Synthesis of Highly Monodisperse Nickel and Nickel Phosphide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
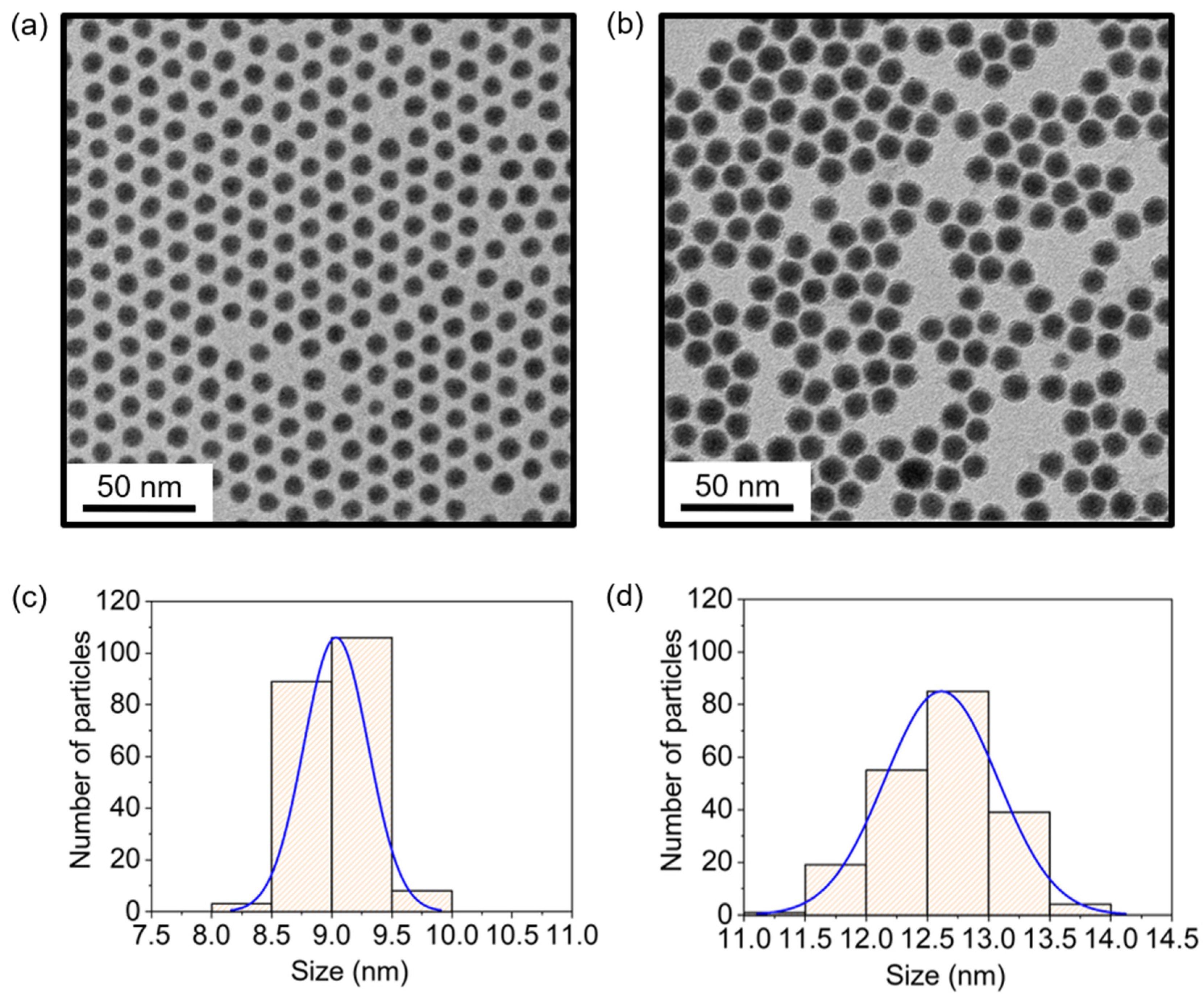
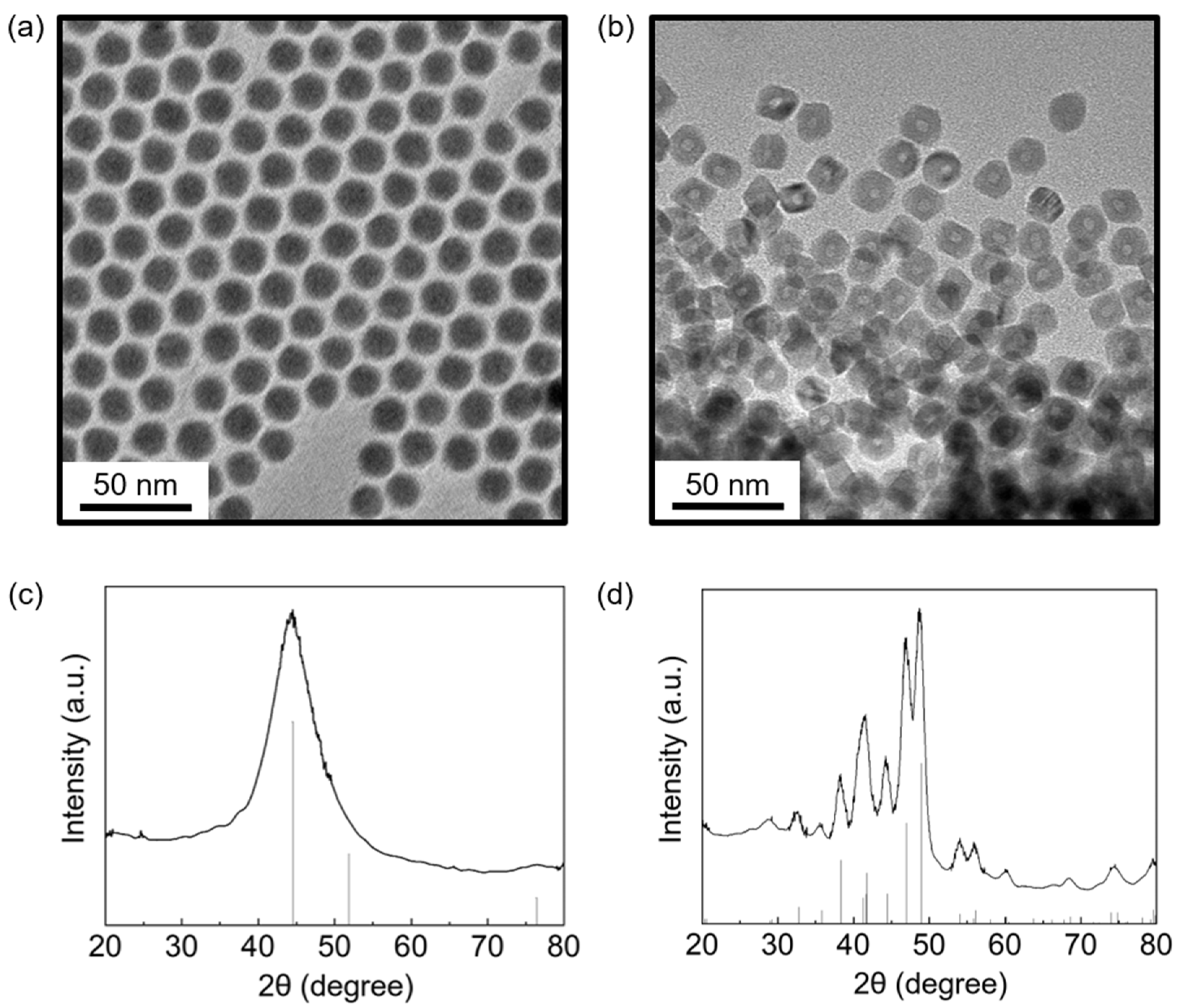
2.2.1. Synthesis of 9 nm Nickel Nanoparticles
2.2.2. Synthesis of 13 nm Nickel Nanoparticles
2.2.3. Synthesis of Ni12P5 Nanoparticles
2.2.4. Ligand Exchange of Nickel/Nickel Oxide (Ni/NiO) Nanoparticles
2.2.5. Reaction of Ni/NiO Nanoparticles with Proteins
2.2.6. Characterization of Materials
3. Results and Discussion
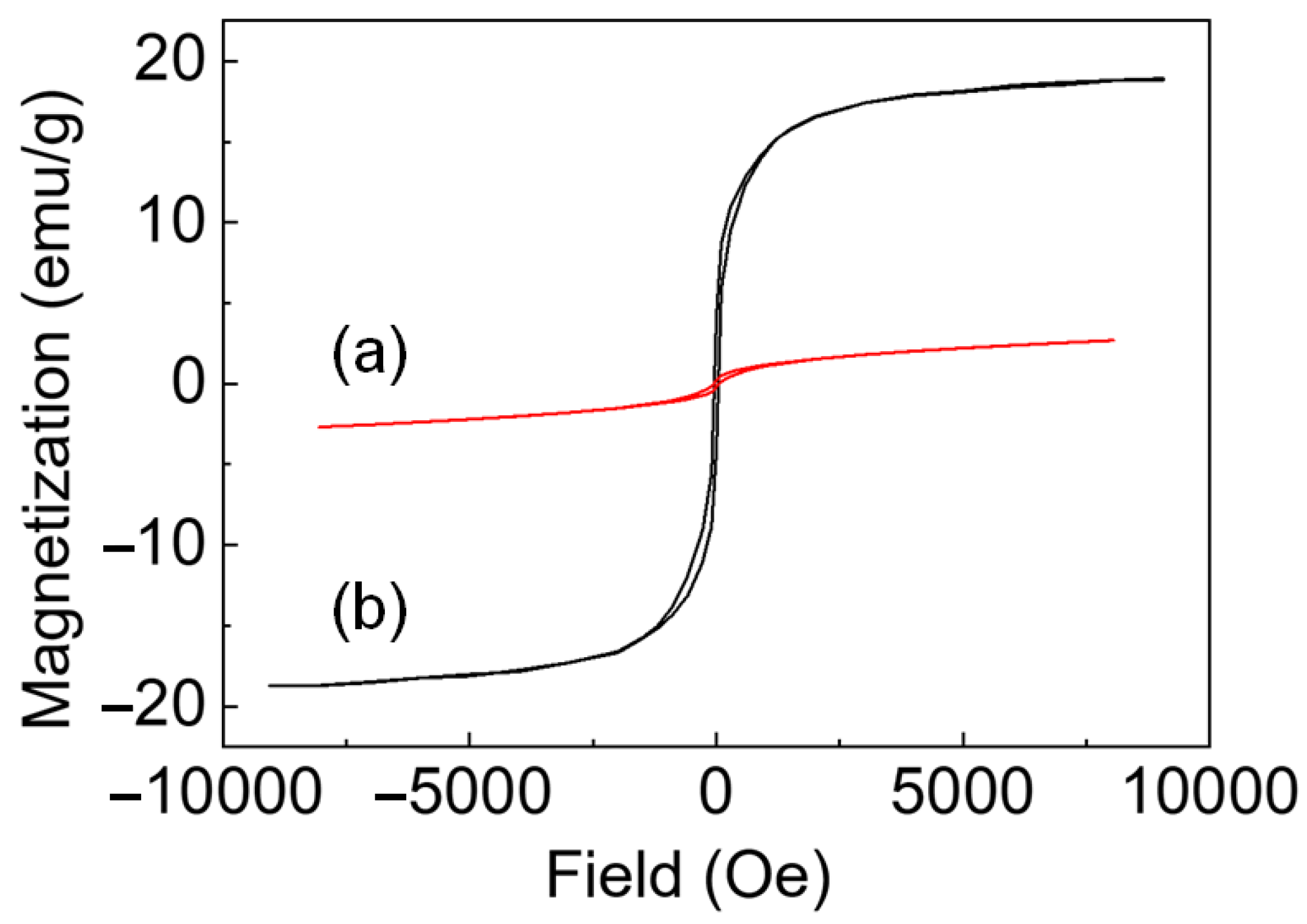
Analysis of Ni Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hyeon, T. Chemical synthesis of magnetic nanoparticles. Chem. Commun. 2003, 34, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Heydari Sheikh Hossein, H.; Jabbari, I.; Zarepour, A.; Zarrabi, A.; Ashrafizadeh, M.; Taherian, A.; Makvandi, P. Functionalization of magnetic nanoparticles by folate as potential MRI contrast agent for breast cancer diagnostics. Molecules 2020, 25, 4053. [Google Scholar] [CrossRef] [PubMed]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Mendoza-Garcia, A.; Li, Q.; Sun, S. Organic phase syntheses of magnetic nanoparticles and their applications. Chem. Rev. 2016, 116, 10473–10512. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, N.; Park, M.; Kim, B.H.; An, K.; Hyeon, T. Synthesis of Uniform Ferrimagnetic Magnetite Nanocubes. J. Am. Chem. Soc. 2009, 131, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H. Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef]
- Song, X.; Gao, L. Facile Synthesis and Hierarchical Assembly of Hollow Nickel Oxide Architectures Bearing Enhanced Photocatalytic Properties. J. Phys. Chem. C 2008, 112, 15299–15305. [Google Scholar] [CrossRef]
