Comparative Analysis of LiMPO4 (M = Fe, Co, Cr, Mn, V) as Cathode Materials for Lithium-Ion Battery Applications—A First-Principle-Based Theoretical Approach
Abstract
1. Introduction
2. Materials and Computational Methodology
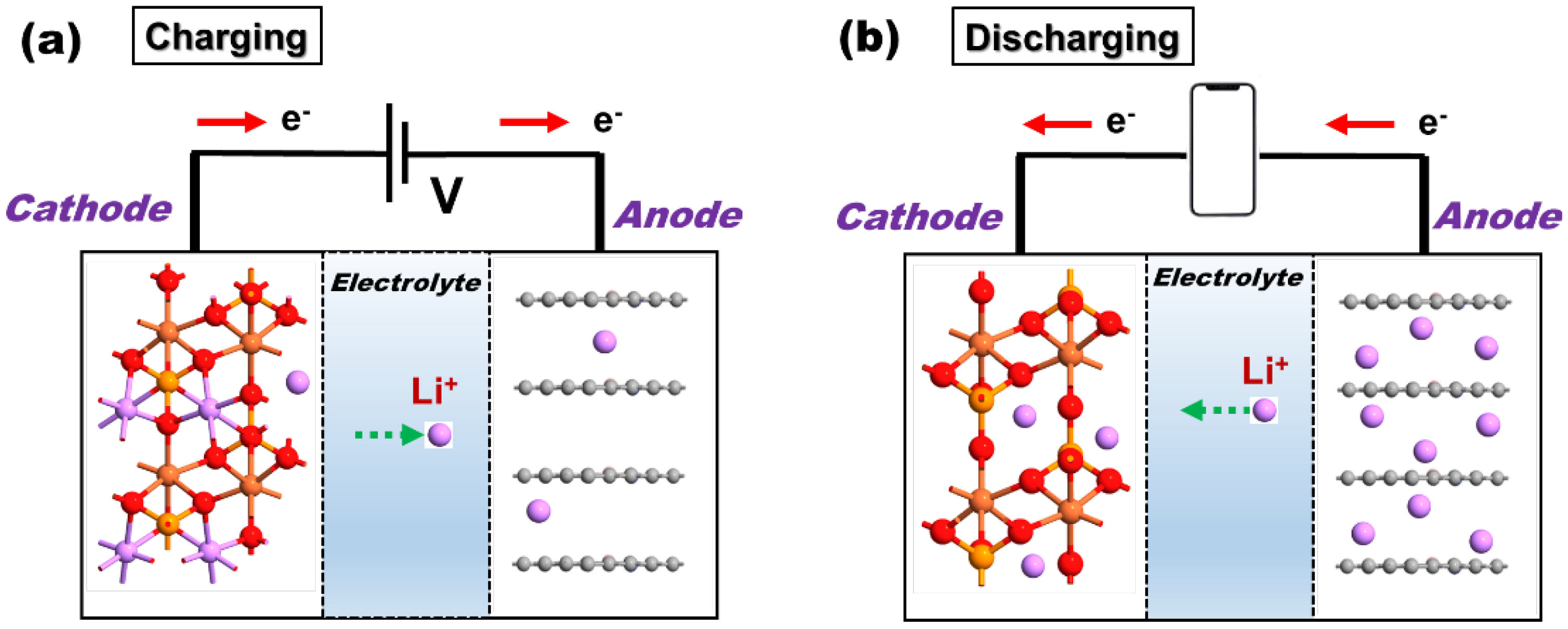
2.1. Battery Mechanism
2.2. Material Specifications
2.3. Simulation Framework
2.4. Parameter Definitions
3. Results and Discussion
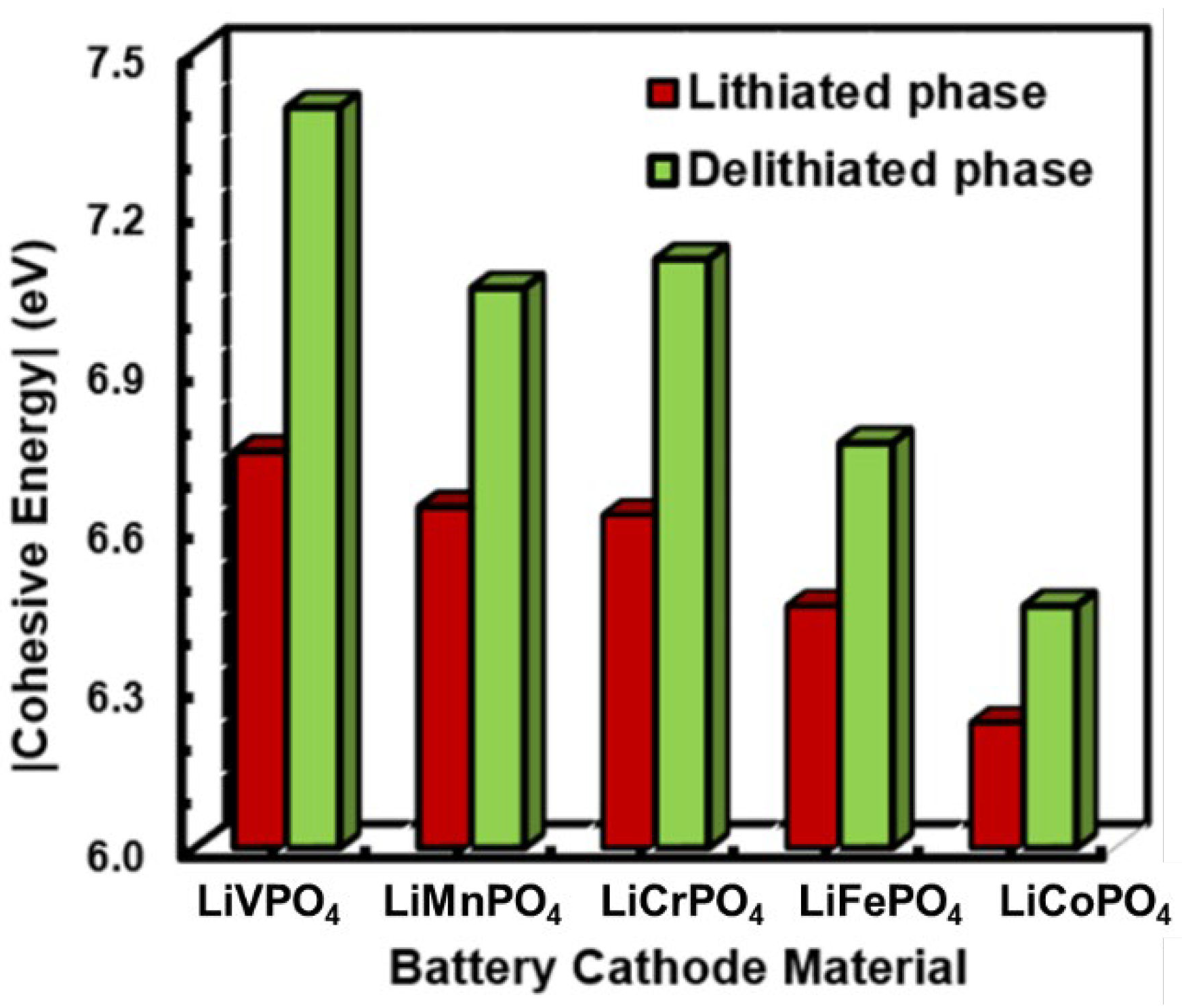
3.1. Structural Properties of LiMPO4 (M = Fe, Co, Cr, Mn, and V)
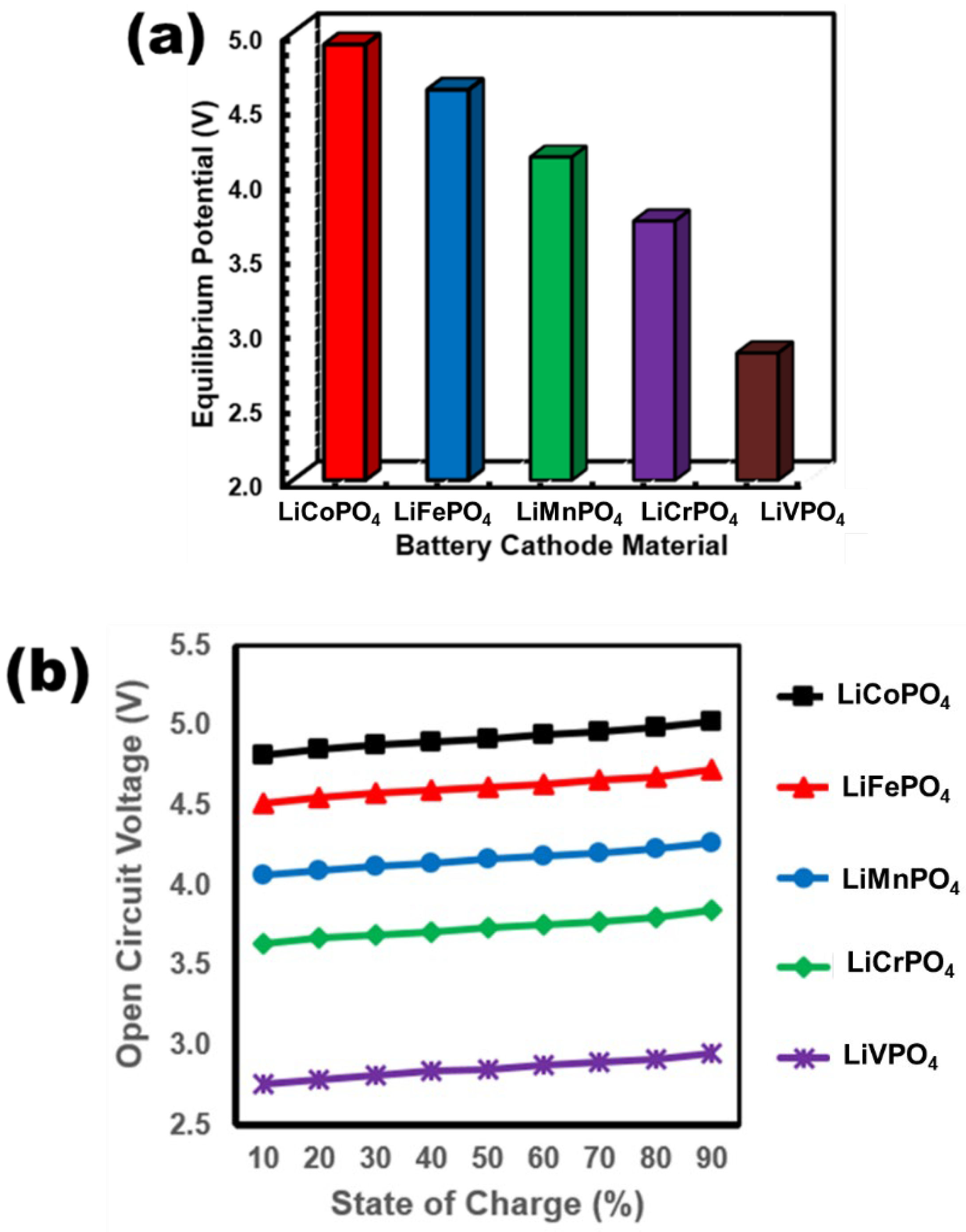
3.2. Electrochemical Properties of LiMPO4 (M = Fe, Co, Cr, Mn, and V)
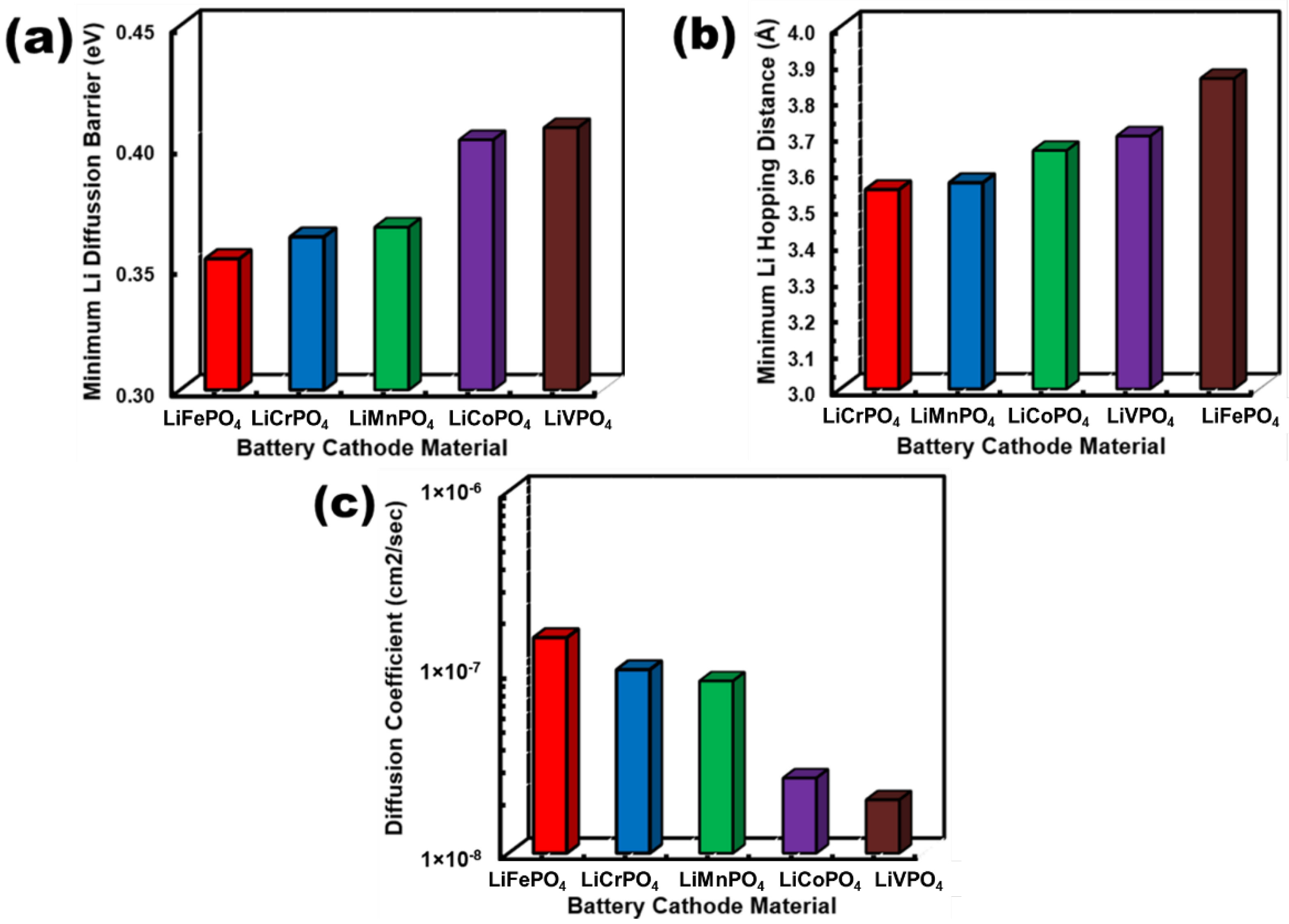
3.3. Electrical Characteristics of LiMPO4 (M = Fe, Co, Cr, Mn, and V)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Islam, M.S.; Fisher, C.A. Lithium and sodium battery cathode materials: Computational insights into voltage, diffusion and nanostructural properties. Chem. Soc. Rev. 2014, 43, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Yamada, S.; Jalem, R.; Kasuga, T. Density functional studies of olivine-type LiFePO4 and NaFePO4 as positive electrode materials for rechargeable lithium and sodium ion batteries. Solid State Ion. 2016, 286, 40–44. [Google Scholar] [CrossRef]
