A Simple Fabrication of Sb2S3/TiO2 Photo-Anode with Long Wavelength Visible Light Absorption for Efficient Photoelectrochemical Water Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of TiO2/ITO Electrode
2.3. Fabrication of Sb2S3/TiO2/ITO Electrode
2.4. Measurement
3. Results
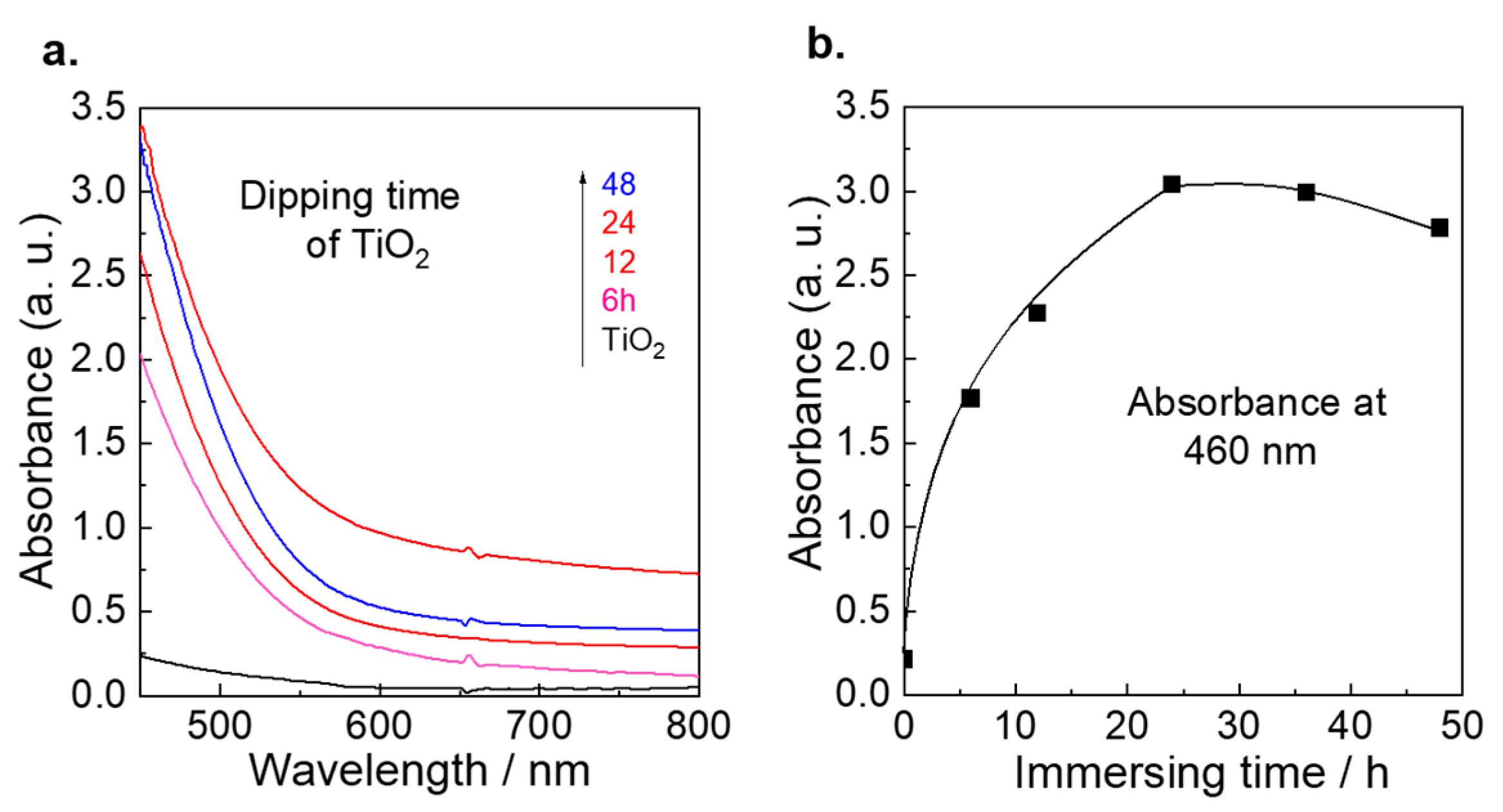
3.1. Optimization of Immersion Time
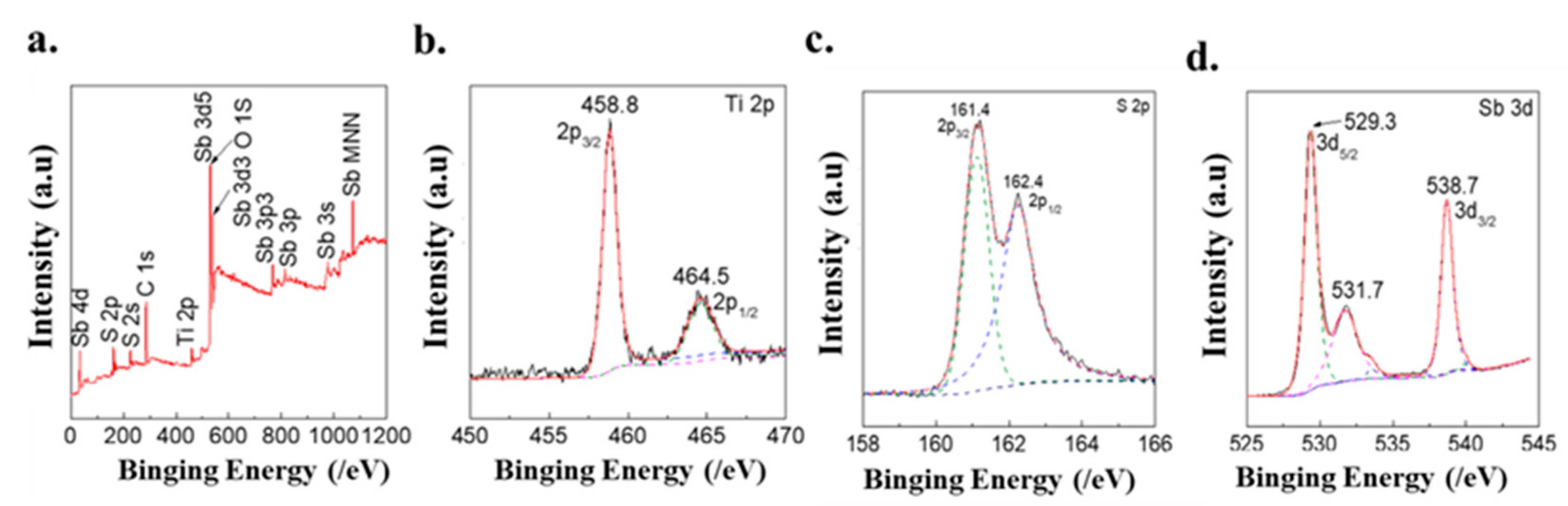
3.2. Characterization Structure of Different Samples
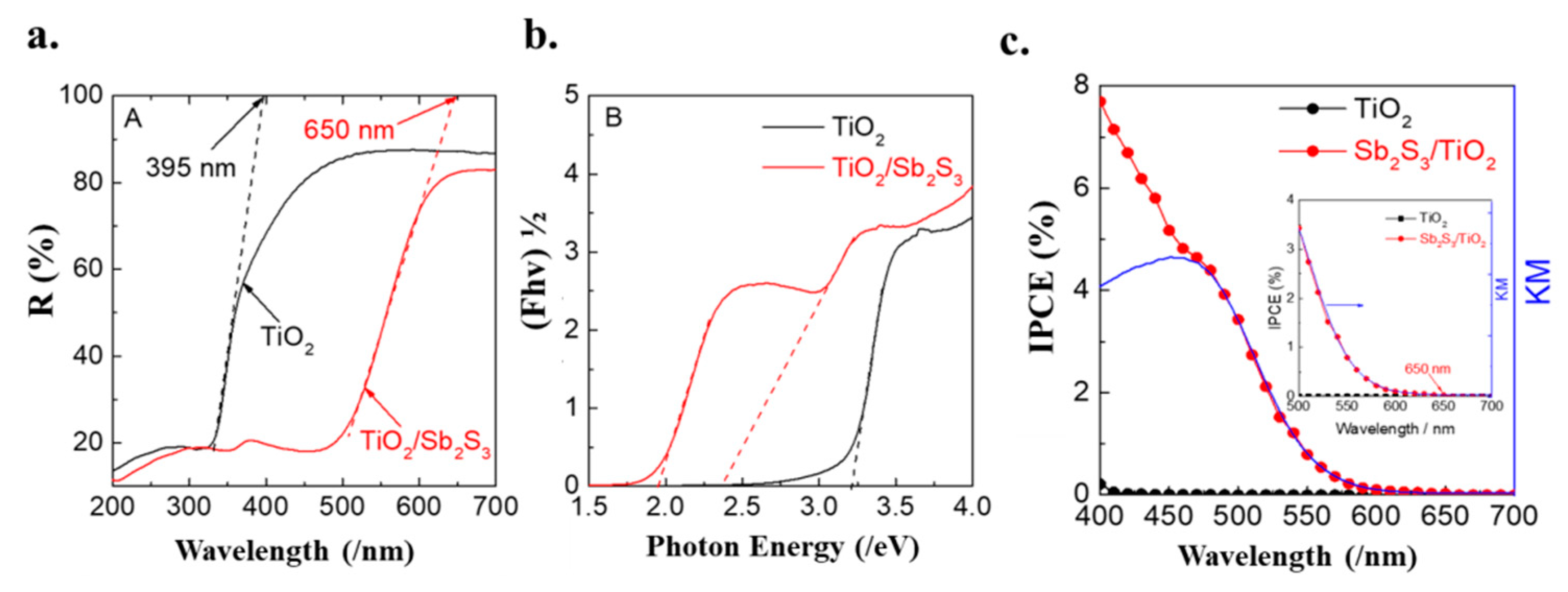
3.3. The Optical Properties of Sb2S3/TiO2
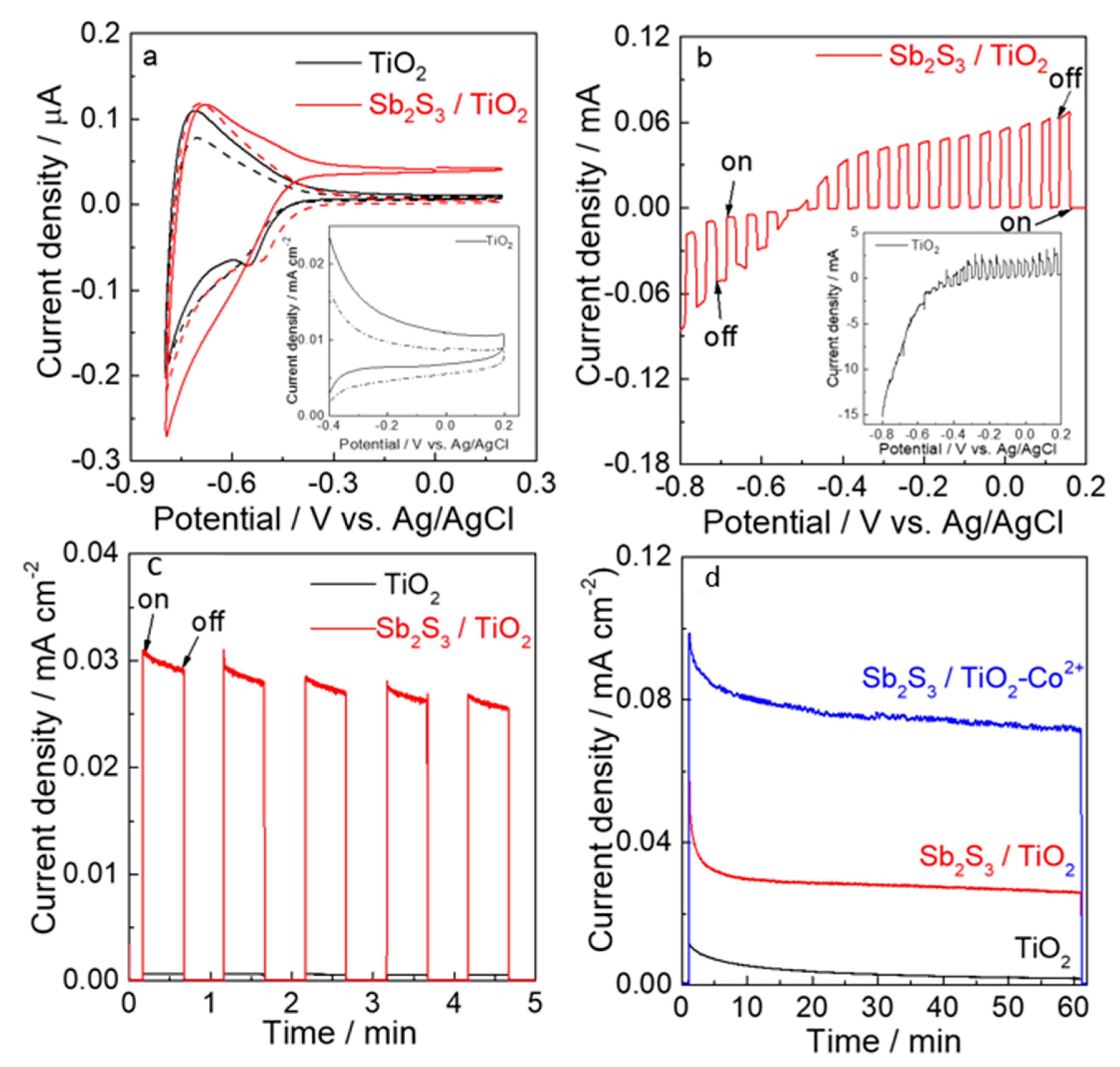
3.4. Photoelectrocatalytic Properties
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting—superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Chandra, D.; Tsuriya, R.; Sato, T.; Takama, D.; Abe, N.; Kajita, M.; Li, D.; Togashi, T.; Kurihara, M.; Saito, K.; et al. Characterization of Interfacial Charge-Transfer Photoexcitation of Polychromium-Oxo-Electrodeposited TiO2 as an Earth-Abundant Photoanode for Water Oxidation Driven by Visible Light. ChemPlusChem 2016, 81, 1116–1122. [Google Scholar] [CrossRef]
- El Rouby, W.M.A.; Antuch, M.; You, S.; Millet, P. Surface sensitization of TiO2 nanorod mats by electrodeposition of ZIF-67 for water photo-oxidation. Electrochim. Acta 2020, 339, 135882–135897. [Google Scholar] [CrossRef]
- Liu, R.; Fang, S.Y.; Tsai, K.C.; Yang, W.D. Enhancing hydrogen evolution of water splitting under solar spectra using Au/TiO2 heterojunction photocatalysts. Int. J. Hydrogen Energy 2021, 46, 28462–28473. [Google Scholar] [CrossRef]
- Park, H.; Choi, W.; Hoffmann, M.R. Effects of the preparation method of the ternary CdS/TiO2/Pt hybrid photocatalysts on visible light-induced hydrogen production. J. Mater. Chem. 2008, 18, 2379–2385. [Google Scholar] [CrossRef]
