Hollow Iron Oxide Nanospheres Obtained through a Combination of Atomic Layer Deposition and Electrospraying Technologies
Abstract
:1. Introduction
2. Experimental Details
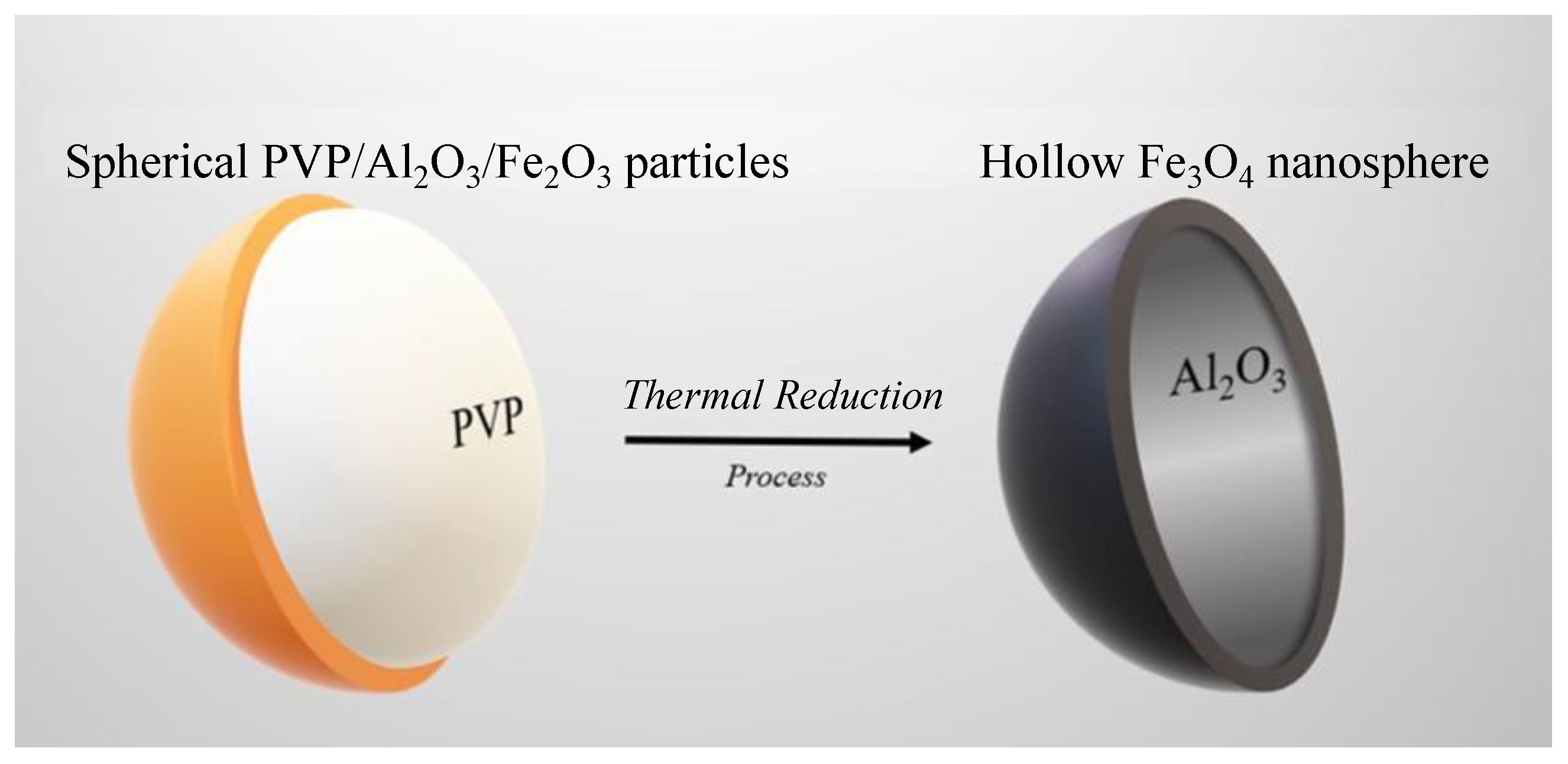
2.1. Development of Hollow Fe3O4 Nanospheres
2.1.1. Electrospraying Process of PVP Spherical Particles
