Modern Synthesis and Sintering Techniques of Calcium Copper Titanium Oxide (CaCu3Ti4O12) Ceramics and Its Current Trend in Prospective Applications: A Mini-Review
Abstract
1. Introduction
2. Advanced Techniques for Synthesis of CCTO Powder
2.1. Microwave Synthesis of Calcium Copper Titanium Oxide Powder
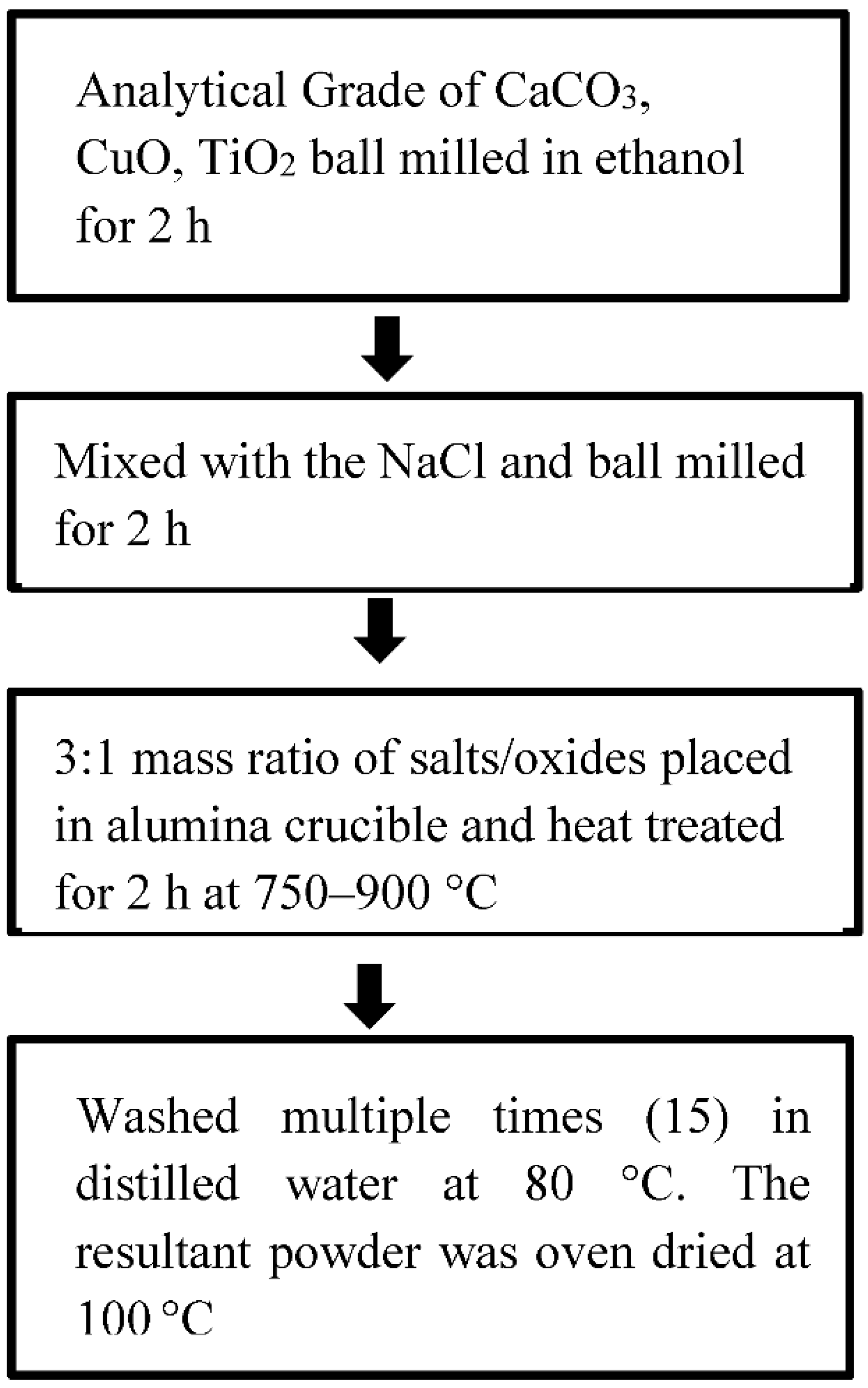
2.2. Molten Salt Synthesis (MSS)
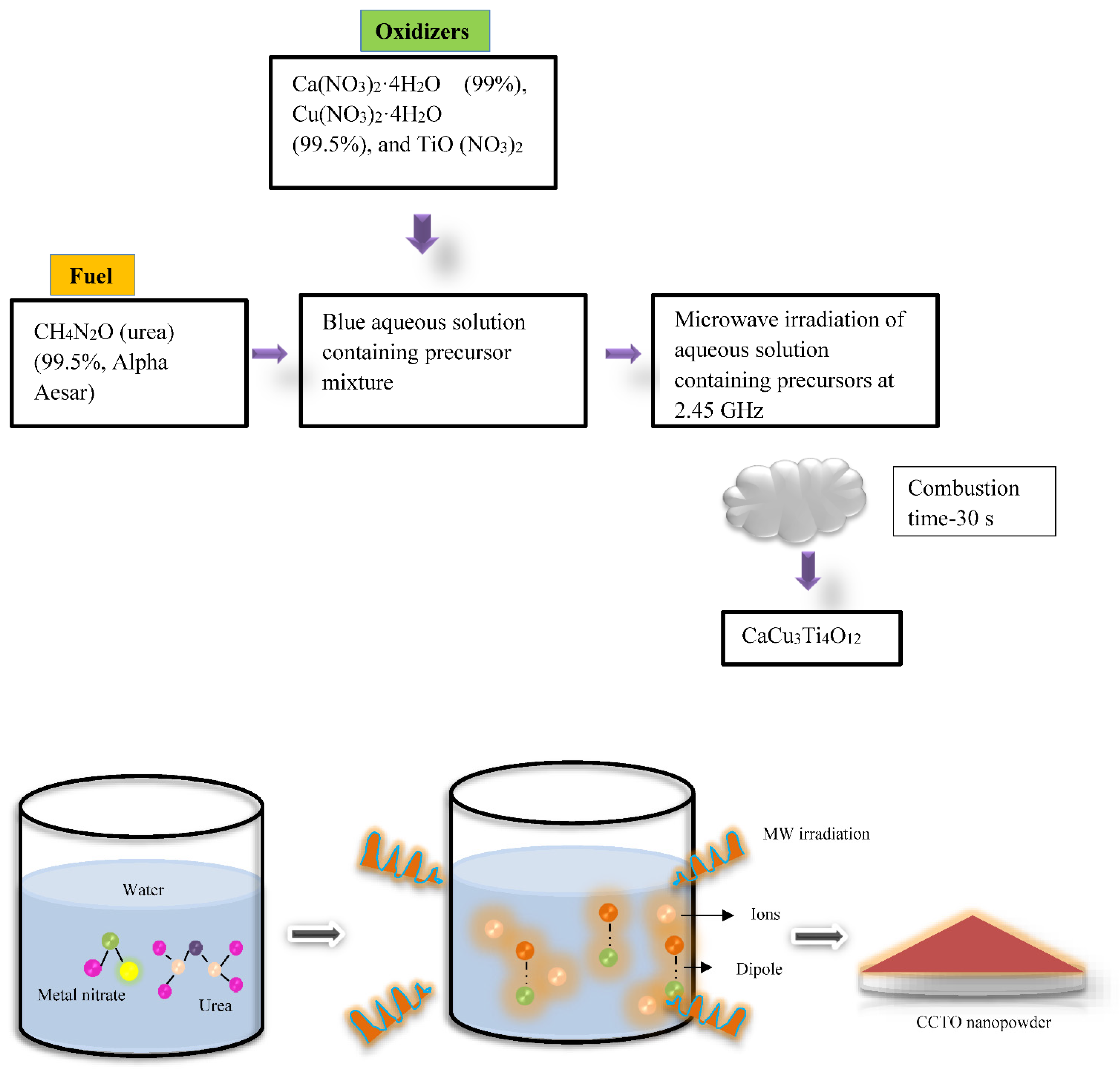
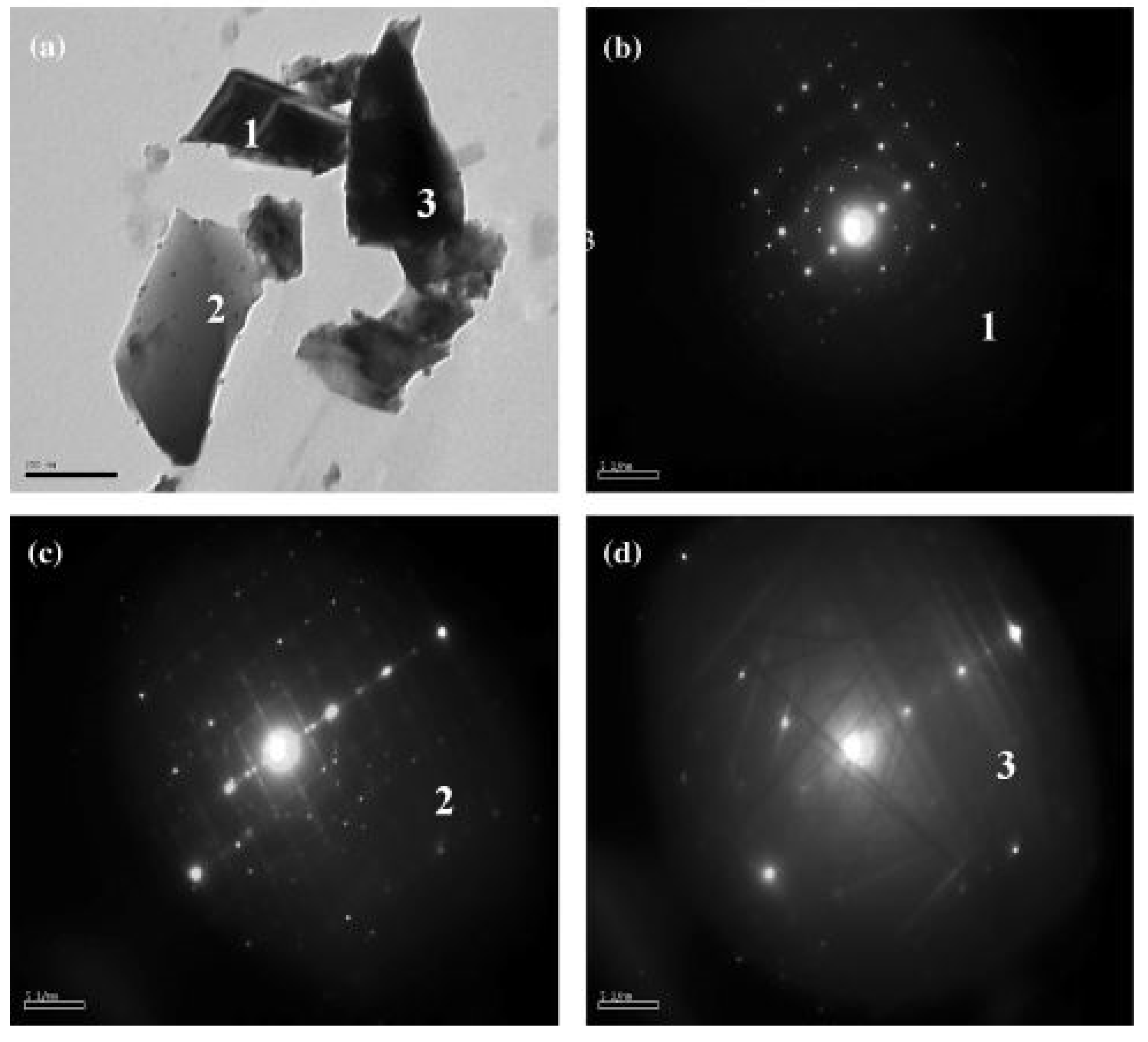
2.3. Microwave Flash Combustion Method
3. Conventional Sintering
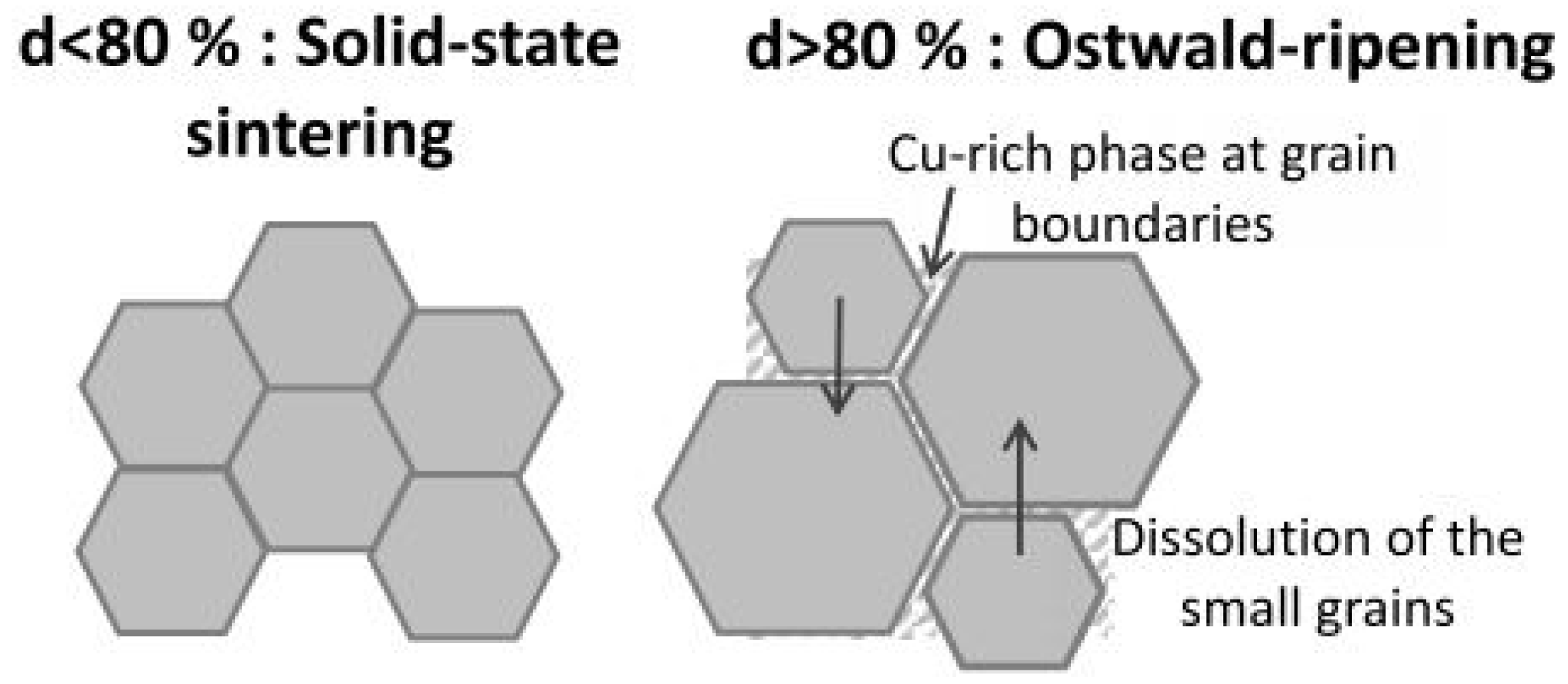
3.1. Grain Growth Mechanism
3.2. Effect of Doping on Electrical and Microstructural Properties
4. Spark Plasma Sintering (SPS)
4.1. Significance of Pressure and Pulsed Current in Rapid Densification
4.2. Effect of Dwell and Annealing Temperature
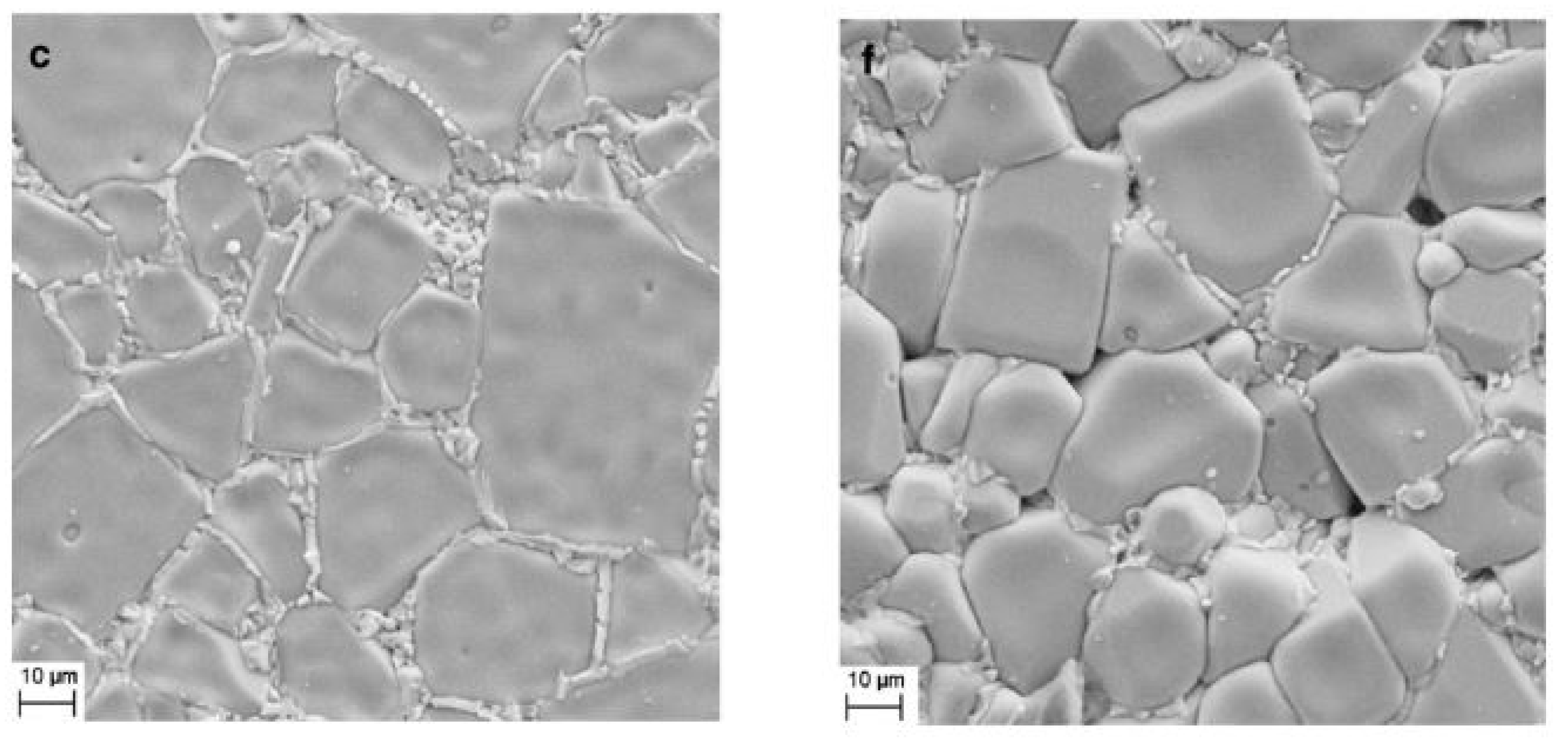
4.3. Influence of Sintering Temperature on the Microstructure and Dielectric Properties
| Samples | Grain Size | ε′ | Tan δ |
|---|---|---|---|
| SPS-800 | 2.23 (±1.56) μm | 7.68 × 103 | 0.119 |
| SPS-850 | 3.66 (±2.24) μm | 1.07 × 104 | 0.141 |
| SPS-900 | 4.68 (±2.45) μm | 1.58 × 104 | 0.490 |
5. Microwave Sintering (MWS)
5.1. Fundamentals of Microwave Sintering
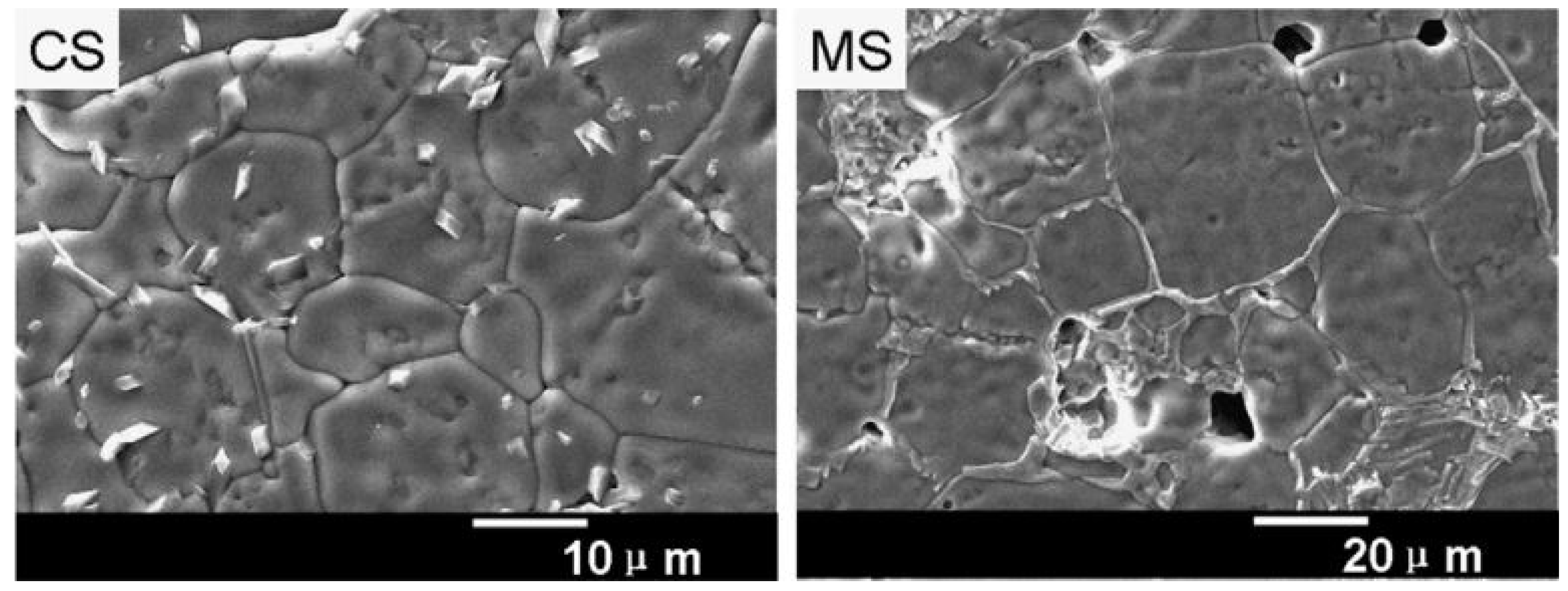
5.2. Comparison of Microwave Sintering over Conventional Sintering
5.3. Effect of MWS on Dielectric Properties
6. Applications
6.1. Paper-Based Zinc–Air Batteries

6.2. Energy Storage and Energy Conversion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramirez, A.; Subramanian, M.; Gardel, M.; Blumberg, G.; Li, D.; Vogt, T.; Shapiro, S. Giant dielectric constant response in a copper-titanate. Solid State Commun. 2000, 115, 217–220. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Li, D.; Duan, N.; Reisner, B.A.; Sleight, A.W. High Dielectric Constant in ACu3Ti4O12 and ACu3Ti3FeO12 Phases. J. Solid State Chem. 2000, 151, 323–325. [Google Scholar] [CrossRef]
- Shao, S.F.; Zhang, J.L.; Zheng, P.; Zhong, W.L.; Wang, C.L. Microstructure and electrical properties of CaCu3Ti4O12 ceramics. J. Appl. Phys. 2006, 99, 084106. [Google Scholar] [CrossRef]
- Ferrarelli, M.C.; Sinclair, D.C.; West, A.R.; Dabkowska, H.A.; Dabkowski, A.; Luke, G.M. Comment on the origin(s) of the giant permittivity effect in CaCu3Ti4O12 single crystals and ceramics. J. Mater. Chem. 2009, 19, 5916–5919. [Google Scholar] [CrossRef]
- Li, Y.; Liang, P.; Chao, X.; Yang, Z. Preparation of CaCu3Ti4O12 ceramics with low dielectric loss and giant dielectric constant by the sol–gel technique. Ceram. Int. 2013, 39, 7879–7889. [Google Scholar] [CrossRef]
- Singh, L.; Rai, U.; Mandal, K. Dielectric properties of zinc doped nanocrystalline calcium copper titanate synthesized by different approach. Mater. Res. Bull. 2013, 48, 2117–2122. [Google Scholar] [CrossRef]
- Bender, B.; Pan, M.-J. The effect of processing on the giant dielectric properties of CaCu3Ti4O12. Mater. Sci. Eng. B 2005, 117, 339–347. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, X.; Ren, L.; Sun, J.; Yang, L.; Guo, J.; Liao, R. Enhanced electrical properties of CaCu3Ti4O12 ceramics by spark plasma sintering: Role of Zn and Al co-doping. J. Alloy. Compd. 2019, 792, 1079–1087. [Google Scholar] [CrossRef]
- Löhnert, R.; Schmidt, R.; Töpfer, J. Effect of sintering conditions on microstructure and dielectric properties of CaCu3Ti4O12 (CCTO) ceramics. J. Electroceramics 2015, 34, 241–248. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, Y.; Wu, K. CCTO Giant Dielectric Ceramic Prepared by Reaction Sintering. Procedia Eng. 2015, 102, 468–474. [Google Scholar] [CrossRef]