- Chen, Y.; Sang, M.; Jiang, W.; Wang, Y.; Zou, Y.; Lu, C.; Ma, Z. Fracture predictions based on a coupled chemo-mechanical model with strain gradient plasticity theory for film electrodes of Li-ion batteries. Eng. Fract. Mech. 2021, 253, 107866. [Google Scholar] [CrossRef]
- Ma, Z.S.; Xie, Z.C.; Wang, Y.; Zhang, P.P.; Pan, Y.; Zhou, Y.C.; Lu, C. Failure modes of hollow core-shell structural active materials during the lithiation-delithiation process. J. Power Sources 2015, 290, 114–122. [Google Scholar] [CrossRef]
- Chen, C.F.; Barai, P.; Mukherjee, P.P. An overview of degradation phenomena modelling in lithium-ion battery electrodes. Curr. Opin. Chem. Eng. 2016, 13, 82–90. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, H.; Wang, Y.; Pan, Y.; Lu, C. An electrochemical-irradiated plasticity model for metallic electrodes in lithium-ion batteries. Int. J. Plast. 2017, 88, 188–203. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and challenges of next-generation “beyond Li-ion” batteries for electric vehicles and grid decarbonization. Chem. Rev. 2020, 121, 1623–1669. [Google Scholar] [CrossRef]
