Microwave Synthesized 2D WO3 Nanosheets for VOCs Gas Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Characterization
2.4. Gas Sensing Measurement
3. Results and Discussion
3.1. Structure Analysis
3.2. Morphology Analysis
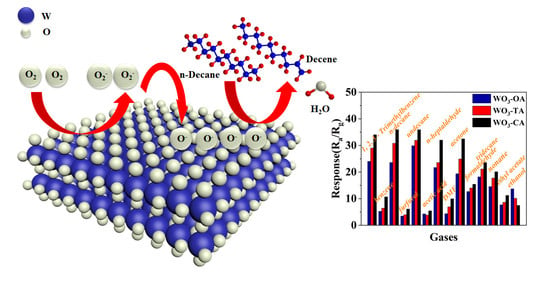
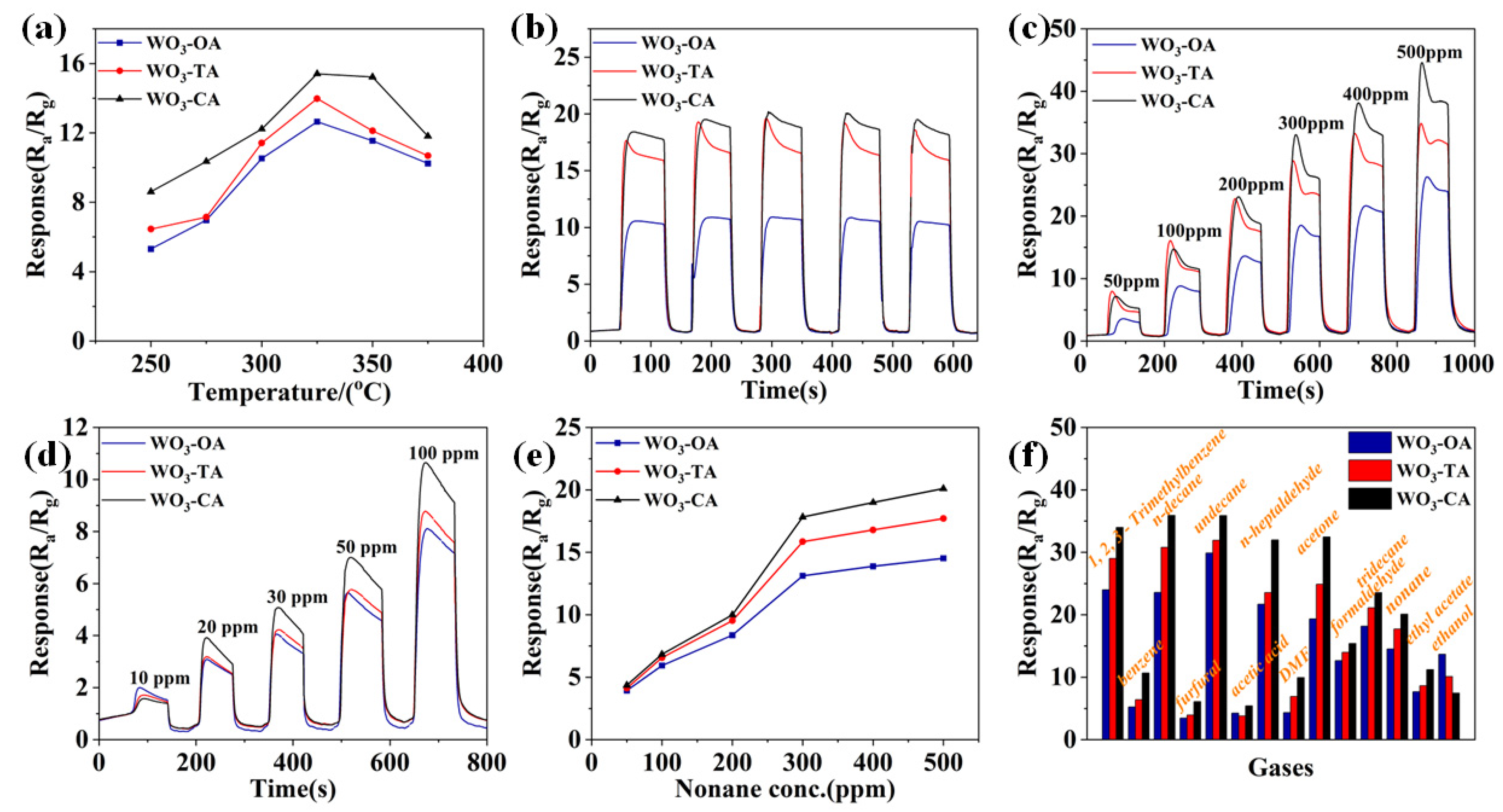
3.3. Gas Sensing Performance and Photoelectric Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinohara, N.; Kai, Y.; Mizukoshi, A.; Fujii, M.; Kumagai, K.; Okuizumi, Y.; Jona, M.; Yanagisawa, Y. On-site passive flux sampler measurement of emission rates of carbonyls and VOCs from multiple indoor sources. Build. Environ. 2009, 44, 859–863. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Zhou, X.; Yuan, M.; Qu, D.; Zheng, Y.; Vishinkin, R.; Khatib, M.; Wu, W.; Haick, H. Disease Detection with Molecular Biomarkers: From Chemistry of Body Fluids to Nature-Inspired Chemical Sensors. Chem. Rev. 2019, 119, 11761–11817. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, Z.; Kumar, U.; Chen, D.D.Y. Review of recent developments in determining volatile organic compounds in exhaled breath as biomarkers for lung cancer diagnosis. Anal. Chim. Acta 2017, 996, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-I.; Hwang, S.-J.; Dai, Z.; Kang, Y.C.; Lee, J.-H. Rh-catalyzed WO3 with anomalous humidity dependence of gas sensing characteristics. RSC Adv. 2014, 4, 53130–53136. [Google Scholar] [CrossRef]
- Phillips, M.; Altorki, N.; Austin, J.H.M.; Cameron, R.; Cataneo, R.; Greenberg, J.; Kloss, R.; Maxfield, R.; Munawar, M.; Pass, H.; et al. Prediction of breast cancer using volatile biomarkers in the breath. Breast Cancer Res. Treat. 2006, 99, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Leja, M.; Funka, K.; Skapars, R.; Sivins, A.; Ancans, G.; Liepniece-Karele, I.; Kikuste, I.; Lasina, I.; Haick, H. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut 2016, 65, 400–407. [Google Scholar] [CrossRef]
- Hua, Q.; Zhu, Y.; Liu, H. Detection of volatile organic compounds in exhaled breath to screen lung cancer: A systematic review. Future Oncol. 2018, 14, 1647–1662. [Google Scholar] [CrossRef]
- Nakhleh, M.K.; Amal, H.; Jeries, R.; Broza, Y.Y.; Aboud, M.; Gharra, A.; Ivgi, H.; Khatib, S.; Badarneh, S.; Har-Shai, L.; et al. Diagnosis and Classification of 17 Diseases from 1404 Subjects via Pattern Analysis of Exhaled Molecules. ACS Nano 2017, 11, 112–125. [Google Scholar] [CrossRef]
