Synergistic Enhanced Thermal Conductivity and Crack Resistance of Reactor Epoxy Insulation with Boron Nitride Nanosheets and Multiwalled Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liquid-Phase Stripping of BNNSs
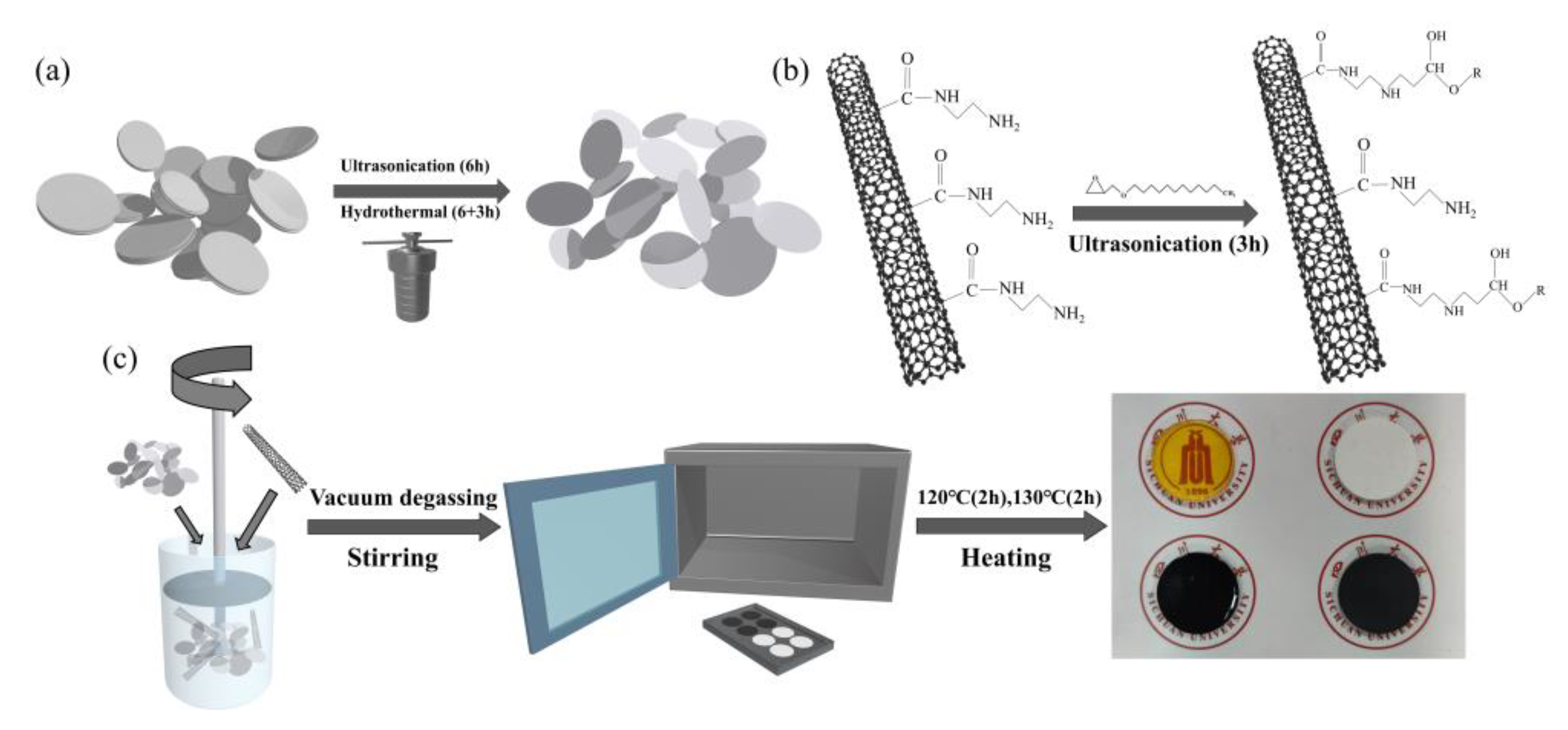
2.3. Preparation of Nano-SiO2@KH560 Nanoparticles and MWCNTs@AGE
2.4. Preparation of BNNSs/MWCNTs@AGE/SiO2@KH560/Epoxy Composites
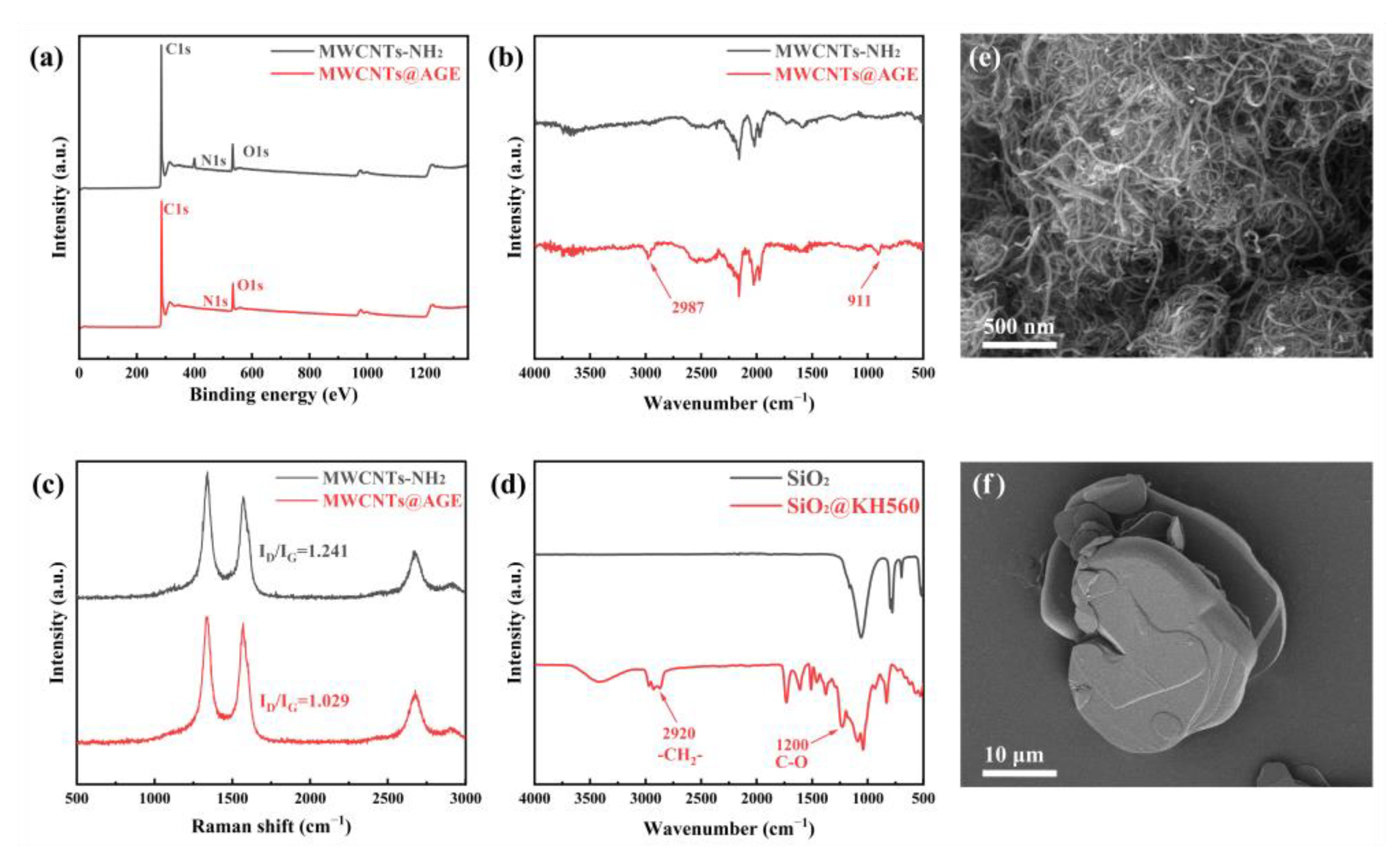
2.5. Characterization
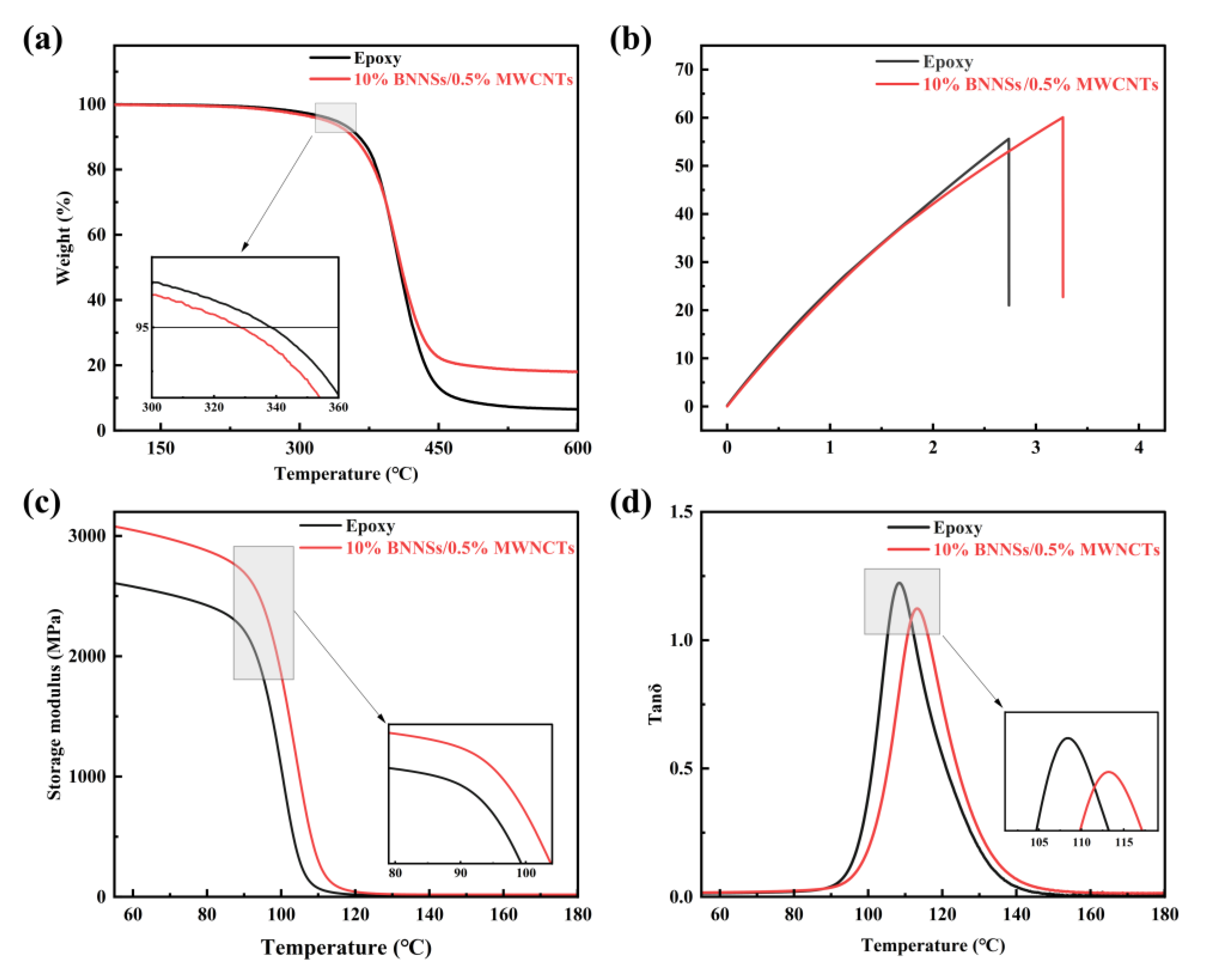
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, Y. Heat dissipation characteristics of anode saturable reactors with high thermal conductivity epoxy resin used for ultra-high-voltage direct current converter valves. High Volt. 2020, 5, 598–604. [Google Scholar] [CrossRef]
- Li, C.; Zhao, C.; Xu, J.; Ji, Y.; Zhang, F.; An, T. A Pole-to-Pole Short-Circuit Fault Current Calculation Method for DC Grids. IEEE Trans. Power Syst. 2017, 32, 4943–4953. [Google Scholar] [CrossRef]
- Sneath, J.; Rajapakse, A.D. Fault Detection and Interruption in an Earthed HVDC Grid Using ROCOV and Hybrid DC Breakers. IEEE Trans. Power Deliv. 2016, 31, 973–981. [Google Scholar] [CrossRef]
- Xu, W.S.; Mansour, Y. Voltage Stability Analysis Using Generic Dynamic Load Models. IEEE Trans. Power Syst. 1994, 9, 479–486. [Google Scholar] [CrossRef]
- de Leon, J.A.D.; Taylor, C.W.; Ieee, I. Understanding and solving short-term voltage stability problems. In Proceedings of the IEEE Power-Engineering-Society Summer Meeting, Chicago, IL, USA, 21 July 2002. Il. [Google Scholar]
- Enohnyaket, M.; Ekman, J. Analysis of Air-Core Reactors From DC to Very High Frequencies Using PEEC Models. IEEE Trans. Power Deliv. 2009, 24, 719–729. [Google Scholar] [CrossRef]