- Late, R.; Rai, H.M.; Saxena, S.K.; Kumar, R.; Sagdeo, P. Effect of hafnium substitution on the dielectric properties of CaCu3Ti4O12. AIP Conf. Proc. 2015, 1665, 140019. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, L.; Chen, X.M. Effects of Nd-substitution on microstructures and dielectric characteristics of CaCu3Ti4O12 ceramics. J. Mater. Sci. Mater. Electron. 2010, 22, 345–350. [Google Scholar] [CrossRef]
- Ren, L.; Yang, L.; Xu, C.; Zhao, X.; Liao, R. Improvement of breakdown field and dielectric properties of CaCu3Ti4O12 ceramics by Bi and Al co-doping. J. Alloy. Compd. 2018, 768, 652–658. [Google Scholar] [CrossRef]
- Chinnathambi, M.; Sakthisabarimoorthi, A.; Jose, M.; Robert, R. Study of the Electrical and Dielectric behaviour of selenium doped CCTO ceramics prepared by a facile sol–gel route. Mater. Chem. Phys. 2021, 272, 124970. [Google Scholar] [CrossRef]
- Huang, Y.; Qiao, Y.; Li, Y.; He, J.; Zeng, H. Zn-Doped Calcium Copper Titanate Synthesized via Rapid Laser Sintering of Sol–gel Derived Precursors. Nanomaterials 2020, 10, 1163. [Google Scholar] [CrossRef] [PubMed]
- Samanta, B.; Kumar, P.; Nanda, D. Effect of Al substitution and secondary CuO phase on dielectric response in microwave-processed CaCu3Ti4−xAlxO12 ceramics. J. Mater. Sci. Mater. Electron. 2022, 33, 1425–1440. [Google Scholar] [CrossRef]
- Abdelal, O.A.; Hassan, A.A.; Ali, M.E.S. Dielectric Properties of Calcium Copper Titanates (CaCu3Ti4O12) Synthesized by Solid State Reaction. Int. J. Adv. Res. Chem. Sci. 2014, 1, 4–10, ISSN 2349-039X (Print) & ISSN 2349-0403 (Online). [Google Scholar]
- Boonlakhorn, J.; Nijpanich, S.; Thongbai, P.; Srepusharawoot, P. High dielectric permittivity and dielectric relaxation behavior in a Y2/3Cu3Ti4O12 ceramic prepared by a modified Sol−Gel route. Ceram. Int. 2022, 48, 15405–15413. [Google Scholar] [CrossRef]
- Aliabadi, T.N.; Alizadeh, P. Microstructure and dielectric properties of CCTO glass-ceramic prepared by the melt-quenching method. Ceram. Int. 2019, 45, 19316–19322. [Google Scholar] [CrossRef]
- Mao, P.; Wang, J.; Zhang, L.; Liu, S.; Zhao, Y.; Sun, Q. Rapid fabrication and improved electrical properties of CaCu3Ti4O12 ceramics by sol–gel and spark plasma sintering techniques. J. Mater. Sci. Mater. Electron. 2019, 30, 13401–13411. [Google Scholar] [CrossRef]
- Riquet, G.; Marinel, S.; Breard, Y.; Harnois, C.; Pautrat, A. Direct and hybrid microwave solid state synthesis of CaCu3Ti4O12 ceramic: Microstructures and dielectric properties. Ceram. Int. 2018, 44, 15228–15235. [Google Scholar] [CrossRef]
- Thomas, P.; Sathapathy, L.N.; Dwarakanath, K.; Varma, K.B.R. Microwave synthesis and sintering characteristics of CaCu3Ti4O12. Bull. Mater. Sci. 2007, 30, 567–570. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Luo, D.; Cao, M. Microwave synthesis of high dielectric constant CaCu3Ti4O12. J. Mater. Process. Technol. 2008, 208, 145–148. [Google Scholar] [CrossRef]
- Ouyang, X.; Cao, P.; Huang, S.; Zhang, W.; Huang, Z.; Gao, W. Microwave-Assisted Synthesis of High Dielectric Constant CaCu3Ti4O12 from Sol–Gel Precursor. J. Electron. Mater. 2015, 44, 2243–2249. [Google Scholar] [CrossRef]
- Chandrasekhar, M.; Kumar, P. Microwave Assisted Sol–gel Synthesis of High Dielectric Constant CCTO and BFN Ceramics for MLC Applica-tions. Processing Appl. Ceram. 2017, 11, 154–159. [Google Scholar]
- Chen, K.P.; He, Y.; Liu, D.Y.; De Liu, Z. Molten Salt Synthesis of CaCu3Ti4O12. Key Eng. Mater. 2008, 368–372, 115–117. [Google Scholar] [CrossRef]
- Prakash, B.S.; Varma, K.B.R. Molten salt synthesis of nanocrystalline phase of high dielectric constant material CaCu3Ti4O12. J. Nanosci. Nanotechnol. 2008, 8, 5762–5769. [Google Scholar] [CrossRef]
