Recent Progress and Perspective: Na Ion Batteries Used at Low Temperatures
Abstract
1. Introduction
2. Cathode Materials
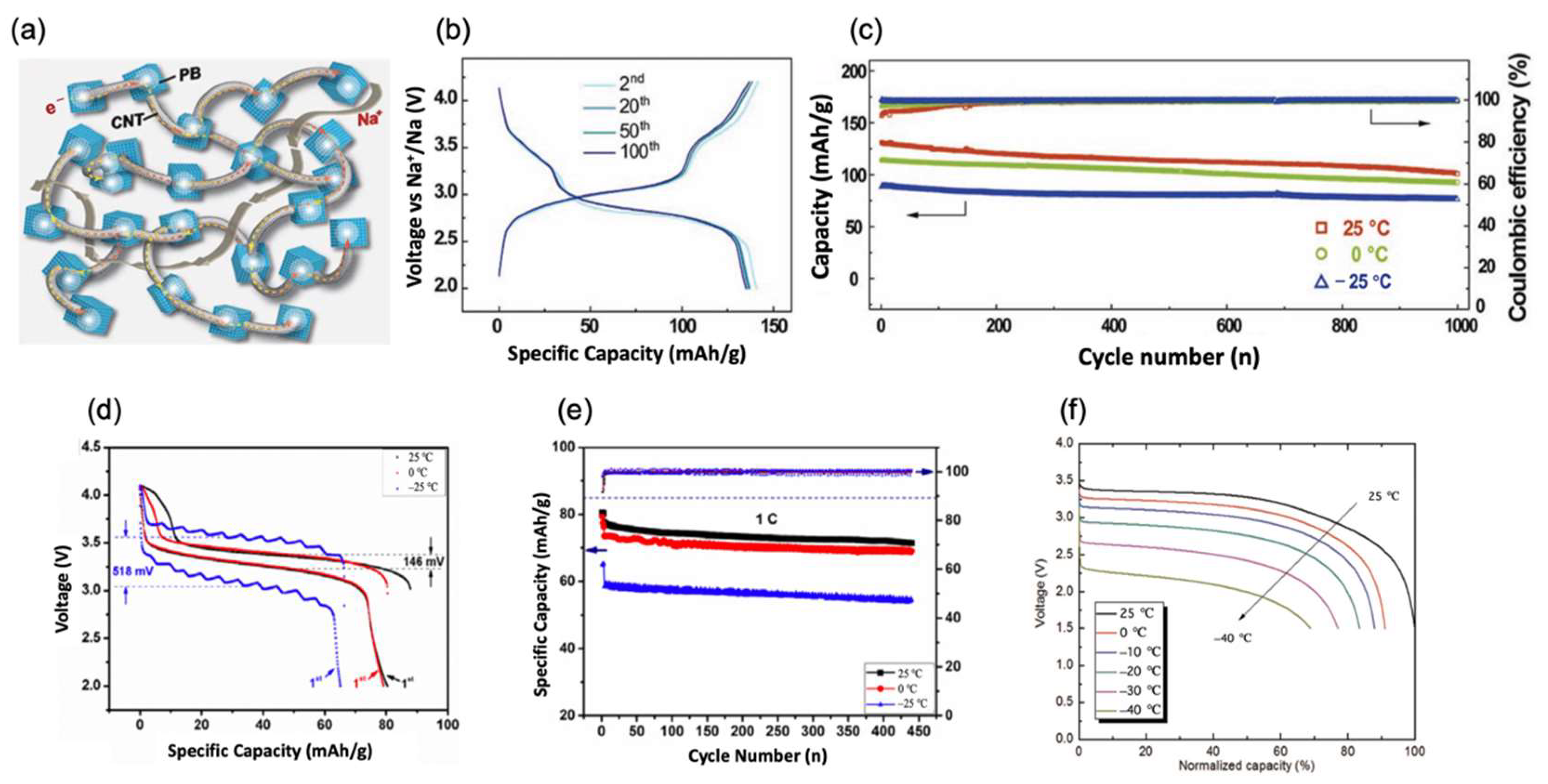
2.1. Prussian Blue (PB) and Prussian Blue Analogues (PBAs)
2.2. Layered Oxides
2.3. Polyanionic-Type Cathodes
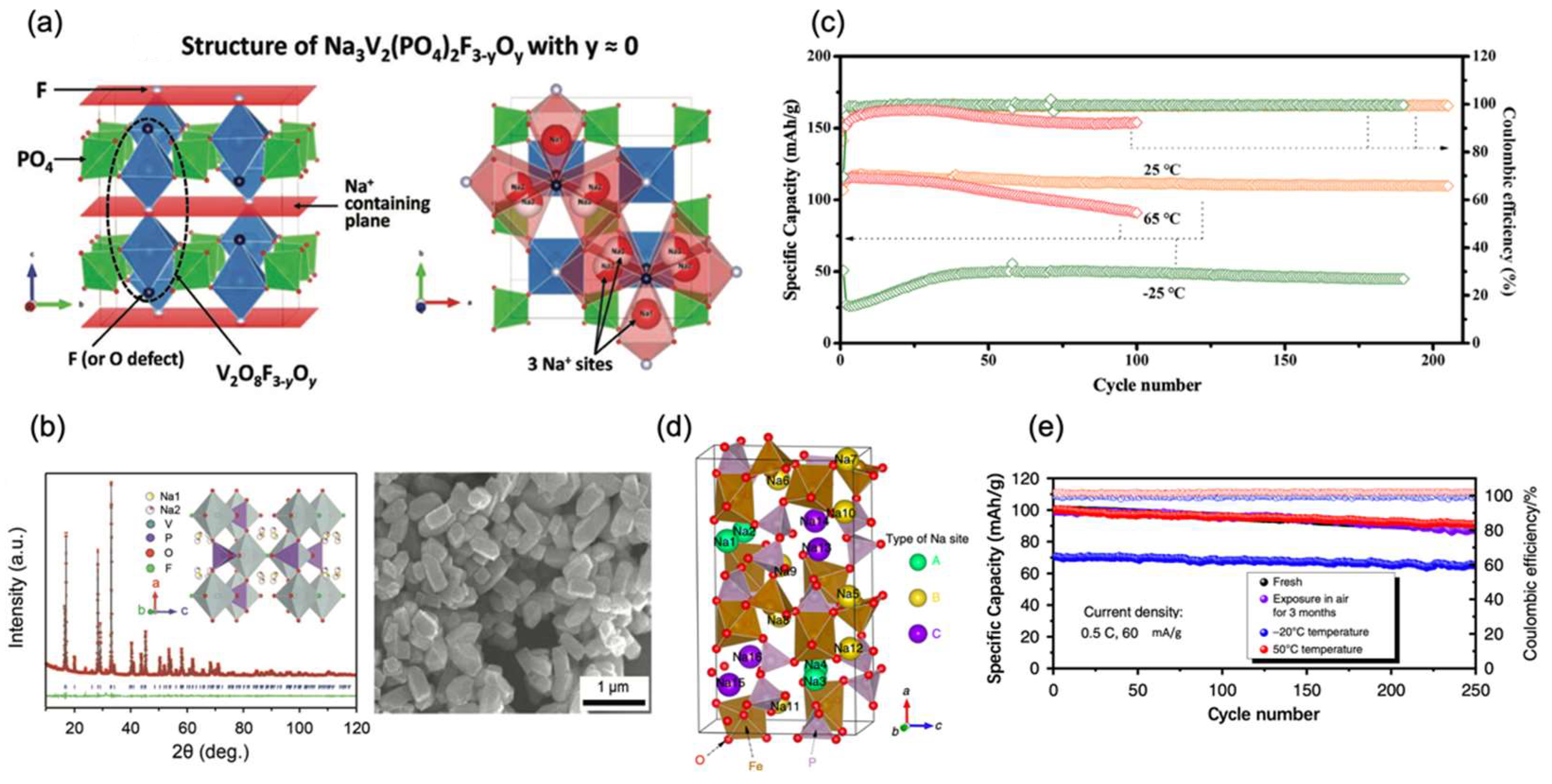
2.3.1. Phosphates
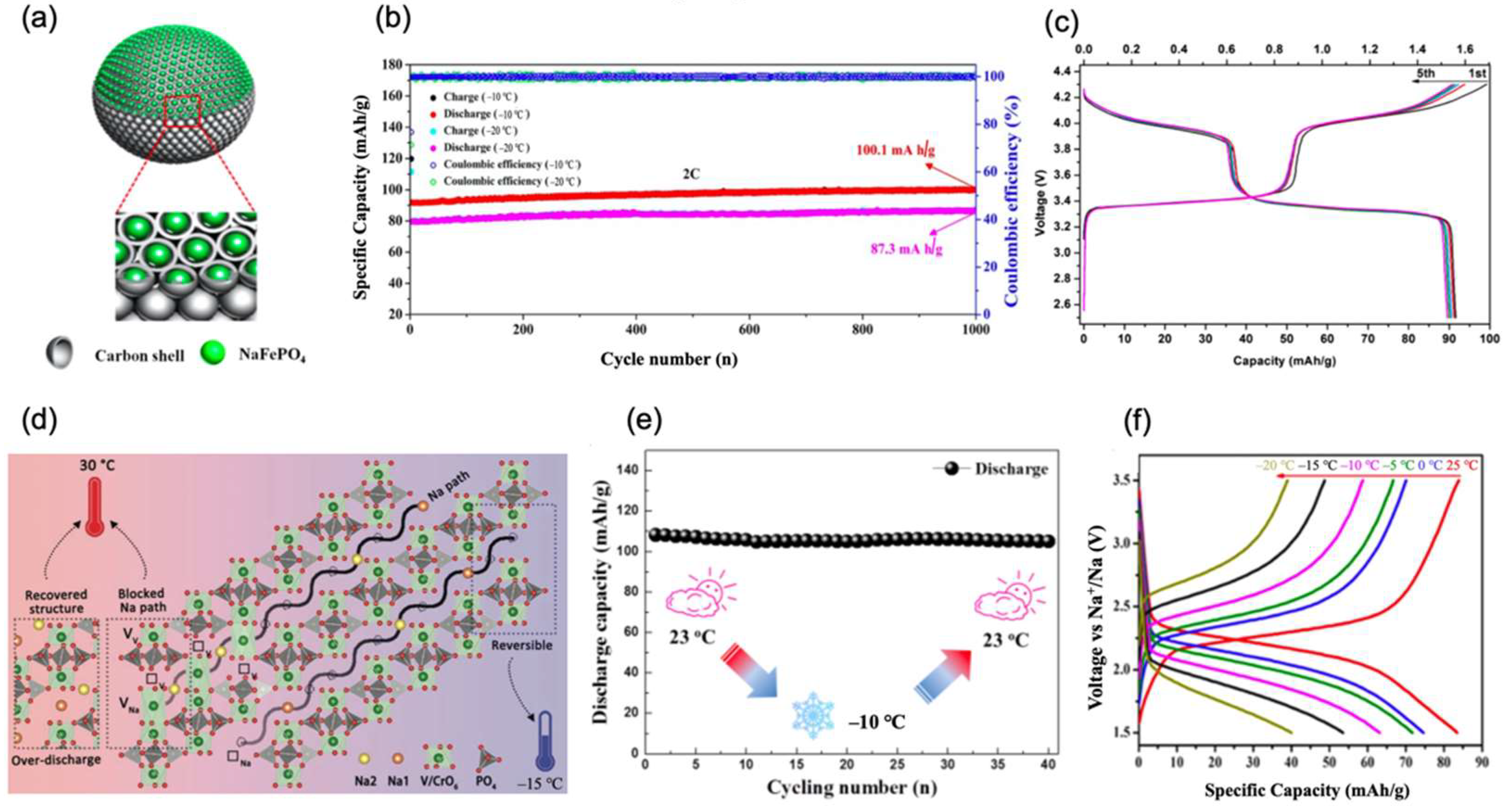
2.3.2. Fluorophosphates
3. Anodes
3.1. Hard Carbon
3.2. Amorphous Selenium and Metal Selenides
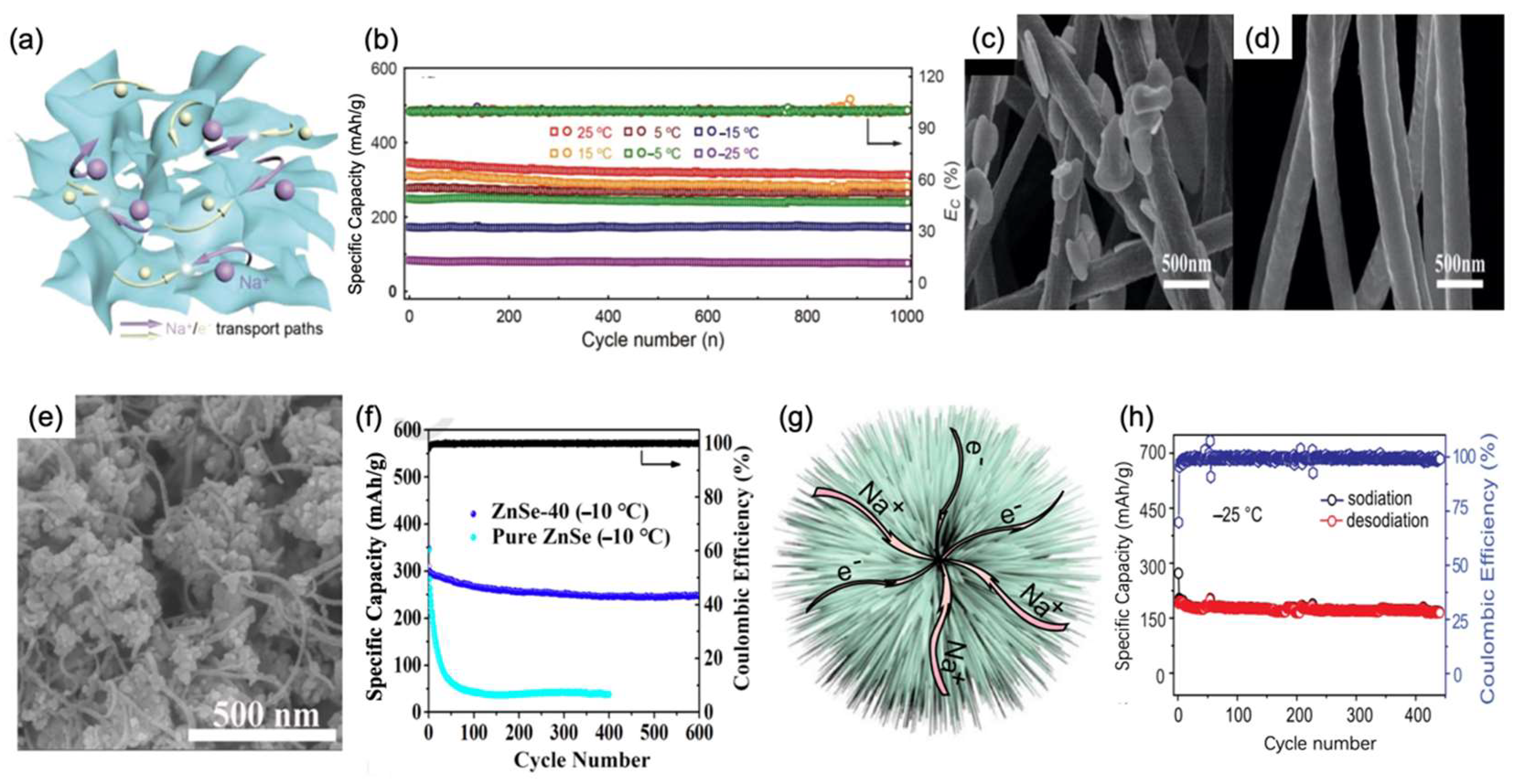
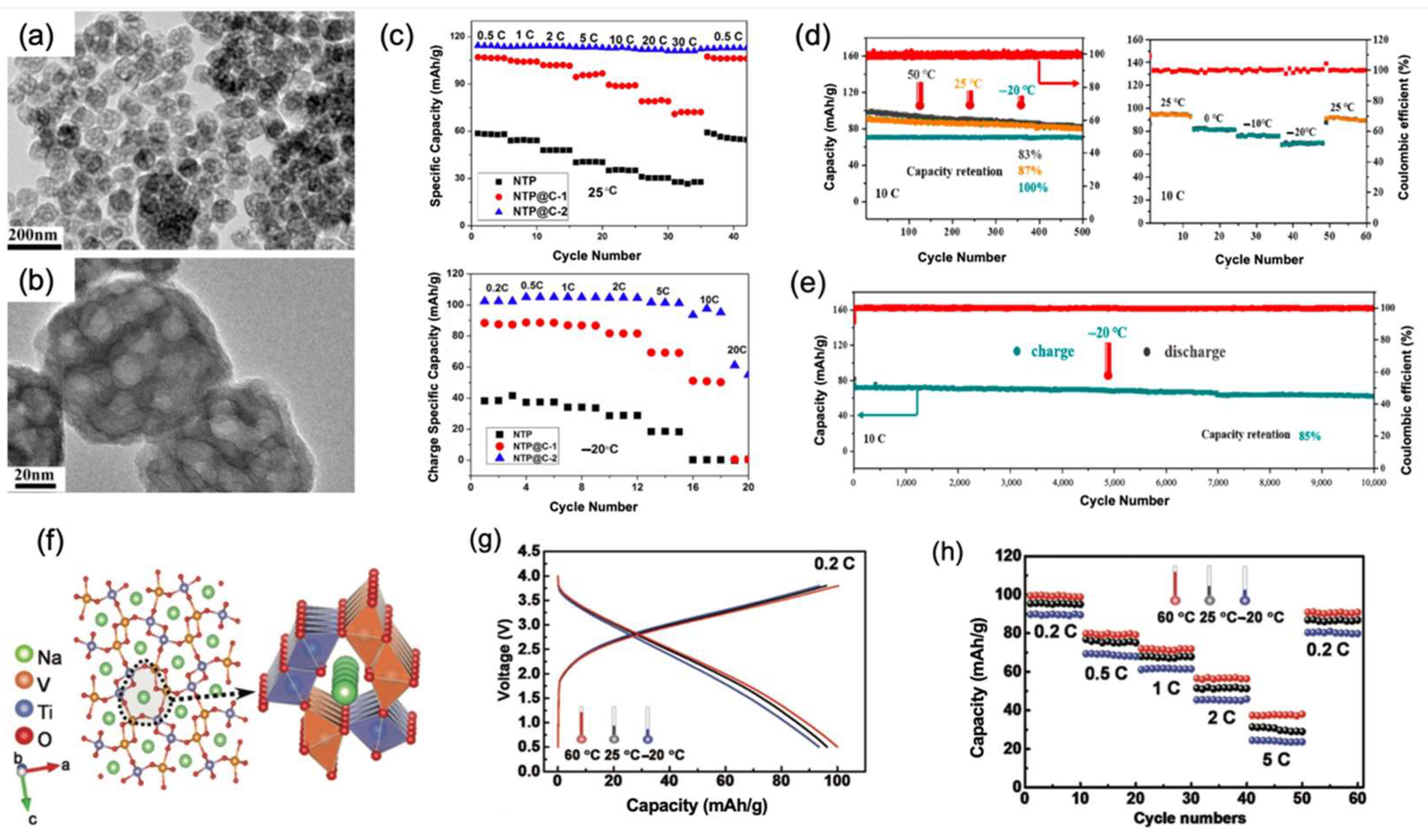
3.3. NaTi2(PO4)3
4. Low-Temperature SIB Electrolytes
4.1. Organic Electrolytes
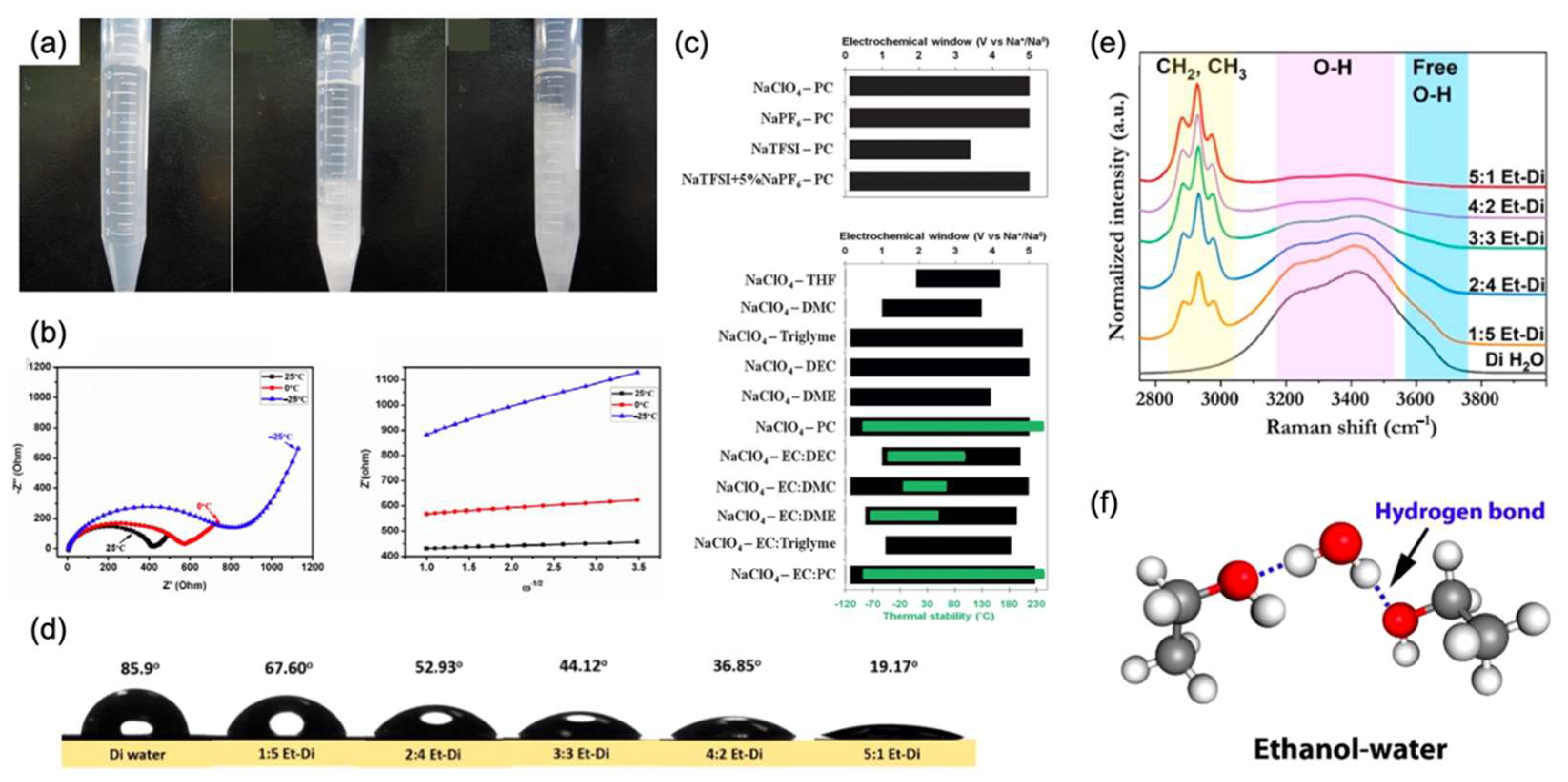
4.2. Ionic Liquid Electrolytes
4.3. Aqueous Electrolytes

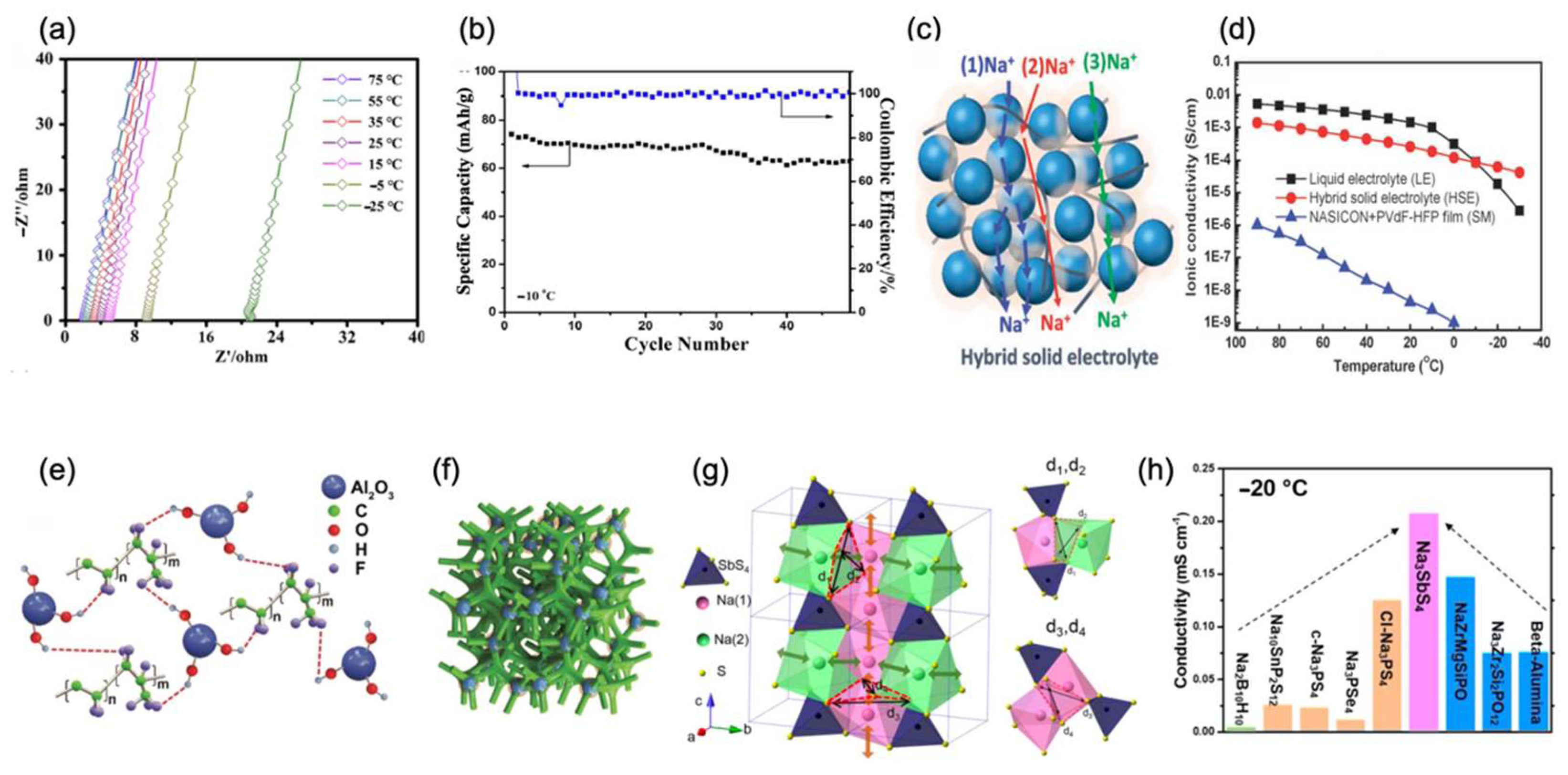
4.4. Solid-State Electrolytes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, C.M.; Barbosa, J.C.; Goncalves, R.; Castro, H.; Del Campo, F.J.; Lanceros-Mendez, S. Recycling and environmental issues of lithium-ion batteries: Advances, challenges and opportunities. Energy Storage Mater. 2021, 37, 433–465. [Google Scholar] [CrossRef]
- Song, J.; Xiao, B.; Lin, Y.; Xu, K.; Li, X. Interphases in sodium-ion batteries. Adv. Energy Mater. 2018, 8, 1703082. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, S.; Zhao, W.; Song, J.; Engelhard, M.H.; Zhang, J.G. Extremely stable sodium metal batteries enabled by localized high-concentration electrolytes. ACS Energy Lett. 2018, 3, 315–321. [Google Scholar] [CrossRef]
- Ponrouch, A.; Dedryvère, R.; Monti, D.; Demet, A.E.; Mba, J.M.A.; Croguennec, L.; Masquelier, C.; Johansson, P.; Palacín, M.R. Towards high energy density sodium ion batteries through electrolyte optimization. Energy Environ. Sci. 2013, 6, 2361–2369. [Google Scholar] [CrossRef]
- Schmitz, C.; Schmidt, H.W.; Thelakkat, M. Lithium−quinolate complexes as emitter and interface materials in organic light-emitting diodes. Chem. Mater. 2000, 12, 3012–3019. [Google Scholar] [CrossRef]
- Chen, W.; Deng, D. Carbonized common filter paper decorated with Sn@ C nanospheres as additive-free electrodes for sodium-ion batteries. Carbon 2015, 87, 70–77. [Google Scholar] [CrossRef]
- Peters, J.; Buchholz, D.; Passerini, S.; Weil, M. Life cycle assessment of sodium-ion batteries. Energy Environ. Sci. 2016, 9, 1744–1751. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Liu, J.; Xu, M.; Cheng, J.; Zhang, D.; Goodenough, J.B. A superior low-cost cathode for a Na-ion battery. Angew. Chem. 2013, 125, 2018–2021. [Google Scholar] [CrossRef]
- Bai, Z.; Lv, X.; Liu, D.H.; Dai, D.; Gu, J.; Yang, L.; Chen, Z. Two-Dimensional NiO@C-N Nanosheets Composite as a Superior Low-Temperature Anode Material for Advanced Lithium-/Sodium-Ion Batteries. ChemElectroChem 2020, 7, 3616–3622. [Google Scholar] [CrossRef]
- Song, B.; Hu, E.; Liu, J.; Zhang, Y.; Yang, X.-Q.; Nanda, J.; Huq, A.; Page, K. A novel P3-type Na 2/3 Mg 1/3 Mn 2/3 O 2 as high capacity sodium-ion cathode using reversible oxygen redox. J. Mater. Chem. A 2019, 7, 1491–1498. [Google Scholar] [CrossRef]
