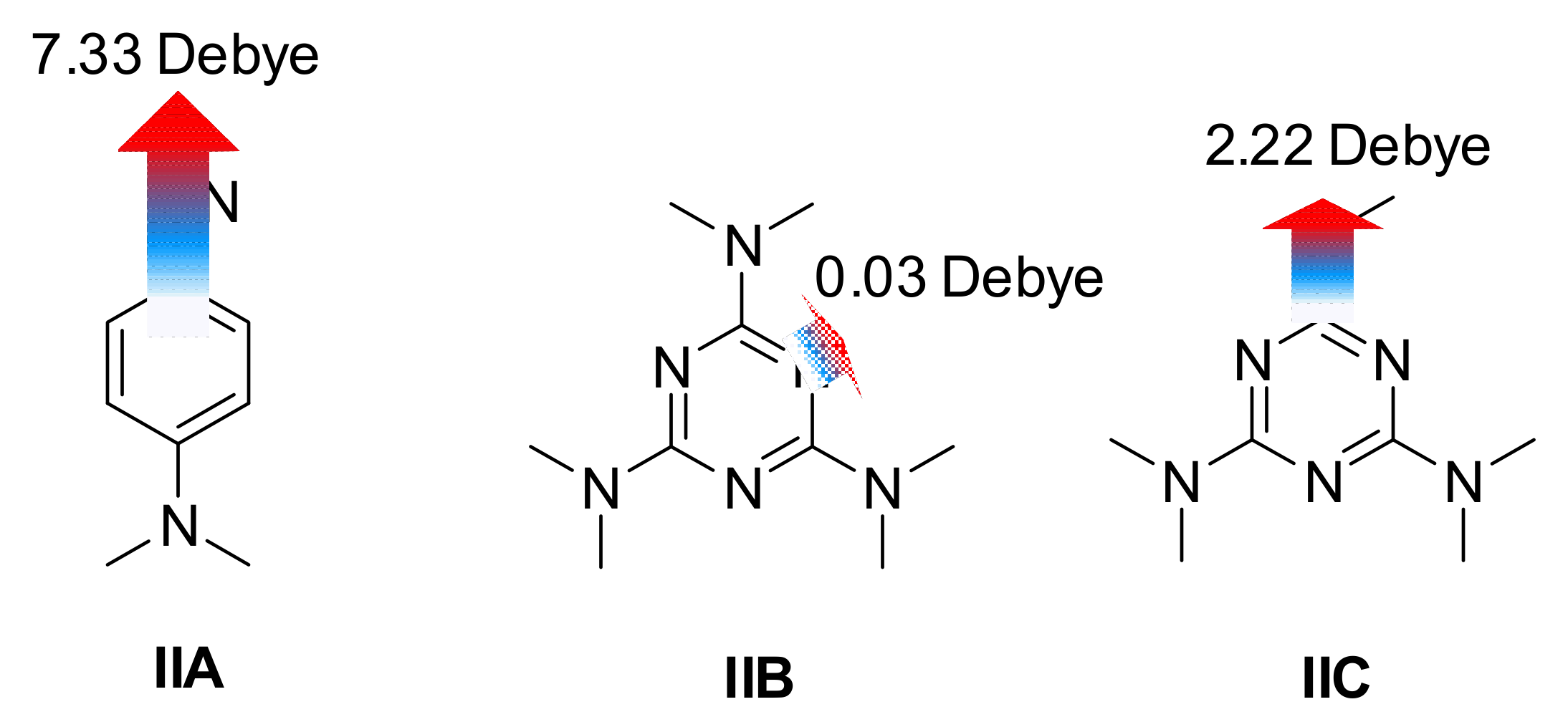
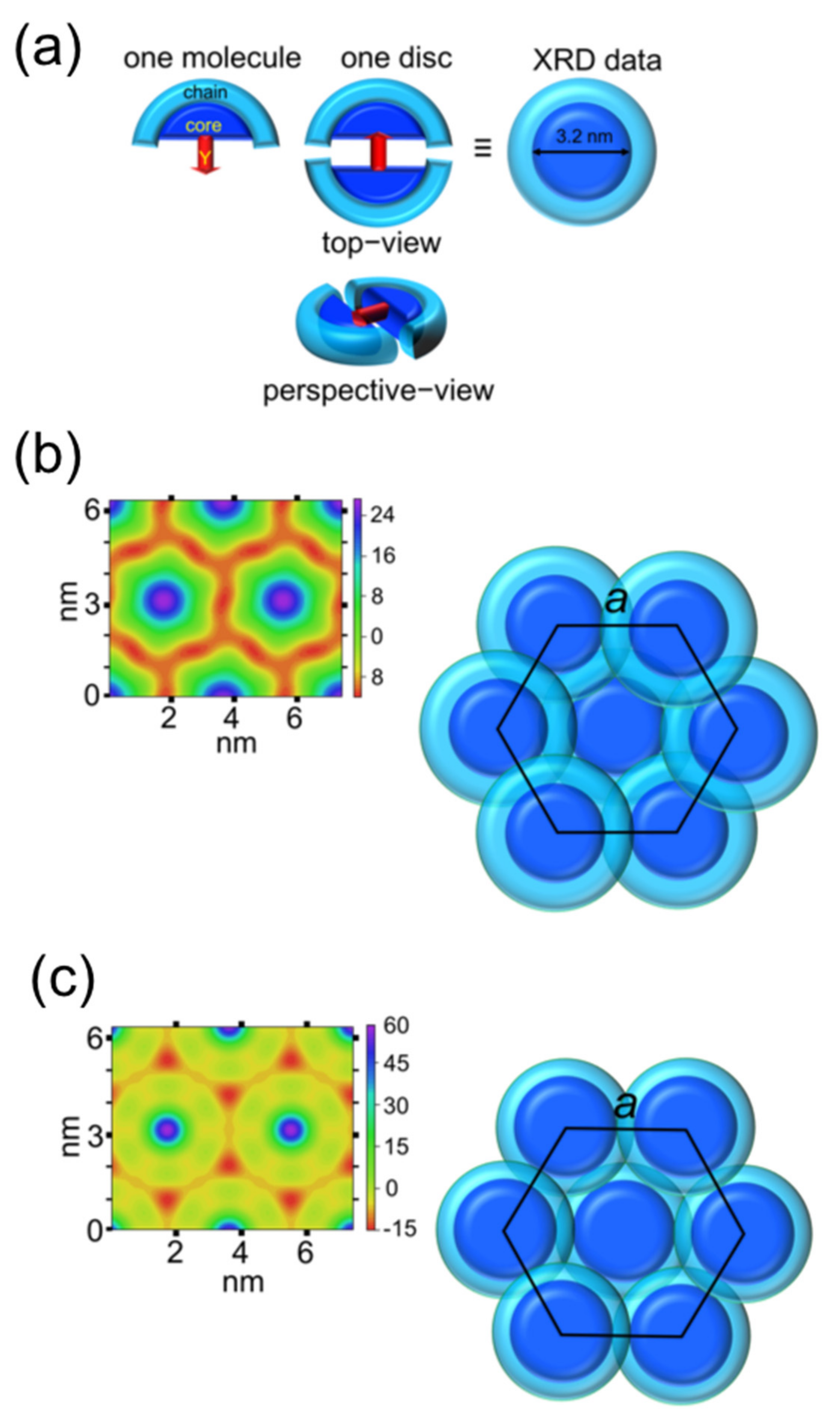
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newkome, G.R.; Moorefield, C.N.; Vögtle, F. Dendrimers and Dendrons: Concepts, Syntheses, Applications; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2001. [Google Scholar]
- Caminade, A.-M.; Turrin, C.-O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. Dendrimers: Towards Catalytic, Material and Biomedical Uses; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional Liquid Crystals towards the Next Generation of Materials. Angew. Chem. Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef]
- Sagara, Y.; Yamane, S.; Mitani, M.; Weder, C.; Kato, T. Mechanoresponsive Luminescence: Mechanoresponsive Luminescent Molecular Assemblies: An Emerging Class of Materials (Adv. Mater. 6/2016). Adv. Mater. 2016, 28, 977. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Cangiotti, M.; Kao, C.-L.; Ottaviani, M.F. EPR Characterization of Copper(II) Complexes of PAMAM-Py Dendrimers for Biocatalysis in the Absence and Presence of Reducing Agents and a Spin Trap. J. Phys. Chem. B 2017, 121, 10498–10507. [Google Scholar] [CrossRef]
- Thompson, S.J.; Soukri, M.; Lail, M. Phosphorus Dendrimer Derived Solid Sorbents for CO2 Capture from Post-Combustion Gas Streams. Energy Fuels 2018, 32, 8658–8667. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Chien, C.-Y.; Hsu, H.-F.; Lai, L.-L. Adsorbing volatile organic chemicals by soluble triazine-based dendrimers under ambient conditions with the adsorption capacity of pyridine up to 946.2 mg/g. Molecules 2021, 26, 4862. [Google Scholar] [CrossRef]
- Lee, C.-H.; Tsai, M.-R.; Chang, Y.-T.; Lai, L.-L.; Lu, K.-L.; Cheng, K.-L. Preparation of Unconventional Dendrimers that Contain Rigid NH—Triazine Linkages and Peripheral tert-Butyl Moieties for CO2-Selective Adsorption. Chem. Eur. J. 2013, 19, 10573–10579. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Soldatov, D.V.; Tzeng, C.-H.; Lai, L.-L.; Lu, K.-L. Design of a Peripheral Building Block for H-Bonded Dendritic Frameworks and Analysis of the Void Space in the Bulk Dendrimers. Sci. Rep. 2017, 7, 3649. [Google Scholar] [CrossRef] [PubMed]
- Connal, L.A.; Vestberg, R.; Hawker, C.J.; Qiao, G.G. Synthesis of Dendron Functionalized Core Cross-linked Star Polymers. Macromolecules 2007, 40, 7855–7863. [Google Scholar] [CrossRef]
