Abstract
Vanadium dioxide (VO2) has attracted interest from researchers because it undergoes a metal–insulator phase transition (MIT), which is accompanied by a reversible and remarkable change in both electrical and optical properties. VO2 exhibits numerous polymorphs and thus it is essential to control the growth of specific monoclinic VO2 (M) and rutile VO2 (R) phases. In this study, we developed a cost-effective and facile method for preparing VO2 nanorods with a highly crystalline monoclinic phase by one-step hydrothermal synthesis, in which only V2O5 and H2C2O4 are used as raw materials. The phase evolution of VO2 during the hydrothermal process was studied. The obtained VO2 nanorods were thoroughly mixed with fluorocarbon resin and homogeneous emulsifier in an ethanol solution to obtain a VO2 dispersion. To prepare VO2 films, screen printing was performed with a stainless steel screen mesh mask on glasses or fabric substrate. The VO2 coating had good thermochromic performance; the infrared transmittance change was greater than 20% @1.5 μm whilst keeping the visible transmittance greater than 50%. Meanwhile, the polyester base coating on the fabric had an emissivity change of up to 22%, which provides a solution for adaptive IR camouflage.
1. Introduction
VO2 is the most common thermal-induced metal–insulator transition (MIT) material, which is characterized by a subtle and reversible structural transformation from VO2 (M) to VO2 (R) at the critical temperature (τc) of ~68 °C [,,] (“M” and “R” are abbreviations for monoclinic and rutile, respectively). The MIT of VO2 displays a significant change in its infrared optical property, which means that VO2 (M) can be transparent to infrared solar irradiation, and that VO2 (R) has an adverse effect on infrared irradiation at a temperature higher than τc. Using this property, it can be observed that VO2 possesses excellent potential for a wide range of applications, including optic switches [,,,], energy-efficient smart windows [,,], and infrared camouflage purposes [,,]. In addition to VO2 (M/R), VO2 can also adopt other kinds of polymorphs, including monoclinic VO2 (B), triclinic VO2 (T), tetragonal VO2 (A), and parametricities VO2 (P). Among them, VO2 (M/R) is the most practical material due to its τc value that is close to ambient temperature. Furthermore, similar to most transition metals, vanadium exists in a wide range of oxidation states, which result in the existence of several intermediate vanadium oxides during the synthesis of VO2. Therefore, the study of a facile and suitable method of preparing the specific VO2 (M/R) phase presents many opportunities for optimization, with the main final goal of controlling the desirable size, morphology, and crystallinity of VO2 to satisfy different specific demands for practical applications. For example, for the sake of good dispersion, the quantity of VO2 (M/R) powder on the nanometer level is important; as regards the infrared modulation, the better the crystallization, the better the regulation ability.
Extensive studies have been conducted to prepare VO2 thin films or nanopowders, such as the sol–gel method [], vapor phase transport [], pulsed-laser deposition [] hydrothermal treatment [], and magnetron-sputter []. By contrast, hydrothermal treatment has been considered the main method used to prepare VO2 because it functions at both a high temperature and pressure that are well-suited to control the morphology, crystallinity, and chemical stoichiometry of the final product []. Unfortunately, to date, hydrothermal reactions in solution have mostly yielded the metastable phases of VO2 (B) or VO2 (A) because the reaction time is not long enough or the reaction temperature is not high enough, as shown in Figure S1. Therefore, the one-step hydrothermal process directly synthesizing thermodynamically stable phases of VO2 (M/R) has attracted significant attention in the research.
