One-Pot Synthesis of Pt Nanobowls Assembled from Ultrafine Nanoparticles for Methanol Oxidation Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Methods
2.3. Characterization
2.4. Electrochemical Measurements
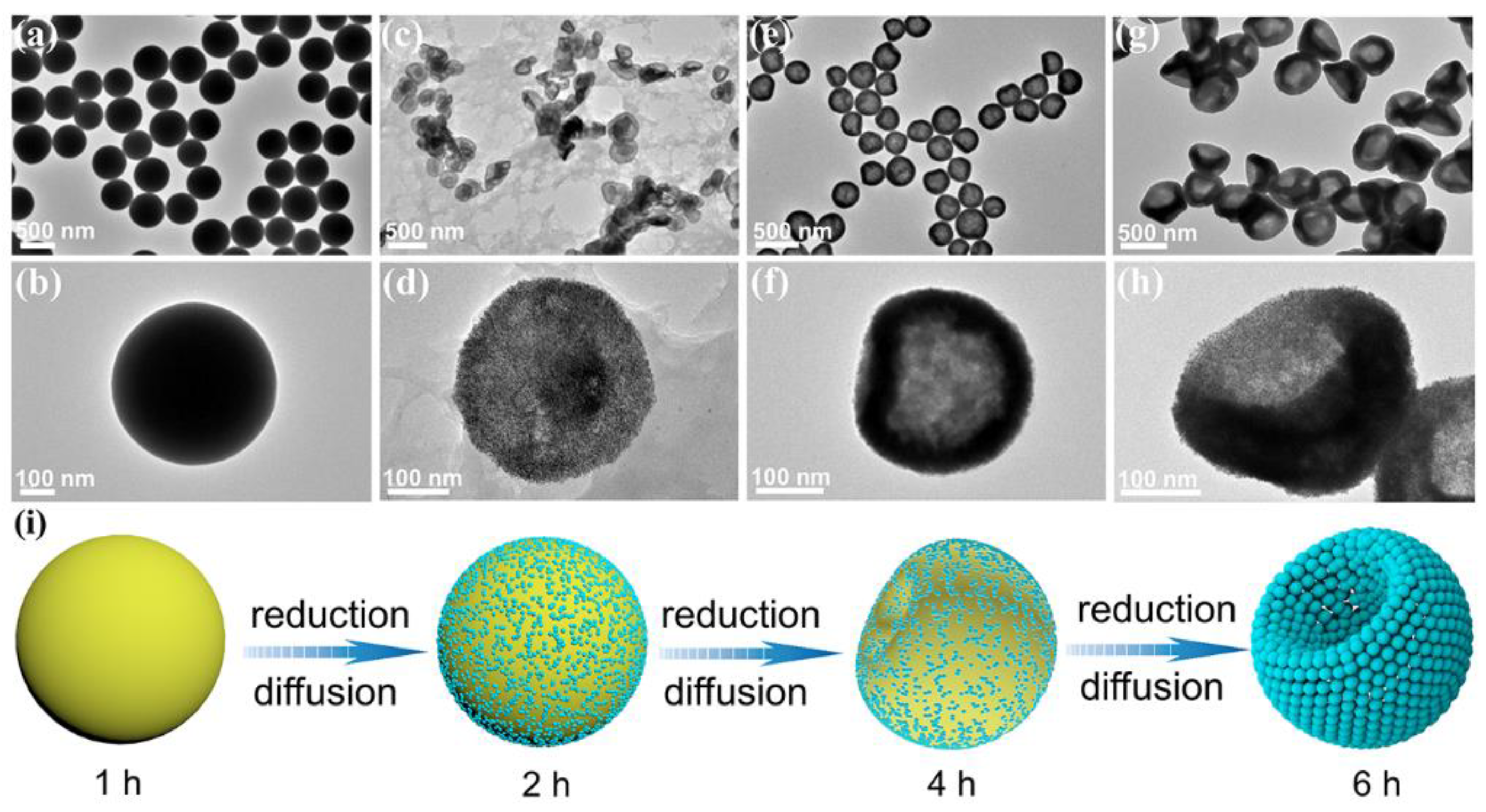
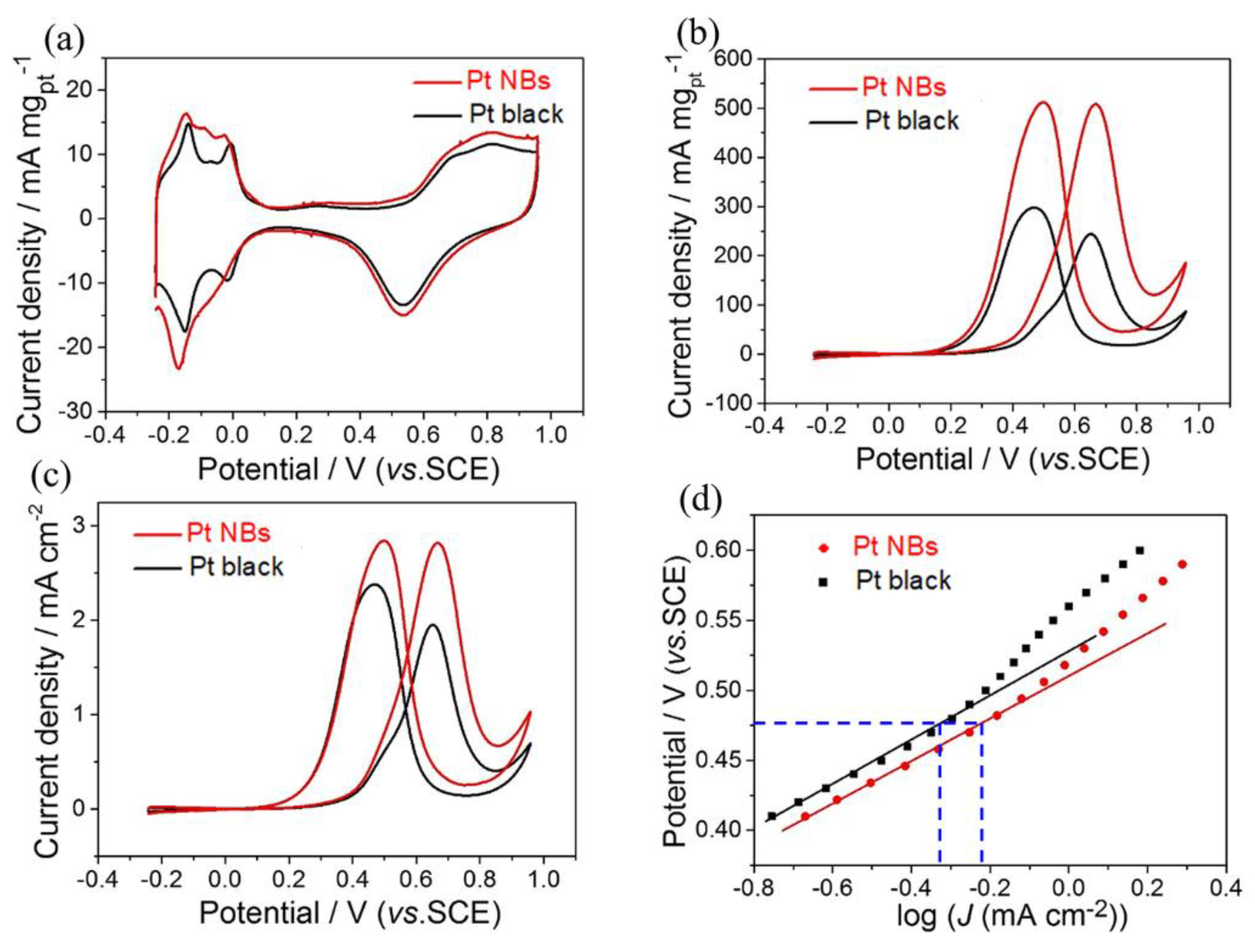
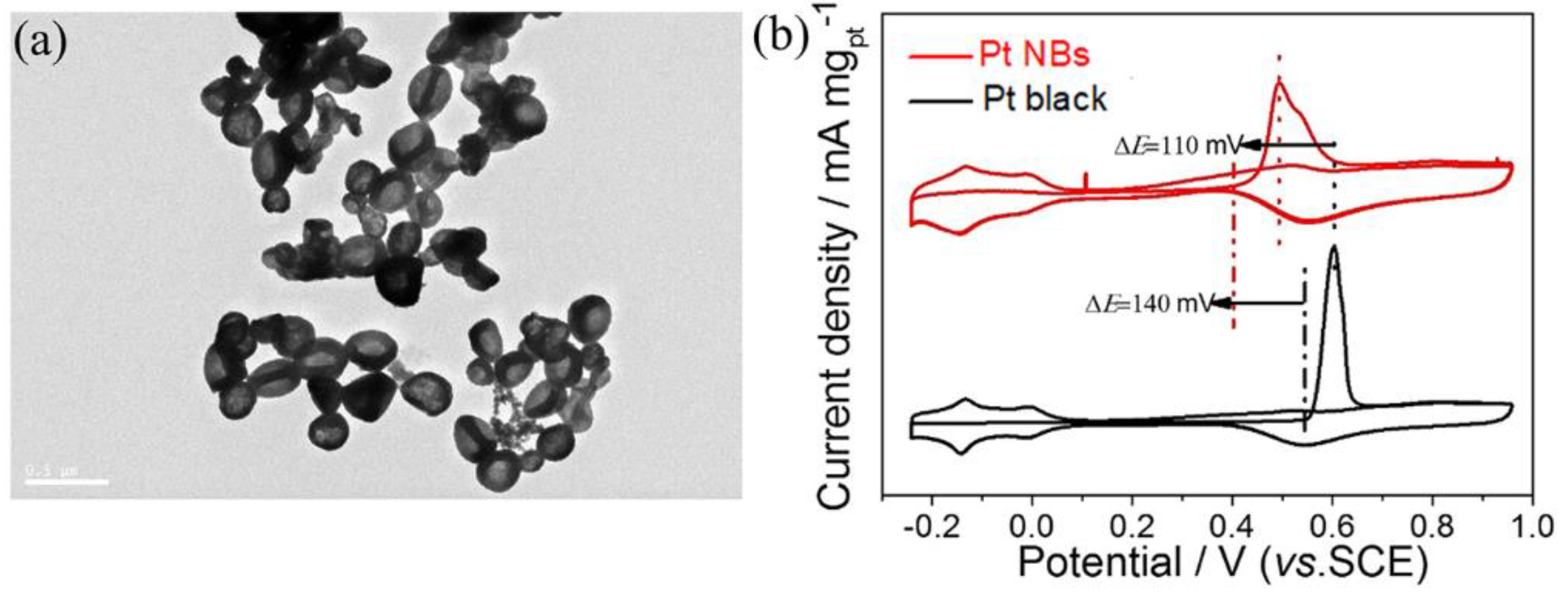
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, B.; Zhong, X.; Zhou, G.; Zhao, N.; Cheng, H.M. Graphene-supported atomically dispersed metals as bifunctional catalysts for next-generation batteries based on conversion reactions. Adv. Mater. 2022, 34, e2105812. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, J.; Zheng, Y.; Xiao, M.; Gao, R.; Zhang, Z.; Wen, G.; Dou, H.; Deng, Y.P.; Yu, A.; et al. Materials engineering toward durable electrocatalysts for proton exchange membrane fuel cells. Adv. Energy Mater. 2021, 12, 122102665. [Google Scholar] [CrossRef]
- Yuda, A.; Ashok, A.; Kumar, A. A comprehensive and critical review on recent progress in anode catalyst for methanol oxidation reaction. Catal. Rev. 2020, 64, 126–228. [Google Scholar] [CrossRef]
- Zheng, F.; Kwong, T.L.; Yung, K.F. Surfactant-free monodispersed pd nanoparticles template for core-shell pd@pdpt nanoparticles as electrocatalyst towards methanol oxidation reaction (mor). Nanomaterials 2022, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.P.; Caracciolo, D.T.; Maswadeh, Y.; Wen, J.; Kong, Z.; Shan, S.; Vargas, J.A.; Yan, S.; Hopkins, E.; Park, K.; et al. Alloying-realloying enabled high durability for pt-pd-3d-transition metal nanoparticle fuel cell catalysts. Nat. Commun. 2021, 12, 859. [Google Scholar] [CrossRef]
- Wu, S.D.; Chiou, A.H. The study on a new method of preparing pmma forming composite bipolar plate. Sci. Rep. 2021, 11, 8753. [Google Scholar] [CrossRef]
- Tong, Y.; Yan, X.; Liang, J.; Dou, S.X. Metal-based electrocatalysts for methanol electro-oxidation: Progress, opportunities, and challenges. Small 2021, 17, e1904126. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Tran, V.V.; Siahaan, A.; Kan, H.C.; Hsu, Y.J.; Hsu, C.C. Free-standing, interwoven tubular graphene mesh-supported binary aupt nanocatalysts: An innovative and high-performance anode methanol oxidation catalyst. Nanomaterials 2022, 12, 1689. [Google Scholar] [CrossRef]