- Whitton, G.; Gillies, E.R. Functional aqueous assemblies of linear-dendron hybrids. J. Polym. Sci. A Polym. Chem. 2015, 53, 148–172. [Google Scholar] [CrossRef]
- Kuchkina, N.V.; Yuzik-Klimova, E.Y.; Sorokina, S.A.; Peregudov, A.S.; Antonov, D.Y.; Gage, S.H.; Boris, B.S.; Nikoshvili, L.Z.; Sulman, E.M.; Morgan, D.G.; et al. Polyphenylenepyridyl Dendrons with Functional Periphery and Focal Points: Syntheses and Applications. Macromolecules 2013, 46, 5890–5898. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, B.; Chen, Y.; Bo, Z.; Chen, Y. Amphiphilic dendrons with a pyrene functional group at the focal point: Synthesis, self-assembly and generation-dependent DNA condensation. Polym. Chem. 2017, 8, 4798–4804. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Q.; Kang, J.; Shen, J.; Wang, B.; Guo, F.; Chen, Z. Hydrophilic triazine-based dendron for copper and lead adsorption in aqueous systems: Performance and mechanism. J. Mol. Liq. 2020, 298, 112031. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Qiu, H.; Sun, X.; Han, M.; Guo, Y. Nanoadsorbents preparing from oligoethylene glycol dendron and citric acid: Enhanced adsorption effect for the removal of heavy metal ions. Colloids Surf. B 2020, 189, 110876. [Google Scholar] [CrossRef]
- Liu, Y.; Mu, S.; Liu, X.; Ling, Q.; Hang, C.; Ruiz, J.; Astruc, D.; Gu, H. Ferrocenyl Janus mixed-dendron stars and their stabilization of Au and Ag nanoparticles. Tetrahedron 2018, 74, 4777–4789. [Google Scholar] [CrossRef]
- Calik, F.; Degirmenci, A.; Eceoglu, M.; Sanyal, A.; Sanyal, R. Dendron–Polymer Conjugate Based Cross-Linked Micelles: A Robust and Versatile Nanosystem for Targeted Delivery. Bioconjug. Chem. 2019, 30, 1087–1097. [Google Scholar] [CrossRef]
- Soberats, B.; Yoshio, M.; Ichikawa, T.; Zeng, X.; Ohno, H.; Ungar, G.; Kato, T. Ionic Switch Induced by a Rectangular–Hexagonal Phase Transition in Benzenammonium Columnar Liquid Crystals. J. Am. Chem. Soc. 2015, 137, 13212–13215. [Google Scholar] [CrossRef]