The growth of VO2 (R) was first reported by the hydrothermal reaction of the V2O3-V2O5-H2O system at temperatures higher than 350 °C []. VO2 (R) nanorods were described by Jin et al. [] in a study for which H2SO4 was used as a very effective addition for the morphology control of VO2. Gao et al. developed a process that could prepare single-crystal VO2 (R) snowflake powders by the hydrothermal treatment of V2O5 and oxalic acid at 240 °C for 7 days []. Micro- and nanocrystals of VO2(M)were prepared via the hydrothermal method using N2H4 as a reducing agent []. Wang et al. [] confirmed that a certain quantity of W dopants could promote a phase transformation from VO2(B) to VO2(M/R) during hydrothermal synthesis; however, excess content can lead to the generation of undesirable crystals and a lower purity of VO2. Zou et al. [] prepared VO2 nanoparticles via the hydrothermal treatment of NH4VO3, using N2H4·H2O as a reducing agent at 340 °C for 6h. The prepared powder was VO2 (M), including nanoparticles with particle sizes between 20 nm and 100 nm, and nanorods that were approximately 200 nm long. A novel, nested, hydrothermal reactor was proposed by Jin et al. in their study, where they separated the oxidant of hydrogen peroxide from the precursor solution; a reaction was performed at 260 °C for 24 h, and VO2(M) nanoparticles with an average size ∼30 nm were prepared []. In general, for the one-step hydrothermal synthesis, toxic hydrazine was extensively used to obtain VO2(M/R); however, sometimes other steps are essential to prepare the hydrothermal precursor. Thus, there still remains an opportunity to develop a facile and environmentally friendly hydrothermal route for synthesizing VO2 (M/R). While many studies report on VO2 nanoparticles, there is less literature concerning the one-step hydrothermal synthesis of VO2 nanorods for the exploration of thermochromic and infrared camouflage properties.
The main purpose of the current study was to directly perform the hydrothermal synthesis of VO2(M/R), in which only V2O5 and H2C2O4 were used as raw materials. To prepare VO2 films, we performed screen printing with a stainless steel screen mesh mask on glasses or a fabric substrate. The VO2 coating had an obvious infrared modulation ability and displayed a good thermochromic performance. Moreover, the polyester base coating on fabric presented an emissivity change of up to 22%, which provides great potential for adaptive infrared camouflage applications.
2. Materials and Methods
2.1. Materials and Synthesis
In the experiments, all of the chemicals, including V2O5 (99.7%, Sarn Chemical Technology, Shanghai, China), C2H2O4·2H2O (analytical purity, Shanghai Qianshun Chemical Reagent, Shanghai, China), C2H5OH (99.7%, Shanghai Titan, Shanghai, China), and Disperbyk-110 (copolymer-containing acid group, 52% nonvolatile component, BYK, Shanghai, China) were used directly, without further purification. In a typical hydrothermal process, 1.82 g of V2O5 powder was added to 60 mL of deionized water at room temperature and stirred thoroughly for 2 h. The solution changed from brownish yellow to a blue-green clarified solution. Then, different quantities of C2H2O4·2H2O were added to the solution and continuously stirred until the C2H2O4·2H2O was completely dissolved. According to the different molar ratios between C2H2O4·2H2O and V2O5 of 0.5:1,1:1,1.5:1,2:1,2.5:1 and 3:1, the samples were labeled as S1, S2, S3, S4, S5, and S6, respectively. The obtained precursor solution was transferred into a 100 mL PPL material-lined stainless-steel autoclave, and the reactor was sealed. The hydrothermal temperature was set to 280 °C and the reaction time was 48 h. The samples were cooled to room temperature in air at the end of the reaction process. A black precipitate was collected by centrifugation and washed three times with deionized water and ethanol in turn. The final black powder solution was obtained after drying in a vacuum oven at 60 °C for 12 h. There was no subsequent annealing treatment.
2.2. Preparation of VO2 Coatings
2.2.1. Preparation of VO2 Coating on Polyester Fabric Surface
A total of 1 g of VO2 powder and 0.1 mL of Disperbyk-110 dispersant were mixed together in 10 mL of ethanol and sonicated for 30 min, and then a high shear emulsifier was added to completely disperse the VO2 powder. A total of 2 g of resin (fluorocarbon resin, PVP, aqueous polyurethane) was used to obtain the ink for printing. The screen-printing technique was used to print the VO2 coating evenly on the polyester fabric. The screen used for the printing process was 200 mesh to ensure that the VO2 nanopowders could be smoothly printed on the surface of the base fabric. The printed fabric was dried on a constant-temperature heating table at 50 °C for 20 min to remove the solvent to form a uniform and firm coating.
2.2.2. Preparation of VO2 Coating on Quartz Surface
A spin coater was used to prepare VO2 optical coatings on the surface of quartz glass using the same ink as mentioned above. The spin-coater speed was set at 400 r/min, acceleration at 100 r/s, and spin-coating time at 65 s. The coatings were prepared with different thicknesses from one to four spin coatings.
