Mussel-Inspired Surface Modification of α-Zirconium Phosphate Nanosheets for Anchoring Efficient and Reusable Ultrasmall Au Nanocatalysts
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Characterizations
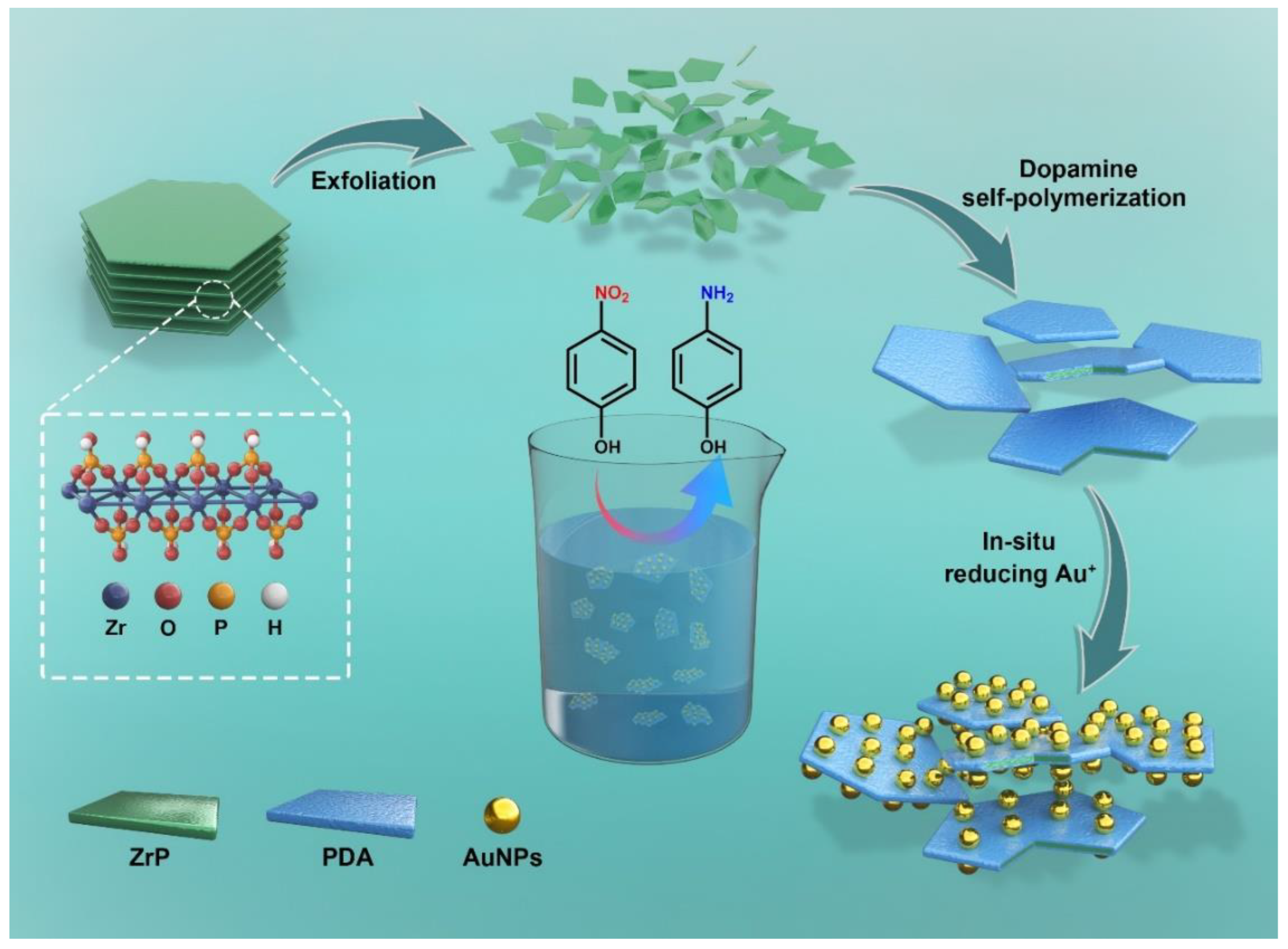
2.3. Preparation of ZrP@PDA/Au Nanocatalyst
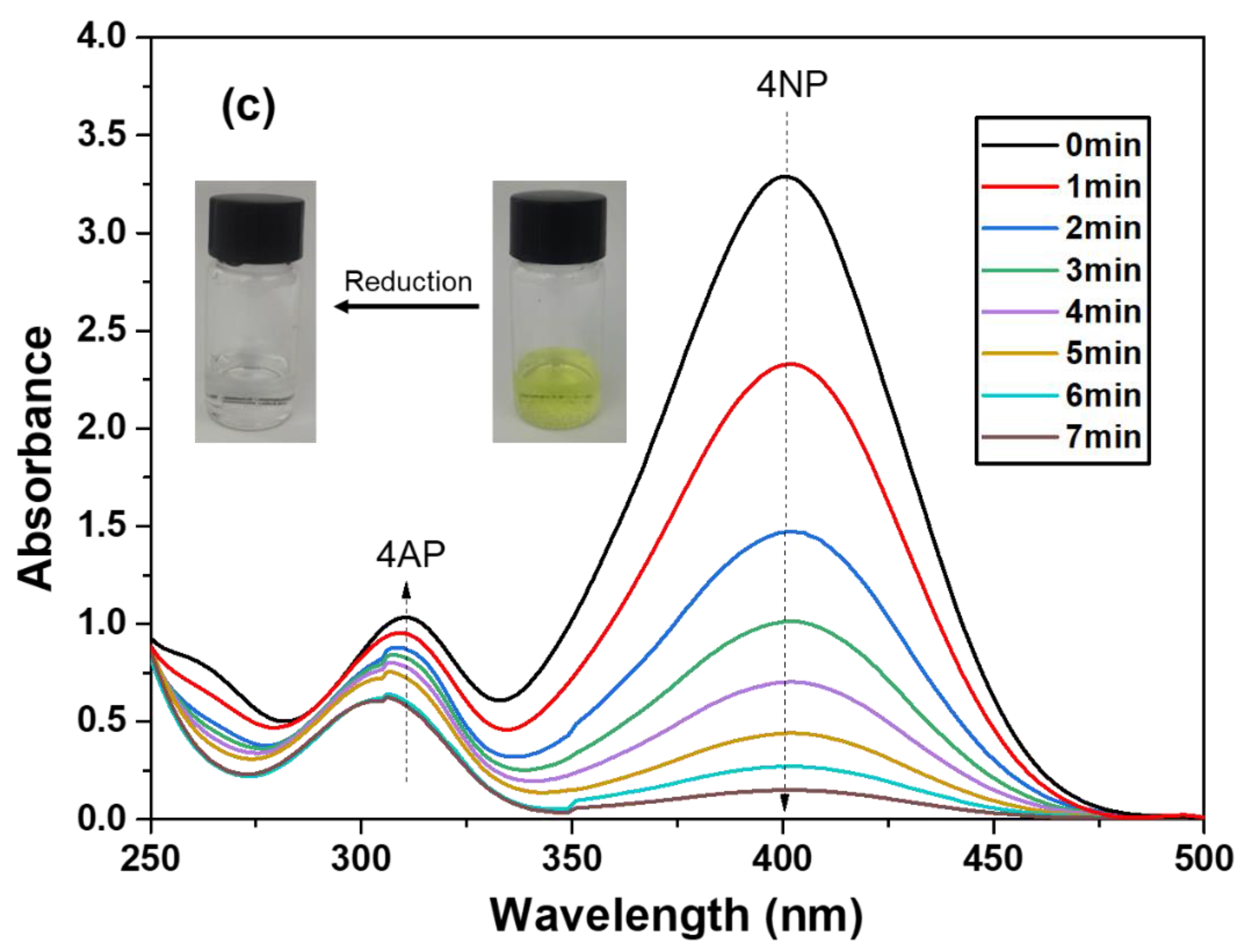
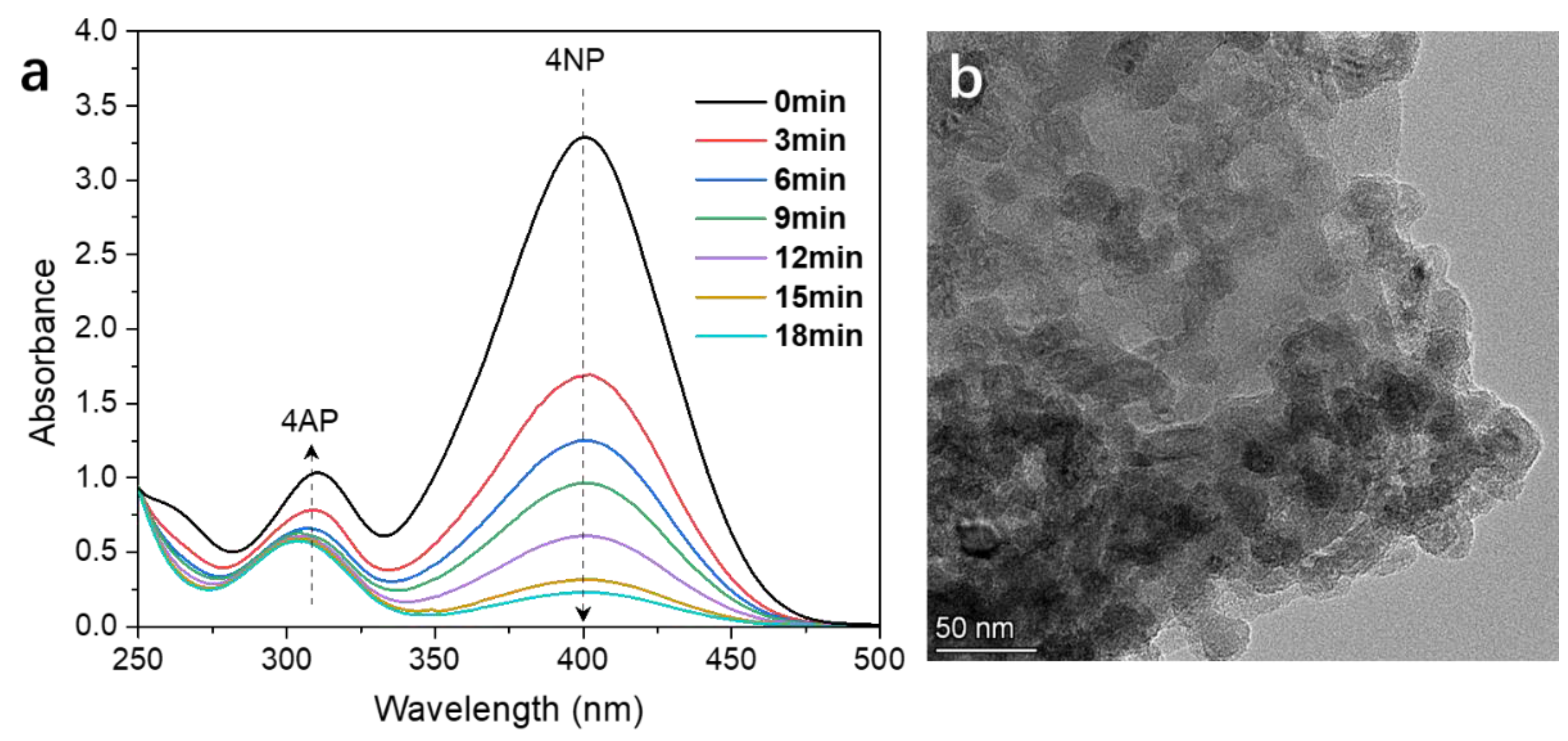
2.4. Catalytic Reduction of 4-Nitrophenol
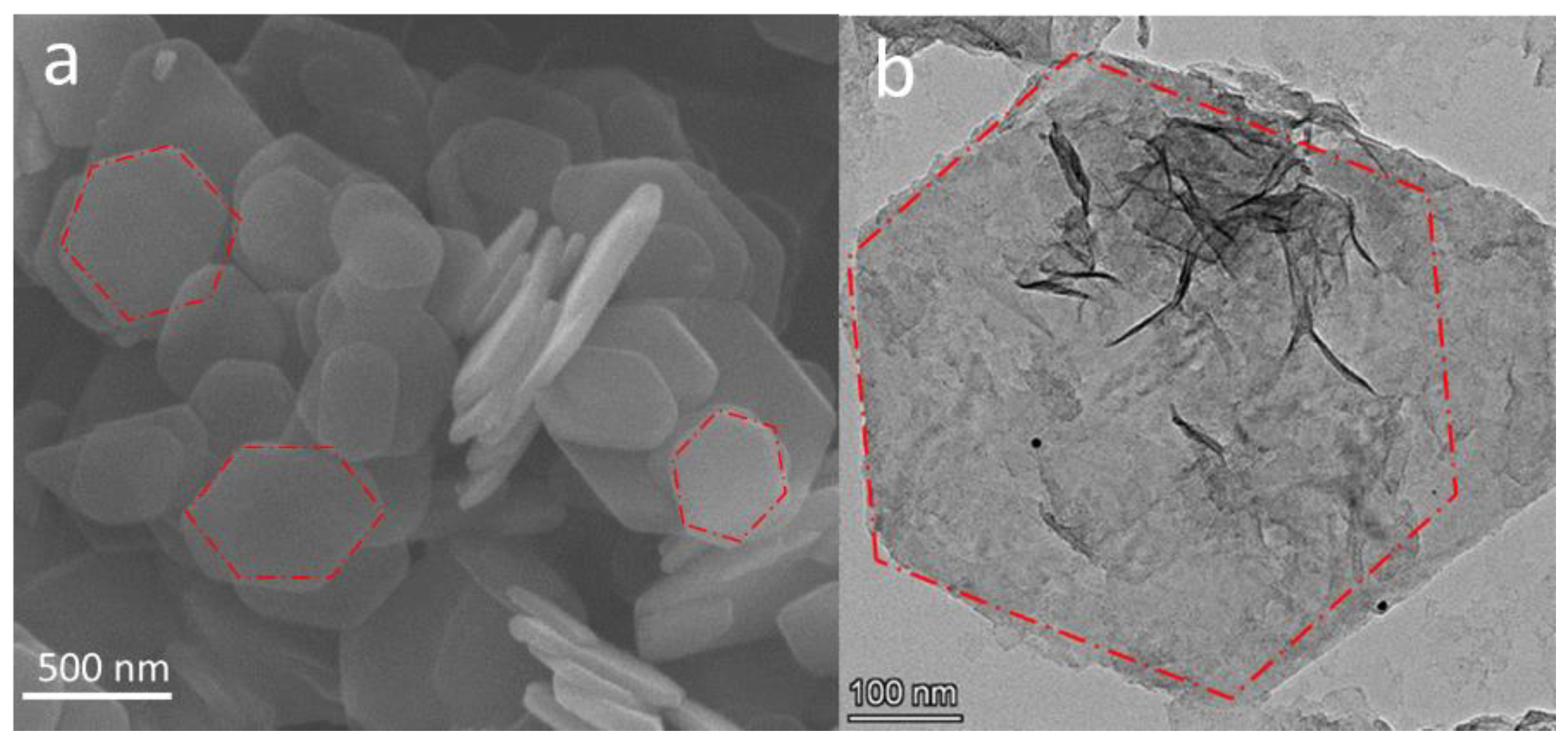
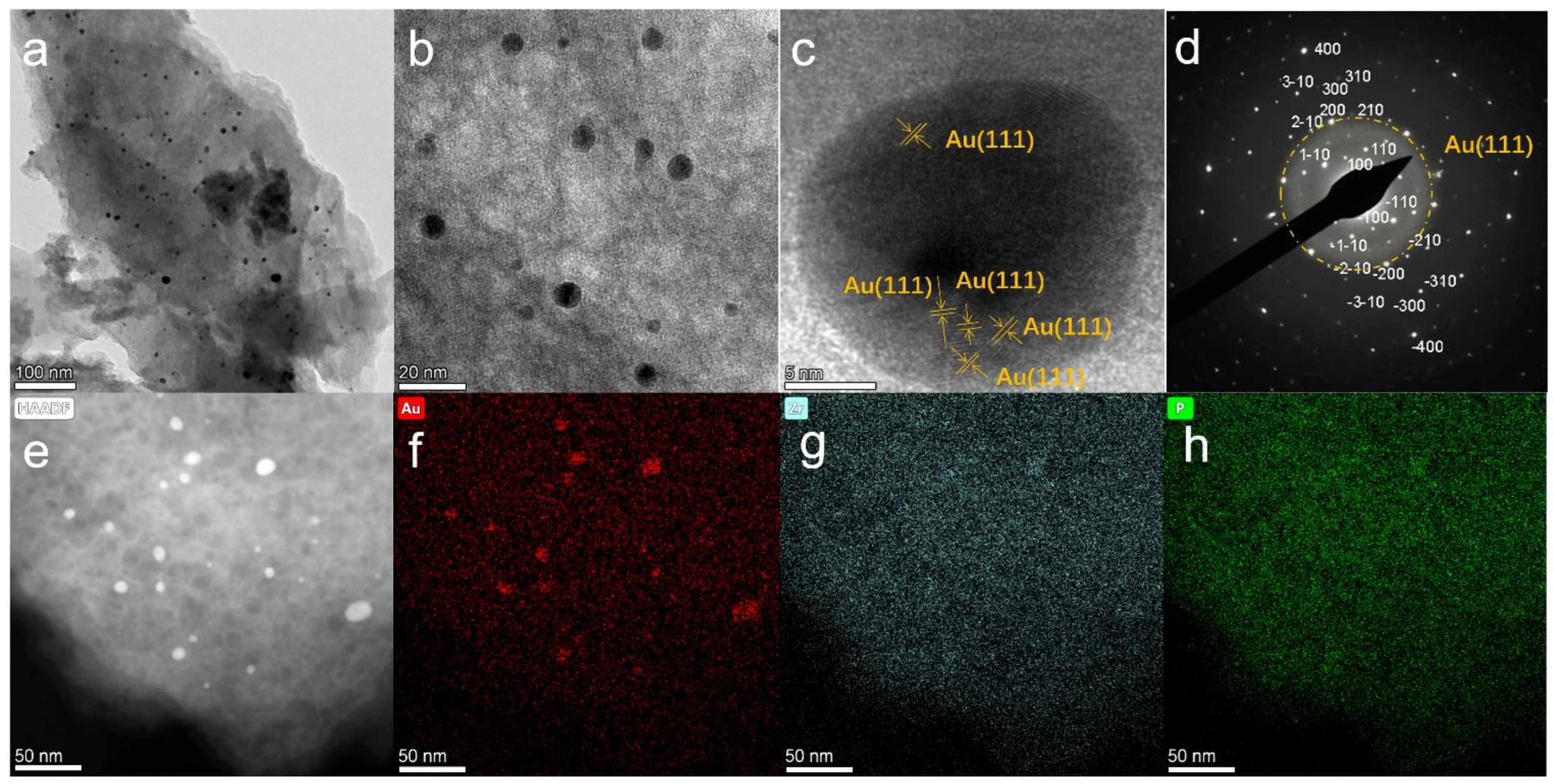
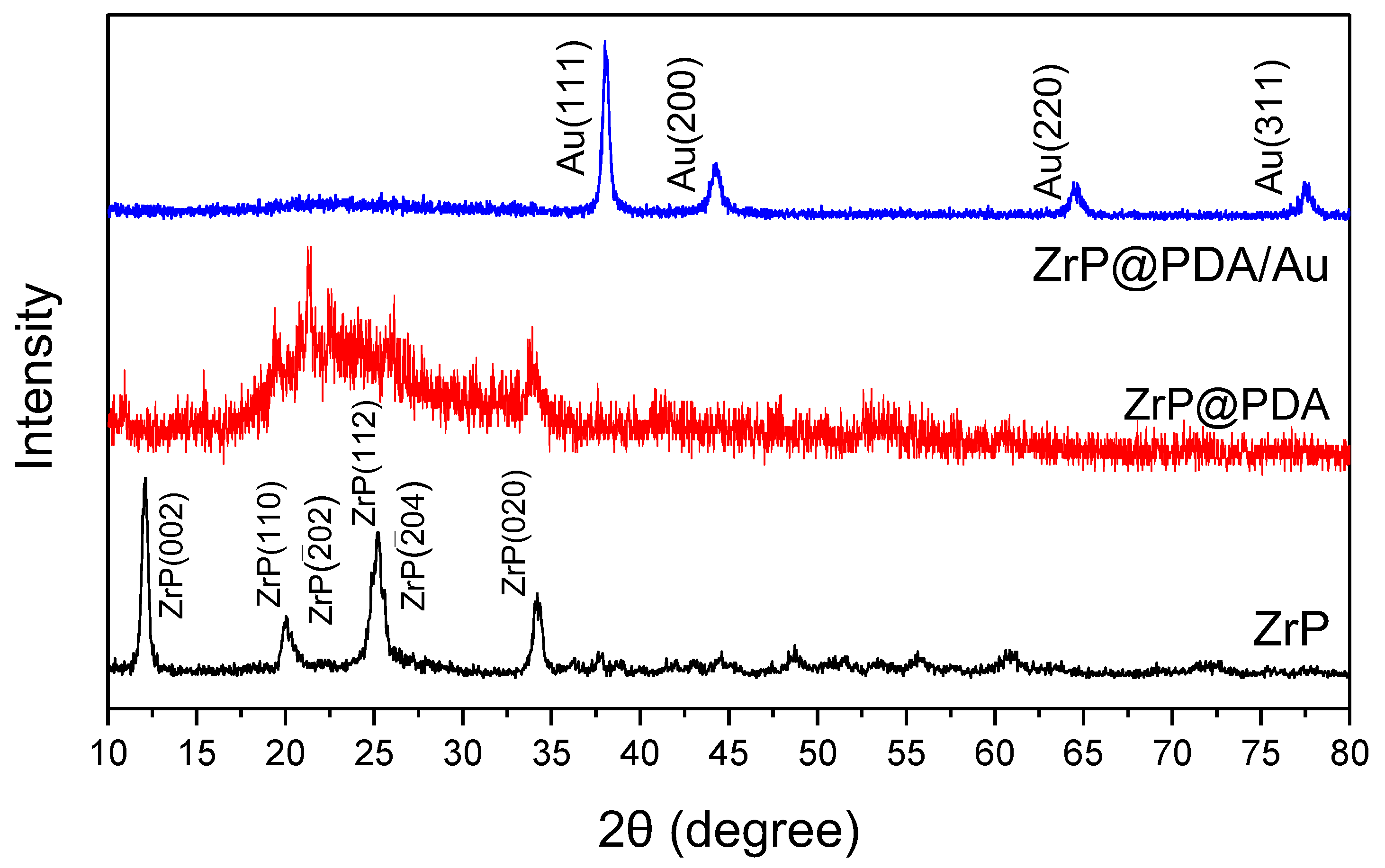
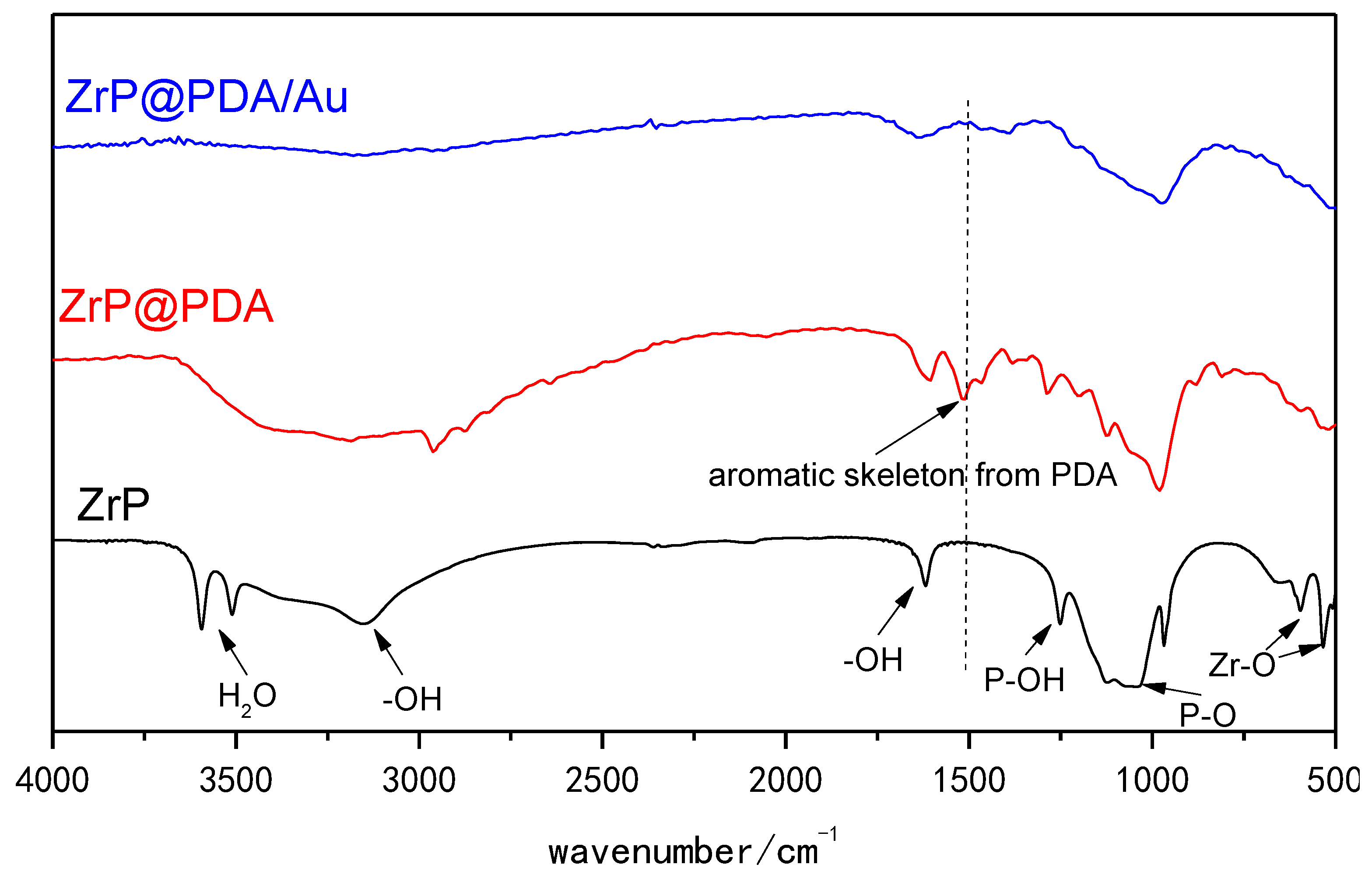
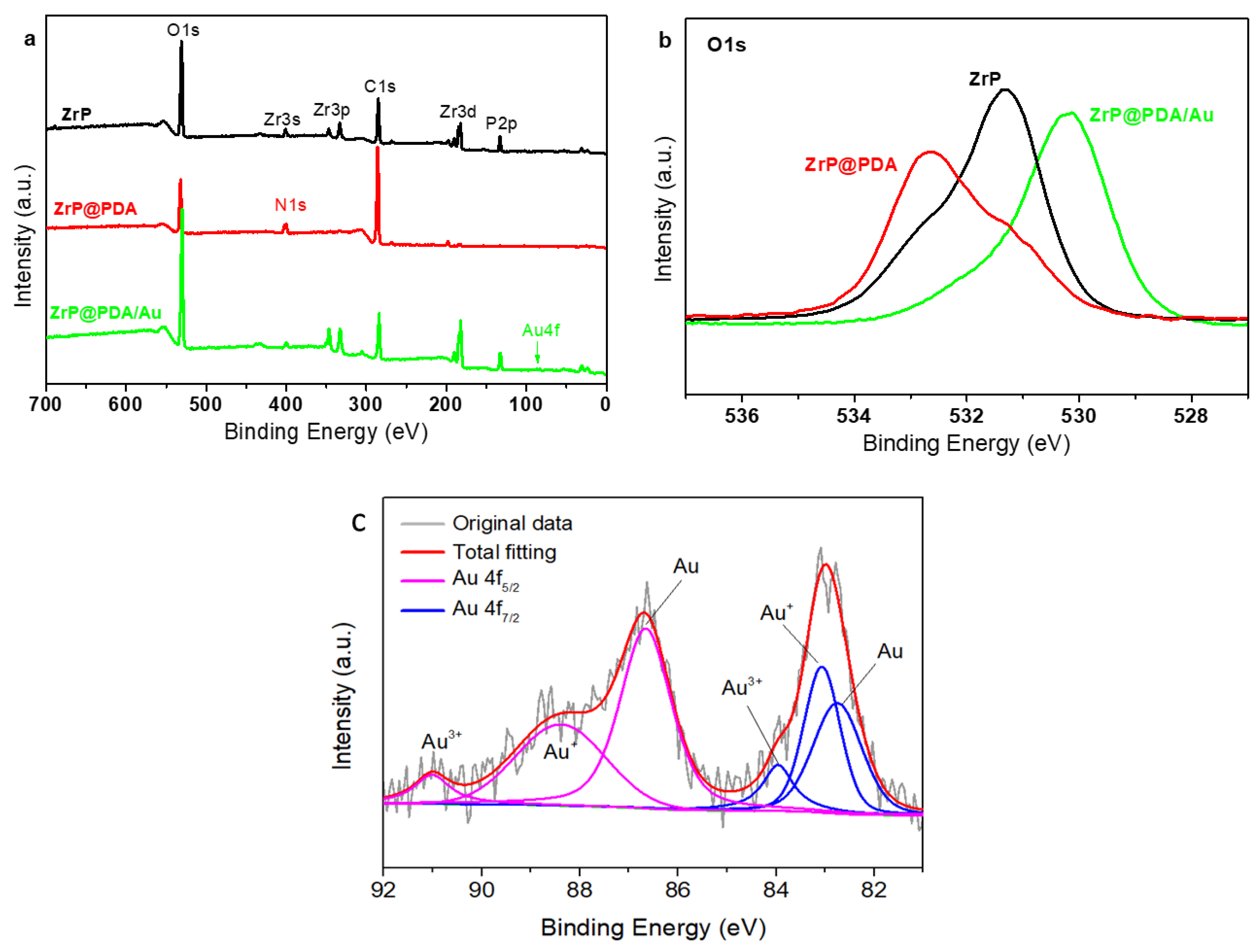
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, H.; Wen, W.; Ou, J.Z. Construction of adsorbents with graphene and its derivatives for wastewater treatment: A review. Environ. Sci.-Nano 2022, 9, 3226–3276. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, Y.; Yuai, Z.; Long, H.; He, Q.; Guo, K.; Zhang, Y.; Chen, D.; Xu, X.; Hu, H. Co-doping g-C3N4 with P and Mo for efficient photocatalytic tetracycline degradation under visible light. Ceram. Int. 2022, 48, 24677–24686. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Huang, H.; Huang, K.; Deng, L.; Hu, H.; Chang, M.; Chen, D.; Wang, Y. Exploration of Highly Porous Biochar Derived from Dead Leaves to Immobilize LiOH·H2O Nanoparticles for Efficient Thermochemical Heat Storage Applications. Energy Technol. 2022, 10, 2101127. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, T.; Yang, Z.; Yu, R.; Zeng, X.; Xu, Y.; Zhang, M.; Hu, H.; Ou, J.Z.; Zheng, L. Crystal phase-driven copolymerization of CO2 and cyclohexene oxide in Prussian blue analogue nanosheets. Appl. Mater. Today 2022, 26, 101352. [Google Scholar] [CrossRef]