- Zeb, S.; Peng, X.; Yuan, G.; Zhao, X.; Qin, C.; Sun, G.; Nie, Y.; Cui, Y.; Jiang, X. Controllable synthesis of ultrathin WO3 nanotubes and nanowires with excellent gas sensing performance. Sens. Actuators B Chem. 2019, 305, 127435. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, C.; Song, L.; Gao, B.; Gong, H.; Xia, W.; Sheng, L.; Wang, T.; He, J. Regulating surface state of WO3 nanosheets by gamma irradiation for suppressing hydrogen evolution reaction in electrochemical N2 fixation. Nano Res. 2020, 13, 2784–2790. [Google Scholar]
- Bi, Z.; Li, X.; Chen, Y.; He, X.; Xu, X.; Gao, X. Large-Scale Multifunctional Electrochromic-Energy Storage Device Based on Tungsten Trioxide Monohydrate Nanosheets and Prussian White. ACS Appl. Mater. Interfaces 2017, 9, 29872–29880. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Shi, G.; Wang, H.; Zhang, Q.; Li, Y. Construction of hydrated tungsten trioxide nanosheet films for efficient electrochromic performance. RSC Adv. 2015, 5, 196–201. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Zhu, L.; Guo, W.; Shen, L.; Wen, S.; Ruan, S. Enhanced toluene sensing performance of gold-functionalized WO3 ·H2O nanosheets. Sens. Actuators B Chem. 2016, 223, 761–767. [Google Scholar] [CrossRef]
- Lee, J.H.; Yim, D.; Park, J.H.; Lee, C.H.; Ju, J.M.; Lee, S.U.; Kim, J.H. Tuning d-band centers by coupling PdO nanoclusters to WO3 nanosheets to promote the oxygen reduction reaction. J. Mater. Chem. A 2020, 8, 13490–13500. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Park, S.; Lee, C. Acetone sensing of Au and Pd-decorated WO3 nanorod sensors. Sens. Actuators B Chem. 2015, 209, 180–185. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Jin, C.; Choi, S.-W.; Kim, S.S.; Lee, C. Enhanced CO gas sensing properties of Pt-functionalized WO3 nanorods. Thermochim. Acta 2012, 542, 69–73. [Google Scholar] [CrossRef]
- Hara, K.; Zhao, Z.; Cui, Y.; Miyauchi, M.; Miyashita, M.; Mori, S. Nanocrystalline electrodes based on nanoporous-walled WO3 nanotubes for organic-dye-sensitized solar cells. Langmuir 2011, 27, 12730–12736. [Google Scholar] [CrossRef]
- Chi, X.; Liu, C.; Liu, L.; Li, Y.; Wang, Z.; Bo, X.; Liu, L.; Su, C. Tungsten trioxide nanotubes with high sensitive and selective properties to acetone. Sens. Actuators B Chem. 2014, 194, 33–37. [Google Scholar] [CrossRef]
- Hieu, N.V.; Quang, V.V.; Hoa, N.D.; Kim, D. Preparing large-scale WO3 nanowire-like structure for high sensitivity NH3 gas sensor through a simple route. Curr. Appl. Phys. 2011, 11, 657–661. [Google Scholar] [CrossRef]