2.3. Characterization
An X-ray diffractometer (RIKEN SmartSab 9kw, Wakō, Japan) was used to characterize the phase composition and crystalline structure of the samples, with a CuKα source, a target voltage of 40 kv, a target current of 30 mA, a scan range of 5°~90°, a scan speed of 5 (°)/min, and a scan length of 0.2°. The surface morphology of the samples was characterized by field-emission electron microscopy (Zeiss MERLIN and FEI Quanta 400 Feg, Jena, Germany). A differential scanning calorimeter (NETZSCH DSC 214, Selb, Germany) was used to characterize the phase-transition behavior of the samples upon a heating and cooling rate of 10 °C/min under an N2 atmosphere. An IR-2 infrared emissometer (Shanghai Institute of Technical Physics, Shanghai, China) was applied to analyze the normal integrated emissivity of fabrics in the 8–14 μm wavelength range. All the emissivity values were obtained as an average of three measurements. The infrared thermogram of the coating was obtained using an infrared thermographic camera (FLIR E5xt, Wilsonville, OR, USA).
3. Results and Discussion
3.1. VO2 Nanorods
Figure 1 presents the XRD plots of S1 and S2 samples; evidently, the insufficient quantity of oxalic acid cannot reduce all V2O5 to VO2 at a ratio lower than 1:1. For S1, it was a mixture phase of V2O5 (JCPDS Card No. 97-015-7988), V4O9 (JCPDS Card No. 97-001-5041), and V3O7 (JCPDS Card No. 97-000-2338). The diffraction peaks at 2θ of 15.424°, 20.352°, 21.787°, 26.221°, 31.070°, and 45.605° correspond to (200), (010), (110), (101), (310), and (411) of V2O5, respectively; peaks at 2θ of 9.860°, 17.575°, 26.292°, 37.143°, 50.211°, and 69.522° belong to (200), (301), (011), (412), (020), and (822) of V4O9, respectively; and the (200), (600), (-111), (006), and (020) crystal planes of V3O7 present diffraction peaks at 8.099°, 24.462°, 24.926°, 29.334°, and 49.512°, respectively. For S2, all of the three phases mentioned above disappeared, and vanadium oxide was reduced further to lower valence states of V6O13 (JCPDS Card No. 01-089-0100) and VO2 (JCPDS Card No. 00-043-1051). The characteristic diffraction peak at 27.796° corresponded to the (011) crystalline plane of VO2 (M), while the peaks at 2θ = 8.873°, 15.125°, 17.800°, 25.359°, and 26.838° can be assigned to the (001), (200), (002), (110), and (003) crystal faces of V6O13, respectively. The crystallographic data of the vanadium oxides observed in S1 and S2 are presented in Table 1.
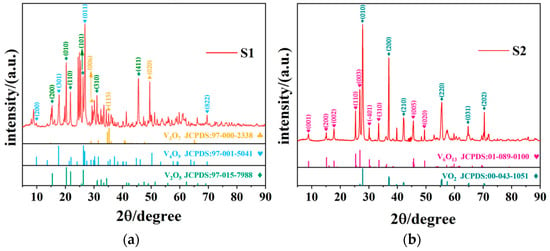
Figure 1.
XRD data of (a) S1 (reactants ratio 0.5:1) and (b) S2 (reactants ratio 1:1) samples.

Table 1.
Crystallographic data of different products in the sample.
Generally, the S1 sample indicates that the low concentration of oxalic acid is not sufficient to completely reduce V2O5, and some unreacted V2O5, as well as intermediate products of V4O9 and V3O7, coexist in S1, while VO2 (M) is evident in S2, but the intermediate product of V6O13 remains present. Therefore, a higher concentration of oxalic acid is necessary to obtain pure VO2.
Figure 2 presents the XRD data for the hydrothermal product when the precursor ratio between C2H2O4·2H2O and V2O5 is greater than 1:1; all of the products are VO2 (M). The diffraction peaks at 2θ = 27.796°, 37.089°, 42.079°, 55.451°, 64.972°, and 70.256° for the four samples corresponded to the (011), (200), (210), (220), (031), and (202) crystalline planes of VO2 (M), respectively, which indicates the purity of the obtained samples. By contrast, the diffraction peak of the S4 sample was higher than that of the other three of samples, implying the good crystalline properties of S4. Meanwhile, as can be observed from S5 and S6, the much higher concentration of oxalic acid is not beneficial to the purity of VO2 and leads to the poor crystallinity of the samples. Overall, the main XRD peaks of these four samples are basically consistent. This kind of consistency is also reflected in their morphology, as presented in the SEM in Figure 3. All of four samples (S2–S5) presented short, rod-like structures, but some differences still remained. For S3, there were obviously some larger micron-scale layers and lumps of crystals, and the particle size was not uniform across the sample. A small amount of flakes close to the micron scale were also observed in S5 and S6, suggesting that excessive oxalic acid was not beneficial to forming uniform samples. The nanorods present in S4 were much more uniform and presented good dispersity properties.