- Chico, R.; de Domingo, E.; Domínguez, C.; Donnio, B.; Heinrich, B.; Termine, R.; Golemme, A.; Coco, S.; Espinet, P. High One-Dimensional Charge Mobility in Semiconducting Columnar Mesophases of Isocyano-Triphenylene Metal Complexes. Chem. Mater. 2017, 29, 7587–7595. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-X.; Wang, H.; Du, J.-Q.; Zhao, K.-Q.; Hu, P.; Wang, B.-Q.; Monobe, H.; Heinrich, B.; Donnio, B. Molecular design of benzothienobenzothiophene-cored columnar mesogens: Facile synthesis, mesomorphism, and charge carrier mobility. J. Mater. Chem. C 2018, 6, 4471–4478. [Google Scholar] [CrossRef]
- Albrecht, K.; Matsuoka, K.; Fujita, K.; Yamamoto, K. A dendrimer emitter doped in a dendrimer host: Efficient thermally activated delayed fluorescence OLEDs with fully-solution processed organic-layers. Mater. Chem. Front. 2018, 2, 1097–1103. [Google Scholar] [CrossRef]
- Gracia, I.; Feringán, B.; Serrano, J.L.; Termine, R.; Golemme, A.; Omenat, A.; Barberá, J. Functional Carbazole Liquid-Crystal Block Codendrimers with Optical and Electronic Properties. Chem. Eur. J. 2015, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Mula, S.; Frein, S.; Russo, V.; Ulrich, G.; Ziessel, R.; Barberá, J.; Deschenaux, R. Red and Blue Liquid-Crystalline Borondipyrromethene Dendrimers. Chem. Mater. 2015, 27, 2332–2342. [Google Scholar] [CrossRef] [Green Version]
- Huitorel, B.; Benito, Q.; Fargues, A.; Garcia, A.; Gacoin, T.; Boilot, J.-P.; Perruchas, S.; Camerel, F. Mechanochromic Luminescence and Liquid Crystallinity of Molecular Copper Clusters. Chem. Mater. 2016, 28, 8190–8200. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, S. Liquid crystals in photovoltaics: A new generation of organic photovoltaics. Polym. J. 2017, 49, 85–111. [Google Scholar] [CrossRef]
- Pieper, P.; Russo, V.; Heinrich, B.; Donnio, B.; Deschenaux, R. Liquid-Crystalline Tris[60]fullerodendrimers. J. Org. Chem. 2018, 83, 3208–3219. [Google Scholar] [CrossRef]
- Eremin, A.; Nádasi, H.; Hirankittiwong, P.; Kiang-Ia, J.; Chattham, N.; Haba, O.; Yonetake, K.; Takezoe, H. Azodendrimers as a photoactive interface for liquid crystals. Liq. Cryst. 2018, 45, 2121–2131. [Google Scholar] [CrossRef]
- Gruzdev, M.S.; Ramazanova, A.G.; Korolev, V.V.; Chervonova, U.V.; Balmasova, O.V.; Kolker, A.M. Phase Transitions in Mesogenic Third Generation Poly(Propyleneimine) Dendrimer and Its Iron(II) Complex. Russ. J. Inorg. Chem. 2020, 65, 640–645. [Google Scholar] [CrossRef]