- Wan, W.; Liu, C.; Sun, H.; Luo, Z.; Yuan, W.-X.; Wu, H.; Qiu, T. Low-toxic gelcasting of giant dielectric-constant CaCu3Ti4O12 ceramics from the molten salt powder. J. Eur. Ceram. Soc. 2015, 35, 3529–3534. [Google Scholar] [CrossRef]
- Wan, W.; Yang, J.; Yuan, W.-X.; Zhao, X.; Liu, C.; Qiu, T. Preparation of Giant Dielectric CaCu3Ti4O12Ceramics via the Molten Salt Method from NaCl Flux. Int. J. Appl. Ceram. Technol. 2015, 13, 382–388. [Google Scholar] [CrossRef]
- Kumar, R.; Zulfequar, M.; Sharma, L.; Singh, V.N.; Senguttuvan, T.D. Growth of Nanocrystalline CaCu3Ti4O12 Ceramic by the Microwave Flash Combustion Method: Structural and Impedance Spectroscopic Studies. Cryst. Growth Des. 2015, 15, 1374–1379. [Google Scholar] [CrossRef]
- Kumar, R.; Zulfequar, M.; Senguttuvan, T.D. Dielectric properties of microwave flash combustion derived and spark plasma sintered CaCu3Ti4O12 ceramic: Role of reduction in grain boundary activation energy. J. Mater. Sci. Mater. Electron. 2015, 26, 6718–6722. [Google Scholar] [CrossRef][Green Version]
- Supriya, D.M.; Rajani, M.R.; Phani, A.R.; Naveen, C.V.S.; Ravishankar, R. Synthesis of CCTO and Doped CCTO Nanopowders and its Applications in the Field of Electronics. Mater. Today Proc. 2017, 4, 12021–12025. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, M.; Tan, Q. Embedding nanostructure and colossal permittivity of TiO2-covered CCTO perovskite materials by a hydrothermal route. J. Alloy. Compd. 2021, 885, 160948. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; He, J.; Lin, C. Dielectric properties of CaCu3Ti4O12 ceramics: Effect of high purity nanometric powders. J. Mater. Sci. Mater. Electron. 2014, 25, 1842–1847. [Google Scholar] [CrossRef]
- Fiorenza, P.; Raineri, V.; Ebbinghaus, S.G.; Nigro, R.L. CaCu3Ti4O12 single crystals: Insights on growth and nanoscopic investigation. CrystEngComm 2011, 13, 3900–3904. [Google Scholar] [CrossRef]
- Ramadan, R.M.; Labeeb, A.M.; Ward, A.; Ibrahim, A.M.H. New approach for synthesis of nano-sized CaCu3Ti4O12 powder by economic and innovative method. J. Mater. Sci. Mater. Electron. 2020, 31, 9065–9075. [Google Scholar] [CrossRef]
- Babu, S.; Govindan, A. Dielectric Properties of CaCu3Ti4O12 (CCTO) Prepared by Modified Solid State Reaction Method. Int. Rev. Appl. Eng. Res. 2014, 4, 275–280. [Google Scholar]
- Zhao, J.; Zhao, H.; Zhu, Z. Influence of sintering conditions and CuO loss on dielectric properties of CaCu3Ti4O12 ceramics. Mater. Res. Bull. 2019, 113, 97–101. [Google Scholar] [CrossRef]
- Riquet, G.; Marinel, S.; Bréard, Y.; Harnois, C. Sintering mechanism and grain growth in CaCu3Ti4O12 ceramics. Ceram. Int. 2019, 45, 9185–9191. [Google Scholar] [CrossRef]
- Wang, X.W.; Jia, P.B.; Zhang, B.H.; Sun, L.Y.; Liu, Q.B. Calcining temperature dependence on structure and dielectric properties of CaCu3Ti4O12 ceramics. J. Mater. Sci. Mater. Electron. 2016, 27, 12134–12140. [Google Scholar] [CrossRef]
- Senda, S.; Rhouma, S.; Torkani, E.; Megriche, A.; Autret, C. Effect of nickel substitution on electrical and microstructural properties of CaCu3Ti4O12 ceramic. J. Alloy. Compd. 2017, 698, 152–158. [Google Scholar] [CrossRef]
- Zhuk, N.; Sekushin, N.; Krzhizhanovskaya, M.; Belyy, V.; Korolev, R. Electrical properties of Ni-doped CaCu3Ti4O12 ceramics. Solid State Ionics 2021, 364, 115633. [Google Scholar] [CrossRef]
- Kafi, Z.; Kompany, A.; Arabi, H.; Zak, A.K. The effect of cobalt-doping on microstructure and dielectric properties of CaCu3Ti4O12 ceramics. J. Alloy. Compd. 2017, 727, 168–176. [Google Scholar] [CrossRef]