2.1.2. Synthesis of Spherical PVP/Al2O3 Particles
2.1.3. Synthesis of Spherical PVP/Al2O3/Fe2O3 Particles
2.1.4. Thermal Reduction Process to Obtain Hollow Fe3O4 Nanospheres
2.2. Characterization Techniques
3. Results and Discussion
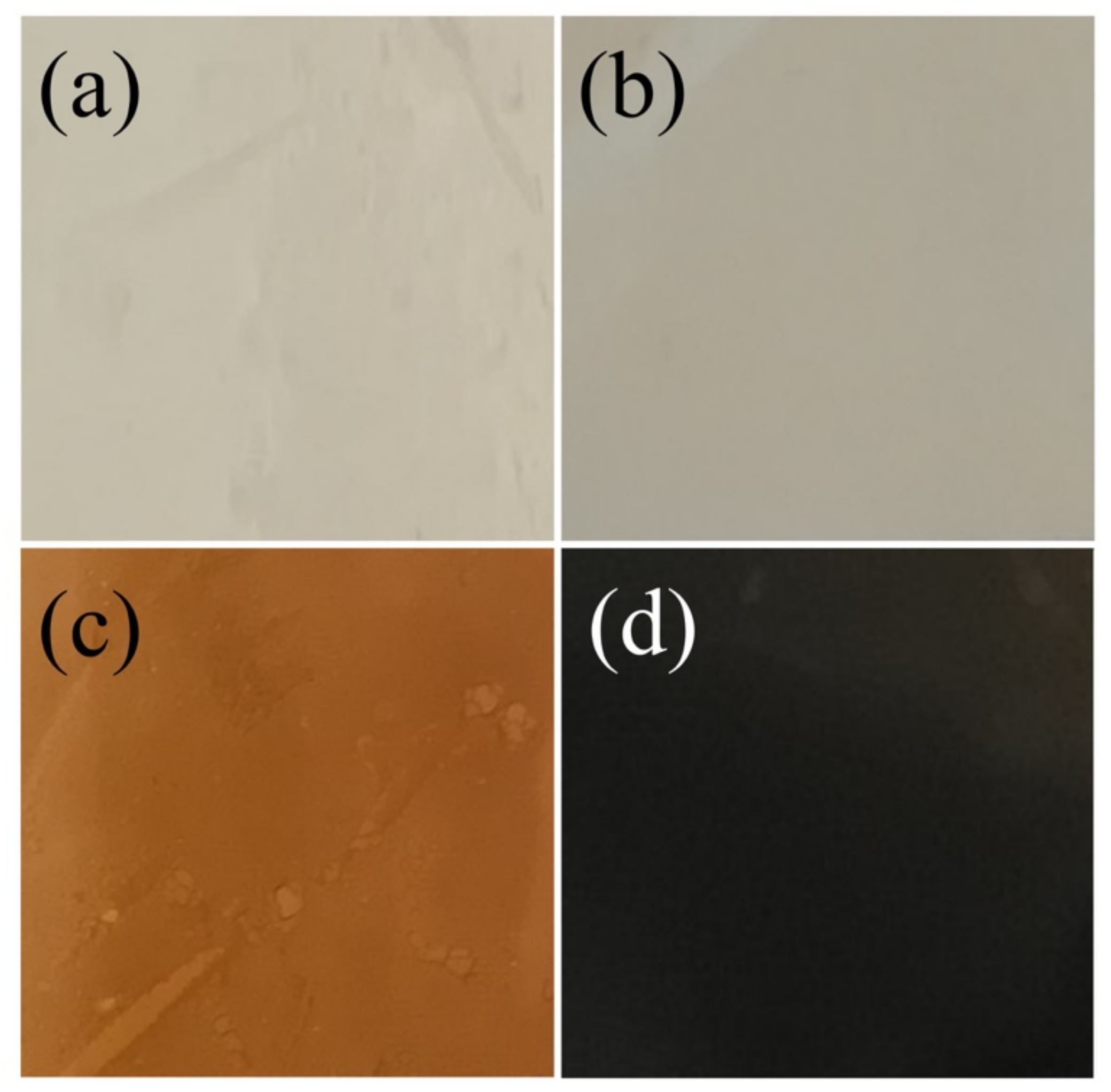
3.1. Macroscopical Characterization
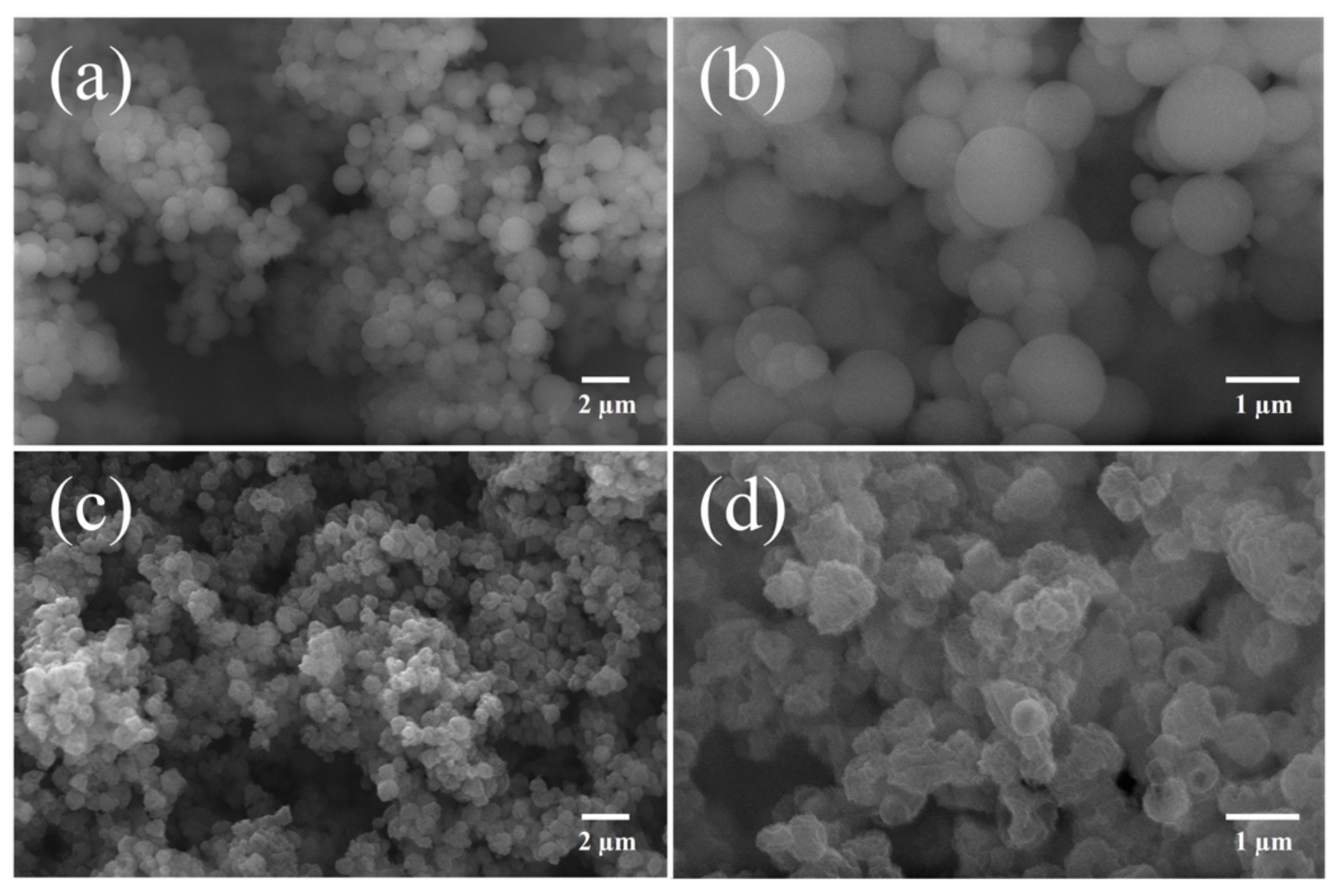
