In Situ Grown Vertically Oriented Graphene Coating on Copper by Plasma-Enhanced CVD to Form Superhydrophobic Surface and Effectively Protect Corrosion
Abstract
:1. Introduction
2. Experimental
2.1. Fabrication and Characterization
2.2. Water Contact Angle Measurement
2.3. Electrochemical Corrosion Test
3. Results and Discussion
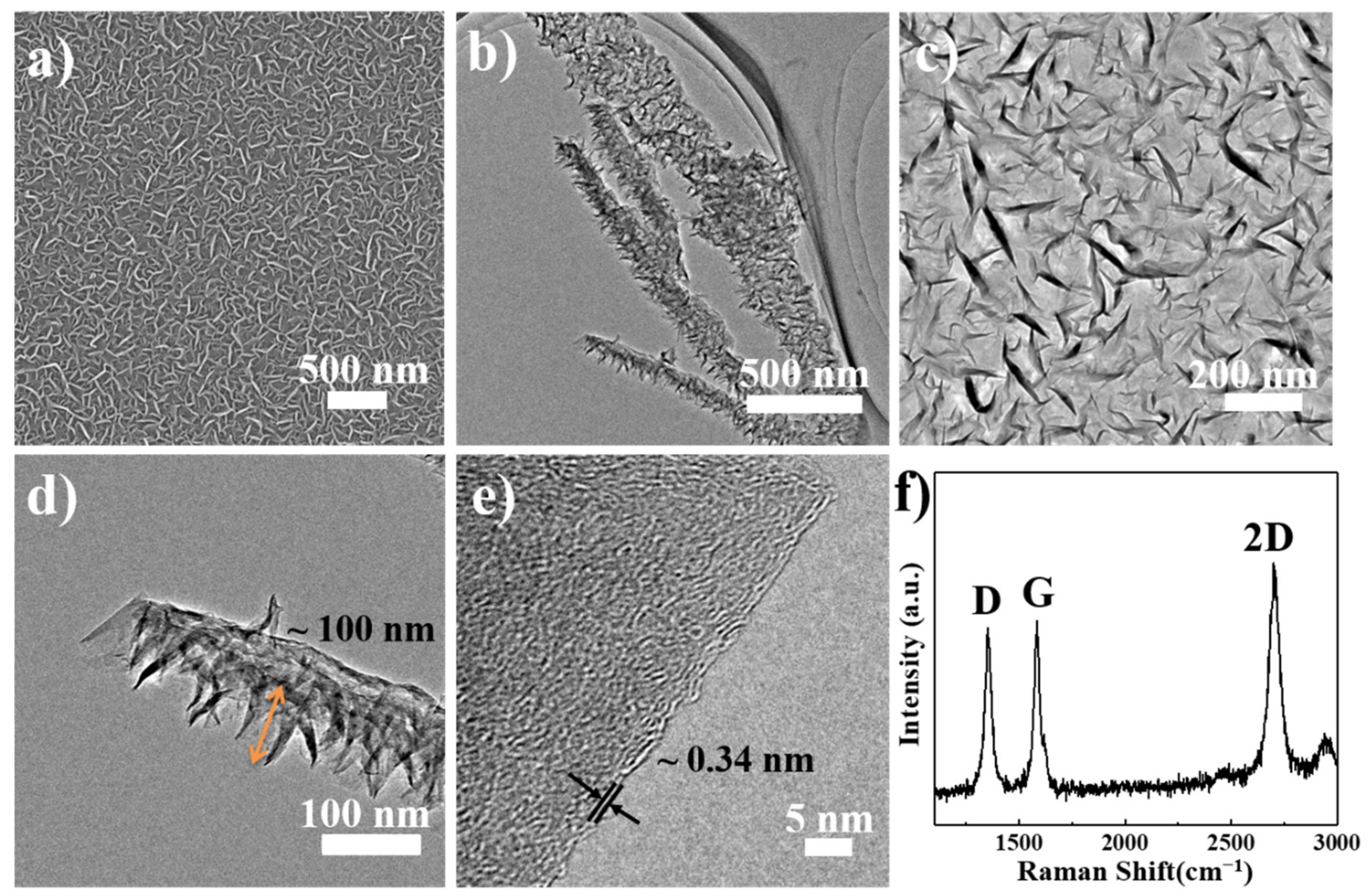
3.1. Microstructure of GS and VFG
3.2. Wettability of GS-Cu and VFG-Cu
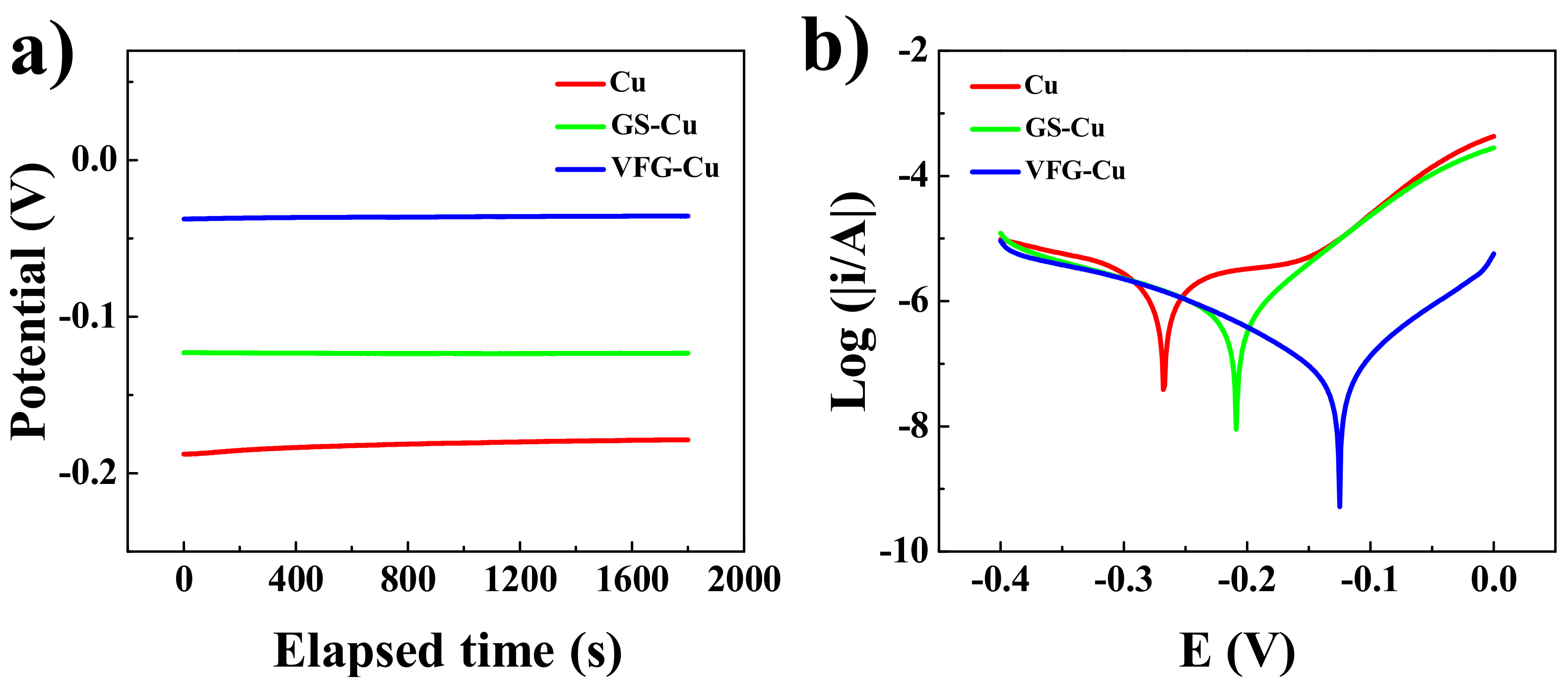
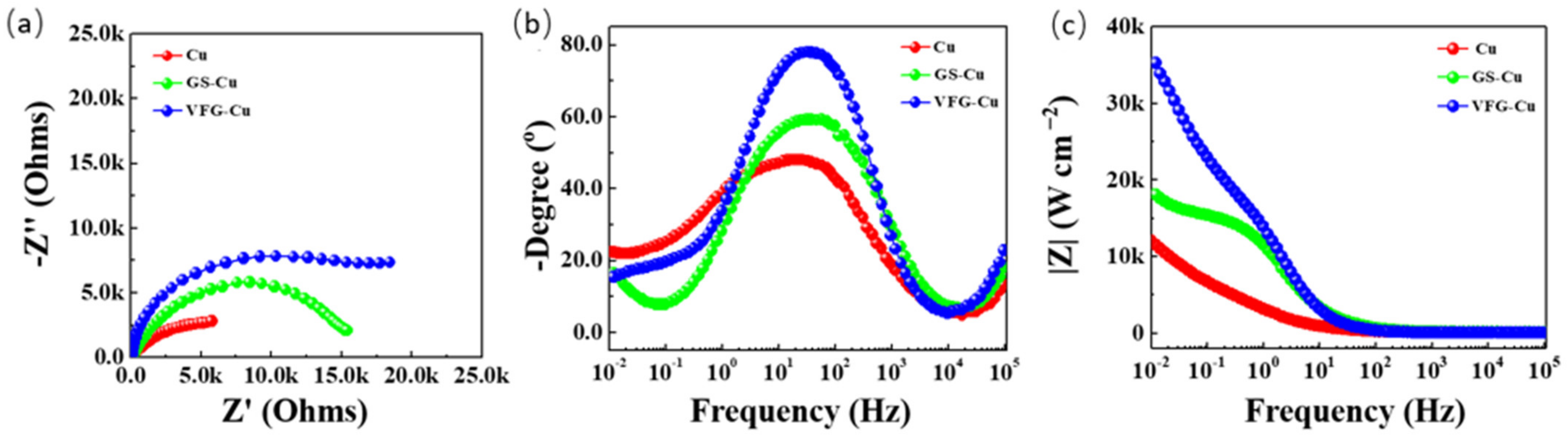
3.3. Electrochemical Corrosion Behavior of GS-Cu and VFG-Cu
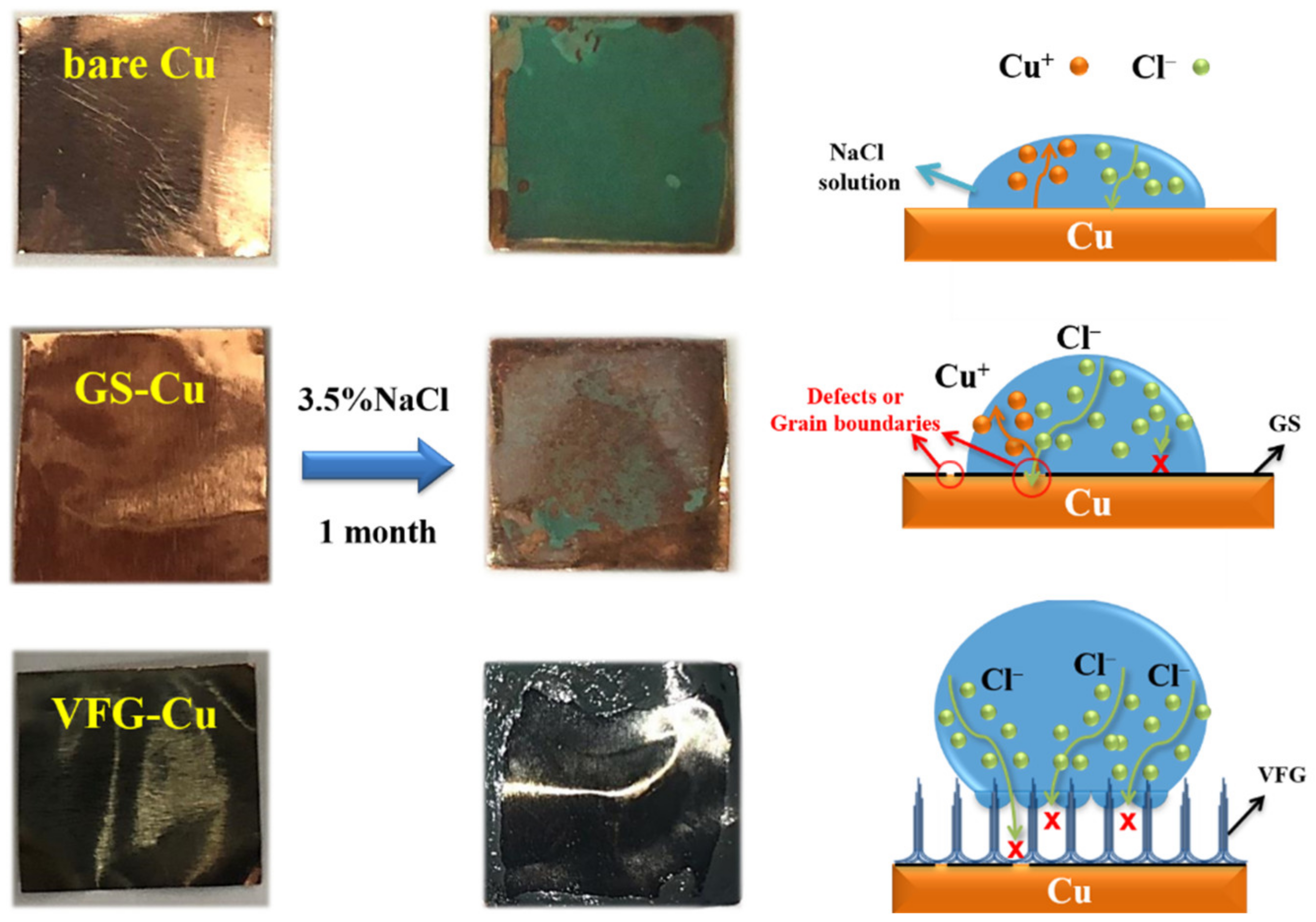
3.4. Morphologies after Long-Term Corrosion
3.5. Corrosion Protection Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wlasny, I.; Dabrowski, P.; Rogala, M.; Kowalczyk, P.J.; Pasternak, I.; Strupinski, W.; Baranowski, J.M.; Klusek, Z. Role of graphene defects in corrosion of graphene-coated Cu(111) surface. Appl. Phys. Lett. 2013, 102, 111601. [Google Scholar] [CrossRef]
- Singh Raman, R.K.; Chakraborty Banerjee, P.; Lobo, D.E.; Gullapalli, H.; Sumandasa, M.; Kumar, A.; Choudhary, L.; Tkacz, R.; Ajayan, P.M.; Majumder, M. Protecting copper from electrochemical degradation by graphene coating. Carbon 2012, 50, 4040–4045. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Q.; Zhou, Q. Corrosion behavior of Cu during graphene growth by CVD. Corros. Sci. 2014, 89, 214–219. [Google Scholar] [CrossRef]
