Exfoliation of MoS2 Quantum Dots: Recent Progress and Challenges
Abstract
:1. Introduction
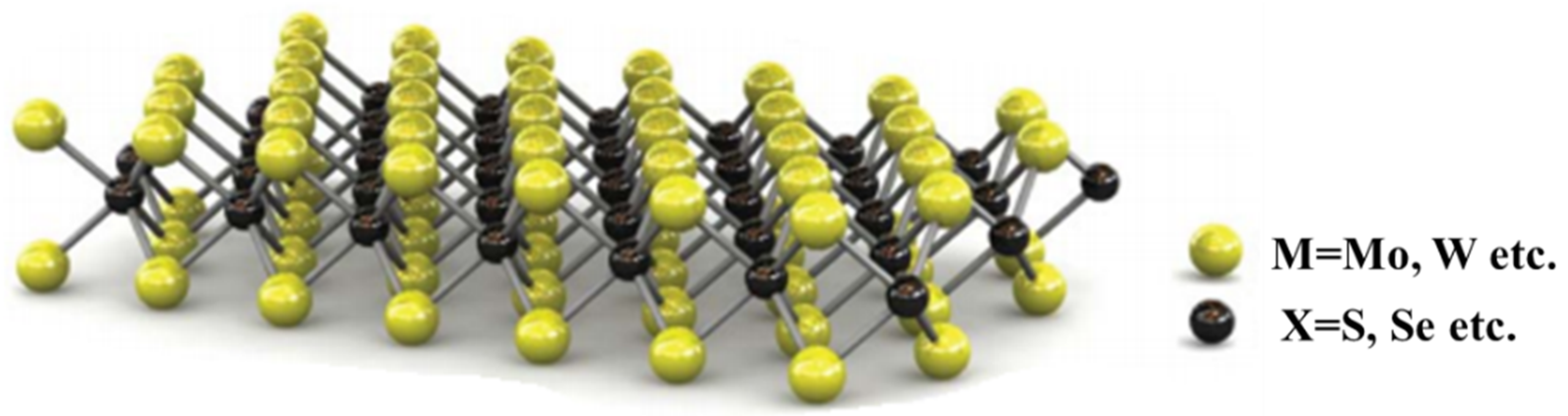
2. MoS2
2.1. Crystal Structure
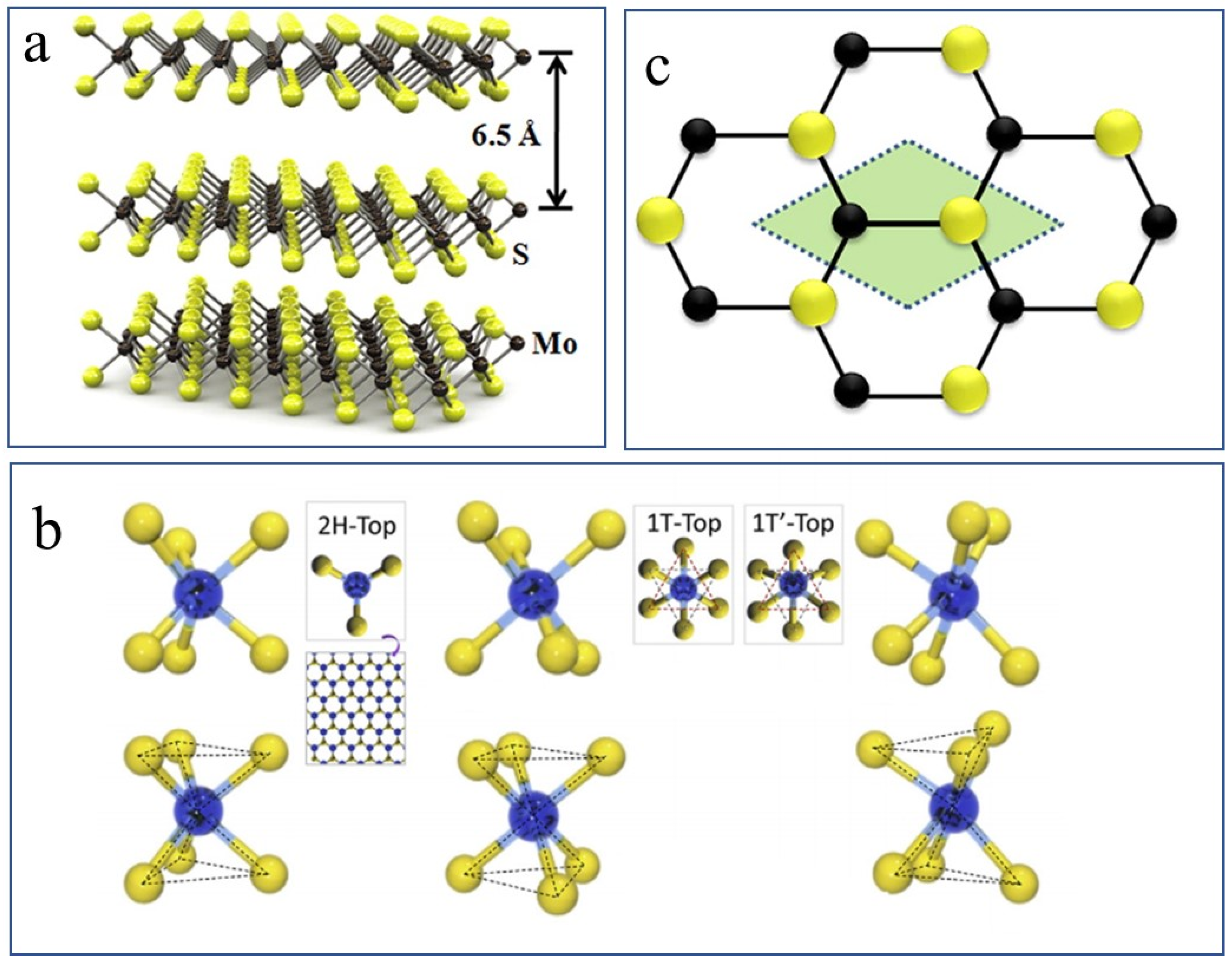
2.2. Properties

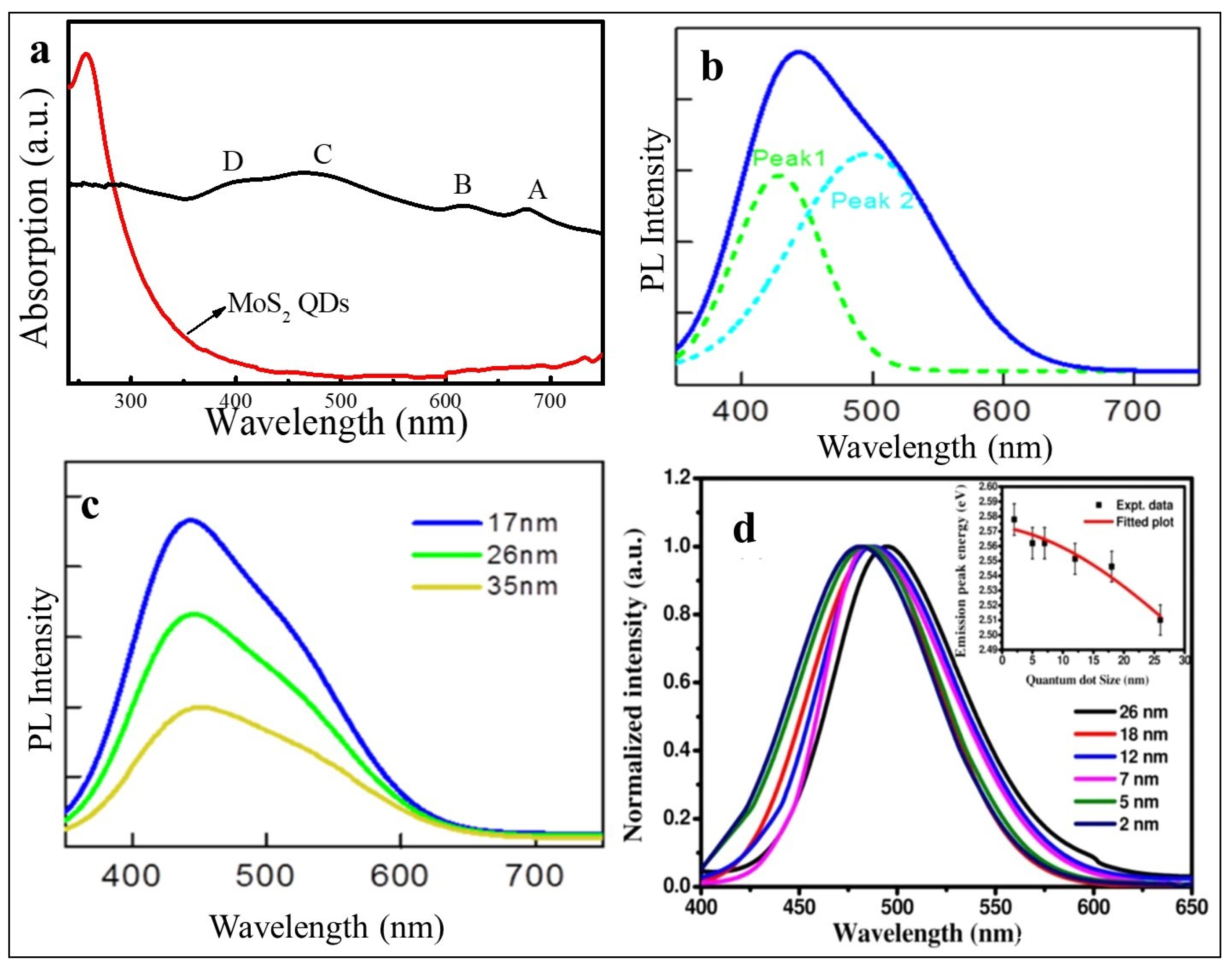
3. What Are Quantum Dots?
3.1. Elimination of van der Waal’s Forces
3.2. Large Surface Area-to-Volume Ratios
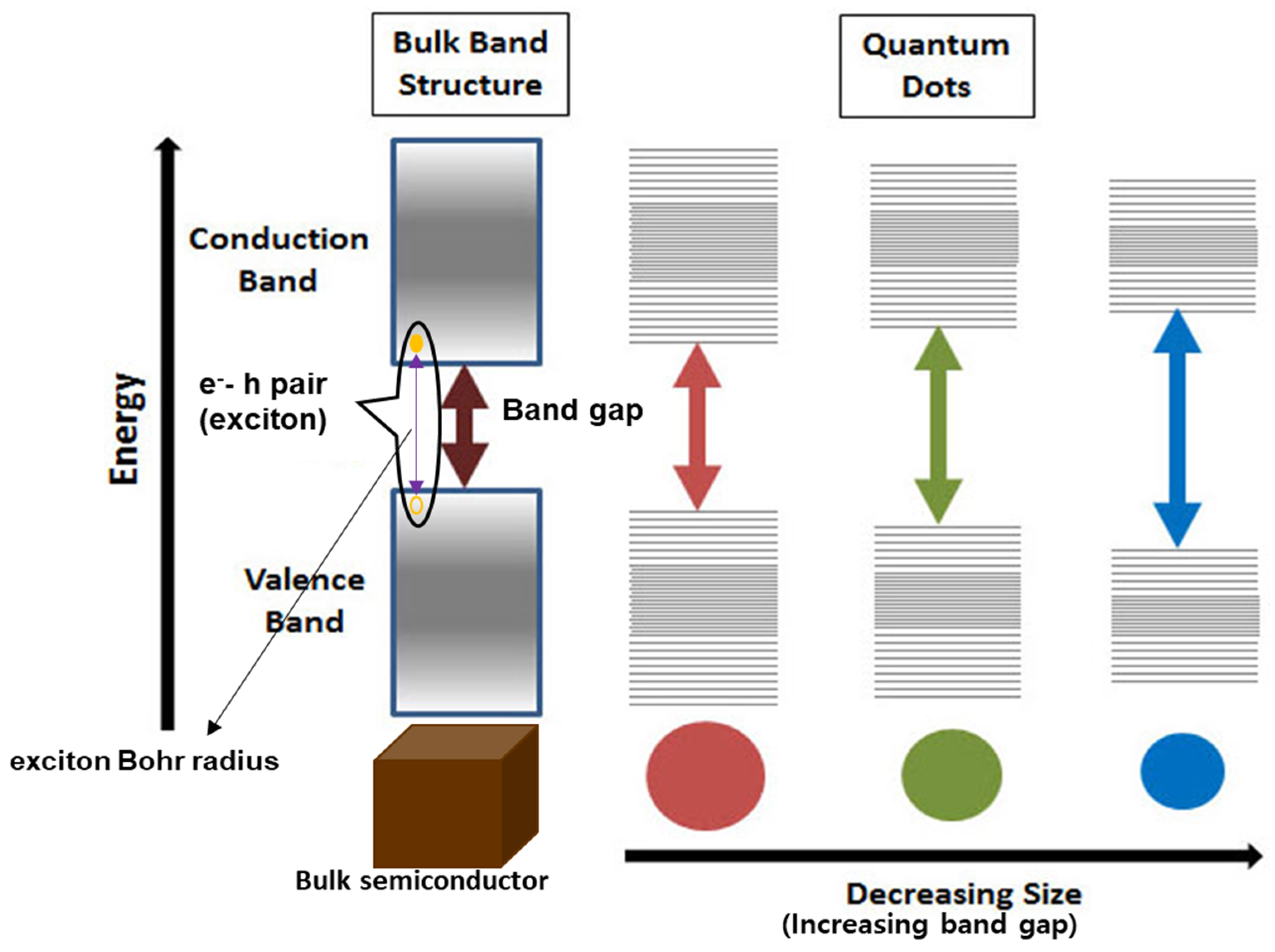
3.3. Quantum Confinement Effect
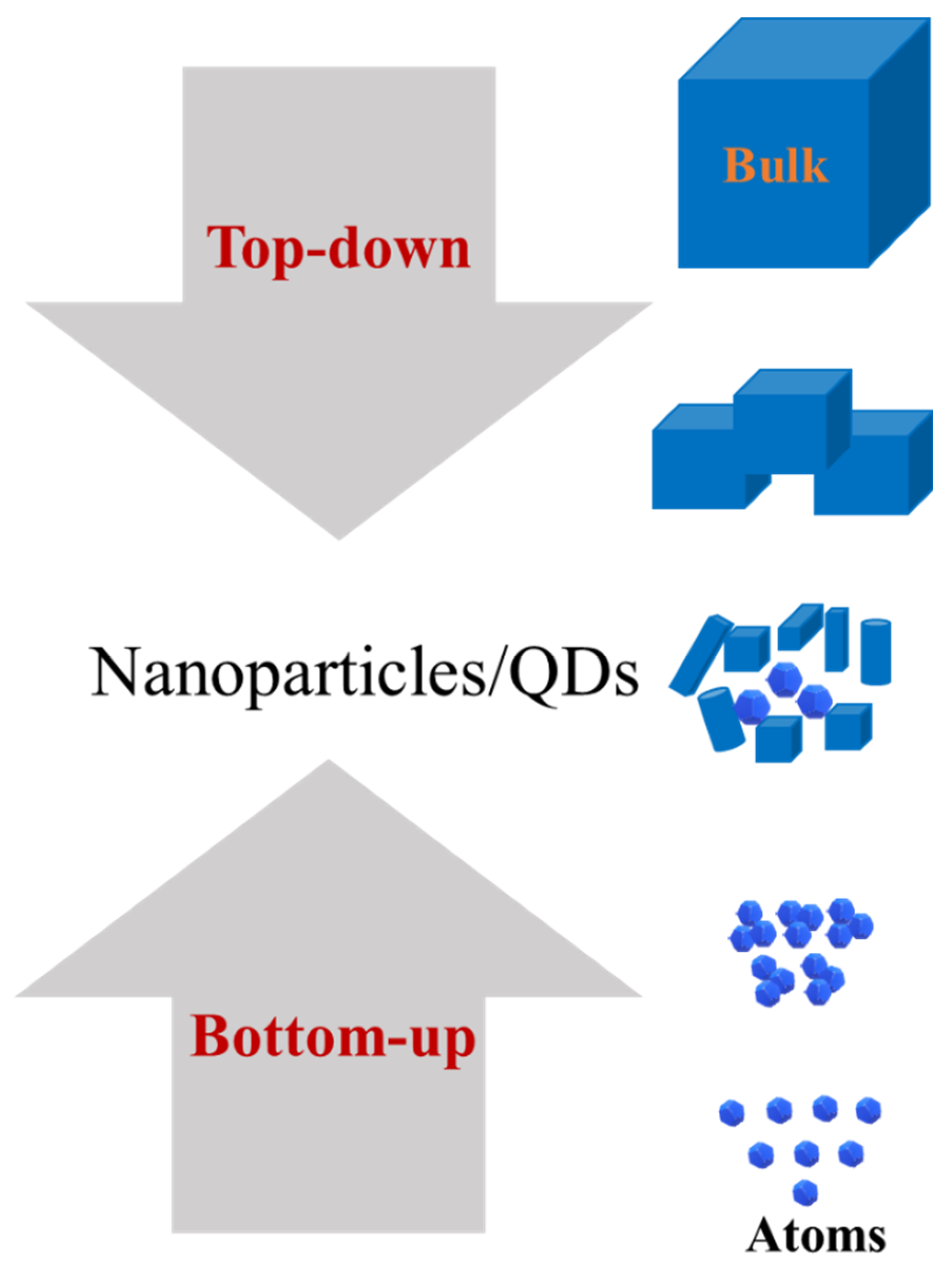
3.4. Fabrication of QDs
3.4.1. Bottom-Up
3.4.2. Top-Down
Mechanical Exfoliation
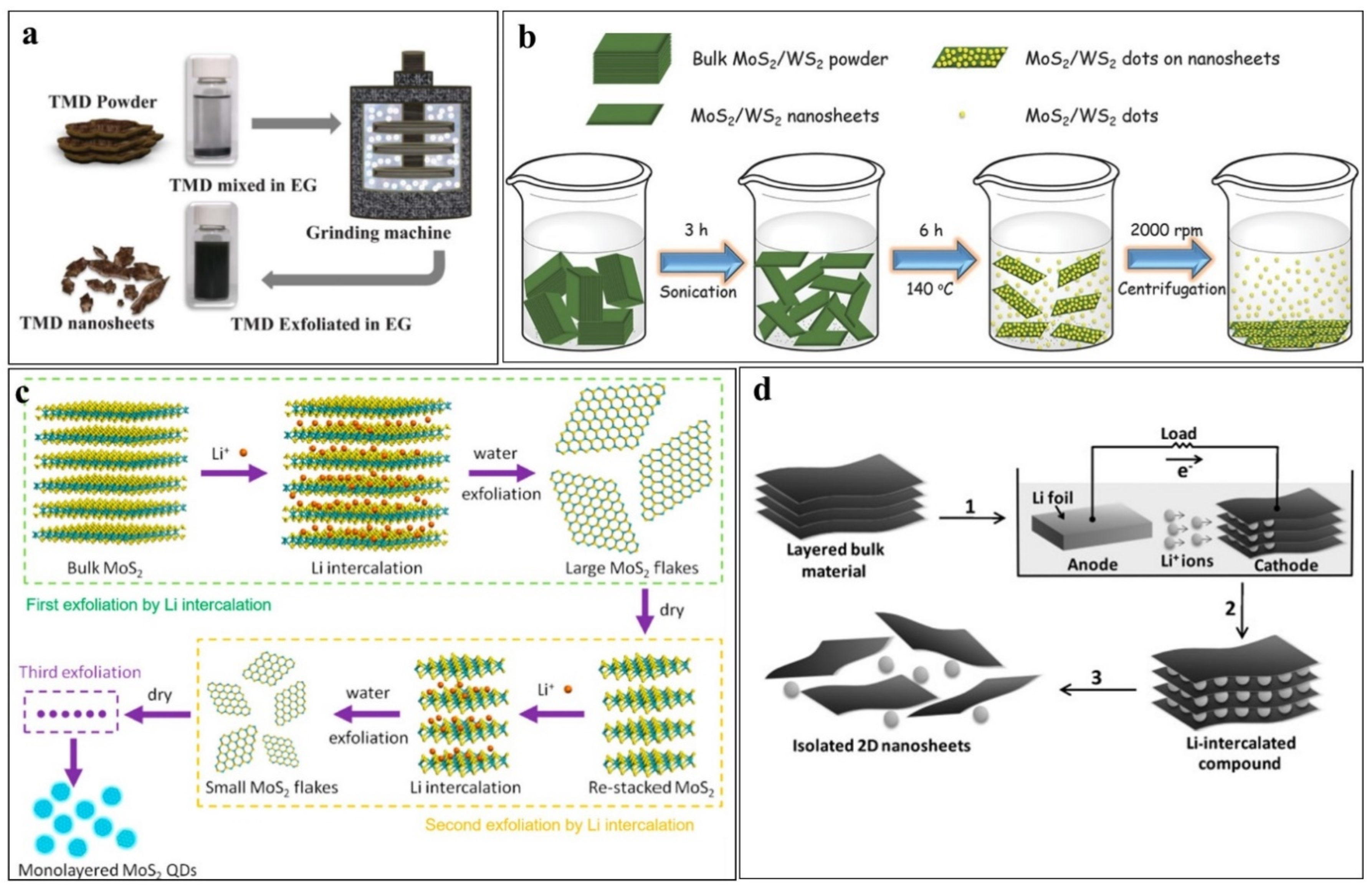
Ultrasonic Exfoliation
Ion-Intercalation Exfoliation
Electrochemical Exfoliation
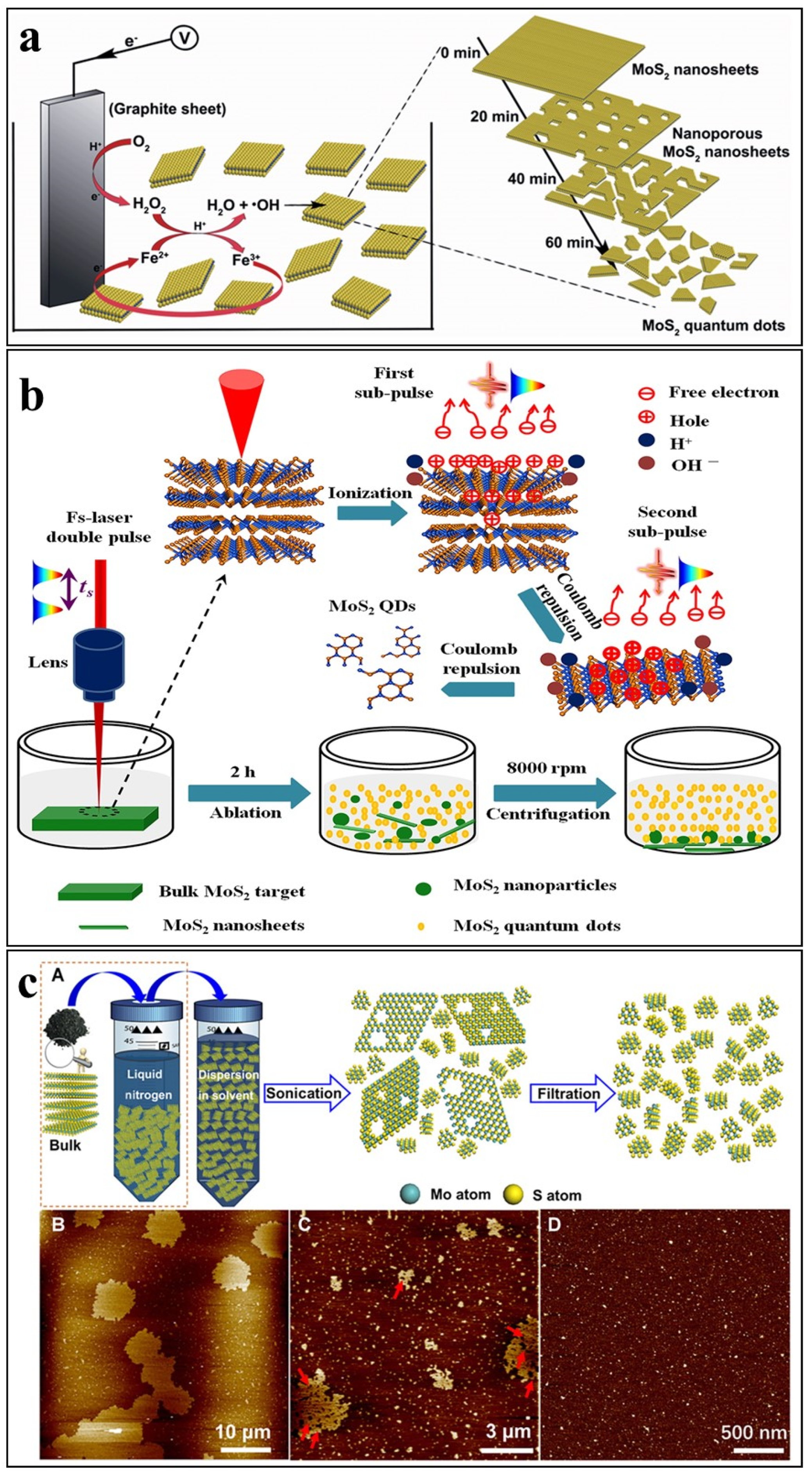
Electro-Fenton Assisted Electrochemical Exfoliation
Microwave-Assisted Exfoliation
Laser Ablation
Cryo-Mediated Exfoliation
3.5. Advantages and Limitations
4. Applications
4.1. Field Effect Transistors (FETs)
4.2. Photodetection
4.3. Photovoltaics
4.4. Sensing
4.5. Composites and Sieving Membranes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, P.R. The band theory of graphite. Phys. Rev. 1947, 71, 622. [Google Scholar] [CrossRef]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451. [Google Scholar] [CrossRef] [Green Version]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, E. Transition metal dichalcogenides and their intercalates. Int. Rev. Phys. Chem. 1983, 3, 177–216. [Google Scholar] [CrossRef]
- Wilson, J.A.; Yoffe, A. The transition metal dichalcogenides discussion and interpretation of the observed optical, electrical and structural properties. Adv. Phys. 1969, 18, 193–335. [Google Scholar] [CrossRef]