- Yanchevskii, O.; V’Yunov, O.; Belous, A.; Kovalenko, L. Dielectric properties of CaCu3Ti4O12 ceramics doped with aluminium and fluorine. J. Alloy. Compd. 2021, 874, 159861. [Google Scholar] [CrossRef]
- Boonlakhorn, J.; Srepusharawoot, P.; Thongbai, P. Distinct roles between complex defect clusters and insulating grain boundary on dielectric loss behaviors of (In3+/Ta5+) co-doped CaCu3Ti4O12 ceramics. Results Phys. 2019, 16, 102886. [Google Scholar] [CrossRef]
- Boonlakhorn, J.; Chanlek, N.; Thongbai, P.; Srepusharawoot, P. Strongly Enhanced Dielectric Response and Structural Investigation of (Sr2+, Ge4+) Co-Doped CCTO Ceramics. J. Phys. Chem. C 2020, 124, 20682–20692. [Google Scholar] [CrossRef]
- Jumpatam, J.; Putasaeng, B.; Chanlek, N.; Manyam, J.; Srepusharawoot, P.; Krongsuk, S.; Thongbai, P. Influence of Sn and F dopants on giant dielectric response and Schottky potential barrier at grain boundaries of CCTO ceramics. Ceram. Int. 2021, 47, 27908–27915. [Google Scholar] [CrossRef]
- Liu, J.; Lu, D.-Y.; Yu, X.-Y.; Liu, Q.; Tao, Q.; Change, H.; Zhu, P.-W. Dielectric Properties of Eu-Doped CaCu3Ti4O12 with Different Compensation Mechanisms. Acta Met. Sin. 2016, 30, 97–103. [Google Scholar] [CrossRef]
- Liu, L.; Fang, L.; Huang, Y.; Li, Y.; Shi, D.; Zheng, S.; Wu, S.; Hu, C. Dielectric and nonlinear current–voltage characteristics of rare–earth doped CaCu3Ti4O12 ceramics. J. Appl. Phys. 2011, 110, 094101. [Google Scholar] [CrossRef]
- Xue, R.; Chen, Z.; Dai, H.; Liu, D.; Li, T.; Zhao, G. Effects of rare earth ionic doping on microstructures and electrical properties of CaCu3Ti4O12 ceramics. Mater. Res. Bull. 2015, 66, 254–261. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, H.; Liu, Y.; Song, Y.; Liu, P. Rare earth doped CaCu3Ti4O12 electronic ceramics for high frequency applications. J. Rare Earths 2010, 28, 43–47. [Google Scholar] [CrossRef]
- Kashyap, R.; Thakur, O.; Tandon, R. Study of structural, dielectric and electrical conduction behaviour of Gd substituted CaCu3Ti4O12 ceramics. Ceram. Int. 2012, 38, 3029–3037. [Google Scholar] [CrossRef]
- Mamedov, V. Spark plasma sintering as advanced PM sintering method. Powder Met. 2002, 45, 322–328. [Google Scholar] [CrossRef]
- Cavaliere, P.; Sadeghi, B.; Shabani, A. Spark Plasma Sintering: Process Fundamentals. In Spark Plasma Sintering of Materials: Advances in Processing and Applications; Cavaliere, P., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–20. [Google Scholar] [CrossRef]
- Suárez, M.; Fernández, A.; Menéndez, J.; Torrecillas, R.; Kessel, H.U.; Hennicke, J.; Kirchner, R.; Kessel, T. Challenges and Opportunities for Spark Plasma Sintering: A Key Technology for a New Generation of Materials. Sinter. Appl. 2013, 13, 319–342. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Munir, Z.A.; Ohyanagi, M. Perspectives on the spark plasma sintering process. J. Mater. Sci. 2020, 56, 1–15. [Google Scholar] [CrossRef]
- de Carvalho, E.; Bertolete, M.; Machado, I.F.; Muccillo, E. Effect of the Dwell Temperature on Spark Plasma Sintered CaCu3Ti4O12. Mater. Sci. Forum 2012, 727–728, 982–987. [Google Scholar] [CrossRef]
- Ruan, X.F.; Yang, Z.; Zhang, Y.; Shi, J.; Xiong, R. The Synthesis of CaCu3Ti4O12 High Dielectric Ceramics by a Spark Plasma Sintering Method. Key Eng. Mater. 2013, 538, 250–253. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Yamada, K. Grain size effect on the giant dielectric constant of CaCu3Ti4O12 nanoceramics prepared by mechanosynthesis and spark plasma sintering. J. Appl. Phys. 2014, 115, 154103. [Google Scholar] [CrossRef]