- Ali, A.; Shen, P.K. Recent advances in graphene-based platinum and palladium electrocatalysts for the methanol oxidation reaction. J. Mater. Chem. A 2019, 7, 22189–22217. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, X.; Wang, X.; Hu, J.; Liu, Y.; Fu, G.; Tang, Y. Concave ptco nanocrosses for methanol oxidation reaction. Appl. Catal. Environ. 2020, 277, 119135. [Google Scholar] [CrossRef]
- Huang, W.; Wang, H.; Zhou, J.; Wang, J.; Duchesne, P.N.; Muir, D.; Zhang, P.; Han, N.; Zhao, F.; Zeng, M.; et al. Highly active and durable methanol oxidation electrocatalyst based on the synergy of platinum-nickel hydroxide-graphene. Nat. Commun. 2015, 6, 10035. [Google Scholar] [CrossRef] [PubMed]
- Daka, M.; Ferrara, M.; Bevilacqua, M.; Pengo, P.; Rajak, P.; Ciancio, R.; Montini, T.; Pasquato, L.; Fornasiero, P. Wet-chemical synthesis of porous multifaceted platinum nanoparticles for oxygen reduction and methanol oxidation reactions. ACS Appl. Nano Mater. 2022, 5, 4710–4720. [Google Scholar] [CrossRef]
- Ahmad, Y.H.; El-Sayed, H.A.; Mohamed, A.T.; Aljaber, A.S.; Al-Qaradawi, S.Y. Rational one-pot synthesis of ternary ptircu nanocrystals as robust electrocatalyst for methanol oxidation reaction. Appl. Surf. Sci. 2020, 534, 147617. [Google Scholar] [CrossRef]
- Guo, K.; Liu, Y.; Han, M.; Xu, D.; Bao, J. Highly branched ultrathin pt-ru nanodendrites. Chem. Commun. 2019, 55, 11131–11134. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Liang, X.; Xu, G.; Li, D.; Li, Y.; Zhang, N.; Chen, Y.; Jiang, X.; Gong, H. Self-supported defect-rich au-based nanostructures as robust bifunctional catalysts for the methanol oxidation reaction and oxygen reduction reaction in an alkaline medium. Nanomaterials 2021, 11, 2193. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, K.; Zhang, Y.; Shi, D.; Wu, Q.; Liang, D.; Hu, C.; Li, H. N-doped carbon confined cofe@pt nanoparticles with robust catalytic performance for the methanol oxidation reaction. J. Mater. Chem. A 2022, 10, 13345–13354. [Google Scholar] [CrossRef]