- Gupta, B.; Melvin, A.A.; Matthews, T.; Dash, S.; Tyagi, A.K. TiO2 modification by gold (Au) for photocatalytic hydrogen (H2) production. Renew. Sust. Energ. Rev. 2016, 58, 1366–1375. [Google Scholar] [CrossRef]
- Patra, K.K.; Gopinath, C.S. Harnessing Visible-Light and Limited Near-IR Photons through Plasmon Effect of Gold Nanorod with AgTiO2. J. Phys. Chem. C 2018, 122, 1206–1214. [Google Scholar] [CrossRef]
- Ibrahim, O.Y.; Gondal, A.M. Visible-light-driven photocatalytic performance of a Z-scheme based TiO2/WO3/g-C3N4 ternary heterojunctions. Mol. Catal. 2021, 505, 111494. [Google Scholar] [CrossRef]
- Ge, M.; Cai, J.; Cao, C.; Huang, J.; Zhang, X.; Shen, J.; Wang, S.; Zhang, S.; Zhang, K.Q.; Lai, Y.; et al. A review of TiO2 nanostructured catalysts for sustainable H2 generation. Int. J. Hydrogen Energy 2017, 42, 8418–8449. [Google Scholar] [CrossRef]
- Chen, S.; Li, C.; Hou, Z. A novel in situ synthesis of TiO2/CdS heterojunction for improving photoelectrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 25473–25485. [Google Scholar] [CrossRef]
- Ding, Q.; Gou, L.; Wei, D.; Xu, D.; Fan, W.; Shi, W. Metal-organic framework derived Co3O4/TiO2 heterostructure nanoarrays for promote photoelectrochemical water splitting. Int. J. Hydrogen Energy 2021, 46, 24965–24976. [Google Scholar] [CrossRef]
- Ismail, M.; Chebaane, M.M.; Bousselmi, L.; Zahraa, O.; Olivier, C.; Toupance, T. Photoelectrochemical properties of WO3 modified anatase TiO2 photoanodes and application for dye-sensitized solar cells. Surf. Interfaces 2021, 27, 101543–101551. [Google Scholar] [CrossRef]