- Zhu, L.; Jiang, Y.; Zheng, J.; Zhang, N.; Yu, C.; Li, Y.; Pao, C.W.; Chen, J.L.; Jin, C.; Lee, J.F. Ultrafine Nanoparticle-Supported Ru Nanoclusters with Ultrahigh Catalytic Activity. Small 2015, 11, 4385–4393. [Google Scholar] [CrossRef]
- Lee, I.S.; Lee, N.; Park, J.; Kim, B.H.; Yi, Y.-W.; Kim, T.; Kim, T.K.; Lee, I.H.; Paik, S.R.; Hyeon, T. Ni/NiO Core/Shell Nanoparticles for Selective Binding and Magnetic Separation of Histidine-Tagged Proteins. J. Am. Chem. Soc. 2006, 128, 10658–10659. [Google Scholar] [CrossRef] [PubMed]
- Oka, K.; Yanagida, T.; Nagashima, K.; Tanaka, H.; Kawai, T. Nonvolatile Bipolar Resistive Memory Switching in Single Crystalline NiO Heterostructured Nanowires. J. Am. Chem. Soc. 2009, 131, 3434–3435. [Google Scholar] [CrossRef]
- Lang, J.-W.; Kong, L.-B.; Wu, W.-J.; Luo, Y.-C.; Kang, L. Facile approach to prepare loose-packed NiO nano-flakes materials for supercapacitors. Chem. Commun. 2008, 35, 4213–4215. [Google Scholar] [CrossRef] [PubMed]
- Koltypin, Y.; Fernandez, A.; Rojas, T.C.; Campora, J.; Palma, P.; Prozorov, R.; Gedanken, A. Encapsulation of Nickel Nanoparticles in Carbon Obtained by the Sonochemical Decomposition of Ni(C8H12)2. Chem. Mater. 1999, 11, 1331–1335. [Google Scholar] [CrossRef]
- Qiao, S.; Feng, C.; Guo, Y.; Chen, T.; Akram, N.; Zhang, Y.; Wang, W.; Yue, F.; Wang, J. CdS nanoparticles modified Ni@ NiO spheres as photocatalyst for oxygen production in water oxidation system and hydrogen production in water reduction system. Chem. Eng. J. 2020, 395, 125068. [Google Scholar] [CrossRef]
- Mateo, D.; Albero, J.; Garcia, H. Graphene supported NiO/Ni nanoparticles as efficient photocatalyst for gas phase CO2 reduction with hydrogen. Appl. Catal. B Environ. 2018, 224, 563–571. [Google Scholar] [CrossRef]