- Iguarbe, V.; Barberá, J.; Serrano, J.L. Functional Janus dendrimers containing carbazole with liquid crystalline, optical and electrochemical properties. Liq. Cryst. 2020, 47, 301–308. [Google Scholar] [CrossRef]
- Concellón, A.; Liang, T.; Schenning, A.P.H.J.; Serrano, J.L.; Romero, P.; Marcos, M. Proton-conductive materials formed by coumarin photocrosslinked ionic liquid crystal dendrimers. J. Mater. Chem. C 2018, 6, 1000–1007. [Google Scholar] [CrossRef] [Green Version]
- Rosen, B.M.; Wilson, C.J.; Wilson, D.A.; Peterca, M.; Imam, M.R.; Percec, V. Dendron-Mediated Self-Assembly, Disassembly, and Self-Organization of Complex Systems. Chem. Rev. 2009, 109, 6275–6540. [Google Scholar] [CrossRef]
- Lyu, X.; Xiao, A.; Shi, D.; Li, Y.; Shen, Z.; Chen, E.-Q.; Zheng, S.; Fan, X.-H.; Zhou, Q.-F. Liquid crystalline polymers: Discovery, development, and the future. Polymer 2020, 202, 122740. [Google Scholar] [CrossRef]
- Park, M.; Kang, D.-G.; Choi, Y.-J.; Yoon, W.-J.; Koo, J.; Park, S.-H.; Ahn, S.; Jeong, K.-U. Kinetically Controlled Polymorphic Superstructures of Pyrene-Based Asymmetric Liquid Crystal Dendron: Relationship Between Hierarchical Superstructures and Photophysical Properties. Chem. Eur. J. 2018, 24, 9015–9021. [Google Scholar] [CrossRef]
- Kuang, G.-C.; Jia, X.-R.; Teng, M.-J.; Chen, E.-Q.; Li, W.-S.; Ji, Y. Organogels and Liquid Crystalline Properties of Amino Acid-Based Dendrons: A Systematic Study on Structure–Property Relationship. Chem. Mater. 2012, 24, 71–80. [Google Scholar] [CrossRef]
- Chuang, W.-T.; Lo, T.-Y.; Huang, Y.-C.; Su, C.-J.; Jeng, U.S.; Sheu, H.-S.; Ho, R.-M. Directing the Interfacial Morphology of Hierarchical Structures of Dendron-Jacketed Block Copolymers via Liquid Crystalline Phases. Macromolecules 2014, 47, 6047–6054. [Google Scholar] [CrossRef]
- Young, C.-M.; Chang, C.L.; Chen, Y.-H.; Chen, C.-Y.; Chang, Y.-F.; Chen, H.-L. Dendrimer-mediated columnar mesophase of surfactants. Soft Matter 2021, 17, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Saez, I.M.; Goodby, J.W. Supermolecular liquid crystals. J. Mater. Chem. 2005, 15, 26–40. [Google Scholar] [CrossRef]
- Hahn, H.; Keith, C.; Lang, H.; Amaranatha Reddy, R.; Tschierske, C. First Example of a Third-Generation Liquid-Crystalline Carbosilane Dendrimer with Peripheral Bent-Core Mesogenic Units: Understanding of “Dark Conglomerate Phases”. Adv. Mater. 2006, 18, 2629–2633. [Google Scholar] [CrossRef]
