Spin-Coating and Aerosol Spray Pyrolysis Processed Zn1−xMgxO Films for UV Detector Applications
Abstract
1. Introduction
2. Sample Preparation and Experimental Details
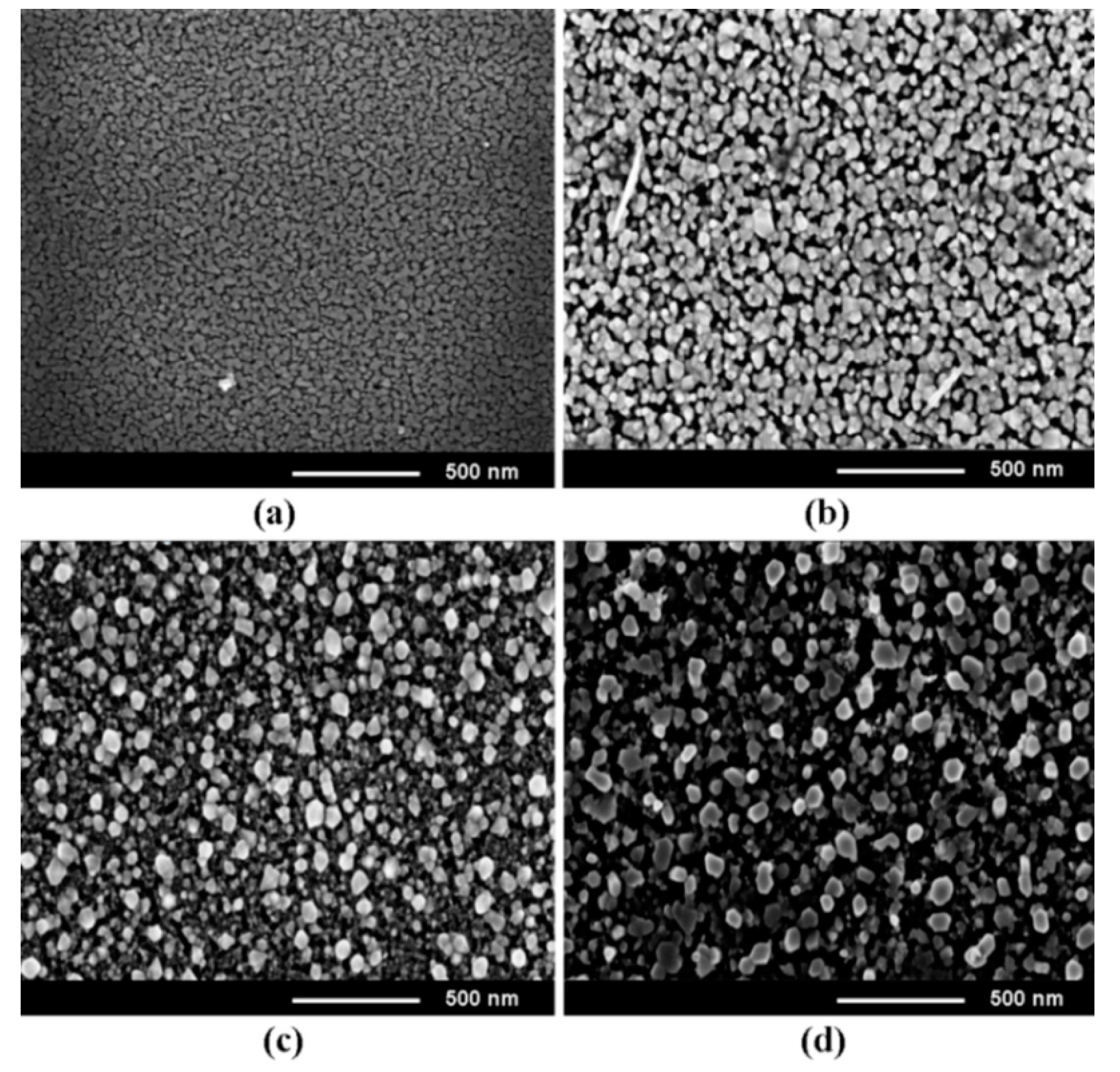
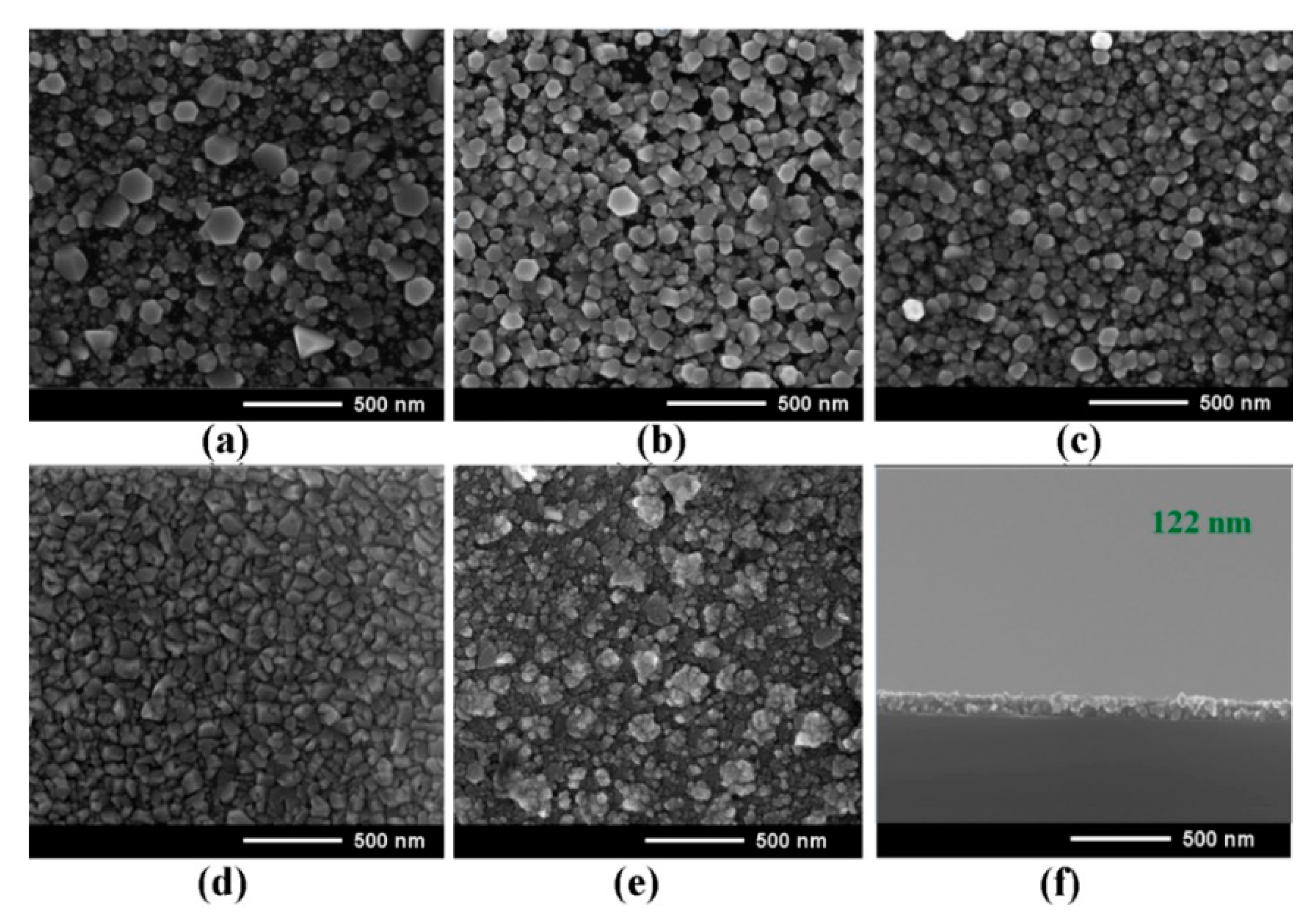
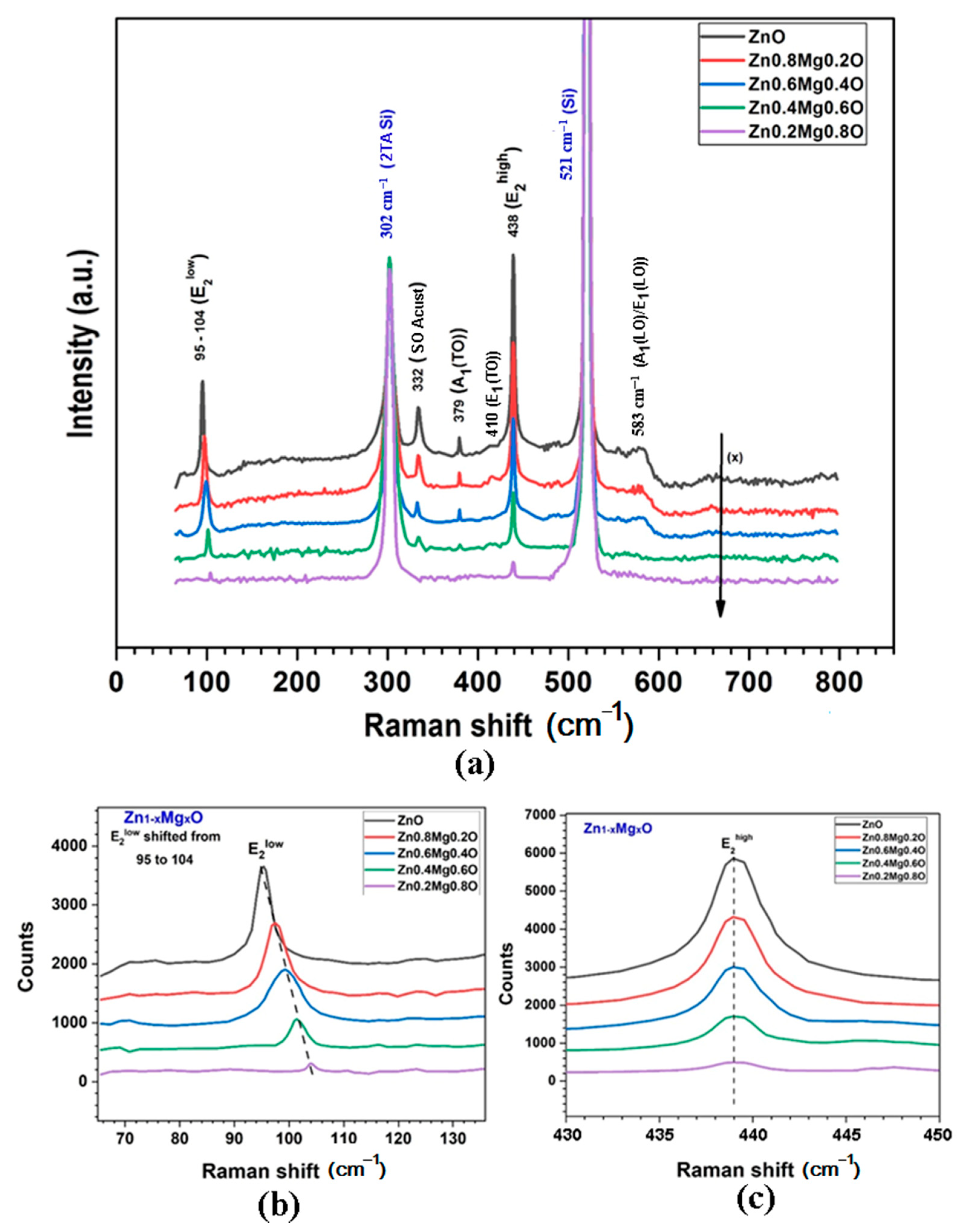
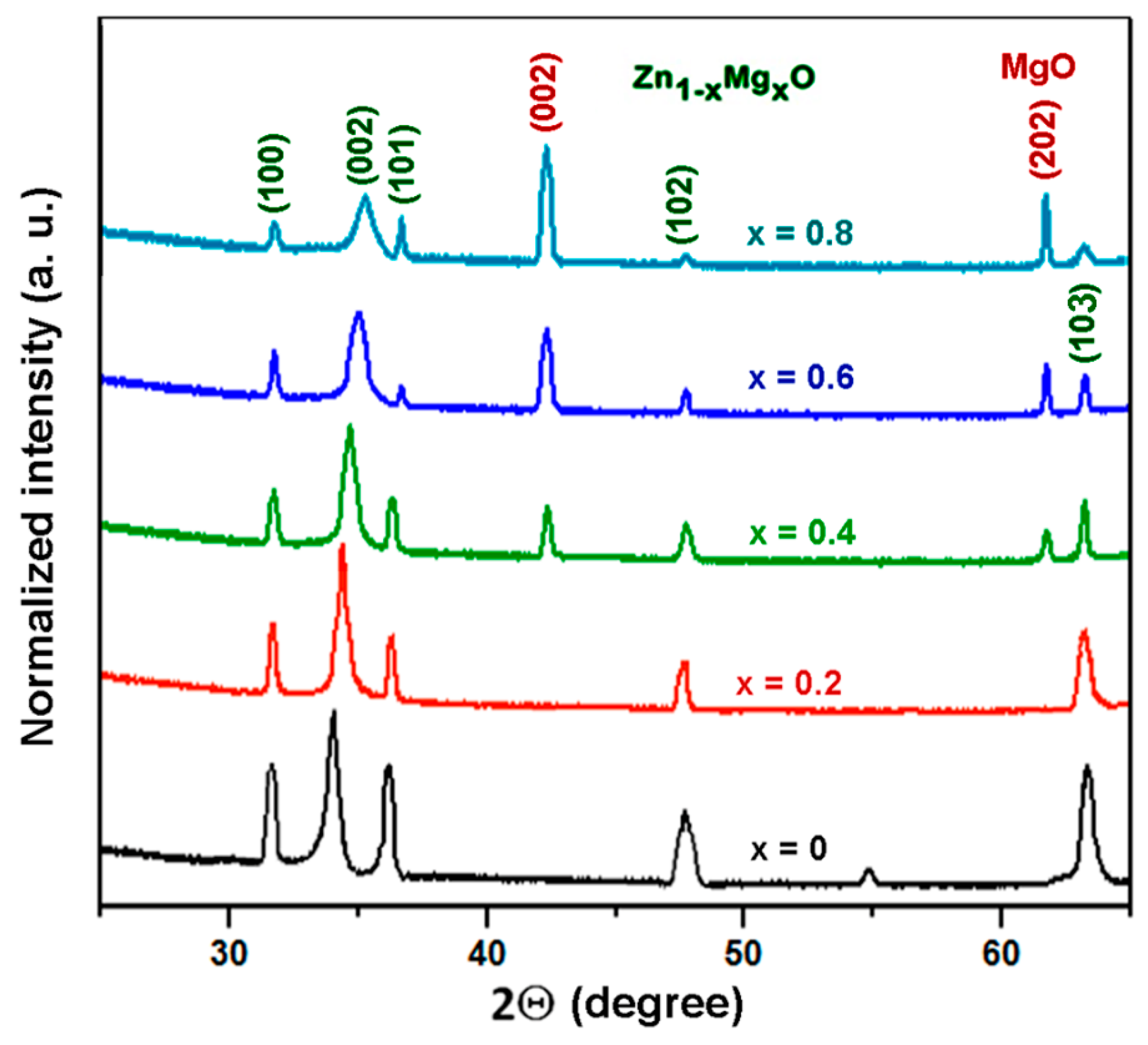
3. Morphology, Composition, and Crystal Structure Characterizations of the Prepared Films
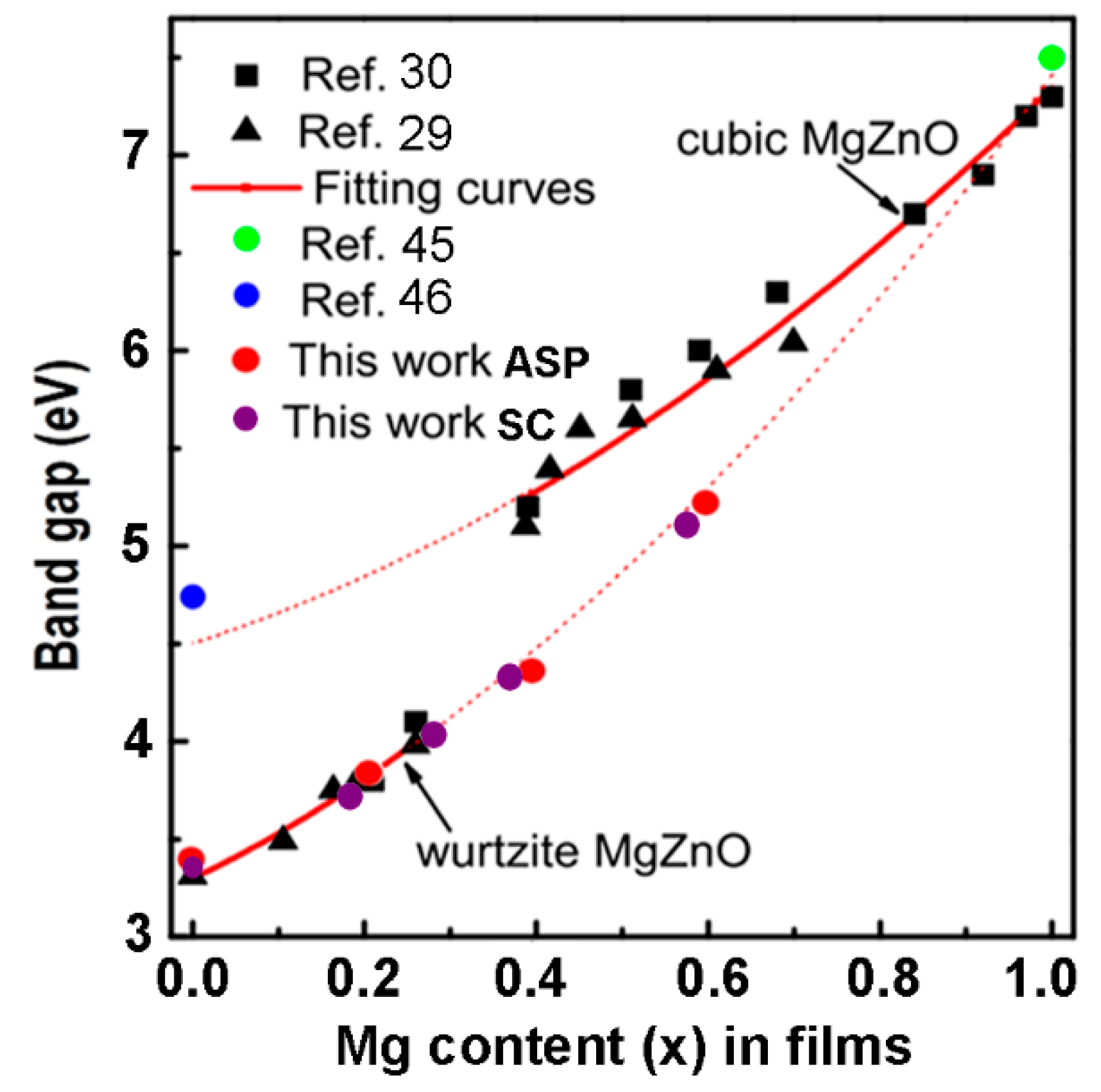
4. Optical Properties and Band Gap Analysis of the Prepared Films
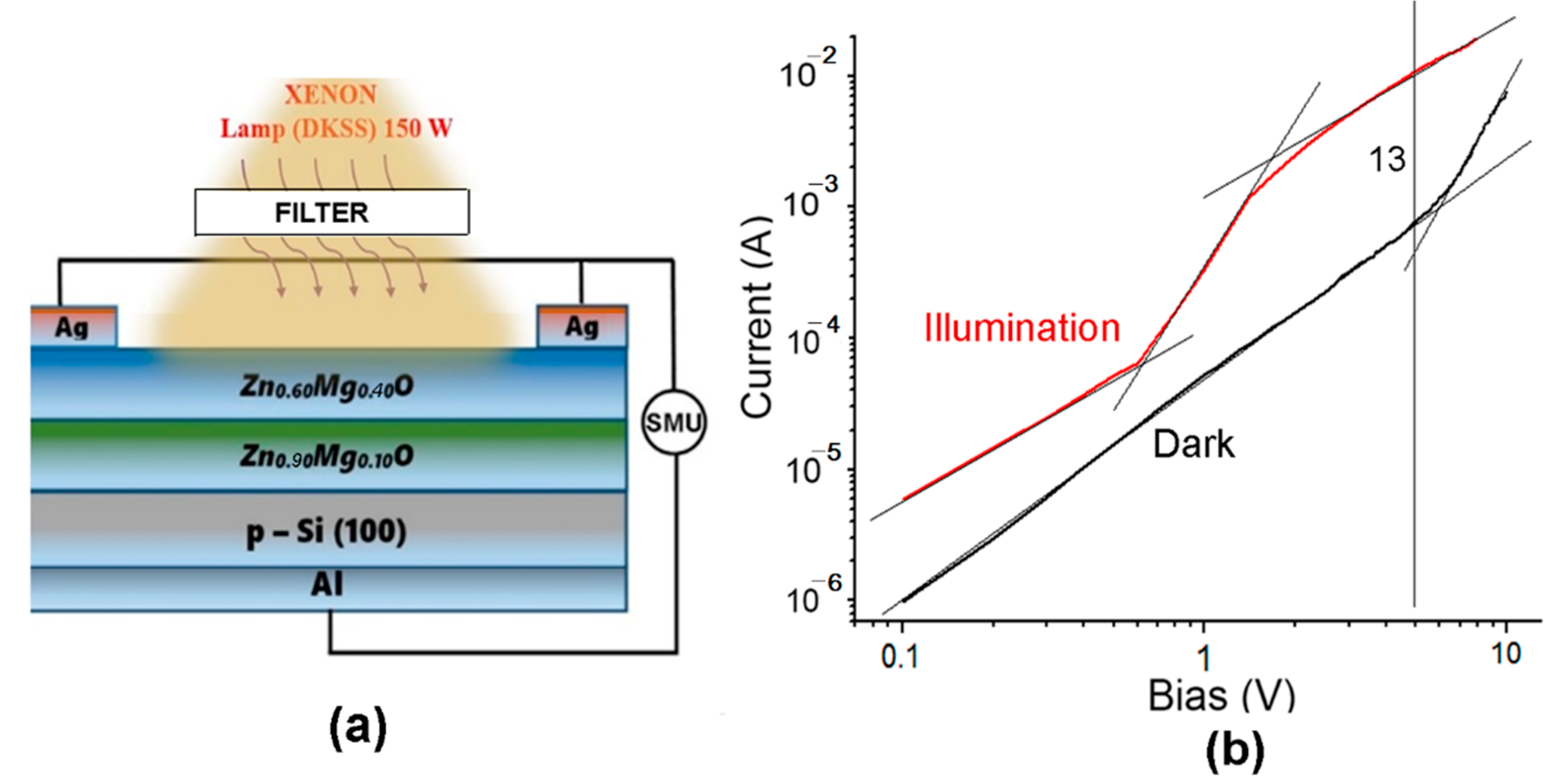
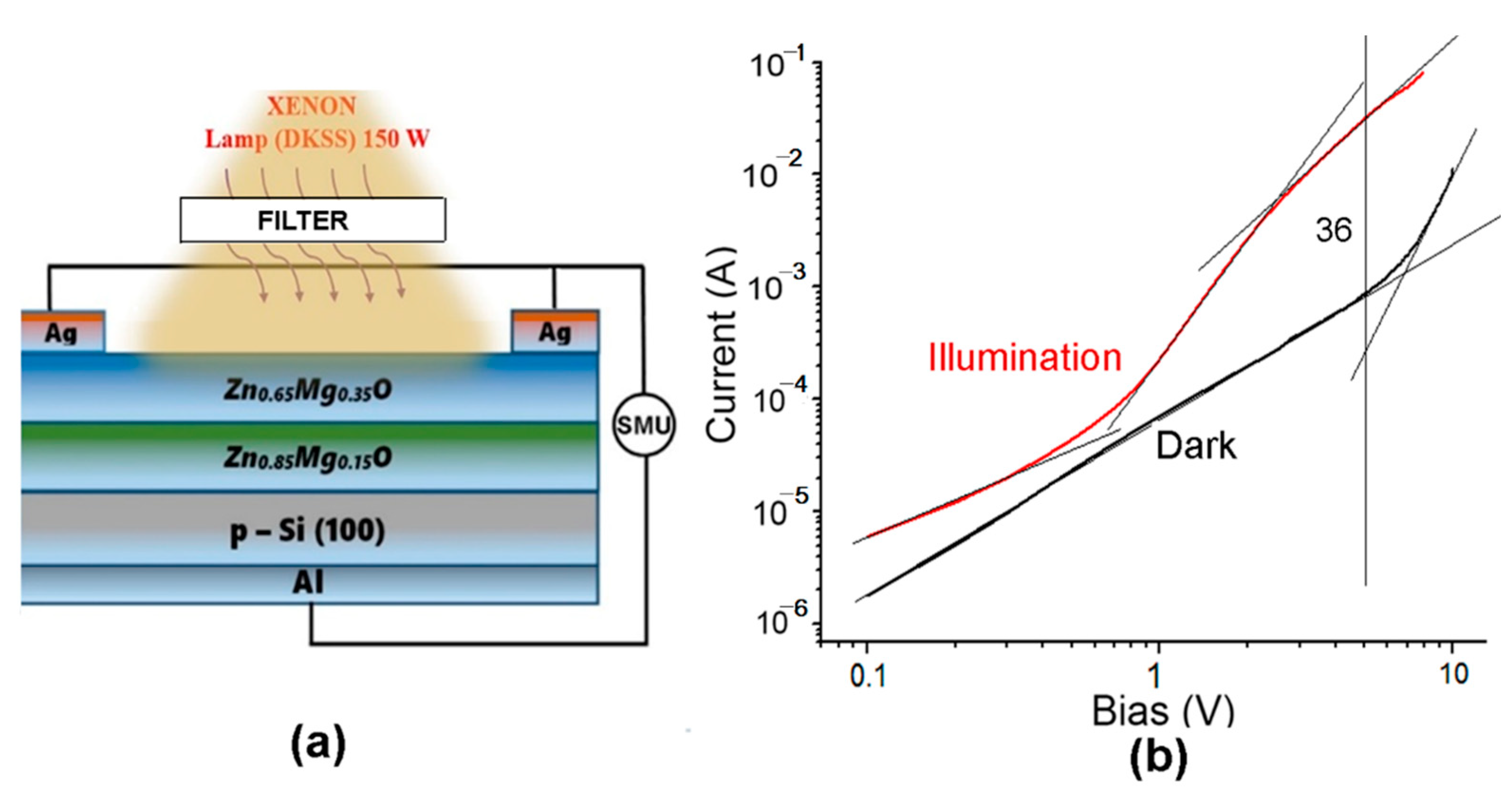
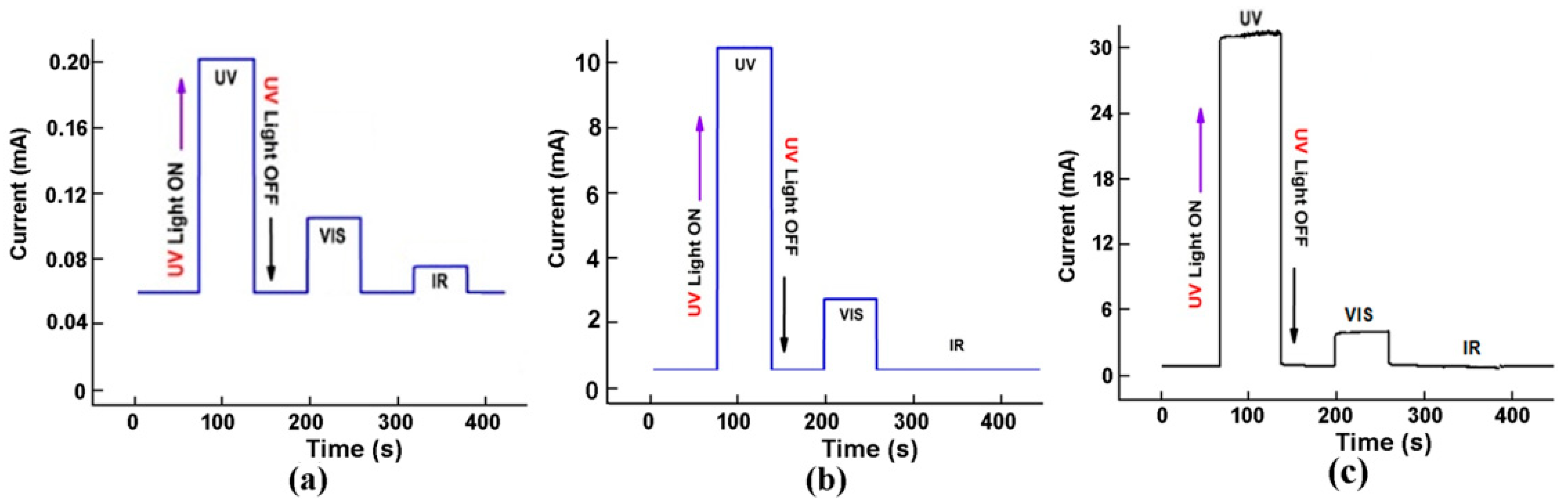
5. Heterostructure Photodetectors Characterization
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, H.K.; Yu, S.F.; Yang, H.Y. ZnO random laser diode arrays for stable single-mode operation at high power. Appl. Phys. Lett. 2010, 97, 241107. [Google Scholar] [CrossRef]
- Vanmaekelbergh, D.; Van Vugt, L.K. ZnO nanowire lasers. Nanoscale 2011, 3, 2783–2800. [Google Scholar] [CrossRef] [PubMed]
- Ursaki, V.V.; Zalamai, V.V.; Tiginyanu, I.M.; Burlacu, A.; Rusu, E.V.; Klingshirn, C. Refractive index dispersion deduced from lasing modes in ZnO microtetrapods. Appl. Phys. Lett. 2009, 95, 171101. [Google Scholar] [CrossRef]
- Zalamai, V.V.; Ursaki, V.V.; Klingshirn, C.; Kalt, H.; Emelchenko, G.A.; Redkin, A.N. Lasing with guided modes in ZnO nanorods and nanowires. Appl. Phys. B 2009, 97, 817–823. [Google Scholar] [CrossRef]
- Lee, B.R.; Lee, S.; Park, J.H.; Jung, E.D.; Yu, J.C.; Nam, Y.S.; Heo, J.; Kim, J.-Y.; Kim, B.-S.; Song, M.H. Amine-Based Interfacial Molecules for Inverted Polymer-Based Optoelectronic Devices. Adv. Mater. 2015, 27, 3553–3559. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F. Zinc oxide light-emitting diodes: A review. Opt. Eng. 2019, 58, 010901. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.Y.; Sun, Y.; Ma, J.G.; Liu, Y.C. ZnO ultraviolet random laser diode on metal copper substrate. Opt. Express 2014, 22, 16731–16737. [Google Scholar] [CrossRef]