- Le, T.T.T.; Tran, D.T.; Danh, T.H. Remarkable enhancement of visible light driven photocatalytic performance of TiO2 by simultaneously doping with C, N, and S. Chem. Phys. 2021, 545, 111144–111153. [Google Scholar] [CrossRef]
- Mollavali, M.; Rohani, S.; Elahifard, M.; Behjatmanesh-Ardakani, R.; Nourany, M. Band gap reduction of (Mo+N) co-doped TiO2 nanotube arrays with a significant enhancement in visible light photo-conversion: A combination of experimental and theoretical study. Int. J. Hydrogen Energy 2021, 46, 21475–21498. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, Z.; Pervaiz, M.; Mehmood, K.; Ejaz, R.; Younas, U.; Nadeem, H.A.; Hussain, S. Enhanced visible light photocatalytic activity of TiO2 co-doped with Fe, Co, and S for degradation of Cango red. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 255, 119644–119655. [Google Scholar] [CrossRef]
- Cerdan-Pasaran, A.; López-Luke, T.; Mathewa, X.; Mathews, N.R. Effect of cobalt doping on the device properties of Sb2S3-sensitized TiO2 solar cells. Sol. Energy 2019, 183, 697–703. [Google Scholar] [CrossRef]
- Elbakkay, M.H.; El Rouby, W.M.A.; Mariño-López, A.; Sousa-Castillo, A.; Salgueiriño, V.; El-Dek, S.I.; Farghali, A.A.; Correa Duarte, M.A.; Millet, P. One-pot synthesis of TiO2/Sb2S3/RGO complex multicomponent heterostructures for highly enhanced photoelectrochemical water splitting. Int. J. Hydrogen Energy 2021, 46, 31216–31227. [Google Scholar]
- Ben Naceur, J.; Ouertani, R.; Chakhari, W.; Chtourou, R. Photo-electrochemical properties of Sb2S3/TiO2 heterostructures integrally synthesis by hydrothermal method. J. Mater. Sci. Mater. Electron. 2019, 30, 5631–5639. [Google Scholar] [CrossRef]
