Annealing Effects on SnO2 Thin Film for H2 Gas Sensing
Abstract
:1. Introduction
2. Materials and Methods
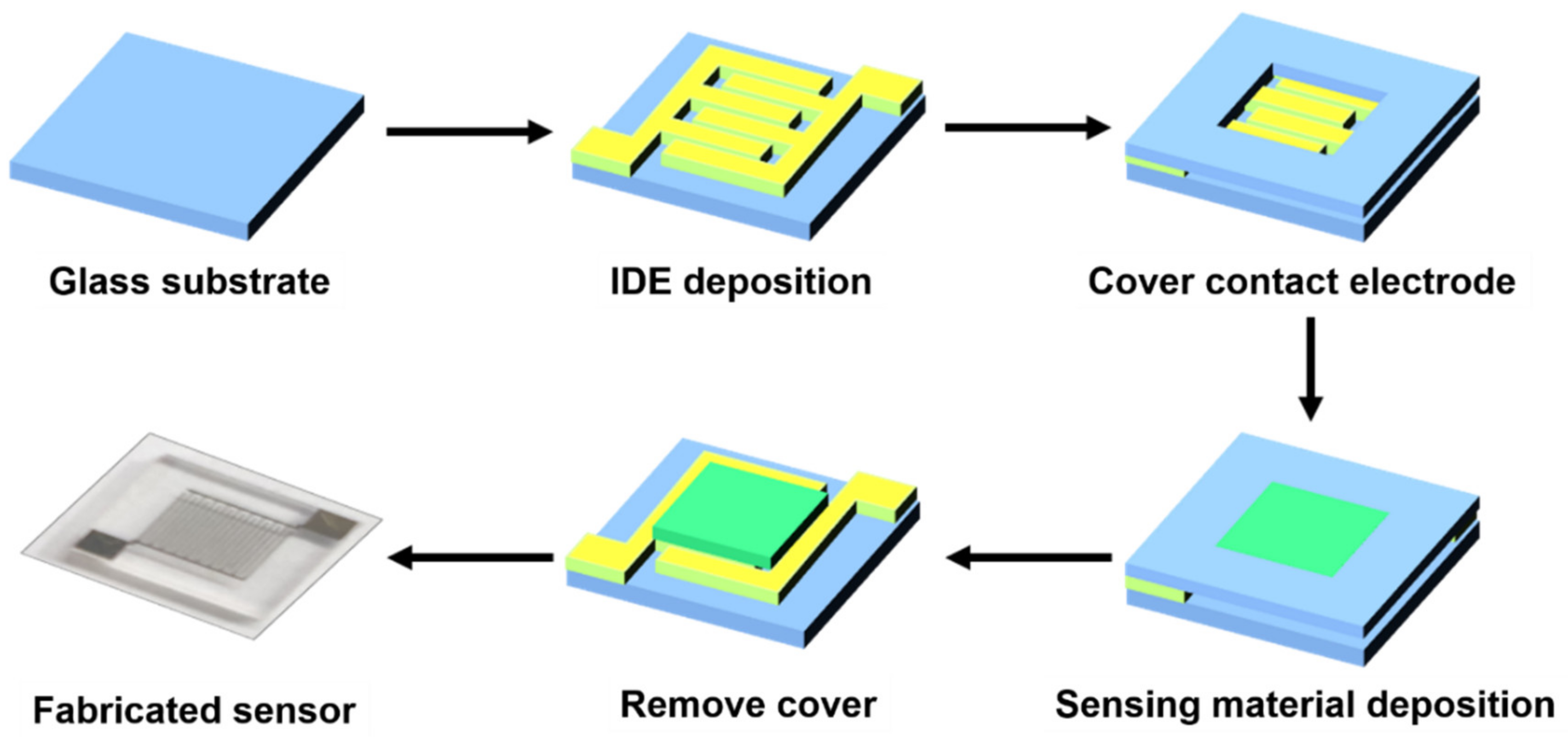
2.1. Sensor Fabrication
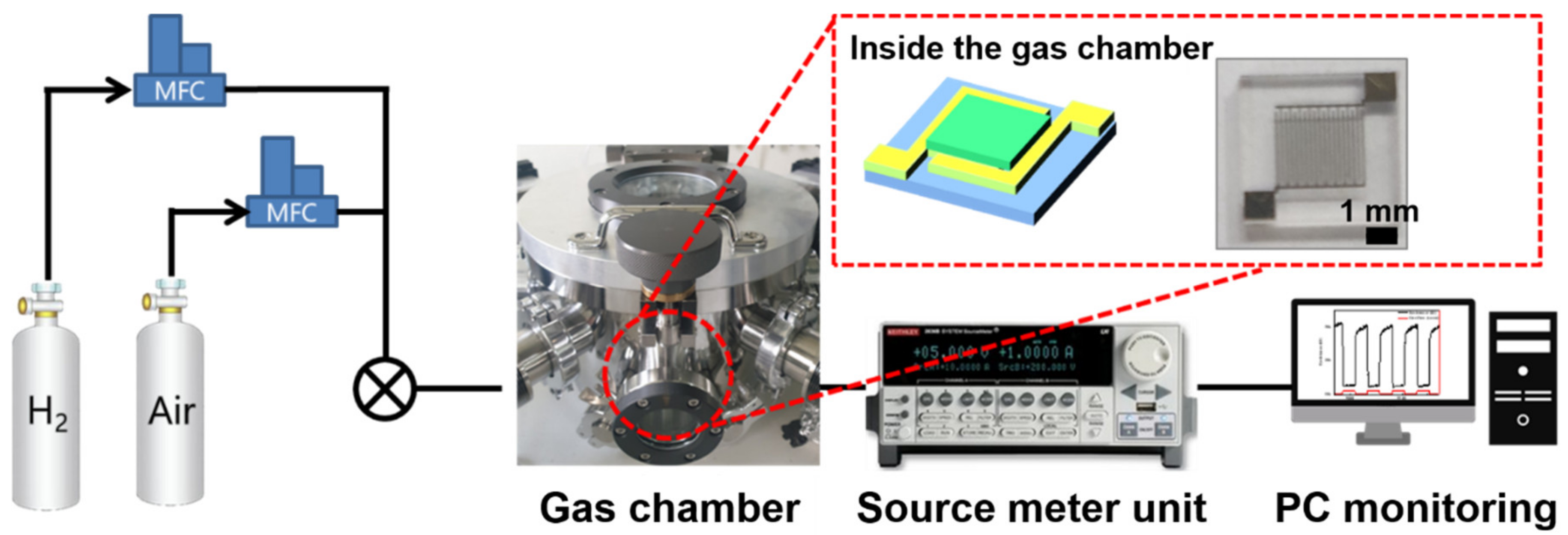
2.2. Sensor Characterization
3. Results and Discussion
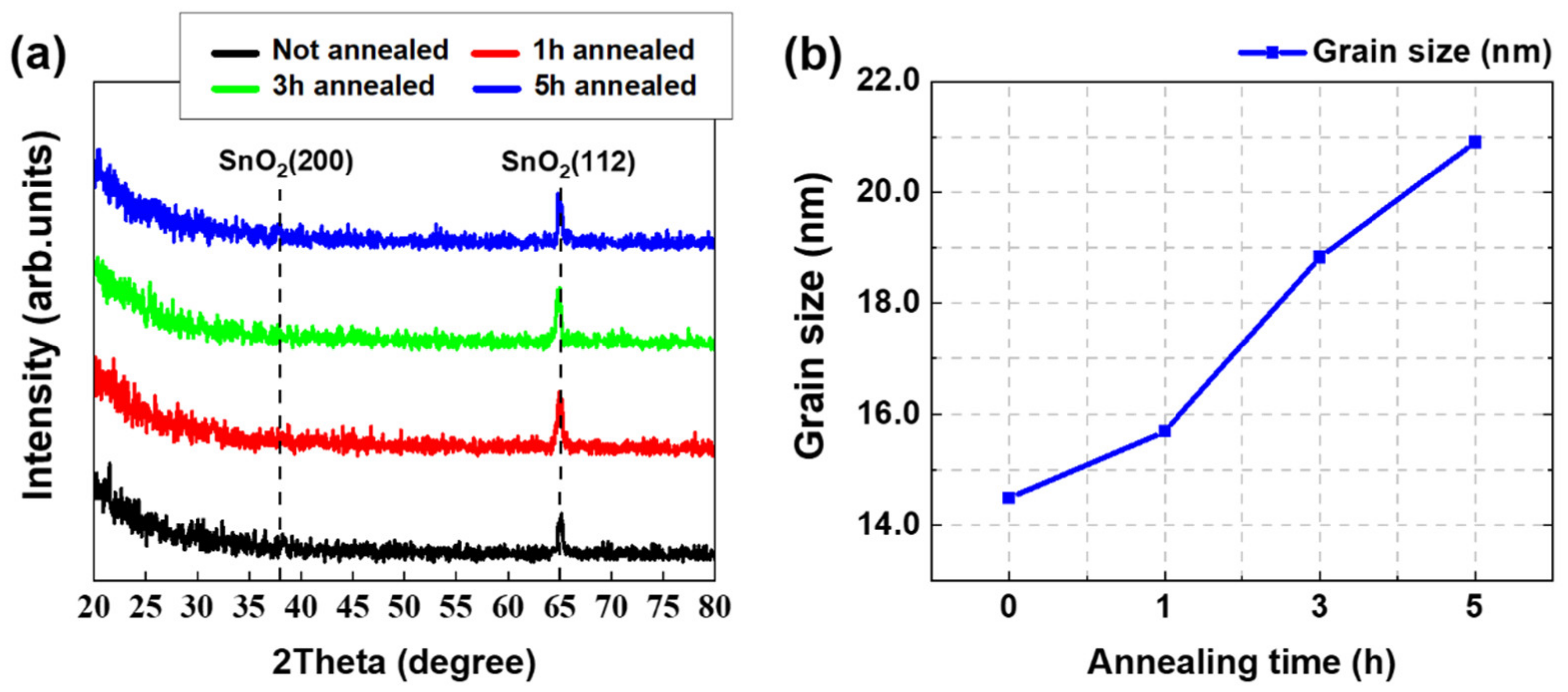
3.1. Structural and Morphological Characteristics
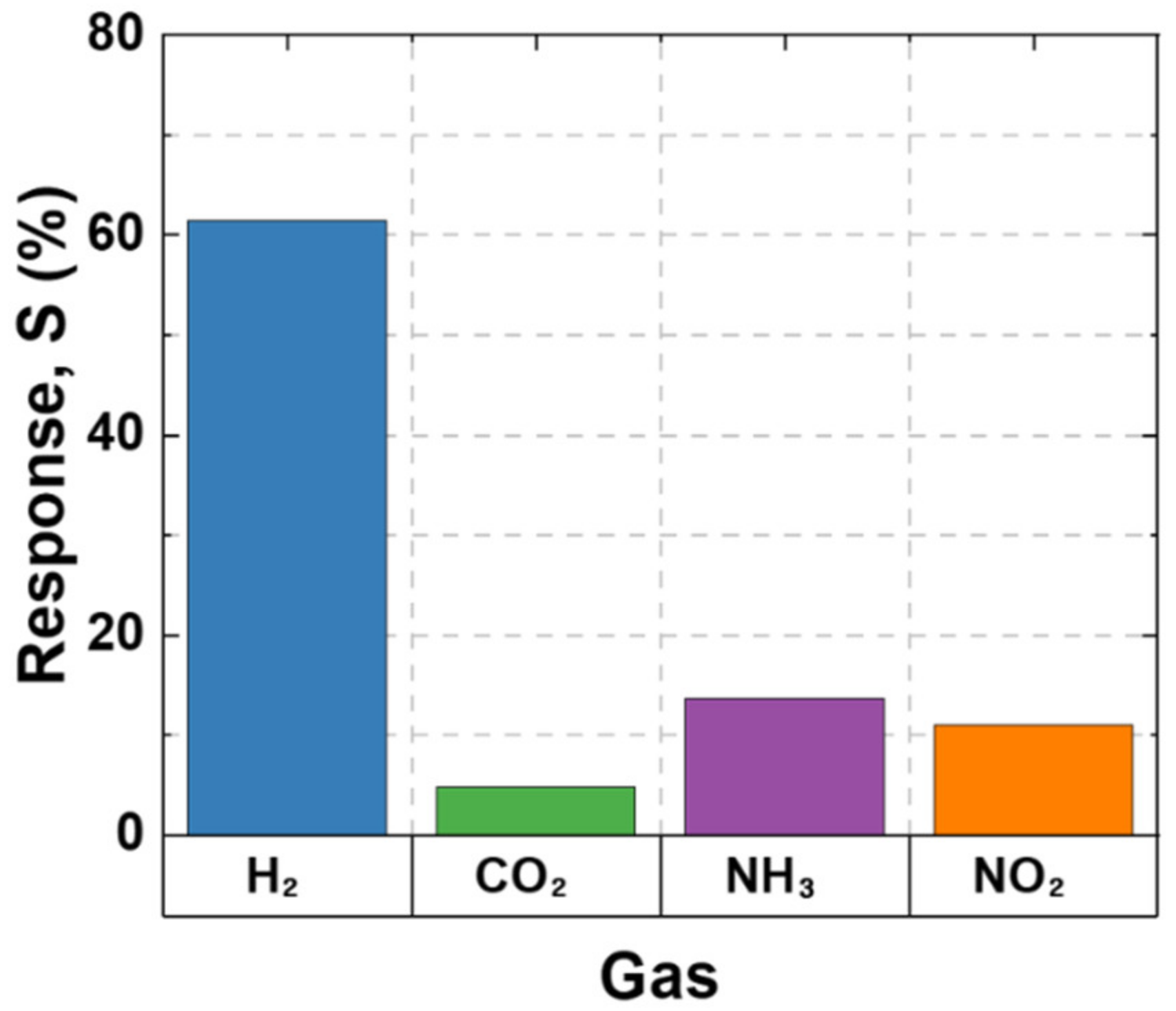
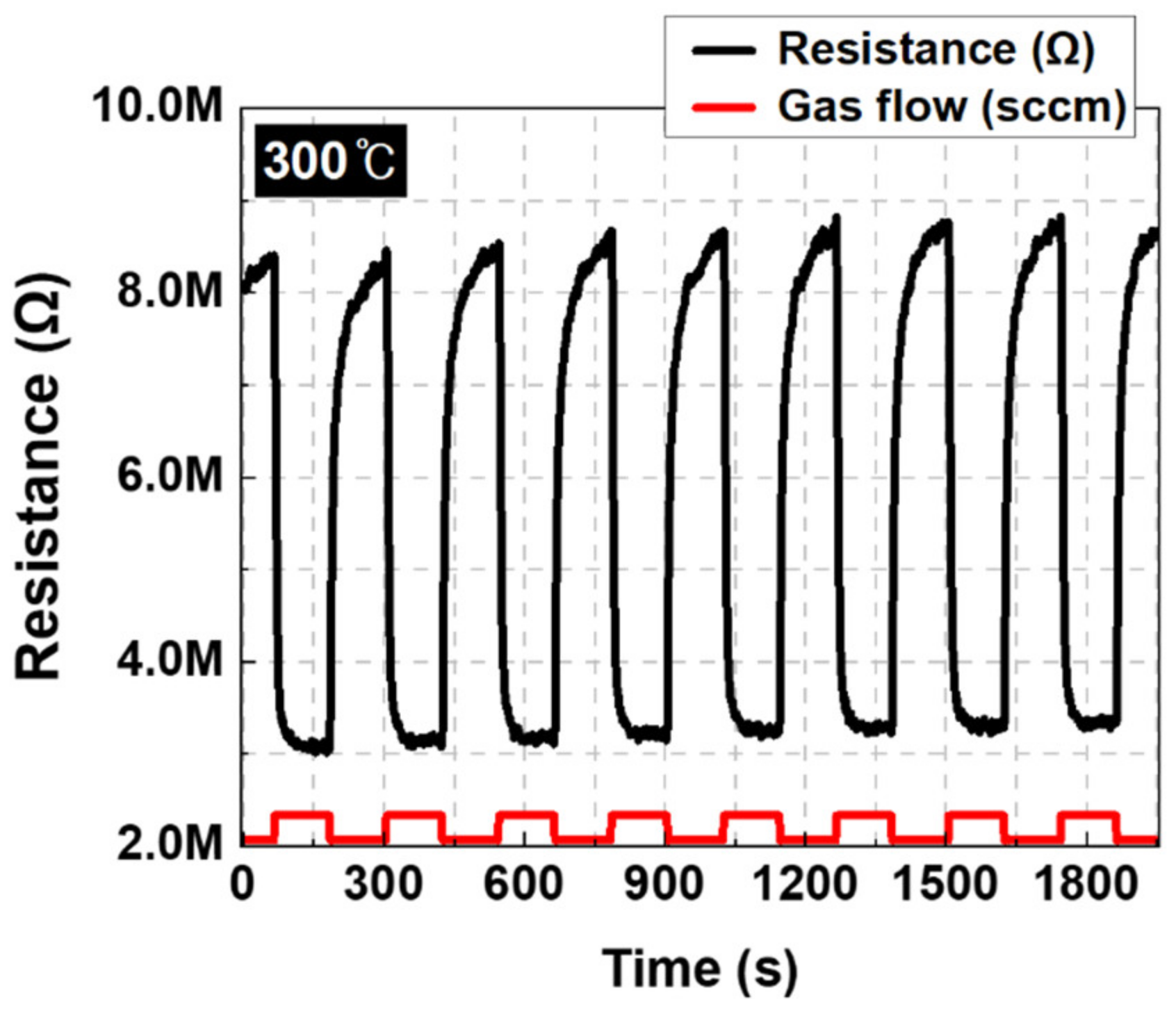
3.2. Sensing Performance
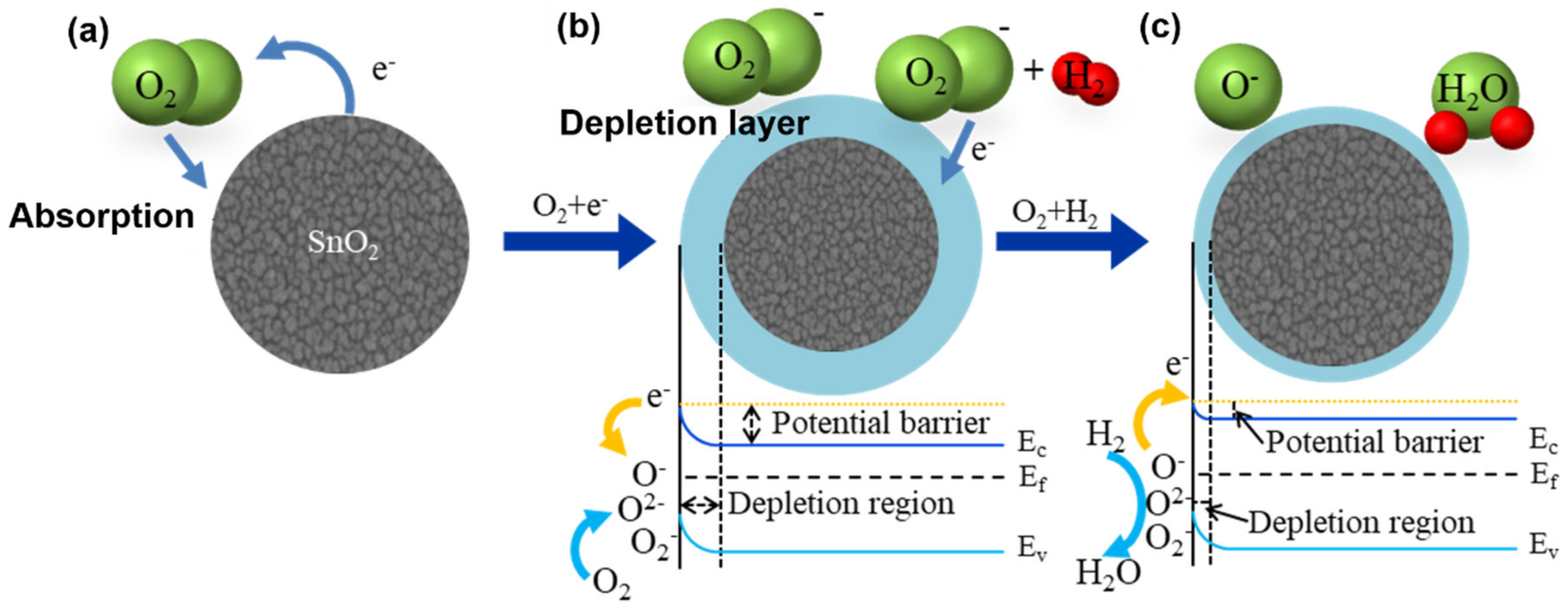
3.3. Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, S.D. Review and new trends of hydrogen gas sensor technologies. J. Korean Sens. Soc. 2010, 19, 67–86. [Google Scholar]
- Kadhim, I.H.; Hassan, H.A.; Abdullah, Q.N. Hydrogen gas sensor based on nanocrystalline SnO2 thin film grown on bare Si substrates. Nano-Micro Lett. 2016, 8, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S. Optimization of Metal Oxide thin Film Thickness and Annealing Conditions for Fabricating High-Sensitivity Hydrogen Sensors for Hydrogen Leakage Detection. Master’s Thesis, Kyungpook National University, Daegu, Korea, 2021. [Google Scholar]