- Mišković-Stanković, V.; Jevremović, I.; Jung, I.; Rhee, K. Electrochemical study of corrosion behavior of graphene coatings on copper and aluminum in a chloride solution. Carbon 2014, 75, 335–344. [Google Scholar] [CrossRef]
- Mondal, J.; Marques, A.; Aarik, L.; Kozlova, J.; Simões, A.; Sammelselg, V. Development of a thin ceramic-graphene nanolaminate coating for corrosion protection of stainless steel. Corros. Sci. 2016, 105, 161–169. [Google Scholar] [CrossRef]
- Mirhashemihaghighi, S.; Światowska, J.; Maurice, V.; Seyeux, A.; Klein, L.H.; Härkönen, E.; Ritala, M.; Marcus, P. Electrochemical and Surface Analysis of the Corrosion Protection of Copper by Nanometer-Thick Alumina Coatings Prepared by Atomic Layer Deposition. J. Electrochem. Soc. 2015, 162, C377–C384. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Rogers, B.R.; Bolotin, K.I. Correction to Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 4540. [Google Scholar] [CrossRef]
- Wlasny, I.; Dabrowski, P.; Rogala, M.; Pasternak, I.; Strupinski, W.; Baranowski, J.M.; Klusek, Z. Impact of electrolyte intercalation on the corrosion of graphene-coated copper. Corros. Sci. 2015, 92, 69–75. [Google Scholar] [CrossRef]
- Mogera, U.; Kurra, N.; Radhakrishnan, D.; Narayana, C.; Kulkarni, G.U. Low cost, rapid synthesis of graphene on Ni: An efficient barrier for corrosion and thermal oxidation. Carbon 2014, 78, 384–391. [Google Scholar] [CrossRef]
- Ming, H.; Wang, J.; Zhang, Z.; Wang, S.; Han, E.; Ke, W. Multilayer Graphene: A Potential Anti-oxidation Barrier in Simulated Primary Water. J. Mater. Sci. Technol. 2014, 30, 1084–1087. [Google Scholar] [CrossRef]