- Urban, A.; Seo, D.H.; Ceder, G. Computational understanding of Li-ion batteries. NPJ Comput. Mater. 2016, 2, 16002. [Google Scholar] [CrossRef]
- Padhi, A.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-Olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Morgan, D.; Ven, A.; Ceder, G. Li conductivity in LixMPO4 (M = Mn, Fe, Co, Ni) olivine materials. Electrochem. Solid-State Lett. 2004, 7, 4032–4037. [Google Scholar] [CrossRef]
- Islam, M.S.; Driscoll, D.J.; Fisher, C.A.; Slater, P.R. Atomic-scale investigation of defects, dopants, and lithium transport in the LiFePO4 olivine-type battery material. Chem. Mater. 2005, 17, 5085–5092. [Google Scholar] [CrossRef]
- Ficher, C.A.J.; Prieto, V.M.H.; Islam, M.S. Lithium battery materials LiMPO4 (M= Mn, Fe, Co and Ni): Insights into defect association, transport mechanisms and doping behaviour. Chem. Mater. 2008, 20, 5907–5915. [Google Scholar] [CrossRef]
- Yang, J.; Tse, J.S. Li ion diffusion mechanisms in LiFePO4: An ab initio molecular dynamics study. J. Phys. Chem. A 2011, 115, 13045–13049. [Google Scholar] [CrossRef]