- Fan, C.; Wen, P.; Li, G.; Li, G.; Gu, J.; Li, Q.; Li, B. Facile synthesis of pt5la nanoalloys as the enhanced electrocatalysts for oxygen reduction reaction and methanol oxidation reaction. J. Alloys Compd. 2022, 894, 161892. [Google Scholar] [CrossRef]
- Zhang, T.; Pu, H.; Dai, H.; Dong, K.; Wang, K.; Zhou, L.; Wang, Y.; Deng, Y. Electrodeposition of pt–rh trioctahedral nanocrystals for the oxidation reactions of methanol and ethanol. ACS Appl. Energy Mater. 2021, 5, 807–814. [Google Scholar] [CrossRef]
- Tian, H.; Wu, D.; Li, J.; Luo, J.; Jia, C.; Liu, Z.; Huang, W.; Chen, Q.; Shim, C.M.; Deng, P.; et al. Rational design ternary platinum based electrocatalysts for effective methanol oxidation reaction. J. Energy Chem. 2022, 70, 230–235. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Ali, A.; Chen, H.; Shen, P.K. Facile one-pot synthesis of a ptrh alloy decorated on ag nanocubes as a trimetallic core–shell catalyst for boosting methanol oxidation reaction. ACS Appl. Energy Mater. 2021, 4, 1085–1092. [Google Scholar] [CrossRef]
- Wang, K.; Huang, D.; Guan, Y.; Liu, F.; He, J.; Ding, Y. Fine-tuning the electronic structure of dealloyed ptcu nanowires for efficient methanol oxidation reaction. ACS Catal. 2021, 11, 14428–14438. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Waterhouse, G.I.N. Complex alloy nanostructures as advanced catalysts for oxygen electrocatalysis: From materials design to applications. J. Mater. Chem. A 2020, 8, 23142–23161. [Google Scholar] [CrossRef]
- Ling, T.; Jaroniec, M.; Qiao, S.Z. Recent progress in engineering the atomic and electronic structure of electrocatalysts via cation exchange reactions. Adv. Mater. 2020, 32, e2001866. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Du, H.; Zhao, Y.; Wang, Q.; Liu, Y.; Li, D.; Feng, J. Recent progress on rational design of bimetallic pd based catalysts and their advanced catalysis. ACS Catal. 2020, 10, 13560–13583. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Du, D.; Lin, Y. Robust noble metal-based electrocatalysts for oxygen evolution reaction. Chem. Soc. Rev. 2019, 48, 3181–3192. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, Z.; Duan, X.; Huang, Y. Nanoscale structure design for high-performance pt-based orr catalysts. Adv. Mater. 