- Sai Pavan, A.S.; Ramanan, S.R. A study on corrosion resistant graphene films on low alloy steel. Appl. Nanosci. 2016, 6, 1175–1181. [Google Scholar] [CrossRef]
- Tan, L.; Wang, C.; Zeng, M.; Fu, L. Graphene: An Outstanding Multifunctional Coating for Conventional Materials. Small 2017, 13, 1603337. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.C.; Samantara, A.K.; Seth, M.; Parwaiz, S.; Singh, B.P.; Rath, P.C.; Jena, B.K. A facile electrochemical approach for development of highly corrosion protective coatings using graphene nanosheets. Electrochem. Commun. 2013, 32, 22–26. [Google Scholar] [CrossRef]
- Qiu, C.; Liu, D.; Jin, K.; Fang, L.; Xie, G.; Robertson, J. Electrochemical functionalization of 316 stainless steel with polyaniline-graphene oxide: Corrosion resistance study. Mater. Chem. Phys. 2017, 198, 90–98. [Google Scholar] [CrossRef]
- Nayak, S.R.; Mohana, K.N.S. Corrosion protection performance of functionalized graphene oxide nanocomposite coating on mild steel. Surf. Interface 2018, 11, 63–73. [Google Scholar] [CrossRef]
- Siddique, J.A.; Attia, N.F.; Geckeler, K.E. Polymer nanoparticles as a tool for the exfoliation of graphene sheets. Mater. Lett. 2015, 158, 186–189. [Google Scholar] [CrossRef]
- Attia, N.F.; Eid, A.M.; Soliman, M.A.; Nagy, M. Exfoliation and decoration of graphene sheets with silver nanoparticles and their antibacterial properties. J. Polym. Environ. 2018, 26, 1072–1077. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, H.; Kim, E.; Sun, H. Recent developments in superhydrophobic surfaces with unique structural and functional properties. Soft Matter 2012, 8, 11217–11231. [Google Scholar] [CrossRef]