- Gui, Y.; Dong, F.; Zhang, Y.; Zhang, Y.; Tian, J. Preparation and gas sensitivity of WO3 hollow microspheres and SnO2 doped heterojunction sensors. Mater. Sci. Semicond. Process. 2013, 16, 1531–1537. [Google Scholar] [CrossRef]
- Zhai, C.; Zhu, M.; Jiang, L.; Yang, T.; Zhao, Q.; Luo, Y.; Zhang, M. Fast triethylamine gas sensing response properties of nanosheets assembled WO3 hollow microspheres. Appl. Surf. Sci. 2019, 463, 1078–1084. [Google Scholar] [CrossRef]
- Mehta, S.S.; Chikhale, L.P.; Mulla, I.S.; Suryavanshi, S.S. Microwave synthesis and acetone sensing properties of WO3 hierarchical nanostructures. J. Mater. Sci.-Mater. Electron. 2017, 28, 17227–17233. [Google Scholar] [CrossRef]
- Sczancoski, J.C.; Cavalcante, L.S.; Joya, M.R.; Varela, J.A.; Pizani, P.S.; Longo, E. SrMoO4 powders processed in microwave-hydrothermal: Synthesis, characterization and optical properties. Chem. Eng. J. 2008, 140, 632–637. [Google Scholar] [CrossRef]
- Hernandez-Uresti, D.B.; Sánchez-Martínez, D.; Martínez, C.A.; Sepúlveda-Guzmán, S. Torres-Martínez, L.M. Characterization and photocatalytic properties of hexagonal and monoclinic WO3 prepared via microwave-assisted hydrothermal synthesis. Ceram. Int. 2017, 40, 4767–4775. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Karthik, M.; Prabhakaran, S. Role of Microwave on Structural, Morphological, Optical and Visible Light Photocatalytic Performance of WO3 Nanostructures. J. Clust. Sci. 2019, 30, 495–506. [Google Scholar] [CrossRef]
- Ding, Q.; Li, J.; Zou, Z.; Sun, K.; Wang, Y.; He, D. Fluoride-assisted highly-active tungsten oxide with modulating exposed facets and defect sites for efficient ppb-level acetone detection. Appl. Surf. Sci. 2022, 584, 152554. [Google Scholar] [CrossRef]
- Wu, J.; Qiao, P.; Li, H.; Ren, L.; Xu, Y.; Tian, G.; Li, M.; Pan, K.; Zhou, W. Surface-oxygen vacancy defect-promoted electron-hole separation of defective tungsten trioxide ultrathin nanosheets and their enhanced solar-driven photocatalytic performance. J. Colloid Interface Sci. 2019, 557, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ren, Y.; Zhou, X.; Liu, L.; Zhu, Y.; Cheng, X.; Xu, P.; Li, X.; Deng, Y.; Zhao, D. Pt Nanoparticles Sensitized Ordered Mesoporous WO3 Semiconductor: Gas Sensing Performance and Mechanism Study. Adv. Funct. Mater. 2017, 28, 1705268. [Google Scholar] [CrossRef]
