Cinchona officinalis Phytochemicals-Loaded Iron Oxide Nanoparticles Induce Cytotoxicity and Stimulate Apoptosis in MCF-7 Human Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cinchona officinalis Bark Methanol Extract
2.2. GC–MS Analysis of the Stem Bark Methanol Extract
2.3. Biosynthesis of Iron Oxide Nanoparticles
2.4. Characterization of Cinchona Officinalis Extract-Loaded Iron Oxide Nanoparticles (CO-NPs)
2.5. Cell Lines and Cell Culture Materials
Cell and Nuclear Staining Agent, cDNA Synthesis Kit and Chemicals
2.6. In-Vitro Cytotoxicity Analysis
2.7. Microscopic Studies
2.7.1. Determination of Apoptotic Morphology Using Propidium Iodide Staining
2.7.2. Early and Late Apoptosis Determination Using AO/EtBr Staining
2.7.3. Determination of Mitochondrial Membrane Potential
2.8. Quantitative-Real Time PCR Analysis
2.9. Statistical Analysis
3. Results
3.1. Characterization of Cinchona officinalis Stem Bark Extract-Loaded Iron Oxide Nanoparticles (CO-NPs)
3.2. GC–MS Profile of the Stem Bark Methanol Extract of Cinchona officinalis
3.3. In Vitro Cytotoxicity
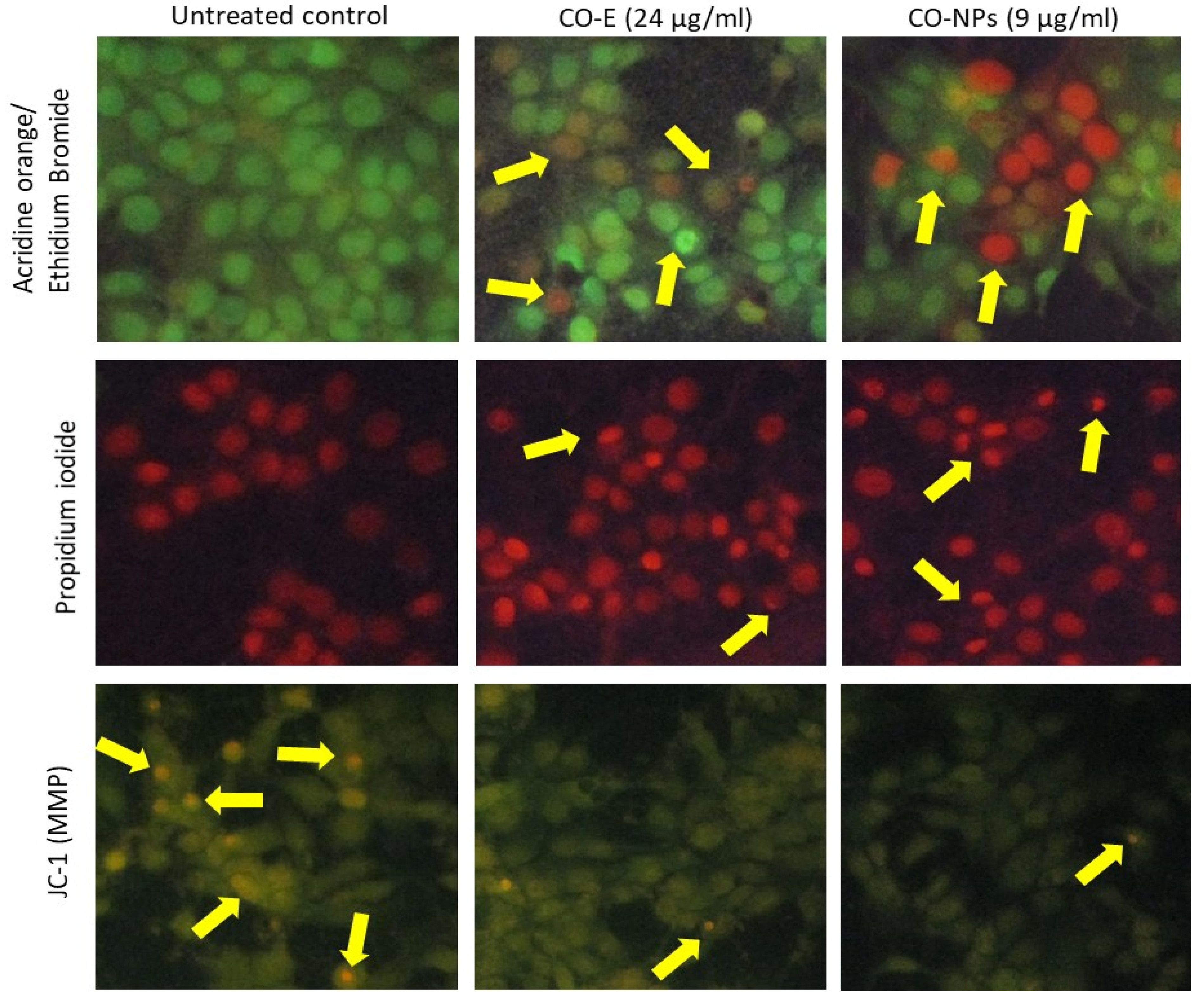
3.4. Cell and Nuclear Morphology
3.5. JC-1 Staining to Determine the Mitochondrial Membrane Potential (Δψm)
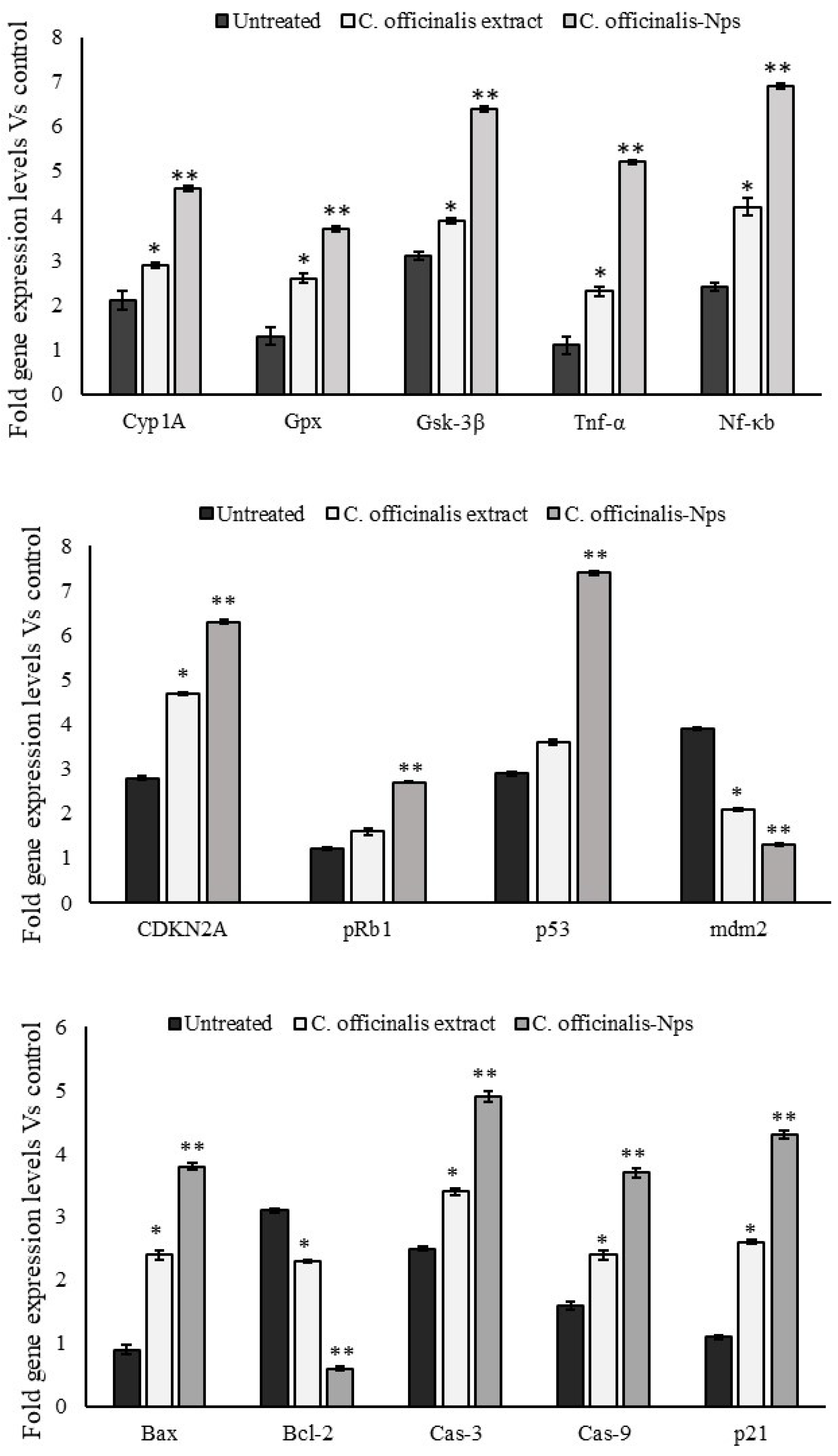
3.6. Expressions Levels of Oxido-Redox and Tumor Suppressor Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Breast Cancer; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer#:~:text=Scope%20of%20the%20problem,the%20world’s%20most%20prevalent%20cancer (accessed on 20 March 2022).