- Cao, J.; Ji, F.; Liu, J.; Chen, P. Valve saturable reactor iron losses of ultrahigh-voltage direct current converter. IET Sci. Meas. Technol. 2016, 10, 77–83. [Google Scholar] [CrossRef]
- Liang, N.; Liu, C.; Liu, K.; Wang, Z.; Zou, Y. Root Cause of Anode Reactor Failure in HVDC Project. In Proceedings of the 3rd International Conference on Information Technologies and Electrical Engineering, Changde, China, 3 December 2020; pp. 246–249. [Google Scholar]
- Sela, R.; Bychanok, D.; Padrez, Y.; Adamchuk, D.; Ksenevich, V.; Naveh, N.; Kuzhir, P. Electromagnetic properties of chloroprene rubber after long-term ultraviolet ageing, oil immersion and thermal degradation. Mater. Res. Express 2019, 6, 075327. [Google Scholar] [CrossRef]
- Feng, Y.; Han, G.; Wang, B.; Zhou, X.; Ma, J.; Ye, Y.; Liu, C.; Xie, X. Multiple synergistic effects of graphene-based hybrid and hexagonal born nitride in enhancing thermal conductivity and flame retardancy of epoxy. Chem. Eng. J. 2020, 379, 122402. [Google Scholar] [CrossRef]
- Elserougi, A.A.; Massoud, A.M.; Ahmed, S. A HVDC shunt tap based on unidirectional hybrid modular DC–DC converter with simultaneous charging and sequential discharging of capacitors. Electr. Power Syst. Res. 2018, 158, 37–44. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; Roman, F.; Folch, A. Epoxy-Thiol Systems Filled with Boron Nitride for High Thermal Conductivity Applications. Polymers 2018, 10, 340. [Google Scholar] [CrossRef]