- Li, Q.; Yang, D.; Chen, H.; Lv, X.; Jiang, Y.; Feng, Y.; Rui, X.; Yu, Y. Advances in metal phosphides for sodium-ion batteries. SusMat 2021, 1, 359–392. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Ming, F.; Alshareef, H.N. Sodium-ion battery anodes: Status and future trends. EnergyChem 2019, 1, 100012. [Google Scholar] [CrossRef]
- Qian, J.; Wu, C.; Cao, Y.; Ma, Z.; Huang, Y.; Ai, X.; Yang, H. Prussian blue cathode materials for sodium-ion batteries and other ion batteries. Adv. Energy Mater. 2018, 8, 1702619. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, S.; Wang, B.; Li, Y.; Sun, W.; Lu, Y.; Yan, M.; Song, B.; Dou, S. Prussian blue@C composite as an ultrahigh-rate and long-life sodium-ion battery cathode. Adv. Funct. Mater. 2016, 26, 5315–5321. [Google Scholar] [CrossRef]
- Peng, J.; Wang, J.; Yi, H.; Hu, W.; Yu, Y.; Yin, J.; Shen, Y.; Liu, Y.; Luo, J.; Xu, Y. A dual-insertion type sodium-ion full cell based on high-quality ternary-metal Prussian blue analogs. Adv. Energy Mater. 2018, 8, 1702856. [Google Scholar] [CrossRef]
- You, Y.; Yao, H.R.; Xin, S.; Yin, Y.X.; Zuo, T.T.; Yang, C.P.; Guo, Y.G.; Cui, Y.; Wan, L.J.; Goodenough, J.B. Subzero-temperature cathode for a sodium-ion battery. Adv. Mater. 2016, 28, 7243–7248. [Google Scholar] [CrossRef]
- Ma, X.H.; Wei, Y.Y.; Wu, Y.D.; Wang, J.; Jia, W.; Zhou, J.H.; Zi, Z.F.; Dai, J.M. High crystalline Na2Ni[Fe(CN)6] particles for a high-stability and low-temperature sodium-ion batteries cathode. Electrochim. Acta 2019, 297, 392–397. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Tang, Y.; Xu, B.B.; Liang, C.; Yan, M.; Jiang, Y. Manganese hexacyanoferrate reinforced by PEDOT coating towards high-rate and long-life sodium-ion battery cathode. J. Mater. Chem. A 2020, 8, 3222–3227. [Google Scholar] [CrossRef]
- Bauer, A.; Song, J.; Vail, S.; Pan, W.; Barker, J.; Lu, Y. The scale-up and commercialization of nonaqueous Na-ion battery technologies. Adv. Energy Mater. 2018, 8, 1702869. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Q.; Yin, X.; Wang, J.; Wang, J.; Zhao, Y.; Zhang, J. Construction nasicon-type NaTi2(PO4)3 nanoshell on the surface of P2-type Na0.67Co0.2Mn0.8O2 cathode for superior room/low-temperature sodium storage. Chem. Eng. J. 2020, 402, 126181. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Chao, D.; Yan, Q.; Fan, H.J. Nonaqueous hybrid lithium-ion and sodium-ion capacitors. Adv. Mater. 2017, 29, 1702093. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Liu, Y.; Wang, Z.; Huang, Y.; Jin, Y.; Liu, Y.; Sun, S.; Qiu, Y.; Peng, J.; Xu, Y. Porous NaTi2(PO4) 3/C hierarchical nanofibers for ultrafast electrochemical energy storage. ACS Appl. Mater. Interfaces 2018, 10, 27039–27046. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, W.; Denis, D.K.; Zhang, J.; Hou, L.; Liu, Y.; Yuan, C. Comparative investigations of high-rate NaCrO2 cathodes towards wide-temperature-tolerant pouch-type Na-ion batteries from −15 to 55 °C: Nanowires vs. bulk. J. Mater. Chem. A 2019, 7, 11915–11927. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Oh, S.M.; Myung, S.T.; Chung, K.Y.; Belharouak, I.; Sun, Y.K. Radially aligned hierarchical columnar structure as a cathode material for high energy density sodium-ion batteries. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Feng, X.; Wang, X.; Shi, Q.; Wang, J.; Wang, J.; Zhang, J.; Hou, Y. A durable P2-type layered oxide cathode with superior low-temperature performance for sodium-ion batteries. Sci. China Mater. 2022, 65, 328–336. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Q.; Li, L.; Zhang, L.; Jin, Z.; Wang, C.; Su, Z. Achieving highly electrochemically active maricite NaFePO4 with ultrafine NaFePO4@ C subunits for high rate and low temperature sodium-ion batteries. Chem. Eng. J. 2021, 405, 126689. [Google Scholar] [CrossRef]
- Liu, R.; Xu, G.; Li, Q.; Zheng, S.; Zheng, G.; Gong, Z.; Li, Y.; Kruskop, E.; Fu, R.; Chen, Z. Exploring highly reversible 1.5-electron reactions(V3+/V4+/V5+) in Na3VCr(PO4)3 cathode for sodium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 43632–43639. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, S.; Yuan, Y.; Yu, P.; Liang, Z.; Zha, W.; Shahbazian-Yassar, R.; Ding, J.; Lu, J.; Yang, Y. Counter-Intuitive Structural Instability Aroused by Transition Metal Migration in Polyanionic Sodium Ion Host. Adv. Energy Mater. 2021, 11, 2003256. [Google Scholar] [CrossRef]
- Liu, T.; Wang, B.; Gu, X.; Wang, L.; Ling, M.; Liu, G.; Wang, D.; Zhang, S. All-climate sodium ion batteries based on the NASICON electrode materials. Nano Energy 2016, 30, 756–761. [Google Scholar] [CrossRef]
- Wang, C.; Du, D.; Song, M.; Wang, Y.; Li, F. A high-power Na3V2(PO4) 3-Bi sodium-ion full battery in a wide temperature range. Adv. Energy Mater. 2019, 9, 1900022. [Google Scholar] [CrossRef]
- Hwang, J.; Matsumoto, K.; Hagiwara, R. Na3V2(PO4)3/C positive electrodes with high energy and power densities for sodium secondary batteries with ionic liquid electrolytes that operate across wide temperature ranges. Adv. Sustain. Syst. 2018, 2, 1700171. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Seo, D.H.; Shi, T.; Chen, S.; Tian, Y.; Kim, H.; Ceder, G. A High-Energy NASICON-Type Cathode Material for Na-Ion Batteries. Adv. Energy Mater. 2020, 10, 1903968. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Zhao, D.; Xia, X.; Zhang, L.; Zhang, J.; Yang, H.; Xia, Y. Highly stable Na3Fe2(PO4)3@ hard carbon sodium-ion full cell for low-cost energy storage. ACS Sustain. Chem. Eng. 2019, 8, 1380–1387. [Google Scholar] [CrossRef]
- Liang, L.W.; Li, X.Y.; Zhao, F.; Zhang, J.Y.; Liu, Y.; Hou, L.R.; Yuan, C.Z. Construction and Operating Mechanism of High-Rate Mo-Doped Na3V2(PO4)3@C Nanowires toward Practicable Wide-Temperature-Tolerance Na-Ion and Hybrid Li/Na-Ion Batteries. Adv. Energy Mater. 2021, 11, 2100287. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Zhang, B.; Qin, K.; Liu, H.; Ma, Z.F. Mn-doped Na4Fe3(PO4)2(P2O7) facilitating Na+ migration at low temperature as a high-performance cathode material of sodium ion batteries. J. Power Sources 2022, 521, 230922. [Google Scholar] [CrossRef]