- Suja, M.; Bashar, S.B.; Debnath, B.; Su, L.; Shi, W.; Lake, R.; Liu, J. Electrically driven deep ultraviolet MgZnO lasers at room temperature. Sci. Rep. 2017, 7, 2677. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Mai, C.; Mu, L.; Li, M.; Wang, J.; Xu, W.; Peng, J. Effects of ZnMgO Electron Transport Layer on the Performance of InP-Based Inverted Quantum Dot Light-Emitting Diodes. Nanomaterials 2021, 11, 1246. [Google Scholar] [CrossRef]
- Galdámez-Martinez, A.; Santana, G.; Güell, F.; Martínez-Alanis, P.R.; Dutt, A. Photoluminescence of ZnO Nanowires: A Review. Nanomaterials 2020, 10, 857. [Google Scholar] [CrossRef]
- Sahoo, A.; Miryala, M.; Dixit, T.; Klimkowicz, A.; Francis, B.; Murakami, M.; Rao, M.S.R.; Krishnan, S. Femtosecond Pulse Ablation Assisted Mg-ZnO Nanoparticles for UV-Only Emission. Nanomaterials 2020, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Z.; Ji, L.W.; Liu, C.H.; Peng, S.M.; Young, S.J.; Lam, K.T.; Huang, C.J. Ultraviolet photodetectors based on MgZnO thin films. J. Vac. Sci. Technol. A Vac. Surf. Film. 2011, 29, 03A118. [Google Scholar] [CrossRef]
- Hou, Y.N.; Mei, Z.X.; Liang, H.L.; Ye, D.Q.; Gu, C.Z.; Du, X.L. Dual-band MgZnO ultraviolet photodetector integrated with Si. Appl. Phys. Lett. 2013, 102, 153510. [Google Scholar] [CrossRef]
- Yang, J.L.; Liu, K.W.; Shen, D.Z. Recent progress of ZnMgO ultraviolet photodetector. Chin. Phys. B 2017, 26, 47308. [Google Scholar] [CrossRef]
- Shiang, J.S.; Brahma, S.; Liu, C.P.; Huang, J.L. Ultraviolet photodetectors based on MgZnO thin film grown by RF magnetron sputtering. Thin Solid Films 2016, 620, 170–174. [Google Scholar] [CrossRef]