- Kang, W. Enhanced hydrogen gas sensing properties of ZnO nanowires gas sensor by heat treatment under oxygen atmosphere. J. Korean Inst. Surf. Eng. 2017, 50, 125–130. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro- and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef]
- Kadhim, I.H.; Hassan, H.A.; Ibrahim, F.T. Hydrogen gas sensing based on nanocrystalline SnO2 thin films operating at low temperatures. Int. J. Hydrogen Energy 2020, 45, 25599–25607. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Shi, C.; Li, L.; Qin, H.; Hu, J. Sensing mechanism of SnO2(1 1 0) surface to H2: Density functional theory calculations. Sens. Actuators B Chem. 2015, 220, 279–287. [Google Scholar] [CrossRef]
- Shanmugasundaram, A.; Basak, P.; Satyanarayana, L.S.; Manorama, V. Hierarchical SnO/SnO2 nanocomposites: Formation of in situ p–n junctions and enhanced H2 sensing. Sens. Actuators B Chem. 2013, 185, 265–273. [Google Scholar] [CrossRef]
- Sun, S.H.; Meng, G.W.; Zhang, G.X.; Gao, T.; Geng, B.Y.; Zhang, L.D.; Zou, J. Raman scattering study of rutile SnO2 nanobelts synthesized by thermal evaporation of Sn powders. Chem. Phys. Lett. 2003, 376, 103–107. [Google Scholar] [CrossRef]
- Leng, D.; Wu, L.; Jiang, H.; Zhao, Y.; Zhang, J.; Li, W.; Feng, L. Preparation and Properties of SnO2 Film Deposited by Magnetron Sputtering. Int. J. Photoenergy 2012, 2012, 235971. [Google Scholar] [CrossRef]
- Liu, Y.; Koep, E.; Liu, M. A Highly sensitive and fast-responding SnO2 sensor fabricated by combustion chemical vapor deposition. Chem. Mater. 2005, 17, 3997–4000. [Google Scholar] [CrossRef]
- Min, B.K.; Choi, S.D. SnO2 thin film gas sensor fabricated by ion beam deposition. Sens. Actuators B Chem. 2004, 98, 239–246. [Google Scholar] [CrossRef]
- Khan, A.F.; Mehmood, M.; Rana, A.M.; Bhatti, M.T. Effect of annealing on electrical resistivity of rf-magnetron sputtered nanostructured SnO2 thin films. Appl. Surf. Sci. 2009, 255, 8562–8565. [Google Scholar] [CrossRef]