- Broux, T.; Fauth, F.; Hall, N.; Chatillon, Y.; Bianchini, M.; Bamine, T.; Leriche, J.b.; Suard, E.; Carlier, D.; Reynier, Y. High rate performance for carbon-coated Na3V2(PO4)2F3 in Na-Ion Batteries. Small Methods 2019, 3, 1800215. [Google Scholar] [CrossRef]
- Guo, J.Z.; Wang, P.F.; Wu, X.L.; Zhang, X.H.; Yan, Q.; Chen, H.; Zhang, J.P.; Guo, Y.G. High-energy/power and low-temperature cathode for sodium-ion batteries: In situ XRD study and superior full-cell performance. Adv. Mater. 2017, 29, 1701968. [Google Scholar] [CrossRef]
- Li, J.; Du, G.; Huang, T.; Zhang, F.; Xie, M.; Qi, Y.; Bao, S.J.; Xu, M. Na3V2O2(PO4)2F Cathode for High-Performance Quasi-Solid-State Sodium-Ion Batteries with a Wide Workable Temperature Range. Energy Technol. 2020, 8, 2000494. [Google Scholar] [CrossRef]
- Chen, M.; Hua, W.; Xiao, J.; Cortie, D.; Chen, W.; Wang, E.; Hu, Z.; Gu, Q.; Wang, X.; Indris, S. NASICON-type air-stable and all-climate cathode for sodium-ion batteries with low cost and high-power density. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Ponrouch, A.; Palacín, M. On the high and low temperature performances of Na-ion battery materials: Hard carbon as a case study. Electrochem. Commun. 2015, 54, 51–54. [Google Scholar] [CrossRef]
- Hou, B.H.; Wang, Y.Y.; Ning, Q.L.; Li, W.H.; Xi, X.T.; Yang, X.; Liang, H.J.; Feng, X.; Wu, X.L. Self-supporting, flexible, additive-free, and scalable hard carbon paper self-interwoven by 1D microbelts: Superb room/low-temperature sodium storage and working mechanism. Adv. Mater. 2019, 31, 1903125. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Du, X.; Tsui, P.S.; Huang, J.Q.; Tan, H.; Zhang, B. Exploring room-and low-temperature performance of hard carbon material in half and full Na-ion batteries. Electrochim. Acta 2019, 316, 60–68. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Dai, W.; Lian, X.; Cui, X.; Zhang, W.; Zhang, K.; Lin, M.; Zou, R.; Loh, K.P. From micropores to ultra-micropores inside hard carbon: Toward enhanced capacity in room-/low-temperature sodium-ion storage. Nano-Micro Lett. 2021, 13, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hou, B.H.; Guo, J.Z.; Ning, Q.L.; Pang, W.L.; Wang, J.; Lv, C.L.; Wu, X.L. An ultralong lifespan and low-temperature workable sodium-ion full battery for stationary energy storage. Adv. Energy Mater. 2018, 8, 1703252. [Google Scholar] [CrossRef]
- Zhou, L.F.; Gao, X.W.; Du, T.; Gong, H.; Liu, L.Y.; Luo, W.B. Two-dimensional NbSSe as anode material for low-temperature sodium-ion batteries. Chem. Eng. J. 2022, 435, 134838. [Google Scholar] [CrossRef]
- Zhou, X.; Ding, S.; He, H.; Huang, Z.; Cai, M.; Cai, Y.; Zhang, M. Encapsulated SnSe in carbon nanofibers as anode of sodium ion batteries with improved properties. Ionics 2020, 26, 3937–3946. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, X.; Fan, A.; Shang, Y.; Xiong, K.; Guo, J.; Jin, S.; Cai, S.; Zheng, C. ZnSe nanoparticles combined with uniform 3D interconnected MWCNTs conductive network as high-rate and freeze-resistant anode materials for sodium-ion batteries. Appl. Surf. Sci. 2021, 538, 148194. [Google Scholar] [CrossRef]