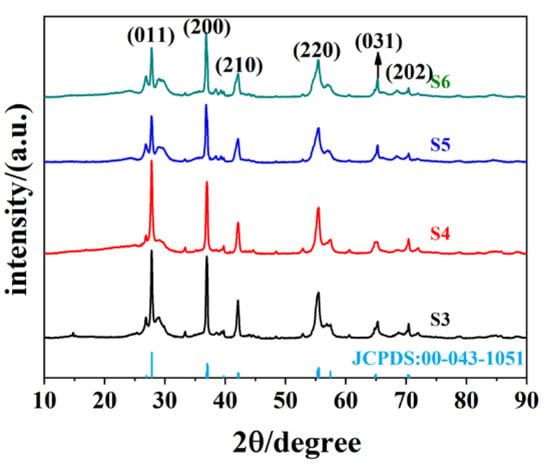
Figure 2.
XRD data for the hydrothermal product with different molar ratios between C2H2O4·2H2O and V2O5, and ratios for S3–S6 are 1.5:1, 2:1, 2.5:1, and 3:1, respectively.
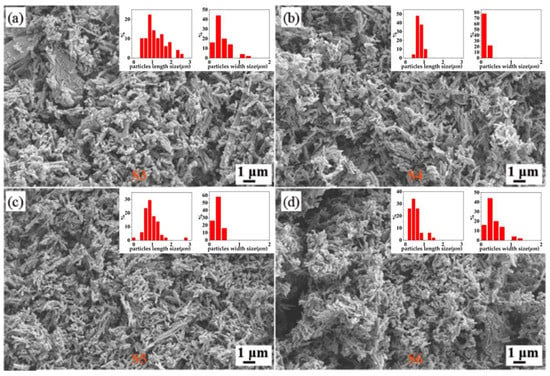
Figure 3.
SEM images of (a) S3 (reactants ratio 1.5:1), (b) S4 (reactants ratio 2:1), (c) S5 (reactants ratio 2.5:1), and (d) S6 (reactants ratio 3:1) samples.
Figure 4 presents the DSC data obtained for samples of S1–S6. Distinct endothermic and exothermic peaks were observed during the heating and cooling cycles, respectively, for samples S2–S6. The thermal hysteresis indicates fully reversible and first-order phase-transition features. The phase-transition temperatures and enthalpies of the samples were determined by particular software (NETZSCH Proteus Thermal Analysis). As shown in Figure 4b, τc is mainly concentrated at approximately ~70 °C. It can be observed that sample S4 presents the highest τc of 72.2 °C, indicating the good crystallinity, which is consistent with the XRD data. The underlying mechanism was mainly related to the defects that can act as the nucleation site to trigger the phase transition from VO2(M) to VO2(R) []. S4 presents relatively fewer defects and thus produces a higher τc value. The highest phase-change enthalpy (ΔH) for S4 also indicates that it presents the best crystallinity (Figure 4b). The higher enthalpy presented during the phase transition indicates that the infrared modulation property of sample S4 will considerably change, which is reflected by the fact that its emissivity changes more rapidly than the other samples. Therefore, in the subsequent experiment, sample S4 was selected for the preparation of a VO2 coating.
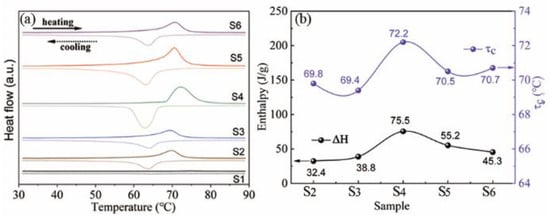
Figure 4.
(a) DSC data of samples with different oxalic acid concentrations; (b) phase-change enthalpy (ΔH) and transition temperature () versus hydrothermal duration.