- Das, S.; Chen, H.-Y.; Penumatcha, A.V.; Appenzeller, J. High performance multilayer MoS2 transistors with scandium contacts. Nano Lett. 2013, 13, 100–105. [Google Scholar] [CrossRef]
- Lee, G.-H.; Cui, X.; Kim, Y.D.; Arefe, G.; Zhang, X.; Lee, C.-H.; Ye, F.; Watanabe, K.; Taniguchi, T.; Kim, P. Highly stable, dual-gated MoS2 transistors encapsulated by hexagonal boron nitride with gate-controllable contact, resistance, and threshold voltage. ACS Nano 2015, 9, 7019–7026. [Google Scholar] [CrossRef]
- Castellanos-Gomez, A.; Poot, M.; Steele, G.A.; Van Der Zant, H.S.; Agraït, N.; Rubio-Bollinger, G. Elastic properties of freely suspended MoS2 nanosheets. Adv. Mater. 2012, 24, 772–775. [Google Scholar] [CrossRef] [Green Version]
- Bertolazzi, S.; Brivio, J.; Kis, A. Stretching and breaking of ultrathin MoS2. ACS Nano 2011, 5, 9703–9709. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical Properties of Monolayer Graphene Oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef] [PubMed]
- Piper, J.R.; Fan, S. Broadband absorption enhancement in solar cells with an atomically thin active layer. ACS Photonics 2016, 3, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amani, M.; Lien, D.-H.; Kiriya, D.; Xiao, J.; Azcatl, A.; Noh, J.; Madhvapathy, S.R.; Addou, R.; Kc, S.; Dubey, M.; et al. Near-unity photoluminescence quantum yield in MoS2. Science 2015, 350, 1065. [Google Scholar] [CrossRef] [Green Version]
- Yan, R.; Simpson, J.R.; Bertolazzi, S.; Brivio, J.; Watson, M.; Wu, X.; Kis, A.; Luo, T.; Hight Walker, A.R.; Xing, H.G. Thermal Conductivity of Monolayer Molybdenum Disulfide Obtained from Temperature-Dependent Raman Spectroscopy. ACS Nano 2014, 8, 986–993. [Google Scholar] [CrossRef]