- Zang, D.; Zhu, R.; Wu, C.; Yu, X.; Zhang, Y. Fabrication of stable superhydrophobic surface with improved anticorrosion property on magnesium alloy. Scripta Mater. 2013, 69, 614–617. [Google Scholar] [CrossRef]
- Xu, W.; Son, J.; Sun, J.; Lu, Y.; Yu, Z. Rapid Fabrication of Large-Area, Corrosion-Resistant Superhydrophobic Mg Alloy Surfaces. ACS Appl. Mater. Interfaces 2011, 3, 4404–4414. [Google Scholar] [CrossRef]
- Arukalam, I.O.; Oguzie, E.E.; Li, Y. Nanostructured superhydrophobic polysiloxane coating for high barrier and anticorrosion applications in marine environment. J. Colloid Interface Sci. 2018, 512, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, J.; Rafiee, M.A.; Yu, Z.; Koratkar, N. Superhydrophobic to Superhydrophilic Wetting Control in Graphene Films. Adv. Mater. 2010, 22, 2151–2215. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.; Kim, P.; Choi, J.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 475, 706–710. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Casiraghi, C.; Pisana, S.; Novoselov, K.S.; Geim, A.K.; Ferrari, A.C. Raman fingerprint of charged impurities in graphene. Appl. Phys. Lett. 2007, 91, 233108. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, B.; Wang, X.; Wang, W.; Shi, H. One-step growth of vertical graphene sheets on carbon nanotubes and their field emission properties. Appl. Phys. Lett. 2013, 103, 53105. [Google Scholar]