- Maity, S.; Sahu, P.P.; Bhunia, C.T. High Photo Sensing Performance With Electro-Optically Efficient Silicon Based ZnO/ZnMgO Heterojunction Structure. IEEE Sens. J. 2018, 18, 6569–6575. [Google Scholar] [CrossRef]
- Ajmal, H.M.S.; Khan, F.; Nam, K.; Kim, H.Y.; Kim, S.D. Ultraviolet Photodetection Based on High-Performance Co-Plus-Ni Doped ZnO Nanorods Grown by Hydrothermal Method on Transparent Plastic Substrate. Nanomaterials 2020, 10, 1225. [Google Scholar] [CrossRef]
- Zheng, G.; Zhu, P.; Sun, L.; Jiang, J.; Liu, J.; Wang, X.; Li, W. Thin film zinc oxide gas sensor fabricated using near-field electrospray. AIP Adv. 2016, 6, 125306. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Chang, S.J.; Ji, L.W.; Hsiao, Y.J.; Tang, I.T.; Lu, H.Y.; Chu, Y.L. High Sensitivity of NO Gas Sensors Based on Novel Ag-Doped ZnO Nanoflowers Enhanced with a UV Light-Emitting Diode. ACS Omega 2018, 3, 13798–13807. [Google Scholar] [CrossRef]
- Cesini, I.; Kowalczyk, M.; Lucantonio, A.; D’Alesio, G.; Kumar, P.; Camboni, D.; Massari, L.; Pingue, P.; De Simone, A.; Morgera, A.F.; et al. Seedless Hydrothermal Growth of ZnO Nanorods as a Promising Route for Flexible Tactile Sensors. Nanomaterials 2020, 10, 977. [Google Scholar] [CrossRef]
- Huang, J.; Yin, Z.; Zheng, Q. Applications of ZnO in organic and hybrid solar cells. Energy Environ. Sci. 2011, 4, 3861–3877. [Google Scholar] [CrossRef]
- Vittal, R.; Ho, K.C. Zinc oxide based dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 70, 920–935. [Google Scholar] [CrossRef]
- Consonni, V.; Briscoe, J.; Kärber, E.; Li, X.; Cossuet, T. ZnO nanowires for solar cells: A comprehensive review. Nanotechnology 2019, 30, 362001. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, J.; Huang, K.; Li, S.; Wang, Q.; Sun, Z.; Mei, T.; Wang, J.; Zhang, L.; Wang, N.; et al. Low-Temperature and Solution-Processable Zinc Oxide Transistors for Transparent Electronics. ACS Omega 2017, 2, 8990–8996. [Google Scholar] [CrossRef]