- Gopalakrishnan, D.; Damien, D.; Shaijumon, M.M. MoS2 Quantum Dot-Interspersed Exfoliated MoS2 Nanosheets. ACS Nano 2014, 8, 5297–5303. [Google Scholar] [CrossRef]
- Li, B.L.; Chen, L.X.; Zou, H.L.; Lei, J.L.; Luo, H.Q.; Li, N.B. Electrochemically induced Fenton reaction of few-layer MoS2 nanosheets: Preparation of luminescent quantum dots via a transition of nanoporous morphology. Nanoscale 2014, 6, 9831–9838. [Google Scholar] [CrossRef]
- Xu, S.; Li, D.; Wu, P. One-pot, facile, and versatile synthesis of monolayer MoS2/WS2 quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Ha, H.D.; Han, D.J.; Choi, J.S.; Park, M.; Seo, T.S. Dual role of blue luminescent MoS2 quantum dots in fluorescence resonance energy transfer phenomenon. Small 2014, 10, 3858–3862. [Google Scholar] [CrossRef]
- Han, B.; Hu, Y.H. MoS2 as a co-catalyst for photocatalytic hydrogen production from water. Energy Sci. Eng. 2016, 4, 285–304. [Google Scholar] [CrossRef]
- Benson, J.; Li, M.; Wang, S.; Wang, P.; Papakonstantinou, P. Electrocatalytic hydrogen evolution reaction on edges of a few layer molybdenum disulfide nanodots. ACS Appl. Mater. Interfaces 2015, 7, 14113–14122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, Y.; Zhang, J.; Wu, J.; Xu, H.; Wen, X.; Zhang, X.; Tiwary, C.S.; Yang, W.; Vajtai, R. Cryo-mediated exfoliation and fracturing of layered materials into 2D quantum dots. Sci. Adv. 2017, 3, e1701500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Jiang, L.; Li, X.; Ran, P.; Zuo, P.; Wang, A.; Qu, L.; Zhao, Y.; Cheng, Z.; Lu, Y. Preparation of monolayer MoS2 quantum dots using temporally shaped femtosecond laser ablation of bulk MoS2 targets in water. Sci. Rep. 2017, 7, 11182. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Hu, C.; Wang, X. A facile one-step method to produce MoS2 quantum dots as promising bio-imaging materials. RSC Adv. 2016, 6, 25605–25610. [Google Scholar] [CrossRef]
- Ali, L.; Bang, S.; Lee, Y.J.; Byeon, C.C. Ion-Intercalation Assisted Solvothermal Synthesis and Optical Characterization of MoS2 Quantum Dots. J. Korean Phys. Soc. 2019, 74, 191–195. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, L.; Li, X.; Xu, H. Fabrication of transition metal dichalcogenides quantum dots based on femtosecond laser ablation. Sci. Rep. 2019, 9, 2931. [Google Scholar] [CrossRef] [Green Version]
- Donley, M.S.; Murray, P.T.; Barber, S.A.; Haas, T.W. Deposition and properties of MoS2 thin films grown by pulsed laser evaporation. Surf. Coat. Technol. 1988, 36, 329–340. [Google Scholar] [CrossRef]
- Zabinski, J.S.; Donley, M.S.; John, P.J.; Dyhouse, V.J.; Safriet, A.J.; McDevitt, N.T. Crystallization of Molybdenum Disulfide Films Deposited by Pulsed Laser Ablation. MRS Proc. 2011, 201, 195–200. [Google Scholar] [CrossRef]
- Jiang, J.-W. Graphene versus MoS2: A short review. Front. Phys. 2015, 10, 287–302. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Guo, J.; Kang, Y.; Ai, Y.; Li, C.M. Two dimensional atomically thin MoS2 nanosheets and their sensing applications. Nanoscale 2015, 7, 19358–19376. [Google Scholar] [CrossRef]
- Schonfeld, B.; Huang, J.J.; Moss, S.C. Anisotropic mean-square displacements (MSD) in single-crystals of 2H- and 3R-MoS2. Acta Crystallogr. Sect. B 1983, 39, 404–407. [Google Scholar] [CrossRef]