- Dathar, G.K.P.; Sheppard, D.; Stevenson, K.J.; Henkelman, G. Calculations of Li-ion diffusion in olivine phosphates. Chem. Mater. 2011, 23, 4032–4037. [Google Scholar] [CrossRef]
- Wu, K.C.; Hsieh, C.H.; Chang, B.K. First principles calculations of lithium diffusion near the surface and in the bulk of Fe-doped LiCoPO4. Phys. Chem. Chem. Phys. 2022, 24, 1147–1155. [Google Scholar] [CrossRef]
- Oukakhou, S.; Maymoun, M.; Elomrani, A.; Sbiaai, K.; Hasnaoui, A. Enhancing the Electrochemical Performance of Olivine LiMnPO4 as Cathode Materials for Li-ion Batteries by Ni-Fe codoping. ACS Appl. Energy Mater. 2022. [Google Scholar] [CrossRef]
- Ping, L.Z.; Ming, Z.Y.; Jun, Z.Y. First-principles studies of Mn-doped LiCoPO4. Chin. Phys. B 2011, 20, 018201. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, Y.; Li, J.; Du, K.; Cao, Y.; Li, J. Selecting substituent element for LiMnPO4 cathode materials combined with density functional theory (DFT) calculations and experiments. J. Alloys Compd. 2019, 793, 360–368. [Google Scholar] [CrossRef]