- Li, X.-Y.; Zha, J.W.; Wang, S.J.; Zhong, S.L.; Zhang, C.; Dang, Z.M. Effect of high-thermal conductivity epoxy resin on heat dissipation performance of saturated reactor. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3898–3905. [Google Scholar] [CrossRef]
- Du, B.X.; Xiao, M.; Zhang, J.W. Effect of Thermal Conductivity on Tracking Failure of Epoxy/BN Composite under Pulse Strength. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 296–302. [Google Scholar] [CrossRef]
- Zhao, L.; Yan, L.; Wei, C.; Li, Q.; Huang, X.; Wang, Z.; Fu, M.; Ren, J. Synergistic Enhanced Thermal Conductivity of Epoxy Composites with Boron Nitride Nanosheets and Microspheres. J. Phys. Chem. C 2020, 124, 12723–12733. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Zhu, Y.; Jiang, P. Cellulose Nanofiber Supported 3D Interconnected BN Nanosheets for Epoxy Nanocomposites with Ultrahigh Thermal Management Capability. Adv. Funct. Mater. 2017, 27, 1604754. [Google Scholar] [CrossRef]
- Godara, A.; Mezzo, L.; Luizi, F.; Warrier, A.; Lomov, S.V.; Van Vuure, A.W.; Gorbatikh, L.; Moldenaers, P.; Verpoest, I. Influence of carbon nanotube reinforcement on the processing and the mechanical behaviour of carbon fiber/epoxy composites. Carbon 2009, 47, 2914–2923. [Google Scholar] [CrossRef]
- Chen, W.-J.; Li, Y.L.; Chiang, C.L.; Kuan, C.F.; Kuan, H.C.; Lin, T.T.; Yip, M.C. Preparation and characterization of carbon nanotubes/epoxy resin nano-prepreg for nanocomposites. J. Phys. Chem. Solids 2010, 71, 431–435. [Google Scholar] [CrossRef]
- Alapati, S.; Joy Thomas, M. Influence of nano-fillers on electrical treeing in epoxy insulation. IET Sci. Meas. Technol. 2012, 6, 21–28. [Google Scholar] [CrossRef]