- Fan, H.H.; Li, H.H.; Wang, Z.W.; Li, W.L.; Guo, J.Z.; Fan, C.Y.; Sun, H.Z.; Wu, X.L.; Zhang, J.P. Tailoring coral-like Fe7Se8@C for superior low-temperature Li/Na-ion half/full batteries: Synthesis, structure, and DFT studies. ACS Appl. Mater. Interfaces 2019, 11, 47886–47893. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Lu, J.; Tang, H.; Wang, X.; Zhang, L.; Hu, P.; Zhou, L.; Wang, Y.; Guo, Y.; Khatoon, R. An ultra-stable anode material for high/low-temperature workable super-fast charging sodium-ion batteries. Chem. Eng. J. 2021, 422, 130054. [Google Scholar] [CrossRef]
- Hu, Q.; Yu, M.; Liao, J.; Wen, Z.; Chen, C. Porous carbon-coated NaTi2(PO4)3 with superior rate and low-temperature properties. J. Mater. Chem. A 2018, 6, 2365–2370. [Google Scholar] [CrossRef]
- Reber, D.; Kühnel, R.S.; Battaglia, C. Suppressing crystallization of water-in-salt electrolytes by asymmetric anions enables low-temperature operation of high-voltage aqueous batteries. ACS Mater. Lett. 2019, 1, 44–51. [Google Scholar] [CrossRef]
- Nian, Q.; Liu, S.; Liu, J.; Zhang, Q.; Shi, J.; Liu, C.; Wang, R.; Tao, Z.; Chen, J. All-climate aqueous dual-ion hybrid battery with ultrahigh rate and ultralong life performance. ACS Appl. Energy Mater. 2019, 2, 4370–4378. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, K.; Li, X.; Qiao, Y.; Zhang, X.; He, P.; Guo, S.; Zhou, H. A High-Crystalline NaV1.25Ti0.75O4 Anode for Wide-Temperature Sodium-Ion Battery. Adv. Energy Mater. 2018, 8, 1801162. [Google Scholar] [CrossRef]
- Vignarooban, K.; Kushagra, R.; Elango, A.; Badami, P.; Mellander, B.-E.; Xu, X.; Tucker, T.; Nam, C.; Kannan, A.M. Current trends and future challenges of electrolytes for sodium-ion batteries. Int. J. Hydrog. Energy 2016, 41, 2829–2846. [Google Scholar] [CrossRef]
- Weadock, N.; Varongchayakul, N.; Wan, J.; Lee, S.; Seog, J.; Hu, L. Determination of mechanical properties of the SEI in sodium ion batteries via colloidal probe microscopy. Nano Energy 2013, 2, 713–719. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-ion batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Ponrouch, A.; Marchante, E.; Courty, M.; Tarascon, J.M.; Palacin, M.R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 2012, 5, 8572–8583. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Qi, X.; Lu, Y.; Wu, F.; Zhao, J.; Yu, Y.; Hu, Y.S.; Chen, L. Solid-state sodium batteries. Adv. Energy Mater. 2018, 8, 1703012. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L. Advances in materials for all-climate sodium-ion batteries. EcoMat 2020, 2, e12043. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, L.; Li, L.; Xie, M.; Wu, F.; Chen, R. Electrolytes and electrolyte/electrode interfaces in sodium-ion batteries: From scientific research to practical application. Adv. Mater. 2019, 31, 1808393. [Google Scholar] [CrossRef] [PubMed]
- Lianmei, W.; Xixi, Y.; Suna, Z.; Jie, Z.; Minchang, W.; Yongmin, Q.; Lijun, W. Progress of low-temperature electrolyte for lithium-ion battery. Energy Storage Sci. Technol. 2017, 6, 69. [Google Scholar]