- Thapa, D.; Huso, J.; Miklos, K.; Wojcik, P.M.; McIlroy, D.N.; Morrison, J.L.; Corolewski, C.; McCluskey, M.D.; Williams, T.J.; Norton, M.G.; et al. UV-luminescent MgZnO semiconductor alloys: Nanostructure and optical properties. J. Mater. Sci. Mater. Electron. 2017, 28, 2511–2520. [Google Scholar] [CrossRef]
- Schoenfeld, W.V.; Wei, M.; Boutwell, R.C.; Liu, H.Y. High response solar-blind MgZnO photodetectors grown by molecular beam epitaxy. In Oxide-Based Materials and Devices V; SPIE: Bellingham, WA, USA, 2014; Volume 8987, pp. 329–340. [Google Scholar] [CrossRef]
- Hierro, A.; Tabares, G.; Lopez-Ponce, M.; Ulloa, J.M.; Kurtz, A.; Munoz, E.; Marin-Borras, V.; Munoz-Sanjose, V.; Chauveau, J.M. ZnMgO-based UV photodiodes: A comparison of films grown by spray pyrolysis and MBE. In Oxide-Based Materials and Devices VII; SPIE: Bellingham, WA, USA, 2016; Volume 9749, pp. 70–75. [Google Scholar] [CrossRef]
- Lee, H.Y.; Tsai, W.H.; Lin, Y.C.; Lee, C.T. Performance enhancement of MgZnO ultraviolet photodetectors using ultrathin Al2O3 inserted layer. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2016, 34, 051207. [Google Scholar] [CrossRef]
- Wang, X.; Saito, K.; Tanaka, T.; Nishio, M.; Guo, Q. Lower temperature growth of single phase MgZnO films in all Mg content range. J. Alloys Compd. 2015, 627, 383–387. [Google Scholar] [CrossRef]
- Wang, X.; Saito, K.; Tanaka, T.; Nishio, M.; Nagaoka, T.; Arita, M.; Guo, Q. Energy band bowing parameter in MgZnO alloys. Appl. Phys. Lett. 2015, 107, 022111. [Google Scholar] [CrossRef]
- Wang, L.K.; Ju, Z.G.; Zhang, J.Y.; Zheng, J.; Shen, D.Z.; Yao, B.; Zhao, D.X.; Zhang, Z.Z.; Li, B.H.; Shan, C.X. Single-crystalline cubic MgZnO films and their application in deep-ultraviolet optoelectronic devices. Appl. Phys. Lett. 2009, 95, 131113. [Google Scholar] [CrossRef]
