Controlling CNT-Based Nanorotors via Hydroxyl Groups
Abstract
:1. Introduction
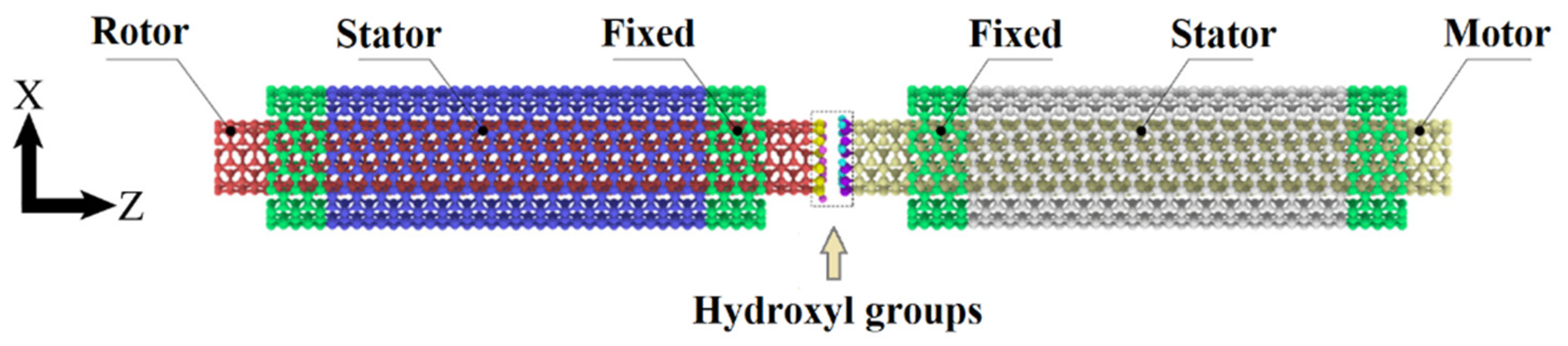
2. Model and Method
3. Results and Discussion
3.1. Transmission
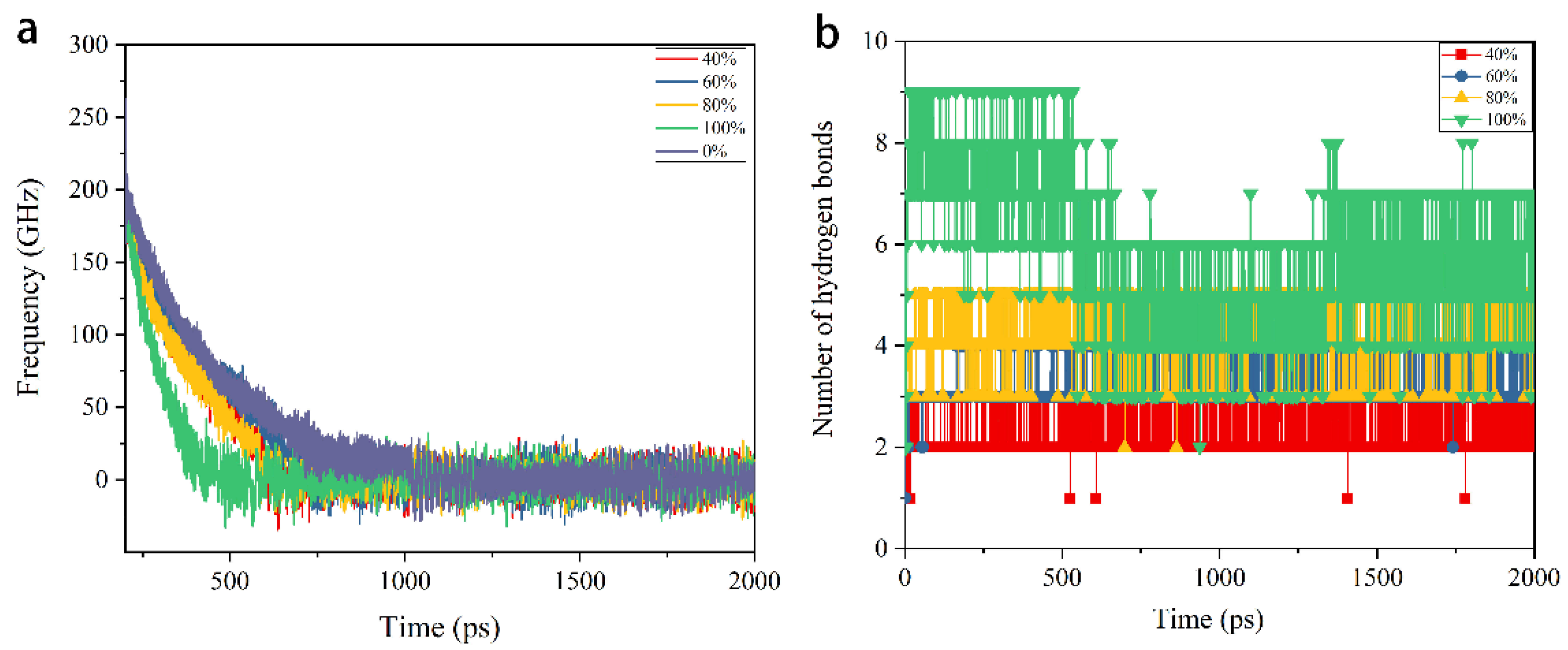
3.2. Braking Process
3.3. Energy Dissipation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirai, Y.; Morin, J.-F.; Sasaki, T.; Guerrero, J.M.; Tour, J.M. Recent progress on nanovehicles. Chem. Soc. Rev. 2006, 35, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Badjić, J.D.; Balzani, V.; Credi, A.; Silvi, S.; Stoddart, J.F. A molecular elevator. Science 2004, 303, 1845. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A.M.; Frochot, C.; Gatti, F.G.; Leigh, D.A.; Mottier, L.C.; Paolucci, F.; Roffia, S.; Wurpel, G.W.H. Photoinduction of fast, reversible translational motion in a hydrogen-bonded molecular shuttle. Science 2001, 291, 2124. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Lieber, C.M. Nanotube nanotweezers. Science 1999, 286, 2148–2150. [Google Scholar] [CrossRef]
- Akita, S.; Nakayama, Y.; Mizooka, S.; Takano, Y.; Okawa, T.; Miyatake, Y.; Yamanaka, S.; Tsuji, M.; Nosaka, T. Nanotweezers consisting of carbon nanotubes operating in an atomic force microscope. Appl. Phys. Lett. 2001, 79, 1691–1693. [Google Scholar] [CrossRef]
- Han, J.; Globus, A.; Jaffe, R.; Deardorff, G. Molecular dynamics simulations of carbon nanotube-based gears. Nanotechnology 1997, 8, 95. [Google Scholar] [CrossRef]
- Zheng, Q.; Jiang, Q. Multiwalled carbon nanotubes as gigahertz oscillators. Phys. Rev. Lett. 2002, 88, 045503. [Google Scholar] [CrossRef]
- Guo, W.; Guo, Y.; Gao, H.; Zheng, Q.; Zhong, W. Energy Dissipation in gigahertz oscillators from multiwalled carbon nanotubes. Phys. Rev. Lett. 2003, 91, 125501. [Google Scholar] [CrossRef]
- Legoas, S.; Coluci, V.; Braga, S.; Coura, P.; Dantas, S.; Galvao, D.S.J. Molecular dynamics simulations of carbon nanotubes as gigahertz oscillators. Phys. Rev. Lett. 2003, 90, 055504. [Google Scholar] [CrossRef]
- Cumings, J.; Zettl, A. Low-friction nanoscale linear bearing realized from multiwall carbon nanotubes. Science 2000, 289, 602–604. [Google Scholar] [CrossRef]
- Bourlon, B.; Glattli, D.C.; Miko, C.; Forró, L.; Bachtold, A. Carbon nanotube based bearing for rotational motions. Nano Lett. 2003, 4, 709–712. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, W.; Yu, T. Energy dissipation of high-speed nanobearings from double-walled carbon nanotubes. Nanotechnology 2008, 19, 465703. [Google Scholar] [CrossRef]
- Cook, E.H.; Buehler, M.J.; Spakovszky, Z.S.; Solids, P.O. Mechanism of friction in rotating carbon nanotube bearings. J. Mech. Phys. Solids 2013, 61, 652–673. [Google Scholar] [CrossRef]