- Lin, H.; He, X.; Gong, Y.; Pang, D.; Yi, Z. Tuning the nonlinear current-voltage behavior of CaCu3Ti4O12 ceramics by spark plasma sintering. Ceram. Int. 2018, 44, 8650–8655. [Google Scholar] [CrossRef]
- Li, T.; Sun, Y.; Dai, H.; Liu, D.; Chen, J.; Xue, R.; Chen, Z. Influence of spark plasma sintering temperature on the microstructures, dielectric and I–V properties of CaCu3Ti4O12 ceramics. J. Alloy. Compd. 2020, 829, 154595. [Google Scholar] [CrossRef]
- Kotb, H.M.; Ahmad, M.M.; Aldabal, S.; Alshoaibi, A.; Aljaafari, A. Structural and dielectric behavior of Al-substituted CaCu3Ti4O12 ceramics with giant dielectric constant by spark plasma sintering. J. Mater. Sci. Mater. Electron. 2019, 30, 18259–18267. [Google Scholar] [CrossRef]
- Chatterjee, A.; Basak, T.; Ayappa, K.G. Analysis of microwave sintering of ceramics. AIChE J. 1998, 44, 2302–2311. [Google Scholar] [CrossRef]
- Das, S.; Mukhopadhyay, A.K.; Datta, S.; Basu, D. Prospects of microwave processing: An overview. Bull. Mater. Sci. 2009, 32, 1–13. [Google Scholar] [CrossRef]
- Agrawal, D.K. Microwave processing of ceramics. Curr. Opin. Solid State Mater. Sci. 1998, 3, 480–485. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Olevsky, E.A.; Krikun, E.V. Microwave Sintering: Fundamentals and Modeling. J. Am. Ceram. Soc. 2013, 96, 1003–1020. [Google Scholar] [CrossRef]
- Boch, P.; Lequeux, N. Do microwaves increase the sinterability of ceramics? Solid State Ionics 1997, 101–103, 1229–1233. [Google Scholar] [CrossRef]
- Borrell, A.; Salvador, M.D. Advanced Ceramic Materials Sintered by Microwave Technology. In Sintering Technology—Method and Application; Malin Liu, M., Ed.; InTech: Vienna, Austria, 2018. [Google Scholar] [CrossRef]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. J. Alloy. Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Sulaiman, M.A. Effect of Calcination Time on the Microstructure and Dielectric Properties of Ca-Cu3Ti4O12 Using Enhanced Microwave Processing. Malays. J. Microsc. 2020, 16, 83–93. [Google Scholar]
- Hutagalung, S.D.; Ibrahim, M.I.M.; Ahmad, Z.A. Microwave assisted sintering of CaCu3Ti4O12. Ceram. Int. 2008, 34, 939–942. [Google Scholar] [CrossRef]
- A Ramírez, M.; Bueno, P.R.; Longo, E.; A Varela, J. Conventional and microwave sintering of CaCu3Ti4O12/CaTiO3ceramic composites: Non-ohmic and dielectric properties. J. Phys. D Appl. Phys. 2008, 41, 152004. [Google Scholar] [CrossRef]
- Kumar, R.; Zulfequar, M.; Singh, V.; Tawale, J.; Senguttuvan, T. Microwave sintering of dielectric CaCu3Ti4O12: An interfacial conductance and dipole relaxation effect. J. Alloy. Compd. 2012, 541, 428–432. [Google Scholar] [CrossRef]
- Jesurani, S.; Kanagesan, S.; Hashim, M.; Ismail, I.; Sabbaghizadeh, R. Influence of Er on microstructural and dielectric properties of CaCu3Ti4O12. J. Mater. Sci. Mater. Electron. 2014, 26, 456–463. [Google Scholar] [CrossRef]
- Evangeline, T.G.; Annamalai, A.R. Influence of heating modes on the microstructural and dielectric properties of calcium copper titanium oxide (CaCu3Ti4O12/CCTO) using conventional and microwave sintering. J. Mater. Sci. Mater. Electron. 2022, 33, 5806–5815. [Google Scholar] [CrossRef]
- Evangeline, T.G.; Annamalai, A.R. Dielectric properties of conventional and microwave-sintered Lanthanum doped CaCu3Ti4O12 ceramics for high-frequency applications. Ceram. Int. 2022, 48, 25705–25713. [Google Scholar] [CrossRef]
- Kawrani, S.; Boulos, M.; Cornu, D.; Bechelany, M. From Synthesis to Applications: Copper Calcium Titanate (CCTO) and its Magnetic and Photocatalytic Properties. ChemistryOpen 2019, 8, 922–950. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Ain, M.F.; Ahmad, Z.A. A Short Review on Copper Calcium Titanate (CCTO) Electroceramic: Synthesis, Dielectric Properties, Film Deposition, and Sensing Application. Nano-Micro Lett. 2016, 8, 291–311. [Google Scholar] [CrossRef]