- Enyashin, A.N.; Yadgarov, L.; Houben, L.; Popov, I.; Weidenbach, M.; Tenne, R.; Bar-Sadan, M.; Seifert, G. New route for stabilization of 1T-WS2 and MoS2 phases. J. Phys. Chem. C 2011, 115, 24586–24591. [Google Scholar] [CrossRef] [Green Version]
- Ataca, C.; Sahin, H.; Ciraci, S. Stable, single-layer MX2 transition-metal oxides and dichalcogenides in a honeycomb-like structure. J. Phys. Chem. C 2012, 116, 8983–8999. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-Like Two-Dimensional Materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Y.; Ni, J.; Shi, L.; Shi, S.; Tang, W. First principles study of structural, vibrational and electronic properties of graphene-like MX2 (M=Mo, Nb, W, Ta; X=S, Se, Te) monolayers. Phys. B Condens. Matter 2011, 406, 2254–2260. [Google Scholar] [CrossRef]
- Kadantsev, E.S.; Hawrylak, P. Electronic structure of a single MoS2 monolayer. Solid State Commun. 2012, 152, 909–913. [Google Scholar] [CrossRef]
- Vikraman, D.; Akbar, K.; Hussain, S.; Yoo, G.; Jang, J.-Y.; Chun, S.-H.; Jung, J.; Park, H.J. Direct synthesis of thickness-tunable MoS2 quantum dot thin layers: Optical, structural and electrical properties and their application to hydrogen evolution. Nano Energy 2017, 35, 101–114. [Google Scholar] [CrossRef]
- Kopaczek, J.; Polak, M.P.; Scharoch, P.; Wu, K.; Chen, B.; Tongay, S.; Kudrawiec, R. Direct optical transitions at K- and H-point of Brillouin zone in bulk MoS2, MoSe2, WS2, and WSe2. J. Appl. Phys. 2016, 119, 235705. [Google Scholar] [CrossRef]
- Ossila. Molybdenum Disulfide (MoS2): Theory & Applications. Available online: https://www.ossila.com/pages/molybdenum-disulfide-mos2 (accessed on 26 September 2022).