- Chalashkanov, N.M.; Dodd, S.J.; Dissado, L.A.; Fothergill, J.C. The role of bulk charge transport processes in electrical tree formation and breakdown mechanisms in epoxy resins. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3256–3266. [Google Scholar] [CrossRef]
- Ding, H.Z.; Varlow, B.R. Thermodynamic model for electrical tree propagation kinetics in combined electrical and mechanical stresses. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 81–89. [Google Scholar] [CrossRef]
- Singha, S.; Thomas, A.J. Reduction of Permittivity in Epoxy Nanocomposites at Low Nano-filler Loadings. In Proceedings of the IEEE Conference on Electrical Insulation and Dielectric Phenomena, Quebec City, QC, Canada, 26–29 October 2008. [Google Scholar]
- Wang, N.; Yang, G.; Wang, H.; Yan, C.; Sun, R.; Wong, C.P. A universal method for large-yield and high-concentration exfoliation of two-dimensional hexagonal boron nitride nanosheets. Mater. Today 2019, 27, 33–42. [Google Scholar] [CrossRef]
- Twu, C.-J.; Ho, J.-R. Molecular-dynamics study of energy flow and the Kapitza conductance across an interface with imperfection formed by two dielectric thin films. Phys. Rev. B 2003, 67, 205422. [Google Scholar] [CrossRef]
- Maiti, A.; Mahan, G.D.; Pantelides, S.T. Dynamical simulations of nonequilibrium processes—Heat flow and the Kapitza resistance across grain boundaries. Solid State Commun. 1997, 102, 517–521. [Google Scholar] [CrossRef]
- Saleem, M.Z.; Akbar, M. Review of the Performance of High-Voltage Composite Insulators. Polymers 2022, 14, 431. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.-H.; Huang, X.; Zhang, G.-J. High thermal conductive polyvinyl alcohol composites with hexagonal boron nitride microplatelets as fillers. Compos. Sci. Technol. 2013, 85, 98–103. [Google Scholar] [CrossRef]