- Ortiz-Bustos, J.; Hierro, I.; de Sánchez-Ruiz, A.; García-Martínez, J.C.; Pérez, Y. Tuning of type-I and type-II mechanisms for visible light degradation in tris(styryl)benzene-sensitized TiO2 nanoparticles. Dyes Pigments 2021, 18, 108802–108816. [Google Scholar] [CrossRef]
- Yu, Y.-X.; Yu, Y.X.; Pan, L.; Son, M.K.; Mayer, M.; Zhang, W.D.; Hagfeldt, A.; Luo, J.; Grätzel, M. Solution Processed Cu2S Photocathodes for Photoelectrochemical Water Splitting. ACS Energy Lett. 2018, 3, 760–766. [Google Scholar] [CrossRef]
- Chandra, M.; Bhunia, K.; Pradhan, D. Controlled Synthesis of CuS/TiO2 Heterostructured Nanocomposites for Enhanced Photocatalytic Hydrogen Generation through Water Splitting. Inorg. Chem. 2018, 57, 4524–4533. [Google Scholar] [CrossRef]
- Hou, Y.; Li, X.; Zhao, Q.; Quan, X.; Chen, G. Fabrication of Cu2O/TiO2 nanotube heterojunction arrays and investigation of its photoelectrochemical behavior. App. Phys. Lett. 2009, 95, 093108. [Google Scholar] [CrossRef]
- Zaban, A.; Mićić, O.; Gregg, B.A.; Nozik, A. Photosensitization of Nanoporous TiO2 Electrodes with InP Quantum Dots. Langmuir 1998, 14, 3153–3156. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, M.; Zhang, B.; Zhu, C.; Wang, F.; Song, J.; Zhong, M.; Ma, L.; Shen, W. InP nanopore arrays for photoelectrochemical hydrogen generation. Nanotechnology 2016, 27, 075704–075715. [Google Scholar] [CrossRef][Green Version]
- Wang, T.; Gong, J. Single-Crystal Semiconductors with Narrow Band Gaps for Solar Water Splitting. Angew. Chem. Int. Edit. 2015, 54, 10718–10732. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, T.; Zhang, J.; Li, C.; Li, H.; Gong, J. Dendritic Hematite Nanoarray Photoanode Modified with a Conformal Titanium Dioxide Interlayer for Effective Charge Collection. Angew. Chem. Int. Edit. 2017, 56, 12878–12882. [Google Scholar] [CrossRef]