- Zheng, X.; Gu, Z.; Fu, J.; Wang, H.; Ye, X.; Huang, L.; Liu, X.; Wu, X.; Luo, W.; Huang, Y. Knocking down the kinetic barriers towards fast-charging and low-temperature sodium metal batteries. Energy Environ. Sci. 2021, 14, 4936–4947. [Google Scholar] [CrossRef]
- Chua, R.; Cai, Y.; Lim, P.Q.; Kumar, S.; Satish, R.; Manalastas Jr, W.; Ren, H.; Verma, V.; Meng, S.; Morris, S.A. Hydrogen-Bonding Interactions in Hybrid Aqueous/Nonaqueous Electrolytes Enable Low-Cost and Long-Lifespan Sodium-Ion Storage. ACS Appl. Mater. Interfaces 2020, 12, 22862–22872. [Google Scholar] [CrossRef] [PubMed]
- Kruk, D.; Jancelewicz, M.; Klimaszyk, A.; Markiewicz, R.; Fojud, Z.; Jurga, S. Internal Dynamics of Ionic Liquids over a Broad Temperature Range—The Role of the Cation Structure. Materials 2021, 15, 216. [Google Scholar] [CrossRef]
- Bellusci, M.; Simonetti, E.; De Francesco, M.; Appetecchi, G.B. Ionic liquid electrolytes for safer and more reliable sodium battery systems. Appl. Sci. 2020, 10, 6323. [Google Scholar] [CrossRef]
- Ding, C.; Nohira, T.; Fukunaga, A.; Hagiwara, R. Charge-discharge performance of an ionic liquid-based sodium secondary battery in a wide temperature range. Electrochemistry 2015, 83, 91–94. [Google Scholar] [CrossRef][Green Version]
- Chen, C.Y.; Matsumoto, K.; Nohira, T.; Hagiwara, R.; Fukunaga, A.; Sakai, S.; Nitta, K.; Inazawa, S. Electrochemical and structural investigation of NaCrO2 as a positive electrode for sodium secondary battery using inorganic ionic liquid NaFSA–KFSA. J. Power Sources 2013, 237, 52–57. [Google Scholar] [CrossRef]
- Moreno, J.S.; Maresca, G.; Panero, S.; Scrosati, B.; Appetecchi, G. Sodium-conducting ionic liquid-based electrolytes. Electrochem. Commun. 2014, 43, 1–4. [Google Scholar] [CrossRef]
- Na, Z.; Feng, W.; Chuan, W.; Ying, B.; Yitong, L. Recent advances of electrolytes for sodium-ion batteries. Energy Storage Sci. Technol. 2016, 5, 285. [Google Scholar]
- Havemeyer, R.N. Freezing point curve of dimethyl sulfoxide—Water solutions. J. Pharm. Sci. 1966, 55, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Nian, Q.; Wang, J.; Liu, S.; Sun, T.; Zheng, S.; Zhang, Y.; Tao, Z.; Chen, J. Aqueous batteries operated at −50 °C. Angew. Chem. Int. Ed. 2019, 58, 16994–16999. [Google Scholar] [CrossRef]
- Bi, H.; Wang, X.; Liu, H.; He, Y.; Wang, W.; Deng, W.; Ma, X.; Wang, Y.; Rao, W.; Chai, Y. A universal approach to aqueous energy storage via ultralow-cost electrolyte with super-concentrated sugar as hydrogen-bond-regulated solute. Adv. Mater. 2020, 32, 2000074. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Zhou, F.; Das, P.; Wen, P.; Zheng, S.; Lu, P.; Yu, Y.; Wu, Z.-S. High-voltage aqueous planar symmetric sodium ion micro-batteries with superior performance at low-temperature of −40 °C. Nano Energy 2021, 82, 105688. [Google Scholar] [CrossRef]