3.2. Infrared Camouflage and Thermochromic Properties
VO2 coating is printed on the surface of polyester fabric using screen-printing technology. Polyester fabric is, at present, the most widely used fabric material for the factory production of camouflage nets because it is a high-strength fiber with good strength and toughness properties that is not easily damaged, and it is heat resistant and not easily deformed. Screen-printing technology is a convenient and practical printing technology that can use various types of inks, such as oil-based, water-based, synthetic-resin emulsion type, and powder; at the same time, it is not limited by the shape of the substrate’s surface and the size of the area, which exhibits a high operability quality and is suitable for a wide range of purposes and uses.
In the current paper, three kinds of resins commonly used to prepare infrared stealth coatings were used to prepare the coatings: aqueous polyurethane (PU) [], PVP [], and fluorocarbon resin []. The advantage of the three resins is that they are all infrared transparent resins. The resins do not affect the infrared properties of the prepared coatings, and the sample powders can be well dispersed in the three resins. However, PU and PVP are waterborne resins, and the coating stability following film formation is not as good as that of fat-soluble fluorocarbon resin. The SEM images of the printed coating are presented in Figure 5a–c. It can be observed from the figure that the coating surface of water-based polyurethane is rough and cannot completely cover the fibers of the polyester base cloth; the coating surface of the PVP resin is smooth but there are a lot of bubbles present. The coating surface of the fluorocarbon resin is more delicate, and the VO2 powder is evenly adhered to the fiber surface. Therefore, the fluorocarbon resin was finally elected as the film-forming substance for the coating preparation. Figure 5c,d present the SEM images of the surface and cross-section of the coating formed by printing once and twice, respectively. The surface fibers of the twice-printed coating are completely covered by the coating, and the surface is fine without cracks and bubbles. From the cross-sectional view, it can be observed that the average thickness of the coating is approximately 10 μm for the once-printed coating and approximately 20 μm for the twice-printed coating, doubling the thickness.
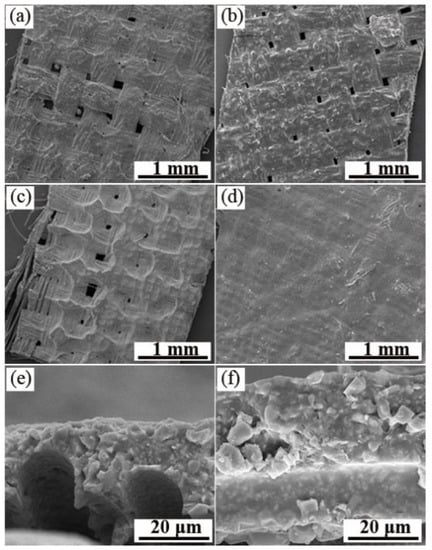
Figure 5.
SEM images of the surface and interface of the coating. Coating surface on which the film-forming substance is present; (a) waterborne polyurethane; (b) PVP; (c) fluorocarbon resin; (d) fluorocarbon-resin-coated surface printed twice; (e) fluorocarbon-resin-coated cross-section printed once; and (f) fluorocarbon-resin-coated cross-section printed twice.
Figure 6a–c presents the values of the surface’s normal, integrated emissivity of the polyester base fabric, and the once- and twice-coated films using an IR-2 dual-band emissivity tester. The emissivity-modulation ability of the coating increased significantly with the increase in the coating’s thickness. The maximum change in the emissivity of the once-printed coating was 0.1, reaching 10.6%; the maximum change in the emissivity of the twice-printed coating was 0.19, reaching 22%; and the surface of the twice-printed coating was smoother and flatter; therefore, its room-temperature emissivity was lower than that of the polyester-based fabric.
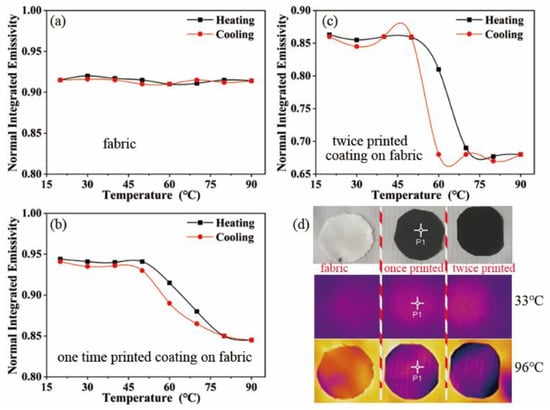
Figure 6.