- Sahoo, D.; Kumar, B.; Sinha, J.; Ghosh, S.; Roy, S.S.; Kaviraj, B. Cost effective liquid phase exfoliation of MoS2 nanosheets and photocatalytic activity for wastewater treatment enforced by visible light. Sci. Rep. 2020, 10, 10759. [Google Scholar] [CrossRef]
- Nguyen, E.P.; Carey, B.J.; Daeneke, T.; Ou, J.Z.; Latham, K.; Zhuiykov, S.; Kalantar-zadeh, K. Investigation of two-solvent grinding-assisted liquid phase exfoliation of layered MoS2. Chem. Mater. 2015, 27, 53–59. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Mukherjee, S.; Mitra, R.K.; Ray, S.K. Size-dependent optical properties of MoS2 nanoparticles and their photo-catalytic applications. Nanotechnology 2020, 31, 145701. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.; Wang, H.-Y.; Grinblat, G.; Huang, Y.-L.; Wang, D.; Ye, X.-H.; Li, X.-B.; Bao, Q.; Wee, A.-S.; et al. Slow cooling and efficient extraction of C-exciton hot carriers in MoS2 monolayer. Nat. Commun. 2017, 8, 13906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikan, V.; Kelley, D.F. Size-Dependent Spectroscopy of MoS2 Nanoclusters. J. Phys. Chem. B 2002, 106, 3794–3804. [Google Scholar] [CrossRef]
- Liu, N.; Kim, P.; Kim, J.H.; Ye, J.H.; Kim, S.; Lee, C.J. Large-Area Atomically Thin MoS2 Nanosheets Prepared Using Electrochemical Exfoliation. ACS Nano 2014, 8, 6902–6910. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Maiti, R.; Katiyar, A.K.; Das, S.; Ray, S.K. Novel Colloidal MoS2 Quantum Dot Heterojunctions on Silicon Platforms for Multifunctional Optoelectronic Devices. Sci. Rep. 2016, 6, 29016. [Google Scholar] [CrossRef] [Green Version]
- Pankratov, V.; Hoszowska, J.; Dousse, J.C.; Huttula, M.; Kis, A.; Krasnozhon, D.; Zhang, M.; Cao, W. Vacuum ultraviolet excitation luminescence spectroscopy of few-layered MoS2. J. Phys. Condens. Matter 2015, 28, 015301. [Google Scholar] [CrossRef]
- Albiter, M.A.; Huirache-Acuña, R.; Paraguay-Delgado, F.; Rico, J.L.; Alonso-Nuñez, G. Synthesis of MoS2 nanorods and their catalytic test in the HDS of dibenzothiophene. Nanotechnology 2006, 17, 3473. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, J.; Fu, W.; Liu, Y.; Zhu, Y.; Wang, Z. A facile route to synthesis of MoS2 nanorods. Mater. Lett. 2005, 59, 3452–3455. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Small Is Different: Shape-, Size-, and Composition-Dependent Properties of Some Colloidal Semiconductor Nanocrystals. Acc. Chem. Res. 2004, 37, 326–333. [Google Scholar] [CrossRef]
- Yin, Y.; Alivisatos, A.P. Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 2004, 437, 664. [Google Scholar] [CrossRef]
- Rossetti, R.; Nakahara, S.; Brus, L.E. Quantum size effects in the redox potentials, resonance Raman spectra, and electronic spectra of CdS crystallites in aqueous solution. J. Chem. Phys. 1983, 79, 1086–1088. [Google Scholar] [CrossRef]
- Kabel, J.; Sharma, S.; Acharya, A.; Zhang, D.; Yap, Y.K. Molybdenum Disulfide Quantum Dots: Properties, Synthesis, and Applications. J. Carbon Res. 2021, 7, 45. [Google Scholar] [CrossRef]
- Mohamed, W.A.A.; El-Gawad, H.A.; Mekkey, S.; Galal, H.; Handal, H.; Mousa, H.; Labib, A. Quantum dots synthetization and future prospect applications. Nanotechnol. Rev. 2021, 10, 1926–1940. [Google Scholar] [CrossRef]
- Sanmartín-Matalobos, J.; Bermejo-Barrera, P.; Aboal-Somoza, M.; Fondo, M.; García-Deibe, A.M.; Corredoira-Vázquez, J.; Alves-Iglesias, Y. Semiconductor Quantum Dots as Target Analytes: Properties, Surface Chemistry and Detection. Nanomaterials 2022, 12, 2501. [Google Scholar] [CrossRef]
- Schmid, G. Large clusters and colloids. Metals in the embryonic state. Chem. Rev. 1992, 92, 1709–1727. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.-Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Zhang, S.; Chen, Z. MoS2 nanoribbons: High stability and unusual electronic and magnetic properties. J. Am. Chem. Soc. 2008, 130, 16739–16744. [Google Scholar] [CrossRef]
- Que, H.; Jiang, H.; Wang, X.; Zhai, P.; Meng, L.; Zhang, P.; Gong, Y. Utilization of the van der Waals Gap of 2D Materials. Acta Physico-Chim. Sin 2021, 37, 2010051-0. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Zhang, F.; Lee, C.-S. A Novel Aluminum–Graphite Dual-Ion Battery. Adv. Energy Mater. 2016, 6, 1502588. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Zhang, Y.; Chen, H.; Xu, N.; Deng, S. Enhanced Performance of a Monolayer MoS2/WSe2 Heterojunction as a Photoelectrochemical Cathode. Nano-Micro Lett. 2018, 10, 60. [Google Scholar] [CrossRef]
- Lu, G.Z.; Wu, M.J.; Lin, T.N.; Chang, C.Y.; Lin, W.L.; Chen, Y.T.; Hou, C.F.; Cheng, H.J.; Lin, T.Y.; Shen, J.L.; et al. Electrically Pumped White-Light-Emitting Diodes Based on Histidine-Doped MoS(2) Quantum Dots. Small 2019, 15, e1901908. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.K.; Ulla, H.; Satyanarayan, M.N.; Rawat, K.; Gaur, A.; Gawali, S.; Hassan, P.A.; Bohidar, H.B. Fluorescent MoS2 Quantum Dot–DNA Nanocomposite Hydrogels for Organic Light-Emitting Diodes. ACS Appl. Nano Mater. 2020, 3, 1289–1297. [Google Scholar] [CrossRef]
- Vadivelmurugan, A.; Anbazhagan, R.; Tsai, H.-C. Preparation of fluorescent MoS2 quantum dots conjugated with various ligands, and its fluorescence imaging. Mater. Lett. 2018, 218, 285–289. [Google Scholar] [CrossRef]
- Swaminathan, H.; Balasubramanian, K. Förster resonance energy transfer between MoS2 quantum dots and polyaniline for turn-on bovine serum albumin sensing. Sens. Actuators B Chem. 2018, 264, 337–343. [Google Scholar] [CrossRef]
- Shi, M.; Dong, L.; Zheng, S.; Hou, P.; Cai, L.; Zhao, M.; Zhang, X.; Wang, Q.; Li, J.; Xu, K. “Bottom-up” preparation of MoS2 quantum dots for tumor imaging and their in vivo behavior study. Biochem. Biophys. Res. Commun. 2019, 516, 1090–1096. [Google Scholar] [CrossRef]
- Chauhan, N.; Maekawa, T.; Kumar, D.N.S. Graphene based biosensors—Accelerating medical diagnostics to new-dimensions. J. Mater. Res. 2017, 32, 2860–2882. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Zhong, L.; Guo, L.; Fu, F.; Chen, G. Seeing diabetes: Visual detection of glucose based on the intrinsic peroxidase-like activity of MoS2 nanosheets. Nanoscale 2014, 6, 11856–11862. [Google Scholar] [CrossRef]
- Solomon, G.; Mazzaro, R.; Morandi, V.; Concina, I.; Vomiero, A. Microwave-Assisted vs. Conventional Hydrothermal Synthesis of MoS2 Nanosheets: Application towards Hydrogen Evolution Reaction. Crystals 2020, 10, 1040. [Google Scholar] [CrossRef]