- Du, G.; Tao, M.; Li, J.; Yang, T.; Gao, W.; Deng, J.; Qi, Y.; Bao, S.J.; Xu, M. Low-operating temperature, high-rate and durable solid-state sodium-ion battery based on polymer electrolyte and Prussian blue cathode. Adv. Energy Mater. 2020, 10, 1903351. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, M.; Chen, Z.; Du, X.; Huang, S.; Tang, B.; Dong, T.; Wu, H.; Yu, Z.; Zhang, J. Flame-retardant quasi-solid polymer electrolyte enabling sodium metal batteries with highly safe characteristic and superior cycling stability. Nano Res. 2019, 12, 2230–2237. [Google Scholar] [CrossRef]
- Wen, P.; Lu, P.; Shi, X.; Yao, Y.; Shi, H.; Liu, H.; Yu, Y.; Wu, Z.S. Photopolymerized gel electrolyte with unprecedented room-temperature ionic conductivity for high-energy-density solid-state sodium metal batteries. Adv. Energy Mater. 2021, 11, 2002930. [Google Scholar] [CrossRef]
- Kim, J.K.; Lim, Y.J.; Kim, H.; Cho, G.B.; Kim, Y. A hybrid solid electrolyte for flexible solid-state sodium batteries. Energy Environ. Sci. 2015, 8, 3589–3596. [Google Scholar] [CrossRef]
- Xie, D.; Zhang, M.; Wu, Y.; Xiang, L.; Tang, Y. A flexible dual-ion battery based on sodium-ion quasi-solid-state electrolyte with long cycling life. Adv. Funct. Mater. 2020, 30, 1906770. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Hood, Z.D.; Keum, J.K.; Pandian, A.S.; Chi, M.; An, K.; Liang, C.; Sunkara, M.K. Revealing the structural stability and Na-ion mobility of 3D superionic conductor Na3SbS4 at extremely low temperatures. ACS Appl. Energy Mater. 2018, 1, 7028–7034. [Google Scholar] [CrossRef]

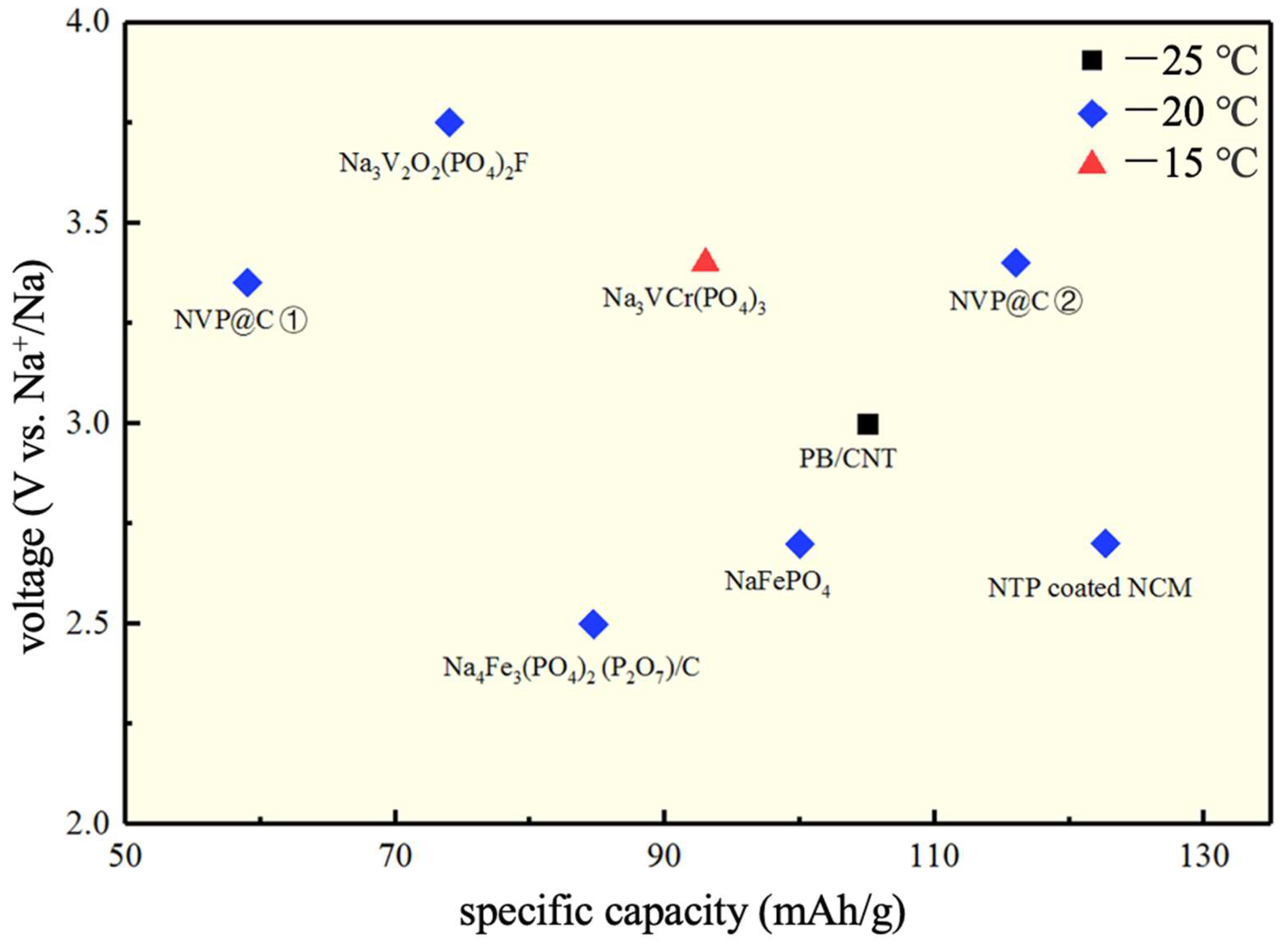
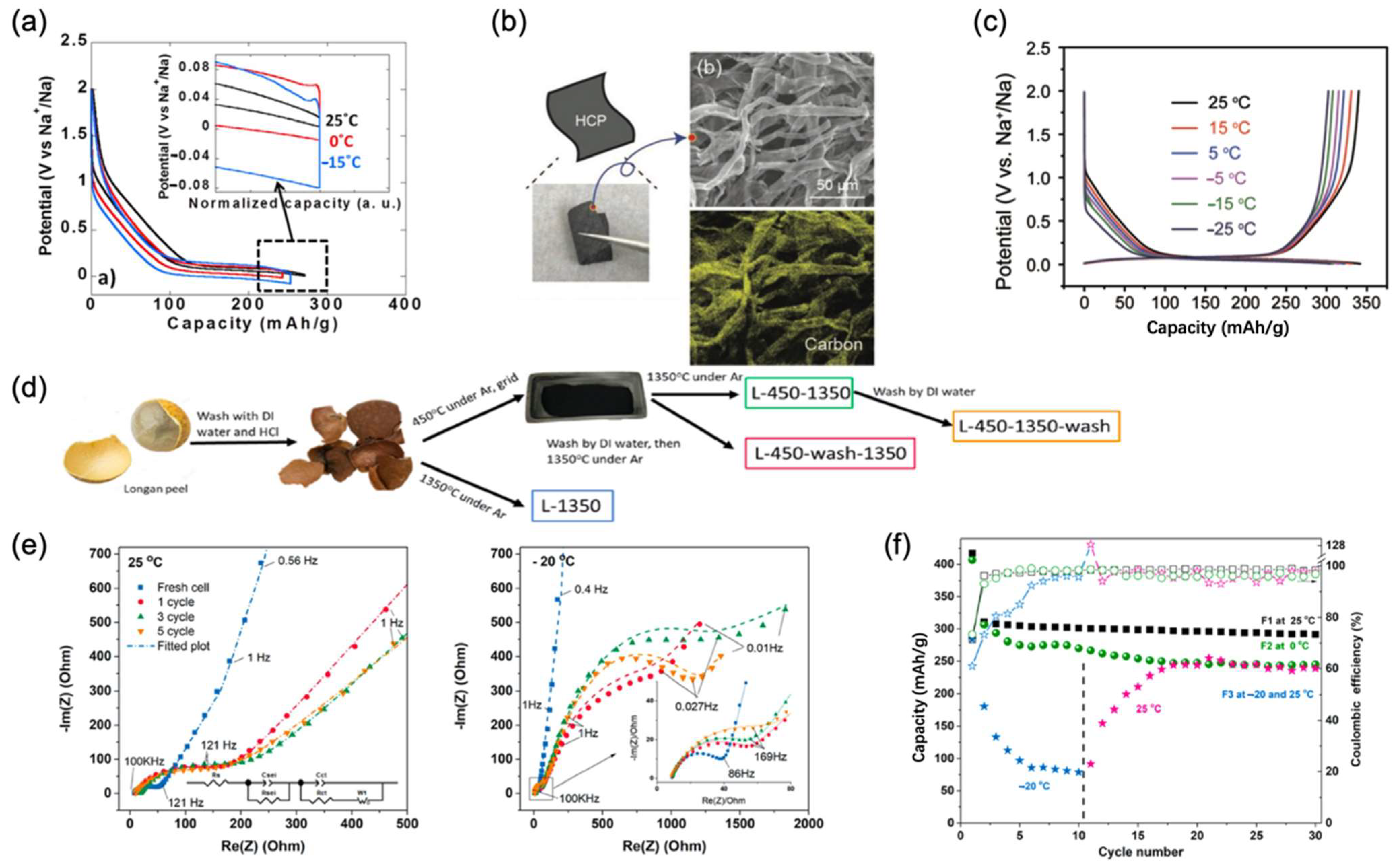
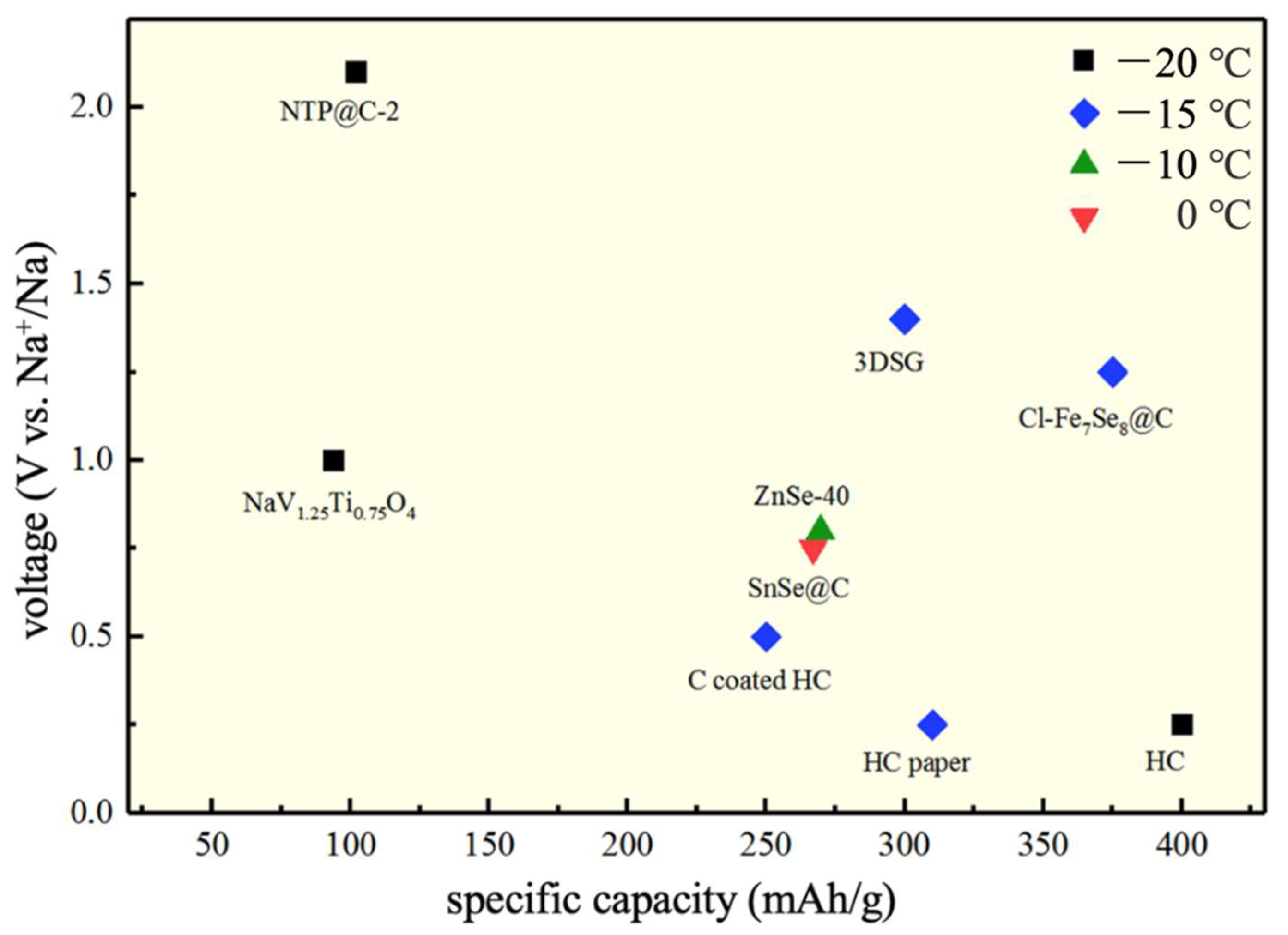
| Electrolytes | Temperature (°C) | Conductivity (S/cm) | Specific Capacity (mAh/g) | Retention Capacity/Number of Cycles | Anode//Cathode |
|---|---|---|---|---|---|
| 1 M NaClO4 in PC 2 vol% FEC Ref. [55] | −20 | / | 94 0.2 C | Na//NaV1.25Ti0.75O4 | |
| −20 | 93 0.2 C | 84%/200 1 C | Na0.8Ni0.4Ti0.6O2//NaV1.25Ti0.75O4 full cell | ||
| 1 M NaClO4 PC with 5 vol % FEC Ref. [28] | −10 | / | 113 0.2 C | 93%/200 0.5 C | Na//NFP@C |
| −20 | 100 0.2 C | 94%/200 0.5 C | |||
| 1 M NaClO4 in EC/PC 5wt% FEC Ref. [46] | −5 | / | 375 20 C | 96.2%/1000 20 C | Na//3DSG composite |
| −15 | 300 20 C | 98%/1000 20 C | |||
| −25 | 250 20 C | 90.4%/1000 20 C | |||
| 1 M NaClO4 in EC/PC 5wt% FEC Ref. [50] | 0 | / | 425 2 C | 96%/80 2 C | Na//cl-Fe7Se8@C |
| −15 | 375 2 C | 82%/80 2 C | |||
| −25 | 350 2 C | 65%/80 2 C | |||
| 1 M NaClO4 in EC/DMC 1:1 (vol) Ref. [19] | 0 | / | 79.3 0.5 C | 87%/440 1 C | Na//PBNi-ES |
| −25 | 65.1 0.5 C | 84%/440 1 C | |||
| 1 M NaPF6 EC/PC 1:1 (wt) 10wt% DMC Ref. [42] | 0 | / | 245 0.1 C | / | HC@C//Na |
| −15 | 150 0.1 C | ||||
| 1 M NaClO4 EC/DEC 1:1 (vol) Ref. [52] | 0 | / | 105 0.266 C | / | Na//NTP@C-2 |
| −10 | / | 110 0.266 C | |||
| −20 | / | 102 0.266 C | |||
| 1 M NaClO4 EC/PC 1:1 (vol) Ref. [6] | 0 | / | 155 0.3 C | 81%/1000 2.4 C | Na//PB/CNT |
| −25 | 105 0.3 C | 86%/1000 2.4 C | |||
| 0.5 M NaPF6 EMS: FEC 98:2 (vol) Ref. [18] | 0 | / | 130 0.75 C | 89%/100 0.75 C | HC//RAHC |
| −20 | 100 0.75 C | 92%/100 0.75 C | |||
| 1 M NaClO4 EC/PC 1:1 (vol) Ref. [31] | 0 | / | 120 0.2 C | Na//NVP@C | |
| −10 | 120 0.2 C | 97.2%/40 1 C | |||