- Chawla, S.; Jayanthi, K.; Chander, H. Enhancement of luminescence in ZnMgO thin-film nanophosphors and application for white light generation. Phys. Status Solidi A 2008, 205, 271–274. [Google Scholar] [CrossRef]
- Morari, V.; Rusu, E.V.; Postolache, V.; Ursachi, V.V.; Tiginyanu, I.M.; Rogachev, A.V.; Semchenko, A.V. Injection photodiode based on an Al–p-Si–n-Zn85Mg15O–n-Zn65Mg35O–Ag structure. Rom. J. Phys. 2021, 66, 609. [Google Scholar]
- Winkler, N.; Wibowo, R.A.; Kautek, W.; Dimopoulos, T. Influence of the aqueous solution composition on the morphology of Zn1−xMgxO films deposited by spray pyrolysis. J. Mater. Chem. C 2019, 7, 3889–3900. [Google Scholar] [CrossRef]
- Tsay, C.Y.; Chen, S.T.; Fan, M.T. Solution-Processed Mg-Substituted ZnO Thin Films for Metal-Semiconductor-Metal Visible-Blind Photodetectors. Coatings 2019, 9, 277. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, X.; Zhang, Q.; Jiang, J.; Jin, X.; Li, F.; Chen, Z.; Zhao, F.; Li, Q. ZnMgO:ZnO composite films for fast electron transport and high charge balance in quantum dot light emitting diodes. Opt. Mater. Express 2018, 8, 909–918. [Google Scholar] [CrossRef]
- Morari, V.; Pantazi, A.; Curmei, N.; Postolache, V.; Rusu, E.V.; Enachescu, M.; Tiginyanu, I.M.; Ursaki, V.V. Band tail state related photoluminescence and photoresponse of ZnMgO solid solution nanostructured films. Beilstein J. Nanotechnol. 2020, 11, 899–910. [Google Scholar] [CrossRef]
- Losev, V.V. Injection photodiodes based on low-resistivity ZnS single crystals. Semiconductors 2009, 43, 1700–1703. [Google Scholar] [CrossRef]
- Mirsagatov, S.A.; Leiderman, A.Y.; Ataboev, O.K. Mechanism of charge transfer in injection photodiodes based on the In-n+-CdS-n-CdSxTe1−x-p-ZnxCd1−xTe-Mo structure. Phys. Solid State 2013, 55, 1635–1646. [Google Scholar] [CrossRef]