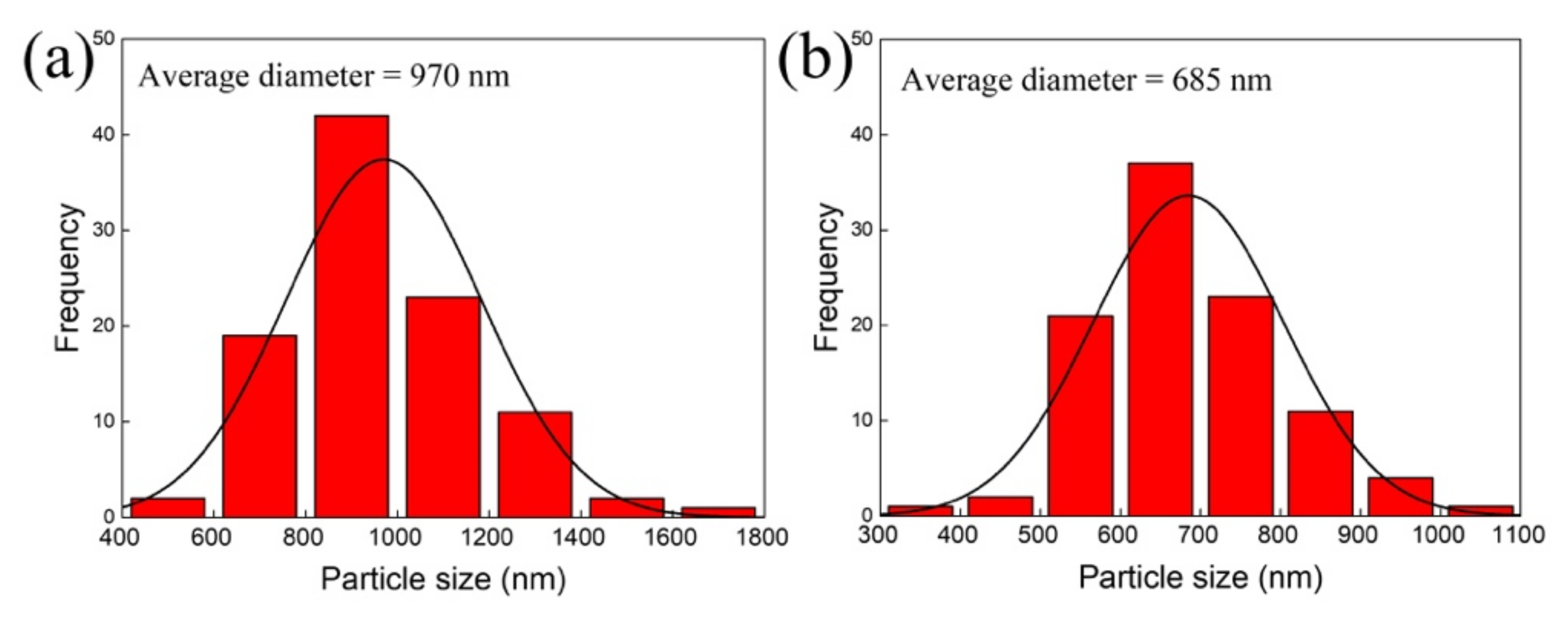
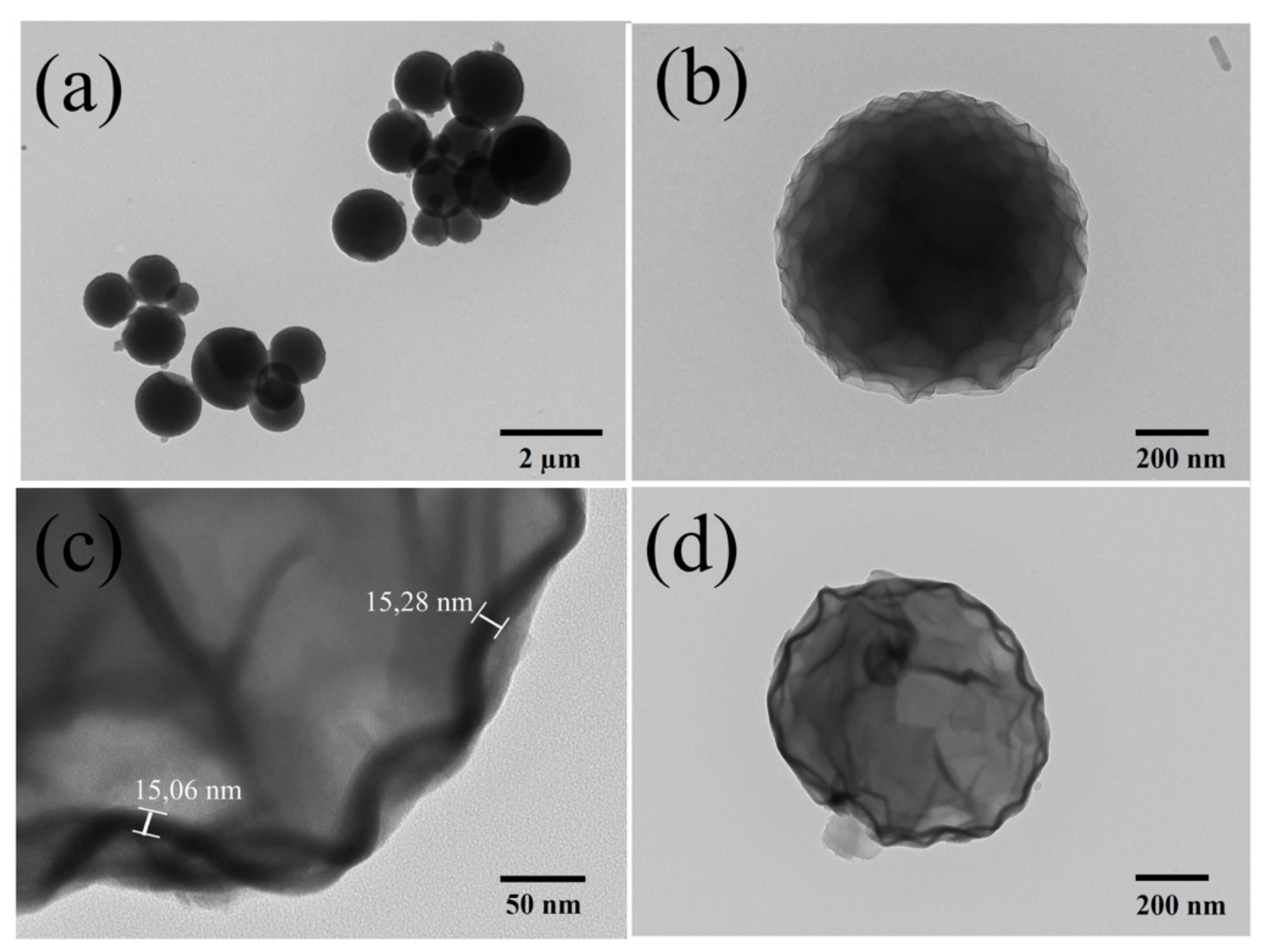
3.2. Morphological Characterization
3.3. Structural Characterization