| −20 | 116 0.2 C | 75.8%/500 10 C | |||
| molar fraction 0.3% DMSO 2 M NaClO4 Ref. [74] | −50 | 1.1 × 10−4 | 68 0.665 C | 95%/100 0.665 C | NaTi2(PO4)3@C//activated carbon |
| 2 M NaClO4 Ref. [54] | 0 | / | 82 10 C | 85%/10,000 10 C | NaTi2(PO4)3@C//nanoNi(OH)2 |
| −10 | 78 10 C | ||||
| −20 | 70 10 C | ||||
| 1 M sodium acetate in ethanol/water 5:1 (vol) Ref. [66] | 0 | / | 44.5 | 94%/50 1 C | Zn//Na0.44MnO2 |
| 0.2NaFSI-0.8EMIFSI Ref. [33] | −10 | / | 78.1 0.1 C | / | Na//NASICON-NVP@C |
| −20 | 58.6 0.1 C | ||||
| 0.2NaFSI-0.8C3C1pyrrFSI Refs. [69,70] | 0 | 9.8 × 10−4 | 100 0.2 C | 95%/500 1 C | Na//NaCrO2 |
| −10 | / | 60 0.2 C | / | ||
| 0 | / | 80 0.2 C | / | Na//Na2FeP2O7 | |
| −10 | 67 0.2 C | ||||
| −20 | 42 0.2 C | ||||
| 0.1NaTFSI-0.9EMIFSI Ref. [68] | −20 | 1.1 × 10−3 | / | / | / |
| 0.1NaTFSI-0.9EMITFSI Ref. [68] | −20 | 3.8 × 10−4 | / | / | / |
| 0.1NaTFSI-0.9PYR14TFSI Ref. [71] | −30 | 2.2 × 10−3 | / | / | / |
| 25m NaFSI-10m NaFTFSI Ref. [53] | −10 | / | 65 0.2 C | 89%/500 0.2 C | NaTi2(PO4)3//Na3(VOPO4)2F |
| PFSA-Na membrane (PFSA-Na/DMF solution with 1 M NaClO4 in EC/DEC) Ref. [77] | −5 | / | 100 | / | Na//HQ-NaFe |
| −15 | 4.88 × 10−5 | 93 | / | ||
| −25 | / | 80 | / | ||
| host: P(MVE-alt-MA) reinforce: BåC plasticier: TEP/VC/NaClO4 abbreviated FRPMM-CPE Ref. [78] | −10 | / | 70 0.1 C | 84.8%/50 0.1 C | Na//Na3V2(PO4)3 |
| −15 | 61.6 0.05 C | / | |||
| PFSA-Na membrane (PFSA-Na powder dissolved in DMF and added with 1 M NaClO4 in EC/DEC) Ref. [40] | −5 | / | 107 1 C | / | Na//PFSA-Na//Na3V2O2 (PO4)2F |
| −15 | 84 1 C | ||||
| −20 | 74 1 C | ||||
| −25 | 4.82 × 10−5 | 58 1 C | 99.6/190 1 C | ||
| −25 | 40.6 1 C | 90%/30 1 C | HC//PFSA-Na//Na3V2O2 (PO4)2F | ||
| PVdF-HFP binder, Na3Zr2Si2PO12-based composite, and 1 M sodium triflate NaCF3SO3/TEGDME liquid electrolyte in 7:1.5:1.5 (wt) Ref. [80] | 0 | 3 × 10−4 | / | / | / |
| −10 | 1 × 10−4 | ||||
| −20 | 2 × 10−5 | ||||
| Na3SbS4 solid electrolyte Ref. [82] | −20 | 2.2 × 10−4 | / | / | / |
| PVDF-HFP membrane with 5 wt% of Al2O3 nanoparticles Ref. [82] | −5 | / | 65 5 C | / | Sn//graphite |
| −20 | 45 5 C | ||||
| ETPTA-NaClO4-QSSE Ref. [79] | 0 | 7 × 10−4 | / | / | / |
| −10 | 8 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Hu, N.; Wang, J.; Wang, S.; Deng, W. Recent Progress and Perspective: Na Ion Batteries Used at Low Temperatures. Nanomaterials 2022, 12, 3529. https://doi.org/10.3390/nano12193529
Li P, Hu N, Wang J, Wang S, Deng W. Recent Progress and Perspective: Na Ion Batteries Used at Low Temperatures. Nanomaterials. 2022; 12(19):3529. https://doi.org/10.3390/nano12193529
Chicago/Turabian StyleLi, Peiyuan, Naiqi Hu, Jiayao Wang, Shuchan Wang, and Wenwen Deng. 2022. "Recent Progress and Perspective: Na Ion Batteries Used at Low Temperatures" Nanomaterials 12, no. 19: 3529. https://doi.org/10.3390/nano12193529
APA StyleLi, P., Hu, N., Wang, J., Wang, S., & Deng, W. (2022). Recent Progress and Perspective: Na Ion Batteries Used at Low Temperatures. Nanomaterials, 12(19), 3529. https://doi.org/10.3390/nano12193529