- Yao, H.; Fu, W.; Yang, H.; Ma, J.; Sun, M.; Chen, Y.; Zhang, W.; Wu, D.; Lv, P.; Li, M. Vertical Growth of Two-Dimensional TiO2 Nanosheets Array Films and Enhanced Photoelectrochemical Properties Sensitized by CdS Quantum Dots. Electrochim. Acta 2014, 125, 258–265. [Google Scholar] [CrossRef]
- Sharma, V.; CDakshinamurthy, A.; Pandey, B.; Roy, S.C.; Sudakar, C. Highly efficient photoelectrochemical ZnO and TiO2 nanorod/Sb2S3 heterostructured photoanodes through one step thermolysis of Sb-MPA complex. Sol. Energy 2021, 225, 333–343. [Google Scholar] [CrossRef]
- Zhong, J.S.; Wang, Q.Y.; Liang, H.; Chen, D.Q.; Ji, Z.G. Improving the visible light photocatalytic activity of TiO2 nanotubes upon decoration with Sb2S3 microcrystalline. Mater. Charact. 2015, 109, 95–99. [Google Scholar] [CrossRef]
- Chang, J.A.; Im, S.H.; Lee, Y.H.; Kim, H.J.; Lim, C.S.; Heo, J.H.; Seok, S.I. Panchromatic photon-harvesting by hole-conducting materials in inorganic-organic heterojunction sensitized-solar cell through the formation of nanostructured electron channels. Nano Lett. 2012, 12, 1863–1867. [Google Scholar] [CrossRef] [PubMed]
- Maiti NIm, S.H.; Lima, C.-S.; Seok, S.I. A chemical precursor for depositing Sb2S3 onto mesoporous TiO2 layers in nonaqueous media and its application to solar cells. Dalton Trans. 2012, 41, 11569–11572. [Google Scholar] [CrossRef]
- Zhu, G.; Pan, L.; Xu, T.; Sun, Z. One-step synthesis of CdS sensitized TiO2 photoanodes for quantum dot-sensitized solar cells by microwave assisted chemical bath deposition method. Acs Appl. Mater. Inter. 2011, 3, 1472–1478. [Google Scholar] [CrossRef]
- Itzhaik, Y.; Itzhaik, Y.; Niitsoo, O.; Page, M.G.; Hodes, G. Sb2S3-Sensitized Nanoporous TiO2 Solar Cells. J. Phys. Chem. C 2013, 113, 4254–4256. [Google Scholar] [CrossRef]