2019, 31, e1802234. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Z.; Sun, M.; Zhang, H.; Wang, H. Ligand-free sub-5 nm platinum nanocatalysts on polydopamine supports: Size-controlled synthesis and size-dictated reaction pathway selection. Nanoscale 2022, 14, 5743–5750. [Google Scholar] [CrossRef]
- Wang, L.; Yin, P.; Zeng, W.-J.; Xu, S.-L.; Chen, P.; Liang, H.-W. Bulky nanodiamond-confined synthesis of sub-5 nanometer ordered intermetallic pd3pb catalysts. Nano Res. 2022, 15, 4973–4979. [Google Scholar] [CrossRef]
- Su, K.; Zhang, H.; Qian, S.; Li, J.; Zhu, J.; Tang, Y.; Qiu, X. Atomic crystal facet engineering of core-shell nanotetrahedrons restricted under sub-10 nanometer region. ACS Nano 2021, 15, 5178–5188. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, X.; Chen, Y.; Wang, S.; Skrabalak, S.E.; Tang, Y. Shape control of monodispersed sub-5 nm pd tetrahedrons and laciniate pd nanourchins by maneuvering the dispersed state of additives for boosting orr performance. Small 2020, 16, e1906026. [Google Scholar] [CrossRef]
- Ni, B.; Zhang, Q.; Ouyang, C.; Zhang, S.; Yu, B.; Zhuang, J.; Gu, L.; Wang, X. The synthesis of sub-nano-thick pd nanobelt–based materials for enhanced hydrogen evolution reaction activity. CCS Chem. 2020, 2, 642–654. [Google Scholar] [CrossRef]
- Cao, S.; Tao, F.F.; Tang, Y.; Li, Y.; Yu, J. Size- and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4765. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jia, Y.; Xie, S.; Xie, Z. Well-faceted noble-metal nanocrystals with nonconvex polyhedral shapes. Chem Soc. Rev. 2016, 45, 3207–3220. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Varela, A.S.; Kühl, S.; Strasser, P.; Cuenya, B.R. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 2016, 1, 16009. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhu, C.; Su, S.; Li, D.; He, Y.; Huang, Q.; Fan, C. Self-Catalyzed, Self-Limiting Growth of Glucose Oxidase-Mimicking Gold Nanoparticles. ACS Nano 2010, 4, 7451–7458. [Google Scholar] [CrossRef]