(a) Emissivity of polyester base fabric at different temperatures; (b) emissivity of once-printed coatings at different temperatures; (c) emissivity of twice-printed coatings at different temperatures; (d) from top to bottom, the image presents the optical image, and normal-temperature and high-temperature thermal infrared images.
The thermal infrared camouflage capability of the coating was characterized using infrared thermography. Figure 6d presents the optical photographs of the polyester base fabric, once-printed coating, as well as the twice-printed coating, with three samples placed on the same constant-temperature heating table. The figure presents the infrared thermal image at room temperature, and the uncoated sample has almost the same thermal radiation intensity as the coated sample. When the temperature reaches 96 °C, the coated sample has a significantly lower thermal radiation intensity and can blend better with the background, achieving the effect of adaptive camouflage.
Figure 7 exhibits the optical transmittance curves of the coatings with different thicknesses formed by spin coating on the surface of quartz glass using the above-selected inks, in which the solid and dashed lines indicate the low- and high-temperature cases, respectively. The four samples were spin-coated 1–4 times, numbered 1–4 and in order, and the transmittance curves present that the low-temperature transmittance decreases with the increase in the coating’s thickness, from approximately 80% to less than 60%, but the optical-transmittance-modulation ability of the coating continuously increases with the increase in the thickness. The amount of infrared transmittance changes for samples 1, 2, 3, and 4 were 14%, 22%, 27%, and 29% @1.5 μm, respectively, while maintaining the visible transmittance at a value greater than 50%. Ru Chen et al. also prepared VO2 films in their study. The infrared transmittance changes in the films printed once and twice were 20% and 23%, respectively []. The thermochromic properties of the films prepared in our study are comparable to those presented in the literature.
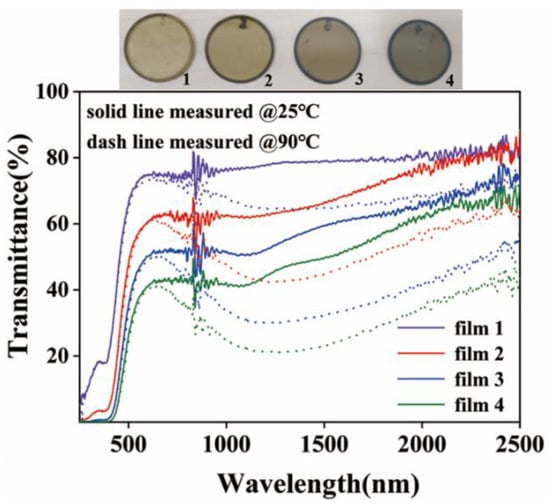
Figure 7.
Images and optical transmittance of samples with different spin-coating times.
4. Conclusions
The VO2 nanorods were synthesized by a one-step hydrothermal method using only V2O5 and H2C2O4 as the raw materials, and the optimum mole ratio between C2H2O4 and V2O5 was 2:1. Three different polymers were used to prepare the VO2 ink that was used to print the coating onto the surface of the polyester base cloth, and fluorocarbon resin was identified as the best dispersant. The flexible VO2 coating can adaptively change the surface’s emissivity property, and the changes in emissivity could reach 0.19, which presented good adaptability to the environment and was expected to be used for producing military tents, camouflage nets, and other equipment. The VO2 coating on the glass substrate also displayed a good thermochromic performance; the amount of infrared transmittance change was greater than 20% @1.5 μm while maintaining the visible transmittance value at a figure greater than 50%.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano12193534/s1, Figure S1: Hydrothermal product of VO2 versus hydrothermal temperature and reaction time; Table S1: the crystallography information of VO2 polymorphs.