- Zhu, W.; Lin, Y.; Kang, W.; Quan, H.; Zhang, Y.; Chang, M.; Wang, K.; Zhang, M.; Zhang, W.; Li, Z.; et al. An aerogel adsorbent with bio-inspired interfacial adhesion between graphene and MoS2 sheets for water treatment. Appl. Surf. Sci. 2020, 512, 145717. [Google Scholar] [CrossRef]
- Hu, H.; Ou, J.Z.; Xu, X.; Lin, Y.; Zhang, Y.; Zhao, H.; Chen, D.; He, M.; Huang, Y.; Deng, L. Graphene-assisted construction of electrocatalysts for carbon dioxide reduction. Chem. Eng. J. 2021, 425, 130587. [Google Scholar] [CrossRef]
- Xu, X.; Huang, T.; Xu, Y.; Hu, H.; Liao, S.; Hu, X.; Chen, D.; Zhang, M. Highly dispersed CeO2–nanoparticles with rich oxygen vacancies enhance photocatalytic performance of g-C3N4 toward methyl orange degradation under visible light irradiation. J. Rare Earths 2022, 40, 1255–1263. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Chiou, J.-C.; Chen, W.-X.; Kan, C.-W.; Lam, T.Y.C.; Hu, H. Poly(hexamethylene biguanide): An efficient pH-tolerant and salt-intensive flocculant in the removal of anionic dyes from wastewater. J. Mater. Sci. 2022, 57, 15662–15673. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Y.; Zhang, Z.; Zhou, L.; Yu, G.; Wen, X.; Chi, T.; Wang, G.; Su, Y.; Deng, F.; et al. Fe-based metal organic frameworks (Fe-MOFs) for organic pollutants removal via photo-Fenton: A review. Chems. Eng. J. 2022, 431, 133932. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, J.; Massey, I.Y.; Peng, T.; Yang, F. Immobilization of Microbes for Biodegradation of Microcystins: A Mini Review. Toxins 2022, 14, 573. [Google Scholar] [CrossRef]
- Hu, H.; Chang, M.; Zhang, M.; Wang, X.; Chen, D. A new insight into PAM/graphene-based adsorption of water-soluble aromatic pollutants. J. Mater. Sci. 2017, 52, 8650–8664. [Google Scholar] [CrossRef]
- Goudjil, S.; Guergazi, S.; Masmoudi, T.; Achour, S. Effect of reactional parameters on the elimination of Congo Red by the combination of coagulation-floculation with aluminum sulphate. Desalin. Water Treat. 2021, 209, 429–436. [Google Scholar] [CrossRef]