- Ely, T.O.; Amiens, C.; Chaudret, B.; Snoeck, E.; Verelst, M.; Respaud, M.; Broto, J.-M. Synthesis of Nickel Nanoparticles. Influence of Aggregation Induced by Modification of Poly(vinylpyrrolidone) Chain Length on Their Magnetic Properties. Chem. Mater. 1999, 11, 526–529. [Google Scholar] [CrossRef]
- Han, M.; Liu, Q.; He, J.; Song, Y.; Xu, Z.; Zhu, J.M. Controllable Synthesis and Magnetic Properties of Cubic and Hexagonal Phase Nickel Nanocrystals. Adv. Mater. 2007, 19, 1096–1100. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, X.; Chen, D. Monodispersed Nickel Nanoparticles with Tunable Phase and Size: Synthesis, Characterization, and Magnetic Properties. J. Phys. Chem. C 2008, 112, 18793–18797. [Google Scholar] [CrossRef]
- Zach, M.P.; Penner, R.M. Nanocrystalline Nickel Nanoparticles. Adv. Mater. 2000, 12, 878–883. [Google Scholar] [CrossRef]
- Winnischofer, H.; Rocha, T.C.R.; Nunes, W.C.; Socolovsky, L.M.; Knobel, M.; Zanchet, D. Chemical Synthesis and Structural Characterization of Highly Disordered Ni Colloidal Nanoparticles. ACS Nano 2008, 2, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Jia, R.; Zhao, J.; Liang, J.; Liu, Y.; Liu, C. Size-controlled synthesis of monodisperse nickel nanoparticles and investigation of their magnetic and catalytic properties. Appl. Surf. Sci. 2014, 316, 276–285. [Google Scholar] [CrossRef]
- Carenco, S.; Boissiere, C.; Nicole, L.; Sanchez, C.; Le Floch, P.; Mézailles, N. Controlled design of size-tunable monodisperse nickel nanoparticles. Chem. Mater. 2010, 22, 1340–1349. [Google Scholar] [CrossRef]