- Gojny, F.H.; Wichmann, M.H.; Fiedler, B.; Kinloch, I.A.; Bauhofer, W.; Windle, A.H.; Schulte, K. Evaluation and identification of electrical and thermal conduction mechanisms in carbon nanotube/epoxy composites. Polymer 2006, 47, 2036–2045. [Google Scholar] [CrossRef]
- Marconnet, A.M.; Yamamoto, N.; Panzer, M.A.; Wardle, B.L.; Goodson, K.E. Thermal conduction in aligned carbon nanotube-polymer nanocomposites with high packing density. ACS Nano 2011, 5, 4818–4825. [Google Scholar] [CrossRef]
- Bryning, M.B.; Milkie, D.E.; Islam, M.F.; Kikkawa, J.M.; Yodh, A.G. Thermal conductivity and interfacial resistance in single-wall carbon nanotube epoxy composites. Appl. Phys. Lett. 2005, 87, 161909. [Google Scholar] [CrossRef]
- Lee, C.; Drelich, J.; Yap, Y. Superhydrophobicity of boron nitride nanotubes grown on silicon substrates. Langmuir 2009, 25, 4853–4860. [Google Scholar] [CrossRef]
- Li, L.H.; Chen, Y. Superhydrophobic properties of nonaligned boron nitride nanotube films. Langmuir 2010, 26, 5135–5140. [Google Scholar] [CrossRef]
- Yum, K.; Yu, M.F. Measurement of wetting properties of individual boron nitride nanotubes with the Wilhelmy method using a nanotube-based force sensor. Nano Lett. 2006, 6, 329–333. [Google Scholar] [CrossRef]
- Shenogin, S.; Xue, L.; Ozisik, R.; Keblinski, P.; Cahill, D.G. Role of thermal boundary resistance on the heat flow in carbon-nanotube composites. J. Appl. Phys. 2004, 95, 8136–8144. [Google Scholar] [CrossRef]