- Mehraj, S.; Ansari, M.S.; Alimuddin. Annealed SnO2 thin films: Structural, electrical and their magnetic properties. Thin Solid Films 2015, 589, 57–65. [Google Scholar] [CrossRef]
- Ozaki, Y.; Suzuki, S.; Morimitsu, M.; Matsunaga, M. Enhanced long-term stability of SnO2-based CO gas sensors modified by sulfuric acid treatment. Sens. Actuators B Chem. 2000, 62, 220–225. [Google Scholar] [CrossRef]
- Hu, K.; Wang, F.; Liu, H.; Li, Y.; Zeng, W. Enhanced hydrogen gas sensing properties of Pd-doped SnO2 nanofibres by Ar plasma treatment. Ceram. Int. 2000, 46, 1609–1614. [Google Scholar] [CrossRef]
- Al-Jumailli, B.E.; Rzaij, J.M.; Ibraheam, A.S. Nanoparticles of CuO thin films for room temperature NO2 gas detection: Annealing time effect. Mater. Today Proc. 2021, 42, 2603–2608. [Google Scholar] [CrossRef]
- Hu, Y.; Tan, O.K.; Pan, J.S.; Huang, H.; Cao, W. The effects of annealing temperature on the sensing properties of low temperature nano-sized SrTiO3 oxygen gas sensor. Sens. Actuators B Chem. 2005, 108, 244–249. [Google Scholar] [CrossRef]