- Hu, H.; Quan, H.; Zhong, B.; Li, Z.; Huang, Y.; Wang, X.; Zhang, M.; Chen, D. A Reduced Graphene Oxide Quantum Dot-Based Adsorbent for Efficiently Binding with Organic Pollutants. ACS Appl. Nano Mater. 2018, 1, 6502–6513. [Google Scholar] [CrossRef]
- Hu, H.; Liang, W.; Zhang, Y.; Wu, S.; Yang, Q.; Wang, Y.; Zhang, M.; Liu, Q. Multipurpose Use of a Corncob Biomass for the Production of Polysaccharides and the Fabrication of a Biosorbent. ACS Sustain. Chem. Eng. 2018, 6, 3830–3839. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, H.; Chang, M.; Wei, H.; Chen, D.; Zhang, M.; Wu, L.; Li, X. Template-free scalable synthesis of TiO2 hollow nanoparticles for excellent photoelectrochemical applications. J. Mater. Sci. 2017, 53, 2102–2114. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, H.; Kang, W.; Qiu, G.; Liang, R.; Deng, L.; Yuan, H. Enhancing hydrogen evolution by photoelectrocatalysis of water splitting over a CdS flowers-loaded TiO2 nanotube array film on the Ti foil substrate. Ceram. Int. 2020, 46, 17606–17613. [Google Scholar] [CrossRef]
- Hu, H.; Chang, M.; Wang, X.; Chen, D. Cotton fabric-based facile solar photocatalytic purification of simulated real dye wastes. J. Mater. Sci. 2017, 52, 9922–9930. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, F.; Chen, M.; Hu, H.; Lin, L.; Wu, J.; Zhang, M. Facile assembly of 2D α-zirconium phosphate supported silver nanoparticles: Superior and recyclable catalysis. New J. Chem. 2020, 44, 9793–9801. [Google Scholar] [CrossRef]
- Hu, H.-W.; Xin, J.H.; Hu, H. Highly Efficient Graphene-Based Ternary Composite Catalyst with Polydopamine Layer and Copper Nanoparticles. ChemPlusChem 2013, 78, 1483–1490. [Google Scholar] [CrossRef]