- Manikandan, A.; Chen, Y.-Z.; Shen, C.-C.; Sher, C.-W.; Kuo, H.-C.; Chueh, Y.-L. A critical review on two-dimensional quantum dots (2D QDs): From synthesis toward applications in energy and optoelectronics. Prog. Quantum Electron. 2019, 68, 100226. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, J.; Ma, Q.; Ma, J.; Bai, H.; Chen, L.; Mu, S. Two-Dimensional MoS2: Structural Properties, Synthesis Methods, and Regulation Strategies toward Oxygen Reduction. Micromachines 2021, 12, 240. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Tolentino, L.; Yang, Z.; Song, X.; Zhang, W.; Chen, Y.; Wong, C.-P. High-Concentration Aqueous Dispersions of MoS2. Adv. Funct. Mater. 2013, 23, 3577–3583. [Google Scholar] [CrossRef]
- Ibrahem, M.A.; Lan, T.-W.; Huang, J.K.; Chen, Y.-Y.; Wei, K.-H.; Li, L.-J.; Chu, C.W. High quantity and quality few-layers transition metal disulfide nanosheets from wet-milling exfoliation. RSC Adv. 2013, 3, 13193–13202. [Google Scholar] [CrossRef]
- Dai, W.; Dong, H.; Fugetsu, B.; Cao, Y.; Lu, H.; Ma, X.; Zhang, X. Tunable Fabrication of Molybdenum Disulfide Quantum Dots for Intracellular MicroRNA Detection and Multiphoton Bioimaging. Small 2015, 11, 4158–4164. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, H.; Zhuo, J.; Zhu, Z.; Papakonstantinou, P.; Lubarsky, G.; Lin, J.; Li, M. Biosensor Based on Ultrasmall MoS2 Nanoparticles for Electrochemical Detection of H2O2 Released by Cells at the Nanomolar Level. Anal. Chem. 2013, 85, 10289–10295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-D.; Zhang, J.; Wang, J.; Yang, J.; Chen, J.; Shen, X.; Deng, J.; Deng, D.; Long, W.; Sun, Y.-M.; et al. Highly Catalytic Nanodots with Renal Clearance for Radiation Protection. ACS Nano 2016, 10, 4511–4519. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lai, Z.; Liu, Z.; Tan, C.; Huang, Y.; Li, B.; Zhao, M.; Xie, L.; Huang, W.; Zhang, H. A Facile and Universal Top-Down Method for Preparation of Monodisperse Transition-Metal Dichalcogenide Nanodots. Angew. Chem. Int. Ed. 2015, 54, 5425–5428. [Google Scholar] [CrossRef]
- Muscuso, L.; Cravanzola, S.; Cesano, F.; Scarano, D.; Zecchina, A. Optical, Vibrational, and Structural Properties of MoS2 Nanoparticles Obtained by Exfoliation and Fragmentation via Ultrasound Cavitation in Isopropyl Alcohol. J. Phys. Chem. C 2015, 119, 3791–3801. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Sohn, W.; Oh, J.H.; Jang, H.W.; Kim, S.Y. Size-Dependent Properties of Two-Dimensional MoS2 and WS2. J. Phys. Chem. C 2016, 120, 10078–10085. [Google Scholar] [CrossRef]
- Stengl, V.; Henych, J. Strongly luminescent monolayered MoS2 prepared by effective ultrasound exfoliation. Nanoscale 2013, 5, 3387–3394. [Google Scholar] [CrossRef]
- Ramakrishna Matte, H.S.S.; Gomathi, A.; Manna, A.K.; Late, D.J.; Datta, R.; Pati, S.K.; Rao, C.N.R. MoS2 and WS2 Analogues of Graphene. Angew. Chem. Int. Ed. 2010, 49, 4059–4062. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Yan, S.; Song, X.; Zhang, X.; Sun, Y.; Chen, X.; Zhong, W.; Du, Y. Monolayer MoS2 quantum dots as catalysts for efficient hydrogen evolution. RSC Adv. 2015, 5, 97696–97701. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Y.; Xia, Z.; Wei, W. As-prepared MoS2 quantum dot as a facile fluorescent probe for long-term tracing of live cells. Nanotechnology 2016, 27, 275101. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. Single-Layer Semiconducting Nanosheets: High-Yield Preparation and Device Fabrication. Angew. Chem. 2011, 50, 11093–11097. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Fan, Y.; Zhang, Y.; Tong, M.; Liao, P. Pd-Catalytic In Situ Generation of H2O2 from H2 and O2 Produced by Water Electrolysis for the Efficient Electro-Fenton Degradation of Rhodamine B. Environ. Sci. Technol. 2011, 45, 8514–8520. [Google Scholar] [CrossRef]
- Rasuli, H.; Rasuli, R.; Alizadeh, M.; BoonTong, G. Microwave-assisted exfoliation and tearing of graphene oxide in the presence of TiO2 nanoparticles. Results Phys. 2020, 18, 103200. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Arunachalam, V.; Vasudevan, S. Liquid-Phase Exfoliation of MoS2 Nanosheets: The Critical Role of Trace Water. J. Phys. Chem. Lett. 2016, 7, 4884–4890. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Yin, Z.; Zhang, H. Preparation and Applications of Mechanically Exfoliated Single-Layer and Multilayer MoS2 and WSe2 Nanosheets. Acc. Chem. Res. 2014, 47, 1067–1075. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Liu, X.; Zhang, S.; Liu, C.; Yan, D.; Zeng, Y.; Pei, Y.; Liu, Y.; Luo, S. Monolayer MoS2 with S vacancies from interlayer spacing expanded counterparts for highly efficient electrochemical hydrogen production. J. Mater. Chem. A 2016, 4, 16524–16530. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Y.; Han, X.; Jiang, G.; Shi, J.; Liu, P.; Khan, M.Z.; Huhtinen, H.; Zhu, J.; Jin, Z.; et al. Ultra-fast growth of cuprate superconducting films: Dual-phase liquid assisted epitaxy and strong flux pinning. Mater. Today Phys. 2021, 18, 100400. [Google Scholar] [CrossRef]
- Meng, B.; Wang, J.; Yang, L.; Chen, M.; Zhu, S.; Wang, F. On the rumpling mechanism in nanocrystalline coatings: Improved by reactive magnetron sputtering with oxygen. J. Mater. Sci. Technol. 2023, 132, 69–80. [Google Scholar] [CrossRef]
- Zeng, Z.; Sun, T.; Zhu, J.; Huang, X.; Yin, Z.; Lu, G.; Fan, Z.; Yan, Q.; Hng, H.H.; Zhang, H. An Effective Method for the Fabrication of Few-Layer-Thick Inorganic Nanosheets. Angew. Chem. Int. Ed. 2012, 51, 9052–9056. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaie, N.; Dorranian, D. Effect of fluence on carbon nanostructures produced by laser ablation in liquid nitrogen. Appl. Phys. A-Mater. Sci. Process. 2016, 122, 558. [Google Scholar] [CrossRef]
- Alam, K.; Lake, R.K. Monolayer MoS2 Transistors Beyond the Technology Road Map. IEEE Trans. Electron Devices 2012, 59, 3250–3254. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, R. Nucleation of CVD-prepared hexagonal boron nitride on Ni(100), Ni(110) and Ni(111) surfaces: A theoretical study. Vacuum 2022, 205, 111396. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, J.; Matsuhashi, Y.; Iwasa, Y. Ambipolar MoS2 thin flake transistors. Nano Lett. 2012, 12, 1136–1140. [Google Scholar] [CrossRef]