- Tong, B.; Deng, Z.; Xu, B.; Meng, G.; Shao, J.; Liu, H.; Dai, T.; Shan, X.; Dong, W.; Wang, S.; et al. Oxygen Vacancy Defects Boosted High Performance p-Type Delafossite CuCrO2 Gas Sensors. ACS Appl. Mater. Interfaces 2018, 10, 34727–34734. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, C.; Mei, Z.; Liu, X.; Qu, X.; Li, Y.; Li, S.; Qi, W.; Zhang, Y.; Ye, J.; et al. Monoclinic Tungsten Oxide with {100} Facet Orientation and Tuned Electronic Band Structure for Enhanced Photocatalytic Oxidations. ACS Appl. Mater. Interfaces 2016, 8, 10367–10374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zai, J.; Xu, M.; Zou, Q.; Su, Y.; Wang, K.; Qian, X. Hierarchical Bi2O2CO3 microspheres with improved visible-light-driven photocatalytic activity. CrystEngComm 2011, 13, 4010–4017. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Yuan, Q.; Yu, X.; Wang, D.; Chen, Y. Ag-Modified Porous Perovskite-Type LaFeO3 for Efficient Ethanol Detection. Nanomaterials 2022, 12, 1768. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic effects in gas sensing semiconducting oxide nano-heterostructures: A review. Sens. Actuators B Chem. 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Yadav, A.A.; Lokhande, A.C.; Kim, J.H.; Lokhande, C.D. Improvement in CO2 sensing characteristics using Pd nanoparticles decorated La2O3 thin films. J. Ind. Eng. Chem. 2017, 49, 76–81. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, Y.; Li, G.; Ma, Z.; Wang, X.; Cheng, Z.; Xu, J. Highly sensitive BTEX sensors based on hexagonal WO3 nanosheets. Sens. Actuators B Chem. 2019, 293, 23–30. [Google Scholar] [CrossRef]
- Yadav, A.A.; Lokhande, A.C.; Kim, J.H.; Lokhande, C.D. Highly sensitive CO2 sensor based on microrods-like La2O3 thin film electrode. RSC Adv. 2016, 6, 106074–106080. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kulkarni, S.B.; Mathe, V.L. A multifunctional ZnO thin film based devises for photoelectrocatalytic degradation of terephthalic acid and CO2 gas sensing applications. Sens. Actuators B Chem. 2018, 274, 1–9. [Google Scholar] [CrossRef]
- Sakthiraj, K.; Balachandrakumar, K. Influence of Ti addition on the room temperature ferromagnetism of tin oxide (SnO2) nanocrystal. J. Magn. Magn. Mater. 2015, 395, 205–212. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Jing, F.; Zhou, J.; Chen, Y.; Sun, D.; Ruan, S. Low-temperature synthesis of WO3 nanolamella and their sensing properties for xylene. RSC Adv. 2015, 5, 85598–85605. [Google Scholar] [CrossRef]
- Li, N.; Fan, Y.; Shi, Y.; Xiang, Q.; Wang, X.; Xu, J. A Low Temperature Formaldehyde Gas Sensor Based on Hierarchical SnO/SnO2 Nano-flowers Assembled from Ultrathin Nanosheets: Synthesis, Sensing Performance and Mechanism. Sens. Actuators B Chem. 2019, 294, 106–115. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Armelao, L.; Mari, C.M.; Polizzi, S.; Ruffo, R.; Scotti, R.; Morazzoni, F. Surface interaction of WO3 nanocrystals with NH3. Role of the exposed crystal surfaces and porous structure in enhancing the electrical response. RSC Adv. 2014, 4, 11012–11022. [Google Scholar] [CrossRef]