- Cai, K.; Yu, J.; Shi, J.; Qin, Q.-H. Robust rotation of rotor in a thermally driven nanomotor. Sci. Rep. 2017, 7, 46159. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Wan, J.; Qin, Q.H.; Shi, J. Quantitative control of a rotary carbon nanotube motor under temperature stimulus. Nanotechnology 2016, 27, 055706. [Google Scholar] [CrossRef]
- Fennimore, A.; Yuzvinsky, T.; Han, W.-Q.; Fuhrer, M.; Cumings, J.; Zettl, A. Rotational actuators based on carbon nanotubes. Nature 2003, 424, 408–410. [Google Scholar] [CrossRef]
- Bailey, S.; Amanatidis, I.; Lambert, C. Carbon nanotube electron windmills: A novel design for nanomotors. Phys. Rev. Lett. 2008, 100, 256802. [Google Scholar] [CrossRef]
- Joseph, S.; Aluru, N.R. Pumping of confined water in carbon nanotubes by rotation-translation coupling. Phys. Rev. Lett. 2008, 101, 064502. [Google Scholar] [CrossRef]
- Barreiro, A.; Rurali, R.; Hernández, E.R.; Moser, J.; Pichler, T.; Forró, L.; Bachtold, A. Subnanometer motion of cargoes driven by thermal gradients along carbon nanotubes. Science 2008, 320, 775. [Google Scholar] [CrossRef]
- Santamaría-Holek, I.; Reguera, D.; Rubi, J.M. Carbon-nanotube-based motor driven by a thermal gradient. J. Phys. Chem. C 2013, 117, 3109–3113. [Google Scholar] [CrossRef]
- Cai, K.; Yin, H.; Wei, N.; Chen, Z.; Shi, J. A stable high-speed rotational transmission system based on nanotubes. Appl. Phys. Lett. 2015, 106, 021909. [Google Scholar] [CrossRef]
- Zhang, X.-N.; Cai, K.; Shi, J.; Qin, Q.-H. Friction effect of stator in a multi-walled CNT-based rotation transmission system. Nanotechnology 2017, 29, 045706. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Jiao, S.; Zheng, C.; Jicheng, Z.; Ning, W. A two-class rotation transmission nanobearing driven by gigahertz rotary nanomotor. Comput. Mater. Sci. 2018, 154, 97–105. [Google Scholar]
- Yin, H.; Cai, K.; Wei, N.; Qin, Q.H.; Shi, J. Study on the dynamics responses of a transmission system made from carbon nanotubes. J. Appl. Phys. 2015, 117, 234305. [Google Scholar] [CrossRef]
- Yin, H.; Cai, K.; Wan, J.; Gao, Z.L.; Chen, Z. Dynamic response of a carbon nanotube-based rotary nano device with different carbon-hydrogen bonding layout. Appl. Surf. Sci. 2016, 365, 352–356. [Google Scholar] [CrossRef]
- Gao, Z.L.; Cai, H.F.; Shi, J.; Liu, L.N.; Chen, Z.; Wang, Y. Effect of hydrogenation and curvature of rotor on the rotation transmission of a curved nanobearing. Comput. Mater. Sci. 2017, 127, 295–300. [Google Scholar] [CrossRef]