- Lee, Y.L.; Tsai, W.C.; Chang, C.H.; Yang, Y.M. Effects of heat annealing on the film characteristics and gas sensing properties of substituted and un-substituted copper phthalocyanine films. Appl. Surf. Sci. 2001, 172, 191–199. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Wang, Y. Ultrafast CO sensor based on flame-annealed porous CeO2 nanosheets for environmental. Appl. J. Inorg. Mater. 2021, 36, 1223–1230. [Google Scholar] [CrossRef]
- Doubi, Y.; Hartiti, B.; Hicham, L.; Fadili, S.; Batan, A.; Tahri, M.; Belfhaili, A.; Thevnin, P. Effect of annealing time on structural and optical proprieties of TiO2 thin films elaborated by spray pyrolysis technique for future gas sensor application. Mater. Today 2020, 30, 823–827. [Google Scholar] [CrossRef]
- Ahmed, N.M.; Sabah, F.A.; Abdulgafour, H.I.; Alsadig, A.; Sulieman, A.; Alkhoaryef, M. The effect of post annealing temperature on grain size of indium-tin-oxide for optical and electrical properties improvement. Results Phys. 2019, 13, 102159. [Google Scholar] [CrossRef]
- Simões, A.Z.; González, A.H.M.; Zaghete, M.A.; Varela, J.A.; Stojanovic, B.D. Effects of annealing on the crystallization and roughness of PLZT thin films. Thin Solid Films 2001, 384, 132–137. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Kang, K.M.; Choi, J.U. Growth characteristic of SnO2 thin film for gas sensor with annealing treatment. J. Kor. Inst. Surf. Eng. 2007, 6, 258–261. [Google Scholar] [CrossRef]
- Patel, S.L.; Chander, S.; Purohit, A.; Kannan, M.D.; Dhaka, M.S. Influence of NH4Cl treatment on physical properties of CdTe thin films for absorber layer applications. J. Phys. Chem. Solids 2018, 123, 216–222. [Google Scholar] [CrossRef]
- Abdulgafour, H.I.; Yusof, Y.; Yam, F.K.; Hassan, Z. Growth of ZnO nanostructures at different temperatures without catalyst by wet thermal oxidation process. Adv. Mater. Res. 2013, 620, 132–136. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Liu, J.; Li, H.Y.; Hu, Z.; Luo, X.; Gao, N.; Zhang, B.; Jiang, J.; Zhong, A.; et al. Sensitive H2 gas sensors based on SnO2 nanowires. Sens. Actuators B Chem. 2021, 345, 130334. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Maeng, B.; Jung, D.G.; Lee, J.; Kim, Y.; Kwon, J.; An, H.K.; Jung, D. Annealing Effects on SnO2 Thin Film for H2 Gas Sensing. Nanomaterials 2022, 12, 3227. https://doi.org/10.3390/nano12183227
Yang Y, Maeng B, Jung DG, Lee J, Kim Y, Kwon J, An HK, Jung D. Annealing Effects on SnO2 Thin Film for H2 Gas Sensing. Nanomaterials. 2022; 12(18):3227. https://doi.org/10.3390/nano12183227
Chicago/Turabian StyleYang, Yijun, Bohee Maeng, Dong Geon Jung, Junyeop Lee, Yeongsam Kim, JinBeom Kwon, Hee Kyung An, and Daewoong Jung. 2022. "Annealing Effects on SnO2 Thin Film for H2 Gas Sensing" Nanomaterials 12, no. 18: 3227. https://doi.org/10.3390/nano12183227
APA StyleYang, Y., Maeng, B., Jung, D. G., Lee, J., Kim, Y., Kwon, J., An, H. K., & Jung, D. (2022). Annealing Effects on SnO2 Thin Film for H2 Gas Sensing. Nanomaterials, 12(18), 3227. https://doi.org/10.3390/nano12183227