- Park, J.; Kang, E.; Son, S.U.; Park, H.M.; Lee, M.K.; Kim, J.; Kim, K.W.; Noh, H.J.; Park, J.H.; Bae, C.J.; et al. Monodisperse Nanoparticles of Ni and NiO: Synthesis, Characterization, Self-Assembled Superlattices, and Catalytic Applications in the Suzuki Coupling Reaction. Adv. Mater. 2005, 17, 429–434. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Li, X.; Li, C.; Zhang, R.; Zhang, Y.; Zhu, H. Sponge-like nickel phosphide–carbon nanotube hybrid electrodes for efficient hydrogen evolution over a wide pH range. Nano Res. 2017, 10, 415–425. [Google Scholar] [CrossRef]
- Xu, J.; Schulte, A.; Schönherr, H.; Jiang, X.; Yang, N. Hierarchical Carbon Nanofibers@ Nickel Phosphide Nanoparticles for High-Performance Supercapacitors. Small Struct. 2022, 3, 2100183. [Google Scholar] [CrossRef]
- Du, W.; Wei, S.; Zhou, K.; Guo, J.; Pang, H.; Qian, X. One-step synthesis and graphene-modification to achieve nickel phosphide nanoparticles with electrochemical properties suitable for supercapacitors. Mater. Res. Bull. 2015, 61, 333–339. [Google Scholar] [CrossRef]
- Sawhill, S.J.; Layman, K.A.; Van Wyk, D.R.; Engelhard, M.H.; Wang, C.; Bussell, M.E. Thiophene hydrodesulfurization over nickel phosphide catalysts: Effect of the precursor composition and support. J. Catal. 2005, 231, 300–313. [Google Scholar] [CrossRef]
- Gregg, K.A.; Perera, S.C.; Lawes, G.; Shinozaki, S.; Brock, S.L. Controlled Synthesis of MnP Nanorods: Effect of Shape Anisotropy on Magnetization. Chem. Mater. 2006, 18, 879–886. [Google Scholar] [CrossRef]
- Park, J.; Koo, B.; Hwang, Y.; Bae, C.; An, K.; Park, J.-G.; Park, H.M.; Hyeon, T. Novel Synthesis of Magnetic Fe2P Nanorods from Thermal Decomposition of Continuously Delivered Precursors using a Syringe Pump. Angew. Chem. Int. Ed. 2004, 43, 2282–2285. [Google Scholar] [CrossRef]
- Park, J.; Koo, B.; Yoon, K.Y.; Hwang, Y.; Kang, M.; Park, J.-G.; Hyeon, T. Generalized Synthesis of Metal Phosphide Nanorods via Thermal Decomposition of Continuously Delivered Metal−Phosphine Complexes Using a Syringe Pump. J. Am. Chem. Soc. 2005, 127, 8433–8440. [Google Scholar] [CrossRef] [PubMed]