- Yang, Y.; Dai, C.; Wu, D.; Liu, Z.; Cheng, D. The size effect of pdcu bimetallic nanoparticles on oxygen reduction reaction activity. ChemElectroChem 2018, 5, 2571–2576. [Google Scholar] [CrossRef]
- Mastronardi, V.; Kim, J.; Veronesi, M.; Pomili, T.; Berti, F.; Udayan, G.; Brescia, R.; Diercks, J.S.; Herranz, J.; Bandiera, T.; et al. Green chemistry and first-principles theory enhance catalysis: Synthesis and 6-fold catalytic activity increase of sub-5 nm pd and pt@pd nanocubes. Nanoscale 2022, 14, 10155–10168. [Google Scholar] [CrossRef]
- Xu, H.; Yan, B.; Zhang, K.; Wang, J.; Li, S.; Wang, C.; Du, Y.; Yang, P. Sub-5nm monodispersed pdcu nanosphere with enhanced catalytic activity towards ethylene glycol electrooxidation. Electrochim. Acta 2018, 261, 521–529. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Huang, T.; Fu, G.; Tang, Y.; Qiu, X.; Zhou, J.; Lee, J.-M. Sub-5 nm palladium nanoparticles in situ embedded in n-doped carbon nanoframes: Facile synthesis, excellent sinter resistance and electrocatalytic properties. J. Mater. Chem. A 2019, 7, 26243–26249. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, L.; Li, J.; Doyle-Davis, K.; Li, R.; Wang, Z.; Sun, X. Advanced support materials and interactions for atomically dispersed noble-metal catalysts: From support effects to design strategies. Adv. Energy Mater. 2021, 12, 2102556. [Google Scholar] [CrossRef]
- Zhou, S.; Liao, W.; Wang, Z.; Chen, M.; Long, J.; Zhou, Q.; Wang, Q. A facile and green eutectic salt-mediated pyrolysis strategy to synthesize hollow ptni alloy nanocubes for oxygen reduction reaction. ACS Appl. Energy Mater. 2022, 5, 6472–6480. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, C.H.; Li, N.W.; Luan, D.; Yu, L.; Lou, X.W.D. Design and synthesis of hollow nanostructures for electrochemical water splitting. Adv. Sci. 2022, 9, e2105135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Liu, S.; Zhou, X.; Xu, L.; Tian, X.; Yang, J.; Tang, Y. Self-templating-oriented manipulation of ultrafine pt3 cu alloyed nanoparticles into asymmetric porous bowl-shaped configuration for high-efficiency methanol electrooxidation. Small 2022, 18, e2202782. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Li, M.; Li, Z.; Liu, Z.; Fu, G.; Tang, Y. Facile synthesis of channel-rich ultrathin palladium-silver nanosheets for highly efficient formic acid electrooxidation. Mater. Today Energy 2021, 19, 100596. [Google Scholar] [CrossRef]