- Malesevic, A.; Vitchev, R.; Schouteden, K.; Volodin, A.; Zhang, L.; Tendeloo, G.V.; Vanhulsel, A.; Haesendonck, C.V. Synthesis of few-layer graphene via microwave plasma-enhanced chemical vapour deposition. Nanotechnology 2008, 19, 305604. [Google Scholar] [CrossRef]
- Qi, J.; Lin, J.; Wang, X.; Guo, J.; Xue, L.; Feng, J.; Fei, W. Low resistance VFG-Microporous hybrid Al-based electrodes for supercapacitors. Nano Energy 2016, 26, 657–667. [Google Scholar] [CrossRef]
- Bo, Z.; Yang, Y.; Chen, J.; Yu, K.; Yan, J.; Cen, K. Plasma-enhanced chemical vapor deposition synthesis of vertically oriented graphene nanosheets. Nanoscale 2013, 5, 5180–5204. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, F.; Wang, X.; Zhang, L.; Cao, J.; Feng, J. Effect of catalyst film thickness on the structures of vertically-oriented few-layer graphene grown by PECVD. RSC Adv. 2014, 4, 44434–44441. [Google Scholar] [CrossRef]
- Jia, B.; Zou, L. Wettability and its influence on graphene nansoheets as electrode material for capacitive deionization. Chem. Phys. Lett. 2012, 548, 23–28. [Google Scholar] [CrossRef]
- Driskill, J.; Vanzo, D.; Bratko, D.; Luzar, A. Wetting transparency of graphene in water. J. Cheml. Phys. 2014, 141, 18C517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ding, J.; Zhou, M.; Yu, H. Enhancing the anticorrosion performance of graphene-epoxy coatings by biomimetic interfacial designs. ACS Appl. Nano Mater. 2021, 4, 6557–6561. [Google Scholar] [CrossRef]