- Yang, L.; Deng, W.; Xu, W.; Tian, Y.; Wang, A.; Wang, B.; Zou, G.; Hou, H.; Deng, W.; Ji, X. Olivine LiMnxFe1−xPo4 cathode materials for lithium ion batteries: Restricted factors of rate performances. J. Mater. Chem. A 2021, 9, 14214–14232. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A. Formation, doping, and lithium incorporation in LiFePO4. AIP Adv. 2022, 12, 045225. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Kim, S.; Park, S.; Lee, S.; Lee, J.; Hwang, J.Y.; Sun, Y.K.; Kim, J. Density functional theory investigation of mixed transition metals in olivine and tavorite cathode materials for Li-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 16376–16386. [Google Scholar] [CrossRef]
- Molenda, J.; Kulka, A.; Milewska, A.; Zając, W.; Świerczek, K. Structural, transport and electrochemical properties of LiFePO4 substituted in lithium and iron sublattices (Al, Zr, W, Mn, Co and Ni). Materials 2013, 6, 1656–1687. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.; Shi, S.; Wang, Z.; Huang, X.; Chen, L. First-principles study of Li ion diffusion in LiFePO4. Phys. Rev. B 2004, 69, 104303. [Google Scholar] [CrossRef]
- “Quantum ATK”. Available online: https://www.synopsys.com/silicon/quantumatk.html (accessed on 1 January 2022).