- Hu, H.; Xin, J.H.; Hu, H. PAM/graphene/Ag ternary hydrogel: Synthesis, characterization and catalytic application. J. Mater. Chem. A 2014, 2, 11319–11333. [Google Scholar] [CrossRef]
- Hu, H.; Xin, J.H.; Hu, H.; Wang, X. Structural and mechanistic understanding of an active and durable graphene carbocatalyst for reduction of 4-nitrophenol at room temperature. Nano Res. 2015, 8, 3992–4006. [Google Scholar] [CrossRef]
- Hu, H.; Wang, X.; Miao, D.; Wang, Y.; Lai, C.; Guo, Y.; Wang, W.; Xin, J.H.; Hu, H. A pH-mediated enhancement of the graphene carbocatalyst activity for the reduction of 4-nitrophenol. Chem. Commun. 2015, 51, 16699–16702. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xin, J.H.; Hu, H.; Wang, X.; Kong, Y. Metal-free graphene-based catalyst—Insight into the catalytic activity: A short review. Appl. Catal. A Gen. 2015, 492, 1–9. [Google Scholar] [CrossRef]
- Hu, H.; Xin, J.H.; Hu, H.; Wang, X.; Miao, D.; Liu, Y. Synthesis and stabilization of metal nanocatalysts for reduction reactions—A review. J. Mater. Chem. A 2015, 3, 11157–11182. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, P.; Matras-Postolek, K. Au Catalyst Decorated Silica Spheres: Synthesis and High-Performance in 4-Nitrophenol Reduction. J. Nanosci. Nanotechnol. 2016, 16, 5966–5974. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, X.; Wang, H.-Y.; Liu, X.; Qin, Y.; Zhang, P.; Guo, Z.-X. Porous gold nanoparticle/graphene oxide composite as efficient catalysts for reduction of 4-nitrophenol. RSC Adv. 2016, 6, 35945–35951. [Google Scholar] [CrossRef]
- Al-Kahtani, A.A.; Almuqati, T.; Alhokbany, N.; Ahamad, T.; Naushad, M.; Alshehri, S.M. A clean approach for the reduction of hazardous 4-nitrophenol using gold nanoparticles decorated multiwalled carbon nanotubes. J. Clean. Prod. 2018, 191, 429–435. [Google Scholar] [CrossRef]