- Marcos, M.; Martín-Rapún, R.; Omenat, A.; Serrano, J.L. Highly congested liquid crystal structures: Dendrimers, dendrons, dendronized and hyperbranched polymers. Chem. Soc. Rev. 2007, 36, 1889–1901. [Google Scholar] [CrossRef]
- Vergara, J.; Gimeno, N.; Cano, M.; Barberá, J.; Romero, P.; Serrano, J.L.; Ros, M.B. Mesomorphism from Bent-Core Based Ionic Dendritic Macromolecules. Chem. Mater. 2011, 23, 4931–4940. [Google Scholar] [CrossRef]
- Olofsson, K.; Andrén, O.C.J.; Malkoch, M. Recent advances on crosslinked dendritic networks. J. Appl. Polym. Sci. 2014, 131, 39876. [Google Scholar] [CrossRef]
- Donnio, B.; Buathong, S.; Bury, I.; Guillon, D. Liquid crystalline dendrimers. Chem. Soc. Rev. 2007, 36, 1495–1513. [Google Scholar] [CrossRef]
- Lai, L.-L.; Hsu, S.-J.; Hsu, H.-C.; Wang, S.-W.; Cheng, K.-L.; Chen, C.-J.; Wang, T.-H.; Hsu, H.-F. Formation of Columnar Liquid Crystals on the Basis of Unconventional Triazine-Based Dendrimers by the C3-Symmetric Approach. Chem. Eur. J. 2012, 18, 6542–6547. [Google Scholar] [CrossRef]
- Lai, L.L.; Wang, S.W.; Cheng, K.L.; Lee, J.J.; Wang, T.H.; Hsu, H.F. Induction of the columnar phase of unconventional dendrimers by breaking the C2 symmetry of molecules. Chem. Eur. J. 2012, 18, 15361–15367. [Google Scholar] [CrossRef]
- Lai, L.-L.; Lee, C.-H.; Wang, L.-Y.; Cheng, K.-L.; Hsu, H.-F. Star-Shaped Mesogens of Triazine-Based Dendrons and Dendrimers as Unconventional Columnar Liquid Crystals. J. Org. Chem. 2008, 73, 485–490. [Google Scholar] [CrossRef]
- Lai, L.-L.; Hsieh, J.-W.; Cheng, K.-L.; Liu, S.-H.; Lee, J.-J.; Hsu, H.-F. A Small Change in Central Linker Has a Profound Effect in Inducing Columnar Phases of Triazine-Based Unconventional Dendrimers. Chem. Eur. J. 2014, 20, 5160–5166. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Hsieh, J.-W.; Lai, L.-L.; Cheng, K.-L.; Liu, S.-H.; Lee, J.-J.; Hsu, H.-F. Converting Nonliquid Crystals into Liquid Crystals by N-Methylation in the Central Linker of Triazine-Based Dendrimers. J. Org. Chem. 2016, 81, 5007–5013. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Huang, C.-C.; Li, C.-Y.; Lai, L.-L.; Lee, J.-J.; Hsu, H.-F. Both increasing the Iso-to-Col transition and lowering the solidifying temperatures of a triazine-based dendrimer by introducing CN polar groups in the dendritic core. J. Mater. Chem. C 2019, 7, 14232–14238. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Hsu, H.-F.; Lai, L.-L. Unconventional Approaches to Prepare Triazine-Based Liquid Crystal Dendrimers. Nanomaterials 2021, 11, 2112. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, W. Poly (vinyl diaminotriazine): From Molecular Recognition to High-Strength Hydrogels. Macromol. Rapid Commun. 2018, 39, e1800190. [Google Scholar] [CrossRef]
- Vinayakumara, D.R.; Kumar, S.; Adhikari, A.V. Supramolecular columnar self-assembly of wedge-shaped rhodanine based dyes: Synthesis and optoelectronic properties. J. Mol. Liq. 2019, 274, 215–222. [Google Scholar] [CrossRef]
- Jber, N.R.; Karam, N.H.; Al-Dujaili, A.H. Supramolecular columnar discotic nematic liquid crystal by hydrogen bonding: Synthesis and characterization. Mol. Cryst. Liq. Cryst. 2018, 675, 29–38. [Google Scholar] [CrossRef]
- Champagne, P.-L.; Ester, D.; Polan, D.; Williams, V.E.; Thangadurai, V.; Ling, C.-C. Amphiphilic Cyclodextrin-Based Liquid Crystals for Proton Conduction. J. Am. Chem. Soc. 2019, 141, 9217–9224. [Google Scholar] [CrossRef] [PubMed]
- Devadiga, D.; Ahipa, T.N. Recent advancements in the mesogens comprising of 1,3,5-triazine core moiety. Liq. Cryst. Rev. 2019, 7, 107–141. [Google Scholar] [CrossRef]
- Mu, B.; Zhao, Y.; Li, X.; Quan, X.; Tian, W. Enhanced Conductivity and Thermochromic Luminescence in Hydrogen Bond-Stabilized Columnar Liquid Crystals. ACS Appl. Mater. Interfaces 2020, 12, 9637–9645. [Google Scholar] [CrossRef] [PubMed]
- Vinayakumara, D.R.; Kumar, S.; Prasad, S.K.; Adhikari, A.V. Self-assembly of taper- and wedge-shaped maleimide derivatives: Synthesis and structure-property relationship. J. Mol. Liq. 2019, 284, 765–772. [Google Scholar] [CrossRef]
- Kaafarani, B.R. Discotic Liquid Crystals for Opto-Electronic Applications. Chem. Mater. 2011, 23, 378–396. [Google Scholar] [CrossRef]
- Kumar, S.; Bisoyi, H.K. Aligned Carbon Nanotubes in the Supramolecular Order of Discotic Liquid Crystals. Angew. Chem. Int. Ed. 2007, 46, 1501–1503. [Google Scholar] [CrossRef]
- Termine, R.; Golemme, A. Charge Mobility in Discotic Liquid Crystals. Int. J. Mol. Sci. 2021, 22, 877. [Google Scholar] [CrossRef]