- Qu, Y.; Wu, Y.; Fan, G.; Xie, P.; Liu, Y.; Zhang, Z.; Xin, J.; Jiang, Q.; Sun, K.; Fan, R. Tunable radio-frequency negative permittivity of Carbon/CaCu3Ti4O12 metacomposites. J. Alloy. Compd. 2020, 834, 155164. [Google Scholar] [CrossRef]
- Ma, R.; Cheng, C.; Qu, Y.; Fan, R. Tailorable negative permittivity of graphene-carbon nanotube/copper calcium titanate metacomposites. Ceram. Int. 2020, 47, 9971–9978. [Google Scholar] [CrossRef]
- Singh, J.P.; Gautam, S.; Kumar, P.; Tripathi, A.; Chen, J.-M.; Chae, K.H.; Asokan, K. Correlation between the dielectric properties and local electronic structure of copper doped calcium titanate. J. Alloy. Compd. 2013, 572, 84–89. [Google Scholar] [CrossRef]
- Mohammed, J.; Carol, T.T.C.; Hafeez, H.; Adamu, B.; Wudil, Y.; Takai, Z.; Godara, S.K.; Srivastava, A. Tuning the dielectric and optical properties of Pr Co–substituted calcium copper titanate for electronics applications. J. Phys. Chem. Solids 2018, 126, 85–92. [Google Scholar] [CrossRef]
- Wang, J.; Chao, X.; Li, G.; Feng, L.; Zhao, K. Fabrication and enhanced characterization of copper powder filled copper calcium titanate/poly(vinylidene difluoride) composite. J. Mater. Sci. Mater. Electron. 2016, 28, 5435–5439. [Google Scholar] [CrossRef]
- Wang, S.; He, X.; Chen, Q.; Chen, Y.; He, W.; Zhou, G.; Zhang, H.; Jin, X.; Su, X. Graphene-coated copper calcium titanate to improve dielectric performance of PPO-based composite. Mater. Lett. 2018, 233, 355–358. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Chen, H.; Xiong, C.; Shi, Z.; Yang, Q. Flexible dielectric nanocomposite films based on chitin/boron nitride/copper calcium titanate with high energy density. Compos. Part. A Appl. Sci. Manuf. 2021, 149, 106554. [Google Scholar] [CrossRef]
- Ding, B.; Lin, J.; Wan, F.; Qu, Y.; Wu, J.; Sun, K.; Fan, R. Communication—Tunable Epsilon-Negative Property of Nickel/Copper Calcium Titanate Cermets. ECS J. Solid State Sci. Technol. 2020, 9, 123004. [Google Scholar] [CrossRef]
- Luangchuang, P.; Chueangchayaphan, N.; Sulaiman, M.A.; Chueangchayaphan, W. Evaluation of cure characteristic, physico-mechanical, and dielectric properties of calcium copper titanate filled acrylonitrile-butadiene rubber composites: Effect of calcium copper titanate loading. J. Appl. Polym. Sci. 2020, 137, 49136. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Nayak, J. Improvement of humidity sensing performance and dielectric response through pH variation in CaCu3Ti4O12 ceramics. Sensors Actuators A Phys. 2022, 341, 113603. [Google Scholar] [CrossRef]
- Tomchenko, A.A.; Harmer, G.P.; Marquis, B.T.; Allen, J.W. Semiconducting metal oxide sensor array for the selective detection of combustion gases. Sensors Actuators B Chem. 2003, 93, 126–134. [Google Scholar] [CrossRef]
- Hilder, M.; Winther-Jensen, B.; Clark, N. Paper-based, printed zinc–air battery. J. Power Sources 2009, 194, 1135–1141. [Google Scholar] [CrossRef]
- Hu, L.; Choi, J.W.; Yang, Y.; Jeong, S.; La Mantia, F.; Cui, L.-F.; Cui, Y. Highly conductive paper for energy-storage devices. Proc. Natl. Acad. Sci. USA 2009, 106, 21490–21494. [Google Scholar] [CrossRef]
- Liu, H.; Crooks, R.M. Paper-Based Electrochemical Sensing Platform with Integral Battery and Electrochromic Read-Out. Anal. Chem. 2012, 84, 2528–2532. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Zhong, J.; Hu, B.; Hu, Q.; Zhou, J.; Wang, Z.L. A paper-based nanogenerator as a power source and active sensor. Energy Environ. Sci. 2013, 6, 1779–1784. [Google Scholar] [CrossRef]