- Beaton, G.; Zacks, J.; Stamplecoskie, K. Al2O3 anchored silver and gold nanoparticles as accessible, stable, and re-usable catalysts. Colloids Surf. A 2022, 646, 128972. [Google Scholar] [CrossRef]
- Qu, H.; Yang, L.; Yu, J.; Wang, L.; Liu, H. Host–Guest Interaction Induced Rapid Self-Assembled Fe3O4@Au Nanoparticles with High Catalytic Activity. Ind. Eng. Chem. Res. 2018, 57, 9448–9456. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, P.; Zhang, X.; Zhuo, R. Gold nanoparticles stabilized by amphiphilic hyperbranched polymers for catalytic reduction of 4-nitrophenol. J. Catal. 2016, 337, 65–71. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, L.; Peng, S.; Zheng, Y.; Sun, X.; Su, H.; Qi, C. Preparation of Au catalysts supported on core-shell SiO2/polypyrrole composites with high catalytic performances in the reduction of 4-nitrophenol. Synth. Met. 2019, 248, 20–26. [Google Scholar] [CrossRef]
- Obulapuram, P.K.; Arfin, T.; Mohammad, F.; Kumari, K.; Khiste, S.K.; Al-Lohedan, H.A.; Chavali, M. Surface-Enhanced Biocompatibility and Adsorption Capacity of a Zirconium Phosphate-Coated Polyaniline Composite. ACS Omega 2021, 6, 33614–33626. [Google Scholar] [CrossRef] [PubMed]
- Pica, M.; Calzuola, S.; Donnadio, A.; Gentili, P.; Nocchetti, M.; Casciola, M. De-Ethylation and Cleavage of Rhodamine B by a Zirconium Phosphate/Silver Bromide Composite Photocatalyst. Catalysts 2018, 9, 3. [Google Scholar] [CrossRef]
- Sanchez, J.; Stevens, M.B.; Young, A.R.; Gallo, A.; Zhao, M.; Liu, Y.; Ramos-Garcés, M.V.; Ben-Naim, M.; Colón, J.L.; Sinclair, R.; et al. Isolating the Electrocatalytic Activity of a Confined NiFe Motif within Zirconium Phosphate. Adv. Energy Mater. 2021, 11, 2003545. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S. Zirconium phosphate (ZrP)-based functional materials: Synthesis, properties and applications. Mater. Des. 2018, 155, 19–35. [Google Scholar] [CrossRef]