- Duong, H.M.; Papavassiliou, D.V.; Lee, L.L.; Mullen, K.J. Random walks in nanotube composites: Improved algorithms and the role of thermal boundary resistance. Appl. Phys. Lett. 2005, 87, 013101. [Google Scholar] [CrossRef]
- Qin, Y.J.; Liu, L.; Shi, J.; Wu, W.; Zhang, J.; Guo, Z.X.; Li, Y.; Zhu, D. Large-scale preparation of solubilized carbon nanotubes. Chem. Mater. 2003, 15, 3256–3260. [Google Scholar] [CrossRef]
- Lau, K.T.; Hui, D. Effectiveness of using carbon nanotubes as nano-reinforcements for advanced composite structures. Carbon 2002, 40, 1605–1606. [Google Scholar] [CrossRef]
- Liu, C.H.; Fan, S.S. Effects of chemical modifications on the thermal conductivity of carbon nanotube composites. Appl. Phys. Lett. 2005, 86, 123106. [Google Scholar] [CrossRef]
- Pan, Z.; Yao, L.; Zhai, J.; Shen, B.; Wang, H. Significantly improved dielectric properties and energy density of polymer nanocomposites via small loaded of BaTiO3 nanotubes. Compos. Sci. Technol. 2017, 147, 30–38. [Google Scholar] [CrossRef]
- Peng, W.; Zhou, W.; Li, T.; Zhou, J.; Yao, T.; Wu, H.; Zhao, X.; Luo, J.; Liu, J.; Zhang, D. Towards inhibiting conductivity of Mo/PVDF composites through building MoO3 shell as an interlayer for enhanced dielectric properties. J. Mater. Sci. Mater. Electron. 2022, 33, 14735–14753. [Google Scholar] [CrossRef]
- Cao, D.; Zhou, W.; Yuan, M.; Li, B.; Li, T.; Li, J.; Liu, D.; Wang, G.; Zhou, J.; Zhang, H. Polymer composites filled with core–shell structured nanofillers: Effects of shell thickness on dielectric and thermal properties of composites. J. Mater. Sci. Mater. Electron. 2022, 33, 5174–5189. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, G.; Chang, J.; Gu, A.; Yuan, L.; Zhang, W. The origin of the electric and dielectric behavior of expanded graphite–carbon nanotube/cyanate ester composites with very high dielectric constant and low dielectric loss. Carbon 2012, 50, 4995–5007. [Google Scholar] [CrossRef]