- Sun, X.; Gong, F.; Hao, M.; Wu, L.; Yin, C.; Sun, Z.; Xiao, R. Enhanced thermal transport and corrosion resistance by coating vertically-aligned graphene on zirconium alloy for nuclear reactor applications. Appl. Surf. Sci. J. Devoted Prop. Interfaces Relat. Synth. Behav. Mater. 2022, 582, 152484. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Q.; Zhou, Q. Time-dependent protection of ground and polished Cu using graphene film. Corros. Sci. 2015, 90, 69–75. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Yang, Y.; Xian, Y.; Chen, H.; Cai, W. In Situ Grown Vertically Oriented Graphene Coating on Copper by Plasma-Enhanced CVD to Form Superhydrophobic Surface and Effectively Protect Corrosion. Nanomaterials 2022, 12, 3202. https://doi.org/10.3390/nano12183202
Zheng X, Yang Y, Xian Y, Chen H, Cai W. In Situ Grown Vertically Oriented Graphene Coating on Copper by Plasma-Enhanced CVD to Form Superhydrophobic Surface and Effectively Protect Corrosion. Nanomaterials. 2022; 12(18):3202. https://doi.org/10.3390/nano12183202
Chicago/Turabian StyleZheng, Xiaohang, Yaqian Yang, Yi Xian, Heng Chen, and Wei Cai. 2022. "In Situ Grown Vertically Oriented Graphene Coating on Copper by Plasma-Enhanced CVD to Form Superhydrophobic Surface and Effectively Protect Corrosion" Nanomaterials 12, no. 18: 3202. https://doi.org/10.3390/nano12183202
APA StyleZheng, X., Yang, Y., Xian, Y., Chen, H., & Cai, W. (2022). In Situ Grown Vertically Oriented Graphene Coating on Copper by Plasma-Enhanced CVD to Form Superhydrophobic Surface and Effectively Protect Corrosion. Nanomaterials, 12(18), 3202. https://doi.org/10.3390/nano12183202