- Campoccia, D.; Ravaioli, S.; Vivani, R.; Donnadio, A.; Vischini, E.; Russo, A.; Visai, L.; Arciola, C.R.; Montanaro, L.; Nocchetti, M. Antibacterial Properties of a Novel Zirconium Phosphate-Glycinediphosphonate Loaded with Either Zinc or Silver. Materials 2019, 12, 3184. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Boo, W.J.; Sue, H.-J.; Clearfield, A. Preparation of alpha-zirconium phosphate nanoplatelets with wide variations in aspect ratios. New J. Chem. 2007, 31, 39–43. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, L.; Zeng, S.; Liu, J.; Xiao, M.; Wang, S.; Meng, Y.; Sun, L. Synthesis of Polylactide Nanocomposites Using an α-Zirconium Phosphate Nanosheet-Supported Zinc Catalyst via in Situ Polymerization. ACS Appl. Polym. Mater. 2019, 1, 1382–1389. [Google Scholar] [CrossRef]
- Shi, Z.; Tang, J.; Chen, L.; Yan, C.; Tanvir, S.; Anderson, W.A.; Berry, R.M.; Tam, K.C. Enhanced colloidal stability and antibacterial performance of silver nanoparticles/cellulose nanocrystal hybrids. J. Mater. Chem. B 2015, 3, 603–611. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.T.; Jaenicke, S.; Chuah, G.-K. Minimalistic Liquid-Assisted Route to Highly Crystalline α-Zirconium Phosphate. ChemSusChem 2017, 10, 3235–3242. [Google Scholar] [CrossRef]
- Thakkar, R.; Patel, H.; Chudasama, U. A comparative study of proton transport properties of zirconium phosphate and its metal exchanged phases. Bull. Mater. Sci. 2007, 30, 205–209. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, A.; Wang, Z.; Chen, M.; Wang, W.; Sun, L.; Liu, X. Titanium functionalized [small alpha]-zirconium phosphate single layer nanosheets for photocatalyst applications. RSC Adv. 2015, 5, 93969–93978. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, R.; Ding, F.; Brittain, A.D.; Liu, J.; Zhang, M.; Xiao, M.; Meng, Y.; Sun, L. Sulfonic acid-functionalized alpha-zirconium phosphate single-layer nanosheets as a strong solid acid for heterogeneous catalysis applications. ACS Appl. Mater. Interfaces 2014, 6, 7417–7425. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Fu, L.; Zuo, X.; Yang, H. Green assembly of stable and uniform silver nanoparticles on 2D silica nanosheets for catalytic reduction of 4-nitrophenol. Appl. Catal. B Environ. 2018, 226, 23–30. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, C.; Zou, P.; Zhang, P.; Zhang, M.; Mu, J.; Guo, Z.; Li, X.; Wang, C.; Liu, Y. In situ assembly of well-dispersed gold nanoparticles on electrospun silica nanotubes for catalytic reduction of 4-nitrophenol. Chem. Commun. 2011, 47, 3906–3908. [Google Scholar] [CrossRef]
- Mao, H.; Ji, C.; Liu, M.; Cao, Z.; Sun, D.; Xing, Z.; Chen, X.; Zhang, Y.; Song, X.-M. Enhanced catalytic activity of Ag nanoparticles supported on polyacrylamide/polypyrrole/graphene oxide nanosheets for the reduction of 4-nitrophenol. Appl. Surf. Sci. 2018, 434, 522–533. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Chaker, M.; Rosei, F.; Ma, D. Gold nanoparticle decorated ceria nanotubes with significantly high catalytic activity for the reduction of nitrophenol and mechanism study. Appl. Catal. B Environ. 2013, 132–133, 107–115. [Google Scholar] [CrossRef]
- Gole, B.; Sanyal, U.; Mukherjee, P.S. A smart approach to achieve an exceptionally high loading of metal nanoparticles supported by functionalized extended frameworks for efficient catalysis. Chem. Commun. 2015, 51, 4872–4875. [Google Scholar] [CrossRef]