- Fu, G.; Zhang, Q.; Wu, J.; Sun, D.; Xu, L.; Tang, Y.; Chen, Y. Arginine mediated synthesis of cube-like platinum nanoassemblies as advanced electrocatalysts. Nano Res. 2015, 8, 3963–3971. [Google Scholar] [CrossRef]
- Li, H.H.; Yu, S.H. Recent advances on controlled synthesis and engineering of hollow alloyed nanotubes for electrocatalysis. Adv. Mater. 2019, 31, e1803503. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, B.; Cheng, B.; Macyk, W.; Kuang, P.; Yu, J. Hollow carbon sphere-supported pt/coo hybrid with excellent hydrogen evolution activity and stability in acidic environment. Appl. Catal. Environ. 2022, 314, 121503. [Google Scholar] [CrossRef]
- Yao, W.; Jiang, X.; Li, M.; Li, Y.; Liu, Y.; Zhan, X.; Fu, G.; Tang, Y. Engineering hollow porous platinum-silver double-shelled nanocages for efficient electro-oxidation of methanol. Appl. Catal. Environ. 2021, 282, 119595. [Google Scholar] [CrossRef]
- Wan, X.K.; Wu, H.B.; Guan, B.Y.; Luan, D.; Lou, X.W.D. Confining sub-nanometer pt clusters in hollow mesoporous carbon spheres for boosting hydrogen evolution activity. Adv. Mater. 2020, 32, e1901349. [Google Scholar] [CrossRef]
- Sui, N.; Wang, K.; Shan, X.; Bai, Q.; Wang, L.; Xiao, H.; Liu, M.; Colvin, V.L.; Yu, W.W. Facile synthesis of hollow dendritic ag/pt alloy nanoparticles for enhanced methanol oxidation efficiency. Dalton Trans. 2017, 46, 15541–15548. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Kou, H.; Ding, S. Complex hollow bowl-like nanostructures: Synthesis, application, and perspective. Adv. Funct. Mater. 2020, 31, 2007801. [Google Scholar] [CrossRef]
- Xu, H.; Song, P.; Yan, B.; Wang, J.; Guo, J.; Du, Y. Surface-plasmon-enhanced photo-electrocatalytic ethylene glycol oxidation based on highly open auag nanobowls. ACS Sustain. Chem. Eng. 2018, 6, 4138–4146. [Google Scholar] [CrossRef]
- Miller, A.V.; Kaichev, V.V.; Prosvirin, I.P.; Bukhtiyarov, V.I. Mechanistic study of methanol decomposition and oxidation on pt (111). J. Phys. Chem. C 2013, 117, 8189–8197. [Google Scholar] [CrossRef]