- Henkes, A.E.; Schaak, R.E. Trioctylphosphine: A General Phosphorus Source for the Low-Temperature Conversion of Metals into Metal Phosphides. Chem. Mater. 2007, 19, 4234–4242. [Google Scholar] [CrossRef]
- Mabayoje, O.; Dunning, S.G.; Kawashima, K.; Wygant, B.R.; Ciufo, R.A.; Humphrey, S.M.; Mullins, C.B. Hydrogen evolution by Ni2P catalysts derived from phosphine MOFs. ACS Appl. Energy Mater. 2019, 3, 176–183. [Google Scholar] [CrossRef]
- Xie, S.; Qiao, M.; Zhou, W.; Luo, G.; He, H.; Fan, K.; Zhao, T.; Yuan, W. Controlled Synthesis, Characterization, and Crystallization of Ni−P Nanospheres. J. Phys. Chem. B 2005, 109, 24361–24368. [Google Scholar] [CrossRef]
- Carenco, S.; Resa, I.; Le Goff, X.; Le Floch, P.; Mézailles, N. White phosphorus as single source of “P” in the synthesis of nickel phosphide. Chem. Commun. 2008, 14, 2568–2570. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kang, H.; Hong, Y.-K.; Cho, G.; Choi, M.; Cho, J.; Ha, D.-H. Influence of the phosphorus source on iron phosphide nanoparticle synthesis for hydrogen evolution reaction catalysis. Int. J. Hydrogen Energy 2020, 45, 32780–32788. [Google Scholar] [CrossRef]
- Chiang, R.-K.; Chiang, R.-T. Formation of Hollow Ni2P Nanoparticles Based on the Nanoscale Kirkendall Effect. Inorg. Chem. 2007, 46, 369–371. [Google Scholar] [CrossRef]
- Henkes, A.E.; Vasquez, Y.; Schaak, R.E. Converting Metals into Phosphides: A General Strategy for the Synthesis of Metal Phosphide Nanocrystals. J. Am. Chem. Soc. 2007, 129, 1896–1897. [Google Scholar] [CrossRef]
- Railsback, J.G.; Johnston-Peck, A.C.; Wang, J.; Tracy, J.B. Size-Dependent Nanoscale Kirkendall Effect During the Oxidation of Nickel Nanoparticles. ACS Nano 2010, 4, 1913–1920. [Google Scholar] [CrossRef]