- Alqahtani, W.S.; Almufareh, N.A.; Domiaty, D.M.; Albasher, G.; Alduwish, M.A.; Alkhalaf, H.; Almuzzaini, B.; AL-marshidy, S.S.; Alfraihi, R.; Elasbali, A.M.; et al. Epidemiology of cancer in Saudi Arabia thu 2010–2019: A systematic review with constrained meta-analysis. AIMS Public Health 2020, 7, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef] [PubMed]
- Comsa, Ş.; Cimpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar] [PubMed]
- Bukhari, A.; Ijaz, I.; Gilani, E.; Nazir, A.; Zain, H.; Saeed, R.; Alarfaji, S.S.; Hussain, S.; Aftab, R.; Naseer, Y. Green synthesis of metal and metal oxide nanoparticles using different plants’ parts for antimicrobial activity and anticancer activity: A review article. Coatings 2021, 11, 1374. [Google Scholar] [CrossRef]
- Evans, E.R.; Bugga, P.; Asthana, V.; Drezek, R. Metallic nanoparticles for cancer immunotherapy. Mater. Today 2018, 21, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin; Husen, A. Biogenic fabrication of iron/iron oxide nanoparticles and their application. Nanoscale Res. Lett. 2016, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Raza, M.A.; Rehman, F.; ur Anwar, S.; Zaha, A.; Rehman, A.; Rashid, E.; Kalsoom, M.; Ilahi, H. The Medicinal Aromatic Activities of cinchona: A Review. Asian J. Adv. Res. 2021, 8, 42–45. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Leite, M.; Quinta-Costa, M.; Leite, P.S.; Guimaraes, J.E. Critical evaluation of techniques to detect and measure cell death–study in a model of UV radiation of the leukaemic cell line HL60. Anal. Cell. Pathol. 1999, 19, 139–151. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real–time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuiyan, M.S.; Miah, M.Y.; Paul, S.C.; Aka, T.D.; Saha, O.; Rahaman, M.M.; Sharif, M.J.I.; Habiba, O.; Ashaduzzaman, M. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef] [PubMed]
- Guivar, J.A.R.; Martínez, A.I.; Ana Osorio Anaya, A.O.; Luis De Los Santos Valladares, L.D.-L.S.; Félix, L.L.; Dominguez, A.B. Structural and magnetic properties of monophasic maghemite (γ-Fe2O3) nanocrystalline Powder. Adv. Nanoparticles 2014, 3, 114–121. [Google Scholar] [CrossRef]
- Liang, C.; Liu, H.; Zhou, J.; Peng, X.; Zhang, H. One-step Synthesis of spherical 𝛾-Fe2O3 panopowders and the evaluation of their thotocatalytic activity for orange I degradation. J. Chem. 2015, 2015, 791829. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Agarwal k Singh, A.K.; Polke, B.G.; Raha, K.C. Characterization of γ- and α-Fe2O3 nano powders synthesized by emulsion precipitation-calcination route and rheological behaviour of α-Fe2O3. Int. J. Eng. Sci. Technol. 2010, 2, 118–126. [Google Scholar]
- Islam, M.S.; Kurawaki, J.; Kusumoto, Y.; Abdulla-Al-Mamun, M.; Bin Mukhlish, M.Z. Hydrothermal Novel Synthesis of Neck-structured Hyperthermia-suitable Magnetic (Fe3O4, γ-Fe2O3 and α-Fe2O3) Nanoparticles. J. Sci. Res. 2012, 4, 99–107. [Google Scholar] [CrossRef]
- Valášková, M.; Tokarský, J.; Pavlovský, J.; Prostějovský, T.; Kočí, K. α-Fe2O3 nanoparticles/vermiculite clay material: Structural, optical and photocatalytic properties. Materials 2019, 12, 1880. [Google Scholar] [CrossRef]