- Song, B.; Cai, K.; Shi, J.; Xie, Y.M.; Qin, Q.H. Coupling effect of van der Waals, centrifugal, and frictional forces on a GHz rotation-translation nano-convertor. Phys. Chem. Chem. Phys. 2019, 21, 359–368. [Google Scholar] [CrossRef]
- Shi, J.; Cao, Z.; Wang, J.B.; Shen, J.H.; Cai, K. Stable rotation transmission of a CNT-based nanogear drive system with intersecting axes at low temperature. Surf. Sci. 2020, 693, 121548. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.; Peng, Q. Tuning the slide-roll motion mode of carbon nanotubes via hydroxyl groups. Nanoscale Res. Lett. 2018, 13, 138. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Xie, L.; Zhu, P.; Li, R.; Peng, Q. Grain size and hydroxyl-coverage dependent tribology of polycrystalline graphene. Nanotechnology 2019, 30, 385701. [Google Scholar] [CrossRef]
- Li, R.; Liu, J.; Zheng, X.; Peng, Q. Achieve 100% transmission via grafting hydroxyl groups on CNT nanomotors. Curr. Appl. Phys. 2021, 29, 59–65. [Google Scholar] [CrossRef]
- Stuart, S.J.; Tutein, A.B.; Harrison, J.A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. [Google Scholar] [CrossRef]
- Hughes, Z.E.; Shearer, C.J.; Shapter, J.; Gale, J.D. Simulation of water transport through functionalized single-walled carbon nanotubes (SWCNTs). J. Phys. Chem. C 2012, 116, 24943. [Google Scholar] [CrossRef]
- Damm, W.; Frontera, A.; Tirado–Rives, J.; Jorgensen, W.L. OPLS all-atom force field for carbohydrates. J. Comput. Chem. 2015, 18, 1955–1970. [Google Scholar] [CrossRef]
- Ruoff, R.; Hickman, A. Van der Waals binding to fullerenes to a graphite plane. J. Phys. Chem. 1993, 97, 2494–2496. [Google Scholar] [CrossRef]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A generic force field for molecular simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Iakoubovskii, K.; Minami, N.; Ueno, T.; Kazaoui, S.; Kataura, H. Optical characterization of double-wall carbon nanotubes: Evidence for inner tube shielding. J. Phys. Chem. C 2008, 112, 11194–11198. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Li, R.; Peng, Q. Controlling CNT-Based Nanorotors via Hydroxyl Groups. Nanomaterials 2022, 12, 3363. https://doi.org/10.3390/nano12193363
Zhang B, Li R, Peng Q. Controlling CNT-Based Nanorotors via Hydroxyl Groups. Nanomaterials. 2022; 12(19):3363. https://doi.org/10.3390/nano12193363
Chicago/Turabian StyleZhang, Boyang, Rui Li, and Qing Peng. 2022. "Controlling CNT-Based Nanorotors via Hydroxyl Groups" Nanomaterials 12, no. 19: 3363. https://doi.org/10.3390/nano12193363
APA StyleZhang, B., Li, R., & Peng, Q. (2022). Controlling CNT-Based Nanorotors via Hydroxyl Groups. Nanomaterials, 12(19), 3363. https://doi.org/10.3390/nano12193363