- Mirsagatov, S.A.; Sapayev, I.B. Injection photodiode based on a p-Si-n-CdS-n+-CdS structure. Semiconductors 2014, 48, 1363–1369. [Google Scholar] [CrossRef]
- Kaschner, A.; Haboeck, U.; Strassburg, M.; Strassburg, M.; Kaczmarczyk, G.; Hoffmann, A.; Thomsen, C.; Zeuner, A.; Alves, H.R.; Hofmann, D.M.; et al. Nitrogen-related local vibrational modes in ZnO:N. Appl. Phys. Lett. 2002, 80, 1909–1911. [Google Scholar] [CrossRef]
- Rajalakshmi, M.; Arora, A.K.; Bendre, B.S.; Mahamuni, S. Optical phonon confinement in zinc oxide nanoparticles. J. Appl. Phys. 2000, 87, 2445–2448. [Google Scholar] [CrossRef]
- Jubu, P.R.; Yam, F.K.; Igba, V.M.; Beh, K.P. Tauc-plot scale and extrapolation effect on bandgap estimation from UV–vis–NIR data—A case study of β-Ga2O3. J. Solid State Chem. 2020, 290, 121576. [Google Scholar] [CrossRef]
- Ekennia, A.C.; Uduagwu, D.N.; Nwaji, N.N.; Oje, O.O.; Emma-Uba, C.O.; Mgbii, S.I.; Olovo, O.J.; Nwanji, O.L. Green Synthesis of Biogenic Zinc Oxide Nanoflower as Dual Agent for Photodegradation of an Organic Dye and Tyrosinase Inhibitor. J. Inorg. Organomet. Polym. Mater. 2021, 31, 886–897. [Google Scholar] [CrossRef]
- Jang, S.H.; Chichibu, S.F. Structural, elastic, and polarization parameters and band structures of wurtzite ZnO and MgO. J. Appl. Phys. 2012, 112, 073503. [Google Scholar] [CrossRef]
- Ni, H.Q.; Lu, Y.F.; Ren, Z.M. Quasiparticle band structures of wurtzite and rock-salt ZnO. J. Appl. Phys. 2002, 91, 1339–1343. [Google Scholar] [CrossRef]
- Liu, Z.L.; Mei, Z.X.; Zhang, T.C.; Liu, Y.P.; Guo, Y.; Du, X.L.; Hallen, A.; Zhu, J.J.; Kuznetsov, A.Y. Solar-blind 4.55 eV band gap Mg0.55Zn0.45O components fabricated using quasi-homo buffers. J. Cryst. Growth 2009, 311, 4356–4359. [Google Scholar] [CrossRef]