- Jiang, X.; Xiong, Y.; Wang, Y.; Wang, J.; Li, N.; Zhou, J.; Fu, G.; Sun, D.; Tang, Y. Treelike two-level pdxagy nanocrystals tailored for bifunctional fuel cell electrocatalysis. J. Mater. Chem. A 2019, 7, 5248–5257. [Google Scholar] [CrossRef]
- Ai, Y.; Hu, Z.; Liu, L.; Zhou, J.; Long, Y.; Li, J.; Ding, M.; Sun, H.B.; Liang, Q. Magnetically hollow pt nanocages with ultrathin walls as a highly integrated nanoreactor for catalytic transfer hydrogenation reaction. Adv. Sci. 2019, 6, 1802132. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Wang, X.; Fu, G.; Tang, Y. The use of amino-based functional molecules for the controllable synthesis of noble-metal nanocrystals: A minireview. Nanoscale Adv. 2021, 3, 1813–1829. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Fu, G.; Tang, Y. Recent advances in amino-based molecules assisted control of noble-metal electrocatalysts. Small 2021, 17, 2007179. [Google Scholar] [CrossRef]
- Lv, H.; Chen, X.; Fu, C.; She, P.; Xu, D.; Liu, B. “Dual-template”-directed synthesis of bowl-shaped mesoporous platinum nanostructures. Inorg. Chem. 2019, 58, 11195–11201. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, P.; Chen, Y.; Yao, W.; Li, Z.; Tang, Y. One-Pot Synthesis of Pt Nanobowls Assembled from Ultrafine Nanoparticles for Methanol Oxidation Reaction. Nanomaterials 2022, 12, 3471. https://doi.org/10.3390/nano12193471
Zhang S, Wang P, Chen Y, Yao W, Li Z, Tang Y. One-Pot Synthesis of Pt Nanobowls Assembled from Ultrafine Nanoparticles for Methanol Oxidation Reaction. Nanomaterials. 2022; 12(19):3471. https://doi.org/10.3390/nano12193471
Chicago/Turabian StyleZhang, Shoulin, Pu Wang, Yaoshun Chen, Wenqing Yao, Zhijuan Li, and Yawen Tang. 2022. "One-Pot Synthesis of Pt Nanobowls Assembled from Ultrafine Nanoparticles for Methanol Oxidation Reaction" Nanomaterials 12, no. 19: 3471. https://doi.org/10.3390/nano12193471
APA StyleZhang, S., Wang, P., Chen, Y., Yao, W., Li, Z., & Tang, Y. (2022). One-Pot Synthesis of Pt Nanobowls Assembled from Ultrafine Nanoparticles for Methanol Oxidation Reaction. Nanomaterials, 12(19), 3471. https://doi.org/10.3390/nano12193471