- Han, X.; Han, X.; Li, L.; Wang, C. Controlling the morphologies of WO3 particles and tuning the gas sensing properties. New J. Chem. 2012, 36, 2205–2208. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, J.; Zu, G.; Che, H.; Lai, C.; Li, H.; Murugadoss, V.; Yan, C.; Fan, J.; Guo, Z. Sandwich structured WO3 nanoplatelets for highly efficient photoelectrochemical water splitting. J. Mater. Chem. A 2019, 7, 26077–26088. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, G.; Yin, L.; Cheng, H.-M. Crystal facet-dependent photocatalytic oxidation and reduction reactivity of monoclinic WO3 for solar energy conversion. J. Mater. Chem. 2012, 22, 6746–6751. [Google Scholar] [CrossRef]
- Xue, D.; Wang, J.; Wang, Y.; Sun, G.; Cao, J.; Bala, H.; Zhang, Z. Enhanced Methane Sensing Properties of WO3 Nanosheets with Dominant Exposed (200) Facet via Loading of SnO2 Nanoparticles. Nanomaterials 2019, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.V.; Kumar, N.; Kumar, M. Strategy and Future Prospects to Develop Room-Temperature-Recoverable NO2 Gas Sensor Based on Two-Dimensional Molybdenum Disulfide. Nano-Micro Lett. 2021, 13, 2–58. [Google Scholar] [CrossRef]
- Carotta, M.C.; Guidi, V.; Martinelli, G.; Nagliati, M.; Puzzovio, D.; Vecchi, D. Sensing of volatile alkanes by metal-oxide semiconductors. Sens. Actuators B Chem. 2008, 130, 497–501. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Duan, L.; Xia, K.; Chen, Y.; Li, Y.; Deng, S.; Xu, J.; Hou, Z. Microwave Synthesized 2D WO3 Nanosheets for VOCs Gas Sensors. Nanomaterials 2022, 12, 3211. https://doi.org/10.3390/nano12183211
Liu H, Duan L, Xia K, Chen Y, Li Y, Deng S, Xu J, Hou Z. Microwave Synthesized 2D WO3 Nanosheets for VOCs Gas Sensors. Nanomaterials. 2022; 12(18):3211. https://doi.org/10.3390/nano12183211
Chicago/Turabian StyleLiu, He, Lingyao Duan, Kedong Xia, Yang Chen, Yunling Li, Shaoxin Deng, Jiaqiang Xu, and Zhenyu Hou. 2022. "Microwave Synthesized 2D WO3 Nanosheets for VOCs Gas Sensors" Nanomaterials 12, no. 18: 3211. https://doi.org/10.3390/nano12183211
APA StyleLiu, H., Duan, L., Xia, K., Chen, Y., Li, Y., Deng, S., Xu, J., & Hou, Z. (2022). Microwave Synthesized 2D WO3 Nanosheets for VOCs Gas Sensors. Nanomaterials, 12(18), 3211. https://doi.org/10.3390/nano12183211