- Alshammari, G.M.; Yagoub, A.E.A.; Subash-Babu, P.; Hassan, A.B.; Al-Nouri, D.M.; Mohammed, M.A.; Yahya, M.A.; Elsayim, R. Inhibition of Lipid Accumulation and Adipokine Levels in Maturing Adipocytes by Bauhinia rufescens (Lam.) Stem Bark Extract Loaded Titanium Oxide Nanoparticles. Molecules 2021, 26, 7238. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Al-Qahtani, A.; Al-Mutairi, A.; Al-Shamri, B.; Aabed, K. Antibacterial and cytotoxic potential of biosyn-thesized silver nanoparticles by some plant extracts. Nanomaterials 2018, 8, 382. [Google Scholar] [CrossRef]
- Hariharan, D.; Srinivasan, K.; Nehu, L. Synthesis and characterization of TiO2 nanoparticles using Cynodon dactylon leaf extract for antibacterial and anticancer (A549 Cell Lines) Activity. J. Nanomed. Res. 2017, 5, 1–5. [Google Scholar]
- Aisida, S.O.; Akpa, P.A.; Ahmad, I.; Ting-kai Zhao, T.K.; Maaza, M.; Ezem, F.I. Bio-inspired encapsulation and functionalization of iron oxide nanoparticles for biomedical applications. Eur. Polym. J. 2020, 122, 109371. [Google Scholar] [CrossRef]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Qasim, S.; Zafar, A.; Saif, M.S.; Ali, Z.; Nazar, M.; Waqas, M.; Haq, A.U.; Tariq, T.; Hassan, S.G.; Iqbal, F.; et al. Green synthesis of iron oxide nanorods using Withania coagulans extract improved photocatalytic degradation and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111784. [Google Scholar] [CrossRef] [PubMed]
- Bala, N.; Saha, S.; Maiti, M.; Sarkar, M.; Das, S.; Nandi, P.; Basu, R. Riboflavin conjugated temperature variant ZnO nanoparticles with potential medicinal application in jaundice. RSC Adv. 2016, 6, 71188–71198. [Google Scholar] [CrossRef]
- English, N.J.; Rahman, M.; Wadnerkar, N.; MacElroy, J.M.D. Photo-active and dynamical properties of hematite (Fe2O3)–water interfaces: An experimental and theoretical study. Phys. Chem. Chem. Phys. 2014, 16, 14445–14454. [Google Scholar] [CrossRef]
- Saniewski, M.; Horbowicz, M.; Kanlayanarat, S. The Biological Activities of Troponoids and Their Use in Agriculture A Review. J. Hortic. Res. 2014, 22, 5–19. [Google Scholar] [CrossRef]
- Cao, F.; Orth, C.; Donlin, M.J.; Adegboyega, P.; Meyers, M.; Murelli, R.P.; Elagawany, M.; Elgendy, B.; Tavis, J.E. Synthesis and Evaluation of Troponoids as a New Class of Antibiotics. ACS Omega 2018, 3, 15125–15133. [Google Scholar] [CrossRef]
- ChEBI. Phenanthidone. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:75292. (accessed on 12 June 2022).
- Insight Drugs. Viridicatol. National Center for Advancing Translational Sciences. Available online: https://drugs.ncats.io/drug/45P12JNE0L (accessed on 12 June 2022).
- Ahmed, N.M.; Youns, M.; Soltan, M.K.; Said, A.M. Design, synthesis, molecular modelling, and biological evaluation of novel substituted pyrimidine derivatives as potential anticancer agents for hepatocellular carcinoma. J. Enzym. Inhib. Med. Chem. 2019, 34, 1110–1120. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, P.; Stanley, C.A.; Hoshi, T.; Li, C. Mechanisms of octanoic acid potentiation of insulin secretion in isolated islets. Islets 2019, 11, 77–88. [Google Scholar] [CrossRef]
- ChEBI. Methyl Nonanoate. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:44499 (accessed on 12 June 2022).
- ChEBI. 2,4-Di-Tert-Butylphenol. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:89188 (accessed on 12 June 2022).
- ChEBI. Methyl Dodecanoate. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:87494 (accessed on 12 June 2022).
- ChEBI. Diethyl Phthalate. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:34698 (accessed on 12 June 2022).
- DrugBank. Norepinephine, (R)-, 4TMS Derivative. Available online: https://go.drugbank.com/drugs/DB00368 (accessed on 12 June 2022).