- Karmhag, R.; Niklasson, G.A.; Nygren, M. Oxidation kinetics of nickel nanoparticles. J. Appl. Phys. 2001, 89, 3012–3017. [Google Scholar] [CrossRef]
- Lee, C.; Schmidt, L.D. Microstructures in Oxidation and Reduction of Small Ni Particles: Bubbles and Clusters. J. Electrochem. Soc. 1989, 136, 2471–2475. [Google Scholar] [CrossRef]
- Yin, Y.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of Hollow Nanocrystals Through the Nanoscale Kirkendall Effect. Science 2004, 304, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.; Stock, S. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: New York, NJ, USA, 2001; pp. 174–177. [Google Scholar]
- Peng, S.; Sun, S. Synthesis and characterization of monodisperse hollow Fe3O4 nanoparticles. Angew. Chem. 2007, 119, 4233–4236. [Google Scholar] [CrossRef]
- Son, S.U.; Jang, Y.; Park, J.; Na, H.B.; Park, H.M.; Yun, H.J.; Lee, J.; Hyeon, T. Designed synthesis of atom-economical Pd/Ni bimetallic nanoparticle-based catalysts for sonogashira coupling reactions. J. Am. Chem. Soc. 2004, 126, 5026–5027. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kang, E.; Bae, C.J.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Park, H.M.; Hyeon, T. Synthesis, characterization, and magnetic properties of uniform-sized MnO nanospheres and nanorods. J. Phys. Chem. B 2004, 108, 13594–13598. [Google Scholar] [CrossRef]
- Chen, D.-H.; Wu, S.-H. Synthesis of nickel nanoparticles in water-in-oil microemulsions. Chem. Mater. 2000, 12, 1354–1360. [Google Scholar] [CrossRef]
- Knecht, M.R.; Garcia-Martinez, J.C.; Crooks, R.M. Synthesis, characterization, and magnetic properties of dendrimer-encapsulated nickel nanoparticles containing <150 atoms. Chem. Mater. 2006, 18, 5039–5044. [Google Scholar]