- Berdiyorov, G.R.; Madjet, M.E.; Mohmoud, K.A. First-Principles Density Functional Theory Calculations of Bilayer Membranes Heterostructures of Ti3C2T2 (MXene)/Graphene and AgNPs. Membranes 2021, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.C.; Mayr, F.; Gagliardi, A. Machine Learning Stability and Bandgaps of Lead-Free Perovskites for Photovoltaics. Adv. Theory Simul. 2020, 3, 1900178. [Google Scholar] [CrossRef]
- Palepu, J.; Anand, P.P.; Parshi, P.; Jain, V.; Tiwari, A.; Bhattacharya, S.; Chakraborthy, S.; Kanungo, S. Compartitive analysis of strain engineering on the electronic properties of homogenous and heterostructure bilayers od MoX2 (X = S, Se, Te). Micro Nanostruct. 2022, 168, 207334. [Google Scholar] [CrossRef]
- Tiwari, A.; Palepu, J.; Choudhury, A.; Bhattacharya, S.; Kanungo, S. Theoretical analysis of the NH3, NO, and NO2 adsorption on boron-nitrogen and boron-phosphorous co-doped monolayer graphene-A comparative study. FlatChem 2022, 34, 100392. [Google Scholar] [CrossRef]
- Yang, C.C.; Li, S. Cohesive energy: The intrinsic dominant of thermal stability and structural evolution in Sn from size scales of bulk to dimer. J. Phys. Chem. C 2009, 113, 14207–14212. [Google Scholar] [CrossRef]
- Klerk, N.J.J.de.; Maas, E.V.D.; Wagemaker, M. Analysis of Diffusion in Solid-State Electrolytes through MD Simulations, improvement of the Li-Ion Conductivity in β-Li3PS4 as an Example. ACS Appl. Energy Mater. 2018, 1, 3230–3242. [Google Scholar] [CrossRef] [PubMed]
- Koettgen, J.; Zacherle, T.; Grieshammer, S.; Martin, M. Ab initio calculation of the attempt frequency of oxygen diffusion in pure and samarium doped ceria. Phys. Chem. Chem. Phys. 2017, 19, 9957–9973. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, G.R.; Islam, M.S. Anti-site defects and ion migration in the LiFe0.5Mn0.5PO4 mixed-metal cathode material. Chem. Mater. 2010, 22, 1242–1248. [Google Scholar] [CrossRef]
- Clementi, E.; Raimondi, D.L.; Reinhardt, W.P. Atomic Screening Constants from SCF Functions. II. Atoms with 37 To 86 Electrons. J. Chem. Phys. 2004, 47, 1300. [Google Scholar] [CrossRef]
- Fan, C.L.; Lin, C.R.; Han, S.C.; Chen, J.; Li, L.F.; Bai, Y.M.; Zhang, K.H.; Zhange, X. Structure, conductive mechanisn and electrochemical of LiFePO4/C doped with Mg2+, Cr3+ and Ti4+ by a carbothermal reduction method. New J. Chem. 2014, 38, 795–801. [Google Scholar] [CrossRef]
- Strobridge, F.C.; Clement, R.J.; Leskes, M.; Middlemiss, D.S.; Borekwicz, O.J.; Wiaderek, K.M.; Chapman, K.W. Identifying the structure of the Intermediate, Li2/3CoPO4, formed during Electrochemical cycling of LiCoPO4. Chem. Mater. 2014, 26, 6193–6205. [Google Scholar] [CrossRef]