- Arshi, A.; Sharifi, F.S.; Ghahfarokhi, M.K.; Faghih, Z.; Doosti, A.; Ostovari, S.; Maymand, E.M.; Seno, M.M.G. Expression Analysis of MALAT1, GAS5, SRA, and NEAT1 lncRNAs in Breast Cancer Tissues from Young Women and Women over 45 Years of Age. Mol. Ther.-Nucleic Acids 2018, 12, 751–757. [Google Scholar] [CrossRef]
- Taherzadeh-Soureshjani, P.; Chehelgerdi, M. Algae-meditated route to cuprous oxide (Cu2O) nanoparticle: Differential expression profile of MALAT1 and GAS5 LncRNAs and cytotoxic effect in human breast cancer. Cancer Nanotechnol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Taherian, A.; Esfandiari, N.; Rouhani, S. Breast cancer drug delivery by novel drug-loaded chitosan-coated magnetic nanoparticles. Cancer Nanotechnol. 2021, 12, 15. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Okur, N.Ü.; Karantas, I.D.; Okur, M.E.; Gündoğdu, E.A. Current update on nanoplatforms as therapeutic and diagnostic tools: A review for the materials used as nanotheranostics and imaging modalities. Asian J. Pharm. Health Sci. 2021, 16, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Kavithaa, K.; Sumathi, S.; Padma, P.R. Intracellular Uptake of PEG-Funtionalized Baicalein Loaded Iron Oxide Nanoparticles Regulates Apoptotic Genes in Triple Negative Breast Cancer Cells: Mitochondrial Pathway Targeted Therapy for Breast Cancer. J. Clust. Sci. 2017, 28, 2057–2073. [Google Scholar] [CrossRef]
- Thenmozhi, T. Functionalization of iron oxide nanoparticles with clove extract to induce apoptosis in MCF-7 breast cancer cells. 3 Biotech 2020, 10, 82. [Google Scholar] [CrossRef]
- Benelli, G. Green synthesized nanoparticles in the fight against mosquito-borne diseases and cancer—A brief review. Enzym. Microb. Technol. 2016, 95, 58–68. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A. Copper ferrite nanoparticle-induced cytotoxicity and oxidative stress in human breast cancer MCF-7 cells. Colloids Surf. B Biointerfaces 2016, 142, 46–54. [Google Scholar] [CrossRef]
- Benelli, G.; Lukehart, C.M. Special Issue: Applications of Green-Synthesized Nanoparticles in Pharmacology, Parasitology and Entomology. J. Clust. Sci. 2017, 28, 1–2. [Google Scholar] [CrossRef]
- Gurung, P.; De, P. Spectrum of biological properties of cinchona alkaloids: A brief review. J. Pharmacogn. Photochem. 2017, 6, 162–166. [Google Scholar]
- Hosseinkazemi, H.; Samani, S.; O’Neill, A.; Soezi, M.; Moghoofei, M.; Azhdari, M.H.; Aavani, F.; Nazbar, A.; Keshel, S.H.; Doroudian, M. Applications of Iron Oxide Nanoparticles against Breast Cancer. J. Nanomater. 2022, 2022, 6493458. [Google Scholar] [CrossRef]
- Alangari, A.; Alqahtani, M.S.; Mateen, A.; Kalam, M.A.; Alshememry, A.; Ali, R.; Kazi, M.; AlGhamdi, K.M.; Syed, R. Iron Oxide Nanoparticles: Preparation, Characterization, and Assessment of Antimicrobial and Anticancer Activity. Adsorpt. Sci. Technol. 2022, 2022, 1562051. [Google Scholar] [CrossRef]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, C.; Sangeetha, B.; Duraipandiyan, V.; Raj, M.K.; Ignacimuthu, S.; Al-Dhabi, N.; Balakrishna, K.; Parthasarathy, K.; Arulmozhi, N.; Arasu, M.V. A flavonoid isolated from Streptomyces sp. (ERINLG-4) induces apoptosis in human lung cancer A549 cells through p53 and cytochrome c release caspase dependant pathway. Chem. Interact. 2014, 224, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Hockenbery, D.M.; Nuñez, G.; Milliman, C.L.; Schreiber, R.D.; Korsmeyer, S.J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990, 348, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, G.M.; Tawfeeq, A.T.; Naji, A.S. Biosynthesis, characterization of magnetic iron oxide nanoparticles and evaluations of the cytotoxicity and DNA damage of human breast carcinoma cell lines. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1215–1229. [Google Scholar] [CrossRef]