- Sidhaye, D.S.; Bala, T.; Srinath, S.; Srikanth, H.; Poddar, P.; Sastry, M.; Prasad, B. Preparation of nearly monodisperse nickel nanoparticles by a facile solution based methodology and their ordered assemblies. J. Phys. Chem. C 2009, 113, 3426–3429. [Google Scholar] [CrossRef]
- Roy, A.; Srinivas, V.; Ram, S.; De Toro, J.; Mizutani, U. Structure and magnetic properties of oxygen-stabilized tetragonal Ni nanoparticles prepared by borohydride reduction method. Phys. Rev. B 2005, 71, 184443. [Google Scholar] [CrossRef]
- Reetz, M.T.; Maase, M. Redox-Controlled Size-Selective Fabrication of Nanostructured Transition Metal Colloids. Adv. Mater. 1999, 11, 773–777. [Google Scholar] [CrossRef]
- Matsuda, J.; Yamamoto, T.; Takahashi, S.; Nakanishi, H.; Sasaki, K.; Matsumura, S. In Situ TEM Investigation of Structural Changes in Ni Nanoparticle Catalysts under Gas Atmospheres: Implications for Catalyst Degradation. ACS Appl. Nano Mater. 2021, 4, 2175–2182. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.; Lee, N.; Kim, B.H. Synthesis of Highly Monodisperse Nickel and Nickel Phosphide Nanoparticles. Nanomaterials 2022, 12, 3198. https://doi.org/10.3390/nano12183198
Cho H, Lee N, Kim BH. Synthesis of Highly Monodisperse Nickel and Nickel Phosphide Nanoparticles. Nanomaterials. 2022; 12(18):3198. https://doi.org/10.3390/nano12183198
Chicago/Turabian StyleCho, Hyungjin, Nohyun Lee, and Byung Hyo Kim. 2022. "Synthesis of Highly Monodisperse Nickel and Nickel Phosphide Nanoparticles" Nanomaterials 12, no. 18: 3198. https://doi.org/10.3390/nano12183198
APA StyleCho, H., Lee, N., & Kim, B. H. (2022). Synthesis of Highly Monodisperse Nickel and Nickel Phosphide Nanoparticles. Nanomaterials, 12(18), 3198. https://doi.org/10.3390/nano12183198