- Bu, Y.; Gwon, O.; Nam, G.; Jang, H.; Kim, S.; Zhong, Q.; Cho, J.; Kim, G. A Highly Efficient and Robust Cation Ordered Perovskite Oxide as a Bifunctional Catalyst for Rechargeable Zinc–air Batteries. ACS Nano 2017, 11, 11594–11601. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Cano, Z.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc–air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2016, 29, 1604685. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Seo, E.; Yang, J.; Lee, Y.; Kim, B.-S.; Song, H.-K. Bifunctional hydrous RuO2 nanocluster electrocatalyst embedded in carbon matrix for efficient and durable operation of rechargeable zinc–air batteries. Sci. Rep. 2017, 7, 7150. [Google Scholar] [CrossRef]
- Bhardwaj, U.; Sharma, A.; Gupta, V.; Batoo, K.M.; Hussain, S.; Kushwaha, H.S. High energy storage capabilities of CaCu3Ti4O12 for paper-based zinc–air battery. Sci. Rep. 2022, 12, 3999. [Google Scholar] [CrossRef]
- Pandey, R.K.; Stapleton, W.A.; Tate, J.; Bandyopadhyay, A.K.; Sutanto, I.; Sprissler, S.; Lin, S. Applications of CCTO supercapacitor in energy storage and electronics. AIP Adv. 2013, 3, 062126. [Google Scholar] [CrossRef]
- Kaur, S.; Kumar, A.; Sharma, A.L.; Singh, D.P. Dielectric and energy storage behavior of CaCu3Ti4O12 nanoparticles for capacitor application. Ceram. Int. 2019, 45, 7743–7747. [Google Scholar] [CrossRef]
- Kushwaha, H.S.; A Madhar, N.; Ilahi, B.; Thomas, P.; Halder, A.; Vaish, R. Efficient Solar Energy Conversion Using CaCu3Ti4O12 Photoanode for Photocatalysis and Photoelectrocatalysis. Sci. Rep. 2016, 6, 18557. [Google Scholar] [CrossRef]
- Kawrani, S.; Boulos, M.; Bekheet, M.F.; Viter, R.; Nada, A.; Riedel, W.; Roualdes, S.; Cornu, D.; Bechelany, M. Segregation of copper oxide on calcium copper titanate surface induced by Graphene Oxide for Water splitting applications. Appl. Surf. Sci. 2020, 516, 146051. [Google Scholar] [CrossRef]
- Irshad, M.; Ain, Q.T.; Zaman, M.; Aslam, M.Z.; Kousar, N.; Asim, M.; Rafique, M.; Siraj, K.; Tabish, A.N.; Usman, M.; et al. Photocatalysis and perovskite oxide-based materials: A remedy for a clean and sustainable future. RSC Adv. 2022, 12, 7009–7039. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.H.; Dyer, M.S.; Palgrave, R.G.; Ireland, C.P.; Darwent, J.R.; Claridge, J.B.; Rosseinsky, M.J. Visible Light Photo-oxidation of Model Pollutants Using CaCu3Ti4O12: An Experimental and Theoretical Study of Optical Properties, Electronic Structure, and Selectivity. J. Am. Chem. Soc. 2010, 133, 1016–1032. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Xu, C.; Feng, L. Sonocatalysis and sono-photocatalysis in CaCu3Ti4O12 ceramics. Ceram. Int. 2022, 48, 11338–11345. [Google Scholar] [CrossRef]
- Saqib, N.U.; Shah, I.; Adnan, R. An Emerging Photocatalyst for Wastewater Remediation: A Mini-Review on CaCu3Ti4O12 Photoca-talysis. Environ. Sci. Pollut. Res. 2022, 29, 40403–40414. [Google Scholar] [CrossRef] [PubMed]


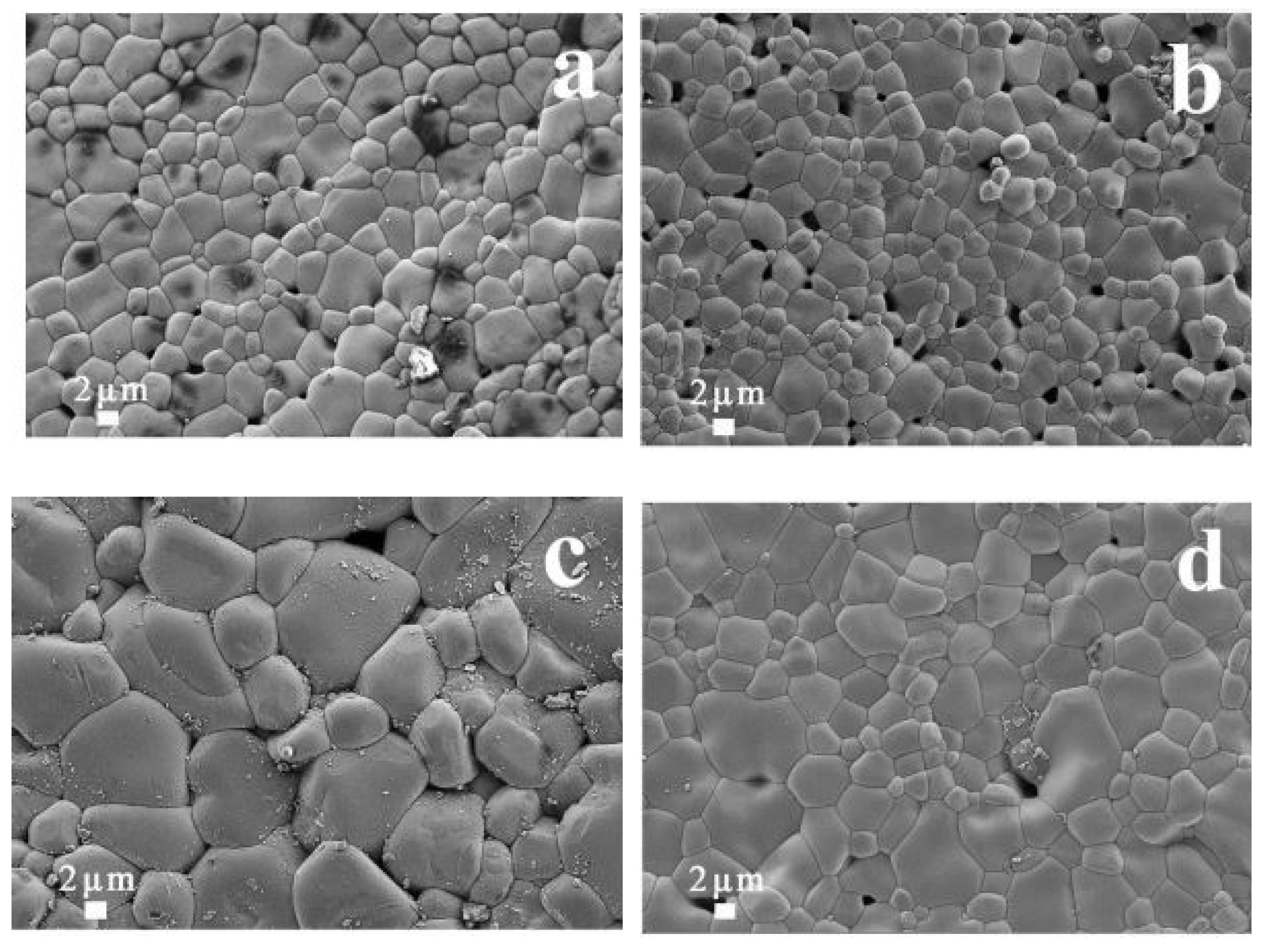


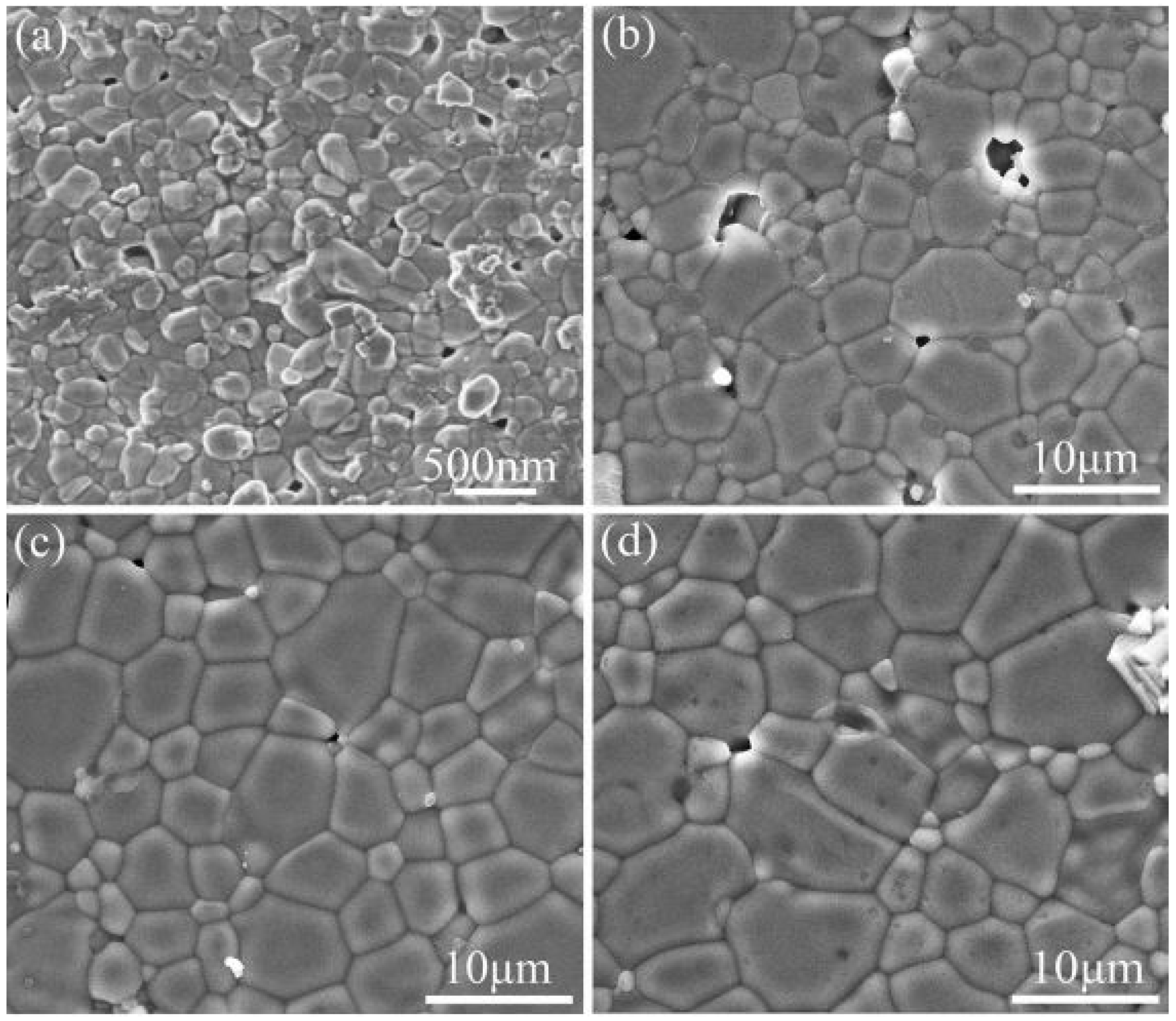

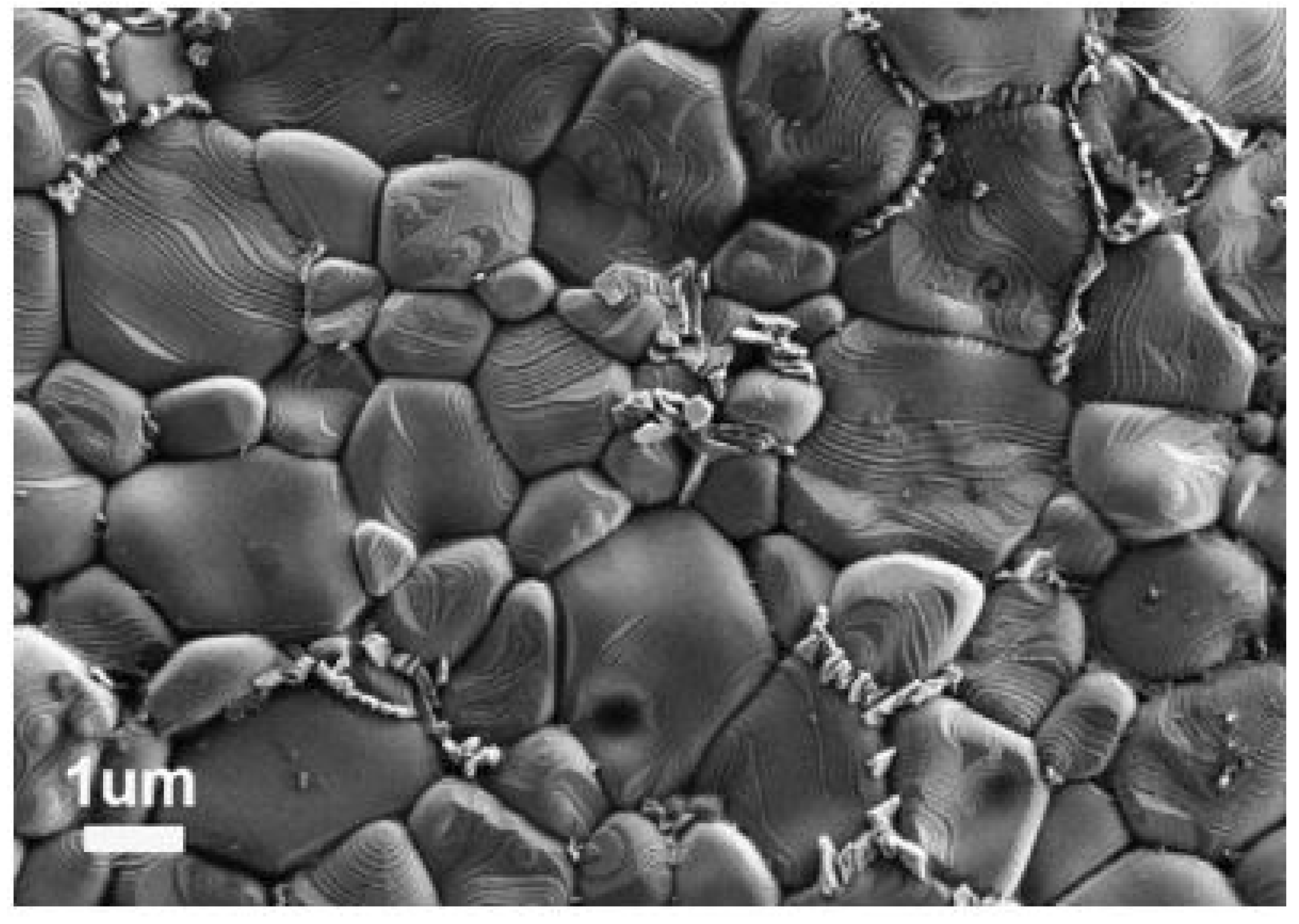
| Synthesis Route | Precursors | Processing Temperature/Time | Advantages | Disadvantages |
|---|---|---|---|---|
| Microwave synthesis | Solid state | 950 °C/20 min | Powders synthesized via this technique yielded a better dielectric constant and subsequently low dielectric at high frequency than the conventional synthesis route. | - [22] |
| Solid state | 800 °C/30 min | Single phase cubic CCTO was obtained with less energy consumption and short processing time. | Repeated calcination is to be ensured to remove secondary traces [23]. | |