- Khan, M.I.; Mohammad, A.; Patil, G.; Naqvi, S.; Chauhan, L.; Ahmad, I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 2012, 33, 1477–1488. [Google Scholar] [CrossRef]
- He, C.; Jiang, S.; Jin, H.; Chen, S.; Lin, G.; Yao, H.; Wang, X.; Mi, P.; Ji, Z.; Lin, Y.; et al. Mitochondrial electron transport chain identified as a novel molecular target of SPIO nanoparticles mediated cancer-specific cytotoxicity. Biomaterials 2016, 83, 102–114. [Google Scholar] [CrossRef]
- Kai, W.; Xiaojun, X.; Ximing, P.; Zhenqing, H.; Qiqing, Z. Cytotoxic effects and the mechanism of three types of magnetic nanoparticles on human hepatoma BEL-7402 cells. Nanoscale Res. Lett. 2011, 6, 480. [Google Scholar] [CrossRef]
- Zhu, M.-T.; Wang, B.; Wang, Y.; Yuan, L.; Wang, H.-J.; Wang, M.; Ouyang, H.; Chai, Z.-F.; Feng, W.-Y.; Zhao, Y.-L. Endothelial dysfunction and inflammation induced by iron oxide nanoparticle exposure: Risk factors for early atherosclerosis. Toxicol. Lett. 2011, 203, 162–171. [Google Scholar] [CrossRef]
- Al-Saran, N.; Subash-Babu, P.; Al-Nouri, D.M.; Alfawaz, H.A.; Alshatwi, A.A. Zinc enhances CDKN2A, pRb1 expression and regulates functional apoptosis via upregulation of p53 and p21 expression in human breast cancer MCF-7 cell. Environ. Toxicol. Pharmacol. 2016, 47, 19–27. [Google Scholar] [CrossRef]
- Stading, R.; Chu, C.; Couroucli, X.; Lingappan, K.; Moorthy, B. Molecular role of cytochrome P4501A enzymes in oxidative stress. Curr. Opin. Toxicol. 2020, 20–21, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ogura, S.; Shimosawa, T. Oxidative Stress and Organ Damages. Curr. Hypertens. Rep. 2014, 16, 452. [Google Scholar] [CrossRef] [PubMed]
- Gathwala, G.; Aggarwal, R. Selenium supplementation for the preterm Indian neonate. Indian J. Public Health 2016, 60, 142–144. [Google Scholar] [CrossRef]
- Tejchman, K.; Kotfis, K.; Sieńko, J. Biomarkers and Mechanisms of Oxidative Stress—Last 20 Years of Research with an Emphasis on Kidney Damage and Renal Transplantation. Int. J. Mol. Sci. 2021, 22, 8010. [Google Scholar] [CrossRef]
- Prabhakar, O. Cerebroprotective effect of resveratrol through antioxidant and anti-inflammatory effects in diabetic rats. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2013, 386, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Heissig, B.; Salama, Y.; Takahashi, S.; Osada, T.; Hattori, K. The multifaceted role of plasminogen in inflammation. Cell Signal. 2020, 75, 109761. [Google Scholar] [CrossRef]
- Liu, W.; Lu, X.; Shi, P.; Yang, G.; Zhou, Z.; Li, W.; Mao, X.; Jiang, D.; Chen, C. TNF-α increases breast cancer stem-like cells through up-regulating TAZ expression via the non-canonical NF-κB pathway. Sci. Rep. 2020, 10, 1804. [Google Scholar] [CrossRef]
- Wu, X.; Wu, M.-Y.; Jiang, M.; Zhi, Q.; Bian, X.; Xu, M.-D.; Gong, F.-R.; Hou, J.; Tao, M.; Shou, L.-M.; et al. TNF-α sensitizes chemotherapy and radiotherapy against breast cancer cells. Cancer Cell Int. 2017, 17, 13. [Google Scholar] [CrossRef]
- Li, J.; Xing, M.; Zhu, M.; Wang, X.; Wang, M.; Zhou, S.; Li, N.; Wu, R.; Zhou, M. Glycogen synthase kinase 3β induces apoptosis in cancer cells through increase of survivin nuclear localization. Cancer Lett. 2008, 272, 91–101. [Google Scholar] [CrossRef]