- Dhondge, A.P.; Chen, J.-Y.; Lin, T.; Yen, F.-M.; Li, K.-W.; Hsieh, H.-C.; Kuo, M.-Y. Di-2-(2-oxindolin-3-ylidene)malononitrile Derivatives for N-Type Air-Stable Organic Field-Effect Transistors. Org. Lett. 2018, 20, 40–43. [Google Scholar] [CrossRef]
- Dheivamalar, S.; Banu, K.B. A DFT study on functionalization of acrolein on Ni-doped (ZnO)6 nanocluster in dye-sensitized solar cells. Heliyon 2019, 5, e02903. [Google Scholar] [CrossRef] [Green Version]
- Amanullah; Ali, U.; Ans, M.; Iqbal, J.; Iqbal, M.A.; Shoaib, M. Benchmark study of benzamide derivatives and four novel theoretically designed (L1, L2, L3, and L4) ligands and evaluation of their biological properties by DFT approaches. J. Mol. Model. 2019, 25, 223. [Google Scholar] [CrossRef]
- Yen, M.-H.; Chaiprapa, J.; Zeng, X.; Liu, Y.; Cseh, L.; Mehl, G.H.; Ungar, G. Added Alkane Allows Thermal Thinning of Supramolecular Columns by Forming Superlattice—An X-ray and Neutron Study. J. Am. Chem. Soc. 2016, 138, 5757–5760. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-C.; Hsu, Y.-T.; Yang, T.-Y.; Liou, I.-C.; Wang, S.-W.; Huang, P.-C.; Lee, J.-J.; Lai, L.-L.; Hsu, H.-F. Converting non-Mesogenic to Mesogenic Stacking of Amino-s-Triazine-Based Dendrons with p-CN Phenyl Unit by Eliminating Peripheral Dipole. Nanomaterials 2022, 12, 185. https://doi.org/10.3390/nano12020185
Lu Y-C, Hsu Y-T, Yang T-Y, Liou I-C, Wang S-W, Huang P-C, Lee J-J, Lai L-L, Hsu H-F. Converting non-Mesogenic to Mesogenic Stacking of Amino-s-Triazine-Based Dendrons with p-CN Phenyl Unit by Eliminating Peripheral Dipole. Nanomaterials. 2022; 12(2):185. https://doi.org/10.3390/nano12020185
Chicago/Turabian StyleLu, Yao-Chih, Yu-Tsz Hsu, Tsung-Yen Yang, I-Chun Liou, Sheng-Wei Wang, Po-Chia Huang, Jey-Jau Lee, Long-Li Lai, and Hsiu-Fu Hsu. 2022. "Converting non-Mesogenic to Mesogenic Stacking of Amino-s-Triazine-Based Dendrons with p-CN Phenyl Unit by Eliminating Peripheral Dipole" Nanomaterials 12, no. 2: 185. https://doi.org/10.3390/nano12020185
APA StyleLu, Y.-C., Hsu, Y.-T., Yang, T.-Y., Liou, I.-C., Wang, S.-W., Huang, P.-C., Lee, J.-J., Lai, L.-L., & Hsu, H.-F. (2022). Converting non-Mesogenic to Mesogenic Stacking of Amino-s-Triazine-Based Dendrons with p-CN Phenyl Unit by Eliminating Peripheral Dipole. Nanomaterials, 12(2), 185. https://doi.org/10.3390/nano12020185