- Tawade, B.V.; Apata, I.E.; Singh, M.; Das, P.; Pradhan, N.; Al-Enizi, A.M.; Karim, A.; Raghavan, D. Recent developments in the synthesis of chemically modified nanomaterials for use in dielectric and electronics applications. Nanotechnology 2021, 32, 142004. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Zhao, C.; Yang, W. High dielectric constant and low dielectric loss hybrid nanocomposites fabricated with ferroelectric polymer matrix and BaTiO3 nanofibers modified with perfluoroalkylsilane. Appl. Surf. Sci. 2014, 305, 531–538. [Google Scholar] [CrossRef]
- Ganguli, S.; Roy, A.K.; Anderson, D.P. Improved thermal conductivity for chemically functionalized exfoliated graphite/epoxy composites. Carbon 2008, 46, 806–817. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Tang, L.C.; Gong, L.X.; Yan, D.; Li, Y.B.; Wu, L.B.; Jiang, J.-X.; Lai, G.-Q. Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon 2014, 69, 467–480. [Google Scholar] [CrossRef]

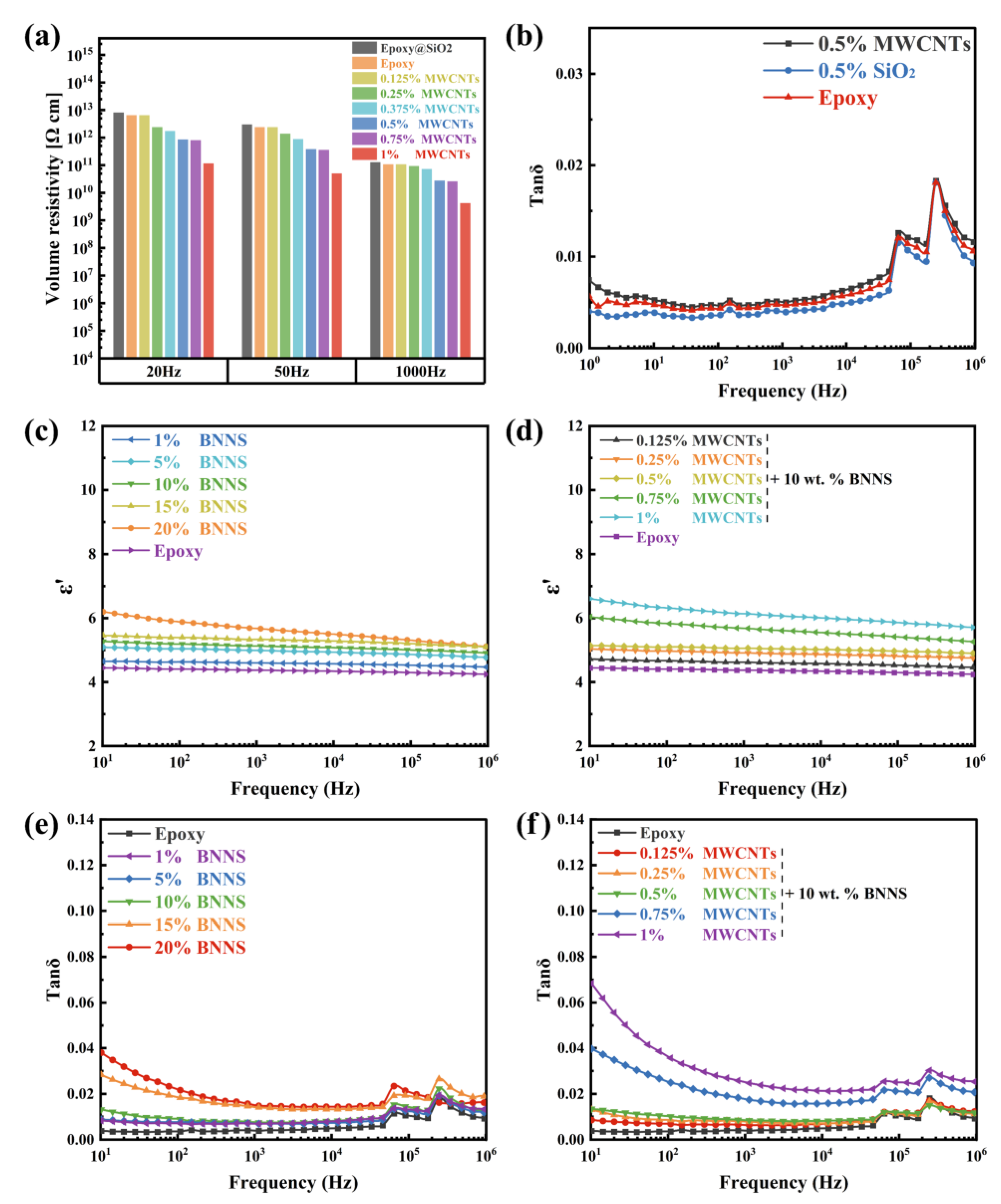
| Composites | Dielectric Constant | Dielectric Loss | Volume Resistivity (Ω cm) |
|---|---|---|---|
| Epoxy | 5.02 | 0.0047 | 1.07819 × 1011 |
| 0.5 wt.% SiO2 | 4.37 | 0.0039 | 1.28016 × 1011 |
| 1% BNNS + 0.5 wt.% SiO2 | 4.61 | 0.0069 | 1.21 × 1010 |
| 5% BNNS + 0.5 wt.% SiO2 | 4.99 | 0.0070 | 3.84 × 1010 |
| 10% BNNS + 0.5 wt.% SiO2 | 5.13 | 0.0075 | 3.95 × 1010 |
| 15% BNNS + 0.5 wt.% SiO2 | 5.34 | 0.0141 | 4.44 × 1010 |