- Culbreth, M.; Aschner, M. GSK-3β, a double-edged sword in Nrf2 regulation: Implications for neurological dysfunction and disease. F1000Research 2018, 7, 1043. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Feng, Z. Tumor suppressor p53 and its gain-of-function mutants in cancer. Acta Biochim. Biophys. Sin. 2013, 46, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Delbridge, A.R.D.; Strasser, A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015, 22, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-Y.; Park, S.-Y.; Park, S.; Lee, Y.R.; Kwak, M.-K.; Kim, J.-A. Effects of geranyl-phloroacetophenone on the induction of apoptosis and chemosensitization of adriamycin-resistant MCF-7 human breast cancer cells. Arch. Pharmacal Res. 2012, 35, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoy-Berard, N.; Aouacheria, A.; Verschelde, C.; Quemeneur, L.; Marçais, A.; Marvel, J. Control of proliferation by Bcl-2 family members. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2004, 1644, 159–168. [Google Scholar] [CrossRef]
- Liebermann, D.A.; Hoffman, B.; Vesely, D. p53 Induced Growth Arrest versus Apoptosis and its Modulation by Survival Cytokines. Cell Cycle 2007, 6, 166–170. [Google Scholar] [CrossRef]
- Lee, Y.K.; Choi, E.-J.; Webster, T.J.; Kim, S.-H.; Khang, D. Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. Int. J. Nanomed. 2014, 10, 97–113. [Google Scholar] [CrossRef]
- Argiris, A.; Cohen, E.; Karrison, T.; Esparaz, B.; Mauer, A.; Ansari, R.; Wong, S.; Lu, Y.; Pins, M.; Dancey, J.; et al. A phase II trial of perifosine, an oral alkylphospholipid, in recurrent or metastatic head and neck cancer. Cancer Biol. Ther. 2006, 5, 766–770. [Google Scholar] [CrossRef]
- O’Donovan, N.; Crown, J.; Stunell, H.; Hill, A.D.; McDermott, E.; O’Higgins, N.; Duffy, M.J. Caspase 3 in breast cancer. Clin. Cancer Res. 2003, 9, 738–742. [Google Scholar]
- Stennicke, H.R.; Salvesen, G.S. Properties of the caspases. Biochim. Biophys. Acta (BBA)—Protein Struct. Mol. Enzym. 1998, 1387, 17–31. [Google Scholar] [CrossRef]
- Mancini, M.; Nicholson, D.W.; Roy, S.; Thornberry, N.A.; Peterson, E.P.; Casciola-Rosen, L.A.; Rosen, A. The Caspase-3 Precursor Has a Cytosolic and Mitochondrial Distribution: Implications for Apoptotic Signaling. J. Cell Biol. 1998, 140, 1485–1495. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.G.; Cullen, S.P.; Sheridan, C.; Lüthi, A.U.; Gerner, C.; Martin, S.J. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc. Natl. Acad. Sci. USA 2008, 105, 12815–12819. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-L.; Chang, C.-C.; Chen, L.-G.; Wang, C.-C. Antitumor Principle Constituents of Myrica rubra Var. acuminata. J. Agric. Food Chem. 2003, 51, 2974–2979. [Google Scholar] [CrossRef]
- Pomerantz, J.H.; Blau, H.M. Tumor suppressors: Enhancers or suppressors of regeneration? Development 2013, 140, 2502–2512. [Google Scholar] [CrossRef]
- ABU, N.; Akhtar, M.N.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Zulfadli, A.J.; Omar, A.R.; Sulaiman, M.R.; Abdullah, M.P.; Alitheen, N.B. Flavokawain A Induces Apoptosis in MCF-7 and MDA-MB231 and Inhibits the Metastatic Process In Vitro. PLoS ONE 2014, 9, e105244. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Othman, S.F.; Curtis, E.T.; Bauer, N.A.; Chauhan, N.; Kumar, D.; Jaggi, M.; Chauhan, S.C. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int. J. Nanomed. 2012, 7, 1761–1779. [Google Scholar] [CrossRef] [Green Version]
- Krishnaveni, M.; Suresh, K. Induction of apoptosis by quinine in human laryngeal carcinoma cell line (KB). Int. J. Curr. Res. Acad. Rev. 2015, 3, 169–178. [Google Scholar]


| No. | RT (min) | Peak Area (%) | Compound Name | Molecular Formula | Molecular Weight (g/mol) | Compound Nature | Bioactivity |
|---|---|---|---|---|---|---|---|
| 1 | 5.36 | 3.18 | 4-Ethoxy-2-(methylamino) tropone | C10H13NO2 | 179.22 | Cyclic aliphatic ketone | Tropone derivatives function as anti-ischemic, insecticidal, bacterial, fungal, and anti-tumor agents. Additionally, they can deactivate polyphenol oxidase and chelate iron [27,28]. |