- Sharavath, V.; Sarkar, S.; Ghosh, S. One-pot hydrothermal synthesis of TiO2/graphene nanocomposite with simultaneous nitrogen-doping for energy storage application. J. Electroanal. Chem. 2018, 829, 208–216. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, W.; Han, Q.; Yang, X.; Lu, L.; Wang, X. Preparation of rod-like Sb2S3 dendrites processed in conventional hydrothermal. Mater. Lett. 2009, 63, 1258–1261. [Google Scholar] [CrossRef]
- Parize, R.; Cossuet, T.; Chaix-Pluchery, O.; Roussel, H.; Appert, E.; Consonni, V. In situ analysis of the crystallization process of Sb2S3 thin films by Raman scattering and X-ray diffraction. Mater. Design 2017, 121, 1–10. [Google Scholar] [CrossRef]
- Kozytskiy, A.V.; Stroyuk, O.L.; Skoryk, M.A.; Dzhagan, V.M.; Kuchmiy, S.Y.; Zahn, D.R.T. Photochemical formation and photoelectrochemical properties of TiO2/Sb2S3 heterostructures. J. Photochem. Photobiol. A 2015, 303–304, 8–16. [Google Scholar] [CrossRef]
- Pal, M.; Mathews, N.R.; Mathew, X. Surfactant-mediated self-assembly of Sb2S3 nanorods during hydrothermal synthesis. J. Mater. Res. 2017, 32, 530–538. [Google Scholar] [CrossRef]
- Avilez Garcia, R.G.; Meza Avendaño, C.A.; Pal, M.; Paraguay Delgado, F.; Mathews, N.R. Antimony sulfide (Sb2S3) thin films by pulse electrodeposition: Effect of thermal treatment on structural, optical and electrical properties. Mat. Sci. Semicon. Proc. 2016, 44, 91–100. [Google Scholar] [CrossRef]
- Lou, W.; Chen, M.; Wang, X.; Liu, W. Novel Single-Source Precursors Approach to Prepare Highly Uniform Bi2S3 and Sb2S3 Nanorods via a Solvothermal Treatment. Chem. Mater. 2007, 19, 872–878. [Google Scholar] [CrossRef]