- Sgroi, M.F.; Lazzaroni, R.; Beljonne, D.; Pullini, D. Doping LiMnPO4 with Cobalt and Nickel: A First Principle study. Batteries 2017, 3, 11. [Google Scholar] [CrossRef]
- Satyavani, T.V.S.L.; Kiran, B.R.; Kumar, V.R.; Kumar, A.S.; Naidu, S.V. Effect on particle size on dc conductivity, activation energy and diffusion coefficient of lithium iron phosphate in Li-ion cells. Eng. Sci. Technol. Int. J. 2016, 19, 40–44. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Z.; Gu, X. The Research on Characteristics of Li-NiMnCo Lithium-Ion Batteries in Electric Vehicles. J. Energy 2020, 2020, 3721047. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, B.; Li, B.; Cao, L.; Lai, Y.; Zheng, W.; Wang, H.; Wang, W.; Wang, M. A Study on the Open Circuit Voltage and State of the Charge Characterization of High Capacity Lithium-Ion Battery Under Different Temperatures. Energies 2018, 11, 2408. [Google Scholar] [CrossRef]




| Materials | Lattice Constant, a (Å) | Lattice Constant, b (Å) | Lattice Constant, c (Å) |
|---|---|---|---|
| LiFePO4 | 9.851 | 5.773 | 4.672 |
| LiCoPO4 | 9.885 | 5.882 | 4.702 |
| LiMnPO4 | 9.953 | 5.830 | 4.689 |
| LiCrPO4 | 10.041 | 5.882 | 4.707 |
| LiVPO4 | 10.276 | 5.981 | 4.709 |
| Materials | Li-O Bond Length Range (Å) | Mulliken Charge on Li (e−) |
|---|---|---|
| LiFePO4 | 2.07–2.13 | 0.009 |
| LiCoPO4 | 2.09–2.13 | 0.009 |
| LiMnPO4 | 2.07–2.17 | 0.052 |
| LiCrPO4 | 2.07–2.20 | 0.054 |
| LiVPO4 | 2.09–2.21 | 0.083 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanungo, S.; Bhattacharjee, A.; Bahadursha, N.; Ghosh, A. Comparative Analysis of LiMPO4 (M = Fe, Co, Cr, Mn, V) as Cathode Materials for Lithium-Ion Battery Applications—A First-Principle-Based Theoretical Approach. Nanomaterials 2022, 12, 3266. https://doi.org/10.3390/nano12193266
Kanungo S, Bhattacharjee A, Bahadursha N, Ghosh A. Comparative Analysis of LiMPO4 (M = Fe, Co, Cr, Mn, V) as Cathode Materials for Lithium-Ion Battery Applications—A First-Principle-Based Theoretical Approach. Nanomaterials. 2022; 12(19):3266. https://doi.org/10.3390/nano12193266
Chicago/Turabian StyleKanungo, Sayan, Ankur Bhattacharjee, Naresh Bahadursha, and Aritra Ghosh. 2022. "Comparative Analysis of LiMPO4 (M = Fe, Co, Cr, Mn, V) as Cathode Materials for Lithium-Ion Battery Applications—A First-Principle-Based Theoretical Approach" Nanomaterials 12, no. 19: 3266. https://doi.org/10.3390/nano12193266
APA StyleKanungo, S., Bhattacharjee, A., Bahadursha, N., & Ghosh, A. (2022). Comparative Analysis of LiMPO4 (M = Fe, Co, Cr, Mn, V) as Cathode Materials for Lithium-Ion Battery Applications—A First-Principle-Based Theoretical Approach. Nanomaterials, 12(19), 3266. https://doi.org/10.3390/nano12193266