| 20 % BNNS + 0.5 wt.% SiO2 | 5.67 | 0.0148 | 7.75 × 1010 |
| 0.125% MWCNTs (10 wt.% BNNS + 0.5 wt% SiO2) | 4.62 | 0.00632 | 1.07815 × 1011 |
| 0.25% MWCNTs (10 wt.% BNNS + 0.5 wt% SiO2) | 4.91 | 0.0078 | 9.4142 × 1011 |
| 0.5% MWCNTs (10 wt.% BNNS + 0.5 wt% SiO2) | 5.68 | 0.0085 | 2.77624 × 1010 |
| 0.75% MWCNT (10 wt.% BNNS + 0.5 wt% SiO2) | 6.14 | 0.0174 | 2.61256 × 1010 |
| 1% MWCNTs (10 wt.% BNNS + 0.5 wt% SiO2) | 6.58 | 0.0247 | 4.21098 × 109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Chen, Z.; Liang, L.; Guan, Z.; Ren, J. Synergistic Enhanced Thermal Conductivity and Crack Resistance of Reactor Epoxy Insulation with Boron Nitride Nanosheets and Multiwalled Carbon Nanotubes. Nanomaterials 2022, 12, 3235. https://doi.org/10.3390/nano12183235
Yang J, Chen Z, Liang L, Guan Z, Ren J. Synergistic Enhanced Thermal Conductivity and Crack Resistance of Reactor Epoxy Insulation with Boron Nitride Nanosheets and Multiwalled Carbon Nanotubes. Nanomaterials. 2022; 12(18):3235. https://doi.org/10.3390/nano12183235
Chicago/Turabian StyleYang, Jun, Zhijie Chen, Longyi Liang, Zhiwen Guan, and Junwen Ren. 2022. "Synergistic Enhanced Thermal Conductivity and Crack Resistance of Reactor Epoxy Insulation with Boron Nitride Nanosheets and Multiwalled Carbon Nanotubes" Nanomaterials 12, no. 18: 3235. https://doi.org/10.3390/nano12183235
APA StyleYang, J., Chen, Z., Liang, L., Guan, Z., & Ren, J. (2022). Synergistic Enhanced Thermal Conductivity and Crack Resistance of Reactor Epoxy Insulation with Boron Nitride Nanosheets and Multiwalled Carbon Nanotubes. Nanomaterials, 12(18), 3235. https://doi.org/10.3390/nano12183235