- Du, X.; Mei, Z.; Liu, Z.; Guo, Y.; Zhang, T.; Hou, Y.; Zhang, Z.; Xue, Q.; Kuznetsov, A.Y. Controlled growth of high-quality ZnO-based films and fabrication of visible-blind and solar-blind ultra-violet detectors. Adv. Mater. 2009, 21, 4625–4630. [Google Scholar] [CrossRef]
- Hou, Y.N.; Mei, Z.X.; Liu, Z.L.; Zhang, T.C.; Du, X.L. Mg0.55Zn0.45O solar-blind ultraviolet detector with high photoresponse performance and large internal gain. Appl. Phys. Lett. 2011, 98, 103506. [Google Scholar] [CrossRef]
- Lampert, M.A. Volume Controlled current injection in insulators. Rep. Prog. Phys. 1964, 27, 329–368. [Google Scholar] [CrossRef]
- Röhr, J.A.; Moia, D.; Saif, A.H.; Kirchartz, T.; Nelson, J. Exploring the validity and limitations of the Mott-Gurney law for charge-carrier mobility determination of semiconducting thin-films. J. Phys. Condens. Matter 2018, 30, 105901. [Google Scholar] [CrossRef]
- Chandra, W.; Ang, L.K.; Pey, K.L.; Ng, C.M. Two-dimensional analytical Mott-Gurney law for a trap-filled solid. Appl. Phys. Lett. 2007, 90, 153505. [Google Scholar] [CrossRef]
- Mirsagatov, S.A.; Kabulov, R.R.; Makhmudov, M.A. Injection photodiode based on an n-CdS/p-CdTe heterostructure. Semiconductors 2013, 47, 825–830. [Google Scholar] [CrossRef]
- Sirkeli, V.P.; Yilmazoglu, O.; Hajo, A.S.; Nedeoglo, N.; Nedeoglo, D.D.; Preu, S.; Kuppers, F.; Hartnagel, H.L. Enhanced Responsivity of ZnSe-Based Metal–Semiconductor–Metal Near-Ultraviolet Photodetector via Impact Ionization. Phys. Status Solidi RRL 2017, 12, 1700418. [Google Scholar] [CrossRef]






| x-Value | Element | Weight % | Atomic % |
|---|---|---|---|
| 0 | O | 22.38 | 51.66 |
| Zn | 77.62 | 48.34 | |
| Mg | 0 | 0 | |
| 0.2 | O | 24.49 | 50.11 |
| Zn | 66.96 | 39.24 | |
| Mg | 8.56 | 10.65 | |
| 0.4 | O | 25.29 | 50.70 |
| Zn | 59.47 | 29.19 | |
| Mg | 15.24 | 20.11 | |
| 0.6 | O | 30.56 | 49.78 |
| Zn | 41.70 | 19.29 | |
| Mg | 27.74 | 30.93 | |
| 0.8 | O | 33.12 | 48.84 |
| Zn | 26.75 | 09.58 | |
| Mg | 40.13 | 41.58 |