| 2 | 6.72 | 3.01 | 6-phenanthidinol, 7,9-dimethyl- | C15H13NO | 223.27 | phenanthidine | A mutagen and immunosuppressive agent [29]. |
| 3 | 9.77 | 11.95 | 2(1H)-Quinolinone, 3-hydroxy-4-(3-hydroxyphenyl)-(Viridicatol) | C15H11NO3 | 253.25 | Alkaloid | Viridicatol acts as an anti-inflammatory agent [30]. |
| 5 | 10.42 | 2.01 | 9-(4-Dimethylaminophenyl)anthracene | C22H19N | 297.4 | Cyclic hydrocarbon | Pyrimidine pyrazoline-anthracene derivatives are active against normal fibroblast cells and hepatocellular carcinoma cells [31]. |
| 6 | 12.05 | 2.33 | Octanoic acid, methyl ester (Methyl octanoate) | C9H18O2 | 158.24 | Fatty acid ester | It is a metabolite. It has a potentiating effect on insulin secretion [32]. |
| 7 | 14.49 | 2.46 | Nonanoic acid, methyl ester (Methyl pelarigonate) | C10H20O2 | 172.26 | Fatty acid ester | It is an epitope, antifungal agent, and antinematodal drug, as well as a plant metabolite [33]. |
| 8 | 20.76 | 12.24 | 2,4-Di-tert-butylphenol | C14H22O | 206.32 | Alkylbenzene and a member of phenols | An auto-toxin, antioxidant, bacterial metabolite, and marine metabolite [34]. |
| 9 | 21.13 | 2.57 | Dodecanoic acid, methyl ester | C14H28O2 | 228.37 | Fatty acid ester | It has a role as a metabolite [35]. |
| 10 | 22.41 | 2.63 | Diethyl Phthalate | C12H14O4 | 222.24 | Phthalate ester | A tetragonic agent, neurotoxin, endocrine disrupter, and a hazardous substance to the environment [36]. |
| 11 | 23.17 | 9.06 | Norepinephrine, (R)-, 4TMS derivative | C20H43NO3Si4 | 457.9 | Catecholamine | The norepinephrine moiety is used in the control of blood pressure [37]. |
| 12 | 26.14 | 6.82 | Methyl 9-methyltetradecanoate | C16H32O2 | 256.42 | Fatty acid ester | Not reported |
| 13 | 26.26 | 9.90 | Heptacos-1-ene | C27H54 | 378.7 | alkene | Not reported |
| 14 | 30.12 | 6.10 | Heptadcanoic acid, methyl ester | C18H36O2 | 284.5 | Fatty acid ester | Not reported |
| 15 | 31.28 | 1.92 | 9-Octadecenoic acid (Z)-, methyl ester (Methyl Oleate) | C19H36O2 | 296.50 | Fatty acid ester | Not reported |
| 16 | 31.37 | 5.52 | Cyclopropaneoctanoic acid, 2-hexyl-, methyl ester | C18H34O2 | 282.5 | Fatty acid ester | Not reported |
| 17 | 34.62 | 2.72 | Eicosanoic acid, methyl ester (Methyl arachidate) | C21H42O2 | 326.6 | Fatty acid ester | Not reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Harbi, L.N.; Al-Shammari, G.M.; Subash-Babu, P.; Mohammed, M.A.; Alkreadees, R.A.; Yagoub, A.E.A. Cinchona officinalis Phytochemicals-Loaded Iron Oxide Nanoparticles Induce Cytotoxicity and Stimulate Apoptosis in MCF-7 Human Breast Cancer Cells. Nanomaterials 2022, 12, 3393. https://doi.org/10.3390/nano12193393
Al-Harbi LN, Al-Shammari GM, Subash-Babu P, Mohammed MA, Alkreadees RA, Yagoub AEA. Cinchona officinalis Phytochemicals-Loaded Iron Oxide Nanoparticles Induce Cytotoxicity and Stimulate Apoptosis in MCF-7 Human Breast Cancer Cells. Nanomaterials. 2022; 12(19):3393. https://doi.org/10.3390/nano12193393
Chicago/Turabian StyleAl-Harbi, Laila Naif, Ghedier M. Al-Shammari, Pandurangan Subash-Babu, Mohammed A. Mohammed, Roaa Ahmed Alkreadees, and Abu ElGasim Ahmed Yagoub. 2022. "Cinchona officinalis Phytochemicals-Loaded Iron Oxide Nanoparticles Induce Cytotoxicity and Stimulate Apoptosis in MCF-7 Human Breast Cancer Cells" Nanomaterials 12, no. 19: 3393. https://doi.org/10.3390/nano12193393
APA StyleAl-Harbi, L. N., Al-Shammari, G. M., Subash-Babu, P., Mohammed, M. A., Alkreadees, R. A., & Yagoub, A. E. A. (2022). Cinchona officinalis Phytochemicals-Loaded Iron Oxide Nanoparticles Induce Cytotoxicity and Stimulate Apoptosis in MCF-7 Human Breast Cancer Cells. Nanomaterials, 12(19), 3393. https://doi.org/10.3390/nano12193393