| Sol–gel | 950 °C/17 min | 89.1 wt% was fabricated in a shorter duration with particles measuring 3.826 microns. The resultant powder demonstrated excellent dielectric performance along with low dielectric loss. | - [24] | |
| Sol–gel | 800 °C/40 min | CCTO powder of desirable grade has been manufactured at low processing temperatures. | - [25] | |
| Molten Salt Synthesis | CaCO3, CuO and TiO2 NaCl/KCl salts | 750–1000 °C/ 1–16 h | High influence of holding time on the particle size. | High temperature and processing time [26]. |
| CaCO3, CuO and TiO2 0 to 20 mol% KCl | 750 °C/10 h | Phase pure CCTO of size ~150 nm has been obtained over conventional routes requiring high temperatures of 1000 °C. KCl flux played a prominent role in reducing the formation temperature of CCTO. | - [27] | |
| CaCO3 (≥99.0%), CuO (≥99.0%) and TiO2 (≥99.0%) NaCl flux | 750–850 °C/2 h | Green compact of high homogeneity and mechanical strength. Gel casting exhibited lower loss (0.2) and a higher dielectric constant than isostatic pressing. | Optimization of dispersant dosage and pH is essential [28]. | |
| CaCO3 (≥99.0%), CuO (≥99.0%) and TiO2 (≥99.0%) NaCl as molten salt | 800 °C/2 h | Better dielectric performance than traditionally synthesized CCTO powder. | - [29] | |
| Microwave flash combustion method | Ca(NO3)2·4H2O (99%, Merck),Cu(NO3)2·4H2O (99.5%, Merck), and TiO(NO3)2 oxidizers urea (CH4N2O) as fuel | 800–900 °C/5 h | CCTO powder of particles size ranging from 50–70 nm was fabricated for the first using this technique. | - [30] |
| Oxidizers (Ca(NO3)24H2O, Cu (NO3)2 4H2O and TiO(NO3)2), urea (CH4N2O) | 900 °C/5 h | Nanocrystalline CCTO particles were formed. | Precautions are to be followed during combustion [31]. |
| Sample | Rg (Ω) | Rgb (Ω) |
|---|---|---|
| CaCu3Co0Ti4O12 | 43 | 5.5 × 105 |
| CaCu2.8Co0.2Ti4O12 | 52 | 9.5 × 106 |
| CaCu2.6Co0.4Ti4O12 | 11 | 3 × 107 |
| CaCu2.4Co0.6Ti4O12 | 77 | 3.2 × 108 |
| Frequency Hz | x = 0 | x = 0.2 | x = 0.5 | |||
|---|---|---|---|---|---|---|
| εr | tan δ | εr | tan δ | εr | tan δ | |
| 50 | 1730 | 0.16 | 5480 | 4.2 | 11,700 | 8.5 |
| 100 | 1633 | 0.15 | 3650 | 3.3 | 8310 | 6.1 |
| 1 k | 1572 | 0.06 | 1850 | 0.9 | 3270 | 1.8 |
| 10 ck | 1527 | 0.06 | 1280 | 0.3 | 1690 | 0.6 |
| 100 ck | 1491 | 0.06 | 1010 | 0.1 | 1050 | 0.3 |
| 1 m | 1462 | 0.17 | 790 | 0.3 | 762 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
T., G.E.; Annamalai, A.R.; Magdaline, T.B. Modern Synthesis and Sintering Techniques of Calcium Copper Titanium Oxide (CaCu3Ti4O12) Ceramics and Its Current Trend in Prospective Applications: A Mini-Review. Nanomaterials 2022, 12, 3181. https://doi.org/10.3390/nano12183181
T. GE, Annamalai AR, Magdaline TB. Modern Synthesis and Sintering Techniques of Calcium Copper Titanium Oxide (CaCu3Ti4O12) Ceramics and Its Current Trend in Prospective Applications: A Mini-Review. Nanomaterials. 2022; 12(18):3181. https://doi.org/10.3390/nano12183181
Chicago/Turabian StyleT., Gecil Evangeline, A. Raja Annamalai, and T. Bonnisa Magdaline. 2022. "Modern Synthesis and Sintering Techniques of Calcium Copper Titanium Oxide (CaCu3Ti4O12) Ceramics and Its Current Trend in Prospective Applications: A Mini-Review" Nanomaterials 12, no. 18: 3181. https://doi.org/10.3390/nano12183181
APA StyleT., G. E., Annamalai, A. R., & Magdaline, T. B. (2022). Modern Synthesis and Sintering Techniques of Calcium Copper Titanium Oxide (CaCu3Ti4O12) Ceramics and Its Current Trend in Prospective Applications: A Mini-Review. Nanomaterials, 12(18), 3181. https://doi.org/10.3390/nano12183181




_Hui.png)