- He, J.; Razzaque, S.; Jin, S.; Hussain, I.; Tan, B. Efficient Synthesis of Ultrafine Gold Nanoparticles with Tunable Sizes in a Hyper-Cross-Linked Polymer for Nitrophenol Reduction. ACS Appl. Nano Mater. 2019, 2, 546–553. [Google Scholar] [CrossRef]


| Catalyst | Completion Time (s) | kapp (10−3 s−1) a | TOF (min−1) b | Reference |
|---|---|---|---|---|
| ZrP@PDA/Au | 420 | 7.4 | 38.10 | this work |
| ZrP@PDA/Ag | 180 | 34.76 | 32.36 | [18] |
| AgNPs/SiNSs | 40 | 80.19 | 3.52 | [44] |
| AuNPs/SNTs | 280 | 10.64 | -- | [45] |
| PLAL-AuNPs/CeO2-NTs | 1200 | 2.25 | 0.367 | [47] |
| Au@MOF-3 | 60 | 68.8 | 0.016 | [48] |
| Au@TR-HCP-TPMT | 480 | 1.67 | 1.19 | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Wen, Y.; Li, L.; Tan, Y.; Yang, P.; Liang, Y.; Xu, Y.; Hu, H.; Xu, Y. Mussel-Inspired Surface Modification of α-Zirconium Phosphate Nanosheets for Anchoring Efficient and Reusable Ultrasmall Au Nanocatalysts. Nanomaterials 2022, 12, 3339. https://doi.org/10.3390/nano12193339
Lin L, Wen Y, Li L, Tan Y, Yang P, Liang Y, Xu Y, Hu H, Xu Y. Mussel-Inspired Surface Modification of α-Zirconium Phosphate Nanosheets for Anchoring Efficient and Reusable Ultrasmall Au Nanocatalysts. Nanomaterials. 2022; 12(19):3339. https://doi.org/10.3390/nano12193339
Chicago/Turabian StyleLin, Limiao, Yi Wen, Lixi Li, Ying Tan, Peng Yang, Yaoheng Liang, Yisheng Xu, Huawen Hu, and Yonghang Xu. 2022. "Mussel-Inspired Surface Modification of α-Zirconium Phosphate Nanosheets for Anchoring Efficient and Reusable Ultrasmall Au Nanocatalysts" Nanomaterials 12, no. 19: 3339. https://doi.org/10.3390/nano12193339
APA StyleLin, L., Wen, Y., Li, L., Tan, Y., Yang, P., Liang, Y., Xu, Y., Hu, H., & Xu, Y. (2022). Mussel-Inspired Surface Modification of α-Zirconium Phosphate Nanosheets for Anchoring Efficient and Reusable Ultrasmall Au Nanocatalysts. Nanomaterials, 12(19), 3339. https://doi.org/10.3390/nano12193339