Author Contributions
Conceptualization, Y.H. and W.X.; methodology, Y.H.; formal analysis, M.L.; investigation, J.Y.; resources, S.W.; data curation, X.Y.; writing—original draft preparation, Y.H.; writing—review and editing, M.L. and H.L.; visualization, Y.H.; project administration, S.W.; funding acquisition, W.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Laboratory Foundation Project of China. Fund number:6142206200205.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rice, T.M.; Brinkman, W.F. Effects of Impurities on the Metal-Insulator Transition. Phys. Rev. B 1972, 5, 4350–4357. [Google Scholar] [CrossRef]
- Lahneman, D.J.; Slusar, T.; Beringer, D.B.; Jiang, H.; Kim, C.-Y.; Kim, H.-T.; Qazilbash, M.M. Insulator-to-Metal Transition in Ultrathin Rutile VO2/TiO2(001). NPJ Quant. Mater. 2022, 7, 72. [Google Scholar] [CrossRef]
- Kim, H.-T.; Chae, B.-G.; Youn, D.-H.; Maeng, S.-L.; Kim, G.; Kang, K.-Y.; Lim, Y.-S. Mechanism and Observation of Mott Transition in VO2-Based Two- and Three-Terminal Devices. New J. Phys. 2004, 6, 52. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Zhang, Y.; Li, J.; Yang, Y.; Zhao, H.; Zheng, C.; Li, J.; Huang, J.; Li, F.; et al. All-optical switchable terahertz spin-photonic devices based on vanadium dioxide integrated metasurfaces. Opt. Commun. 2020, 460, 124986. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, W.; Wang, J.; Jiang, K.; Zhang, J.; Li, W.; Wu, J.; Hu, Z.; Chu, J. The electro-optic mechanism and infrared switching dynamic of the hybrid multilayer VO2/Al:ZnO heterojunctions. Sci. Rep. 2017, 7, 4425. [Google Scholar] [CrossRef]
- Xu, C.; Liu, G.; Li, M.; Li, K.; Luo, Y.; Long, Y.; Li, G. Optical switching and nanothermochromic studies of VO2(M) nanoparticles prepared by mild thermolysis method. Mater. Des. 2020, 187, 108396. [Google Scholar] [CrossRef]
- Kim, J.; Paik, T. Recent Advances in Fabrication of Flexible, Thermochromic Vanadium Dioxide Films for Smart Windows. Nanomaterials 2021, 11, 2674. [Google Scholar] [CrossRef]
- Victor, J.-L.; Gaudon, M.; Penin, N.; Chiron, A.; Chung, U.C.; Viraphong, O.; Rougier, A. Innovative sintering process for fabrication of thermochromic smooth VO2 ceramics. J. Alloys Compd. 2022, 890, 161890. [Google Scholar] [CrossRef]
- Cao, C.; Hu, B.; Tu, G.; Ji, X.; Li, Z.; Xu, F.; Chang, T.; Jin, P.; Cao, X. Sputtering Flexible VO2 Films for Effective Thermal Modulation. ACS Appl. Mater. Interfaces 2022, 14, 28105–28113. [Google Scholar] [CrossRef]
- Ji, H.; Liu, D.; Cheng, H. Infrared optical modulation characteristics of W-doped VO2(M) nanoparticles in the MWIR and LWIR regions. Mater. Sci. Semicond. Proc. 2020, 119, 105141. [Google Scholar] [CrossRef]
- Liu, D.; Ji, H.; Peng, R.; Cheng, H.; Zhang, C. Infrared chameleon-like behavior from VO2(M) thin films prepared by transformation of metastable VO2(B) for adaptive camouflage in both thermal atmospheric windows. Sol. Energy Mater. Sol. Cells 2018, 185, 210–217. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Zhang, X.; Wang, L.; Feng, W.; Li, Q. Beyond the Visible: Bioinspired Infrared Adaptive Materials. Adv. Mater. 2021, 33, 2004754. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Charpentier, P.A. Sol-gel processing of VO2 (M) in supercritical CO2 and supercritical CO2/ ionic liquid biphasic system. J. Supercrit. Fluids 2020, 165, 104989. [Google Scholar] [CrossRef]