- Ota, J.; Srivastava, S.K. Tartaric Acid Assisted Growth of Sb2S3 Nanorods by a Simple Wet Chemical Method. Cryst. Growth Des. 2007, 7, 343–347. [Google Scholar] [CrossRef]
- Kay, A.; Cesar, I.; Grätzel, M. New Benchmark for Water Photooxidation by Nanostructured α-Fe2O3 Films. J. Am. Chem. Soc. 2006, 128, 15714–15721. [Google Scholar] [CrossRef]
- Li, D.; Takeuchi Ryouchi Chandra, D.; Saito, K.; Yui, T.; Yagi, M. Visible Light-Driven Water Oxidation on an In Situ N2-Intercalated WO3 Nanorod Photoanode Synthesized by a Dual-Functional Structure-Directing Agent. ChemSusChem 2018, 11, 1151–1156. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, F.; Ma, S.; Li, D.; Alam, M.M.; Yang, Z. A Simple Fabrication of Sb2S3/TiO2 Photo-Anode with Long Wavelength Visible Light Absorption for Efficient Photoelectrochemical Water Oxidation. Nanomaterials 2022, 12, 3444. https://doi.org/10.3390/nano12193444
Han F, Ma S, Li D, Alam MM, Yang Z. A Simple Fabrication of Sb2S3/TiO2 Photo-Anode with Long Wavelength Visible Light Absorption for Efficient Photoelectrochemical Water Oxidation. Nanomaterials. 2022; 12(19):3444. https://doi.org/10.3390/nano12193444
Chicago/Turabian StyleHan, Fei, Sai Ma, Dong Li, Md Mofasserul Alam, and Zeheng Yang. 2022. "A Simple Fabrication of Sb2S3/TiO2 Photo-Anode with Long Wavelength Visible Light Absorption for Efficient Photoelectrochemical Water Oxidation" Nanomaterials 12, no. 19: 3444. https://doi.org/10.3390/nano12193444
APA StyleHan, F., Ma, S., Li, D., Alam, M. M., & Yang, Z. (2022). A Simple Fabrication of Sb2S3/TiO2 Photo-Anode with Long Wavelength Visible Light Absorption for Efficient Photoelectrochemical Water Oxidation. Nanomaterials, 12(19), 3444. https://doi.org/10.3390/nano12193444