- Lopez-Sanchez, O.; Lembke, D.; Kayci, M.; Radenovic, A.; Kis, A. Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nanotechnol. 2013, 8, 497–501. [Google Scholar] [CrossRef]
- Ulaganathan, R.K.; Yadav, K.; Sankar, R.; Chou, F.C.; Chen, Y.-T. Hybrid InSe Nanosheets and MoS2 Quantum Dots for High-Performance Broadband Photodetectors and Photovoltaic Cells. Adv. Mater. Interfaces 2019, 6, 1801336. [Google Scholar] [CrossRef]
- Bernardi, M.; Palummo, M.; Grossman, J.C. Extraordinary Sunlight Absorption and One Nanometer Thick Photovoltaics Using Two-Dimensional Monolayer Materials. Nano Lett. 2013, 13, 3664–3670. [Google Scholar] [CrossRef]
- Tsai, M.-L.; Su, S.-H.; Chang, J.-K.; Tsai, D.-S.; Chen, C.-H.; Wu, C.-I.; Li, L.-J.; Chen, L.-J.; He, J.-H. Monolayer MoS2 Heterojunction Solar Cells. ACS Nano 2014, 8, 8317–8322. [Google Scholar] [CrossRef] [PubMed]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef]
- Tongay, S.; Zhou, J.; Ataca, C.; Liu, J.; Kang, J.S.; Matthews, T.S.; You, L.; Li, J.; Grossman, J.C.; Wu, J. Broad-Range Modulation of Light Emission in Two-Dimensional Semiconductors by Molecular Physisorption Gating. Nano Lett. 2013, 13, 2831–2836. [Google Scholar] [CrossRef]
- Late, D.J.; Huang, Y.-K.; Liu, B.; Acharya, J.; Shirodkar, S.N.; Luo, J.; Yan, A.; Charles, D.; Waghmare, U.V.; Dravid, V.P.; et al. Sensing Behavior of Atomically Thin-Layered MoS2 Transistors. ACS Nano 2013, 7, 4879–4891. [Google Scholar] [CrossRef] [PubMed]
- Ju, P.; He, Y.; Wang, M.; Han, X.; Jiang, F.; Sun, C.; Wu, C. Enhanced Peroxidase-Like Activity of MoS2 Quantum Dots Functionalized g-C3N4 Nanosheets towards Colorimetric Detection of H2O2. Nanomaterials 2018, 8, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Long, J.; Xie, B.; Kuang, Y.; Chen, X.; Hou, M.; Gao, J.; Liu, H.; He, Y.; Wong, C.-P. One-Step Ultraviolet Laser-Induced Fluorine-Doped Graphene Achieving Superhydrophobic Properties and Its Application in Deicing. ACS Appl. Mater. Interfaces 2022, 14, 4647–4655. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Zhang, X.; Huang, X.; Liao, W.; Zhao, Y. MoS2-based membranes in water treatment and purification. Chem. Eng. J. 2021, 422, 130082. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhao, X.; Shen, Q.; Zhao, W.; Tan, Q.; Zhang, N.; Li, P.; Jiao, L.; Qu, X. Sandwich-Like Heterostructures of MoS2/Graphene with Enlarged Interlayer Spacing and Enhanced Hydrophilicity as High-Performance Cathodes for Aqueous Zinc-Ion Batteries. Adv. Mater. 2021, 33, 2007480. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.-W.; Park, H.S. Mechanical properties of MoS2/graphene heterostructures. Appl. Phys. Lett. 2014, 105, 033108. [Google Scholar] [CrossRef]
- Özuğur Uysal, B.; Nayır, Ş.; Açba, M.; Çıtır, B.; Durmaz, S.; Koçoğlu, Ş.; Yıldız, E.; Pekcan, Ö. 2D Materials (WS2, MoS2, MoSe2) Enhanced Polyacrylamide Gels for Multifunctional Applications. Gels 2022, 8, 465. [Google Scholar] [CrossRef] [PubMed]
| Graphene Family | Graphene | hBN | BCN | Fluorographene | Graphene Oxide | |
|---|---|---|---|---|---|---|
| 2D Chalcogenides | MoS2, WS2, MoSe2, WSe2 | Semiconducting dichalcogenides: MoTe2, WTe2, ZrSe2/S2, etc. | Metallic dichalcogenides; NbSe2/S2, TaS2, TiS2, NiSe2 etc. | |||
| Layered Semiconductors; GaTe/Se, Bi2Se3, InSe, etc. | ||||||
| 2D Oxides | Micas BSCCO | MoO3, WO3 | Perovskites: LaNb2O7, (Ca/Sr)2Nb3O10, Bi4Ti3O12, Ca2Ta2TiO10 etc. | Hydroxides: Eu(OH)2, Ni(OH)2, etc. | ||
| Layered Cu oxides | TiO2, V2O5, TaO3, RuO2, etc. | others | ||||
| Key | ||||||
| Stable materials in ambient conditions | Potentially stable materials in ambient conditions | |||||
| Stable materials in inert conditions only | 3D compounds- have been exfoliated into monolayers | |||||
| Others: includes borides, nitrides, carbides, etc., have been or can be isolated, such as BCN, boron carbon nitride | ||||||
| Precursors | Method | Size (nm) | Yield (%) | Notes | Ref. |
|---|---|---|---|---|---|
| MoS2 powder Organic solvents | Mechanical exfoliation | ~10–60 | - | Simple, green, and cost-effective Low production yield Multi-layered QDs | [65,66,67] |
| MoS2 powder Solvents | Ultrasonic Exfoliation | ~2.5 | ~10 | Facile, inexpensive Commonly used exfoliation process for LTMDs Can be carried out in several solvents | [18,68,69,70,71] |
| MoS2 Li, K, Na salts (LiOH, etc.) Organic solvents (DMF, etc.) | Ion-intercalation Exfoliation | ~4 | 10–20 | Small-sized QDs, good yield Laborious washing processes High catalytic activity | [25,76] |
| MoS2 Electrolyte solvents (LiTFSI, [BMIm]Cl, etc.) | Electrochemical Exfoliation | ~3–6 | - | Small, monolayered QDs can be obtained Tedious post-process washing Harsh reaction conditions | [16,77] |
| MoS2 Fenton reagents | electro-Fenton Reaction | ~5 | - | A good alternative to intercalation and electrochemical exfoliation processes | [17] |
| MoS2 DMF, ethanol | Microwave-assisted Exfoliation | ~4.5 | - | Low production yield Depends on the microwave absorption ability of the solvent | - |
| MoS2 DI water, NMP | Laser Ablation | ~3.0 | Green and facile, produce pure QDs and nanosheets MoS2 powder and pellets can be used Produce poly-crystalline exfoliated nanostructures | [23,81] | |
| MoS2 Liquid Nitrogen | Cryo-mediated Exfoliation | ~2.5 | 1 | Simple, environmentally friendly No by-products Low production yield | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, L.; Subhan, F.; Ayaz, M.; Hassan, S.S.u.; Byeon, C.C.; Kim, J.S.; Bungau, S. Exfoliation of MoS2 Quantum Dots: Recent Progress and Challenges. Nanomaterials 2022, 12, 3465. https://doi.org/10.3390/nano12193465
Ali L, Subhan F, Ayaz M, Hassan SSu, Byeon CC, Kim JS, Bungau S. Exfoliation of MoS2 Quantum Dots: Recent Progress and Challenges. Nanomaterials. 2022; 12(19):3465. https://doi.org/10.3390/nano12193465
Chicago/Turabian StyleAli, Luqman, Fazle Subhan, Muhammad Ayaz, Syed Shams ul Hassan, Clare Chisu Byeon, Jong Su Kim, and Simona Bungau. 2022. "Exfoliation of MoS2 Quantum Dots: Recent Progress and Challenges" Nanomaterials 12, no. 19: 3465. https://doi.org/10.3390/nano12193465
APA StyleAli, L., Subhan, F., Ayaz, M., Hassan, S. S. u., Byeon, C. C., Kim, J. S., & Bungau, S. (2022). Exfoliation of MoS2 Quantum Dots: Recent Progress and Challenges. Nanomaterials, 12(19), 3465. https://doi.org/10.3390/nano12193465