- Shi, R.; Cai, X.; Wang, W.; Wang, J.; Kong, D.; Cai, N.; Chen, P.; He, P.; Wu, Z.; Amini, A.; et al. Single-Crystalline Vanadium Dioxide Actuators. Adv. Funct. Mater. 2019, 29, 1900527. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Liu, J.; Li, B.; Zhou, C.; Sheng, Z. Active and Smart Terahertz Electro-Optic Modulator Based on VO2 Structure. ACS Appl. Mater. Interfaces 2022, 14, 26923–26930. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Fan, W.; Li, D.; Wang, S.; Liu, Z.; Yu, A.; Jiang, X. Smart Textiles: Smart Thermal Management Textiles with Anisotropic and Thermoresponsive Electrical Conductivity. Adv. Mater. Technol. 2020, 5, 2070001. [Google Scholar] [CrossRef]
- Vu, T.D.; Liu, S.; Zeng, X.; Li, C.; Long, Y. High-power impulse magnetron sputtering deposition of high crystallinity vanadium dioxide for thermochromic smart windows applications. Ceram. Int. 2020, 46, 8145–8153. [Google Scholar] [CrossRef]
- Li, M.; Magdassi, S.; Gao, Y.; Long, Y. Hydrothermal Synthesis of VO2 Polymorphs: Advantages, Challenges and Prospects for the Application of Energy Efficient Smart Windows. Small 2017, 13, 1701147. [Google Scholar] [CrossRef]
- Theobald, F. Hydrothermal study of VO2-VO2.5-H2O system. J. Less Common Met. 1977, 53, 55–71. [Google Scholar] [CrossRef]
- Ji, S.; Zhao, Y.; Zhang, F.; Jin, P. Direct formation of single crystal VO2(R) nanorods by one-step hydrothermal treatment. J. Cryst. Growth 2010, 312, 282–286. [Google Scholar] [CrossRef]
- Cao, C.; Gao, Y.; Luo, H. Pure Single-Crystal Rutile Vanadium Dioxide Powders: Synthesis, Mechanism and Phase-Transformation Property. J. Phys. Chem. C 2008, 112, 18810–18814. [Google Scholar] [CrossRef]
- Son, J.H.; Wei, J.; Cobden, D.; Cao, G.; Xia, Y. Hydrothermal Synthesis of Monoclinic VO2 Micro- and Nanocrystals in One Step and Their Use in Fabricating Inverse Opals. Chem. Mater. 2010, 22, 3043–3050. [Google Scholar] [CrossRef]
- Li, Y.; Kong, F.; Wang, B.; Zhao, Y.; Wang, Z. Preparation of shape-controlling VO2(M/R) nanoparticles via one-step hydrothermal synthesis. Front. Optoelectron. 2020, 14, 311–320. [Google Scholar] [CrossRef]
- Zou, J.; Xiao, L.; Zhu, L.; Chen, X. One-step rapid hydrothermal synthesis of monoclinic VO2 nanoparticles with high precursors concentration. J. Sol-Gel Sci. Technol. 2019, 91, 302–309. [Google Scholar] [CrossRef]
- Guo, D.; Ling, C.; Wang, C.; Wang, D.; Li, J.; Zhao, Z.; Wang, Z.; Zhao, Y.; Zhang, J.; Jin, H. Hydrothermal One-Step Synthesis of Highly Dispersed M-Phase VO2 Nanocrystals and Application to Flexible Thermochromic Film. ACS Appl. Mater. Interfaces 2018, 10, 28627–28634. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, X.; Li, L.; Wang, Y.; Li, D.; Pan, J.; Li, S.; Sun, L.; Li, G. Defect-mediated phase transition temperature of VO2 (M) nanoparticles with excellent thermochromic performance and low threshold voltage. J. Mater. Chem. A 2014, 2, 4520–4523. [Google Scholar] [CrossRef]
- Yan, X.; Xu, G. Corrosion and Mechanical Properties of Polyurethane/Al Composite Coatings with Low Infrared Emissivity. J. Alloys Compd. 2010, 491, 649–653. [Google Scholar] [CrossRef]
- Chai, X.; Zhu, D.; Liu, Y.; Qing, Y.; Ren, Z.; Luo, F.; Zhou, W.; Huang, Z.; Li, P. Silver-Modified Chromium(III) Oxide as Multi-Band Compatible Stealth Materials for Visual/Infrared Stealth and Radar Wave Transmission. Compos. Sci. Technol. 2021, 216, 109038. [Google Scholar] [CrossRef]
- Huang, X.; Rao, W.; Chen, Y.; Ding, W.; Zhu, H.; Yu, M.; Chen, J.; Zhang, Q. Infrared Emitting Properties and Environmental Stability Performance of Aluminum/Polymer Composite Coating. J. Mater. Sci. Mater. Electron. 2016, 27, 5543–5548. [Google Scholar] [CrossRef]
- Chen, R.; Miao, L.; Cheng, H.; Nishibori, E.; Liu, C.; Asaka, T.; Iwamoto, Y.; Takata, M.; Tanemura, S. One-Step Hydrothermal Synthesis of V1−xWxO2(M/R) Nanorods with Superior Doping Efficiency and Thermochromic Properties. J. Mater. Chem. A 2015, 3, 3726–3738. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).