| x-Value | Element | Weight % | Atomic % |
|---|---|---|---|
| 0 | O | 21.19 | 51.35 |
| Zn | 78.81 | 48.65 | |
| Mg | 0 | 0 | |
| 0.2 | O | 22.57 | 50.67 |
| Zn | 70.52 | 39.08 | |
| Mg | 6.91 | 10.25 | |
| 0.4 | O | 24.33 | 49.45 |
| Zn | 60.28 | 29.98 | |
| Mg | 15.39 | 20.57 | |
| 0.6 | O | 27.74 | 49.47 |
| Zn | 49.49 | 30.25 | |
| Mg | 22.77 | 20.28 | |
| 0.8 | O | 31.62 | 49.13 |
| Zn | 28.25 | 10.49 | |
| Mg | 40.13 | 40.38 |
| Photodetector Structure Design | Responsivity (R), mA⋅W−1 | Detectivity (D*), cm⋅Hz1/2⋅W−1 |
|---|---|---|
| Zn0.9Mg0.1O/Si | 3.0 | 2.0 × 108 |
| Zn0.8Mg0.2O/Si | 2.2 | 1.8 × 108 |
| Zn0.6Mg0.4O/Si | 0.1 | 2.0 × 107 |
| Zn0.9Mg0.1O/Zn0.6Mg0.4O/Si | 150 | 3.5 × 109 |
| Zn0.85Mg0.15O/Zn0.65Mg0.35O/Si | 460 | 1.0 × 1010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morari, V.; Ursaki, V.V.; Rusu, E.V.; Zalamai, V.V.; Colpo, P.; Tiginyanu, I.M. Spin-Coating and Aerosol Spray Pyrolysis Processed Zn1−xMgxO Films for UV Detector Applications. Nanomaterials 2022, 12, 3209. https://doi.org/10.3390/nano12183209
Morari V, Ursaki VV, Rusu EV, Zalamai VV, Colpo P, Tiginyanu IM. Spin-Coating and Aerosol Spray Pyrolysis Processed Zn1−xMgxO Films for UV Detector Applications. Nanomaterials. 2022; 12(18):3209. https://doi.org/10.3390/nano12183209
Chicago/Turabian StyleMorari, Vadim, Veaceslav V. Ursaki, Emil V. Rusu, Victor V. Zalamai, Pascal Colpo, and Ion M. Tiginyanu. 2022. "Spin-Coating and Aerosol Spray Pyrolysis Processed Zn1−xMgxO Films for UV Detector Applications" Nanomaterials 12, no. 18: 3209. https://doi.org/10.3390/nano12183209
APA StyleMorari, V., Ursaki, V. V., Rusu, E. V., Zalamai, V. V., Colpo, P., & Tiginyanu, I. M. (2022). Spin-Coating and Aerosol Spray Pyrolysis Processed Zn1−xMgxO Films for UV Detector Applications. Nanomaterials, 12(18), 3209. https://doi.org/10.3390/nano12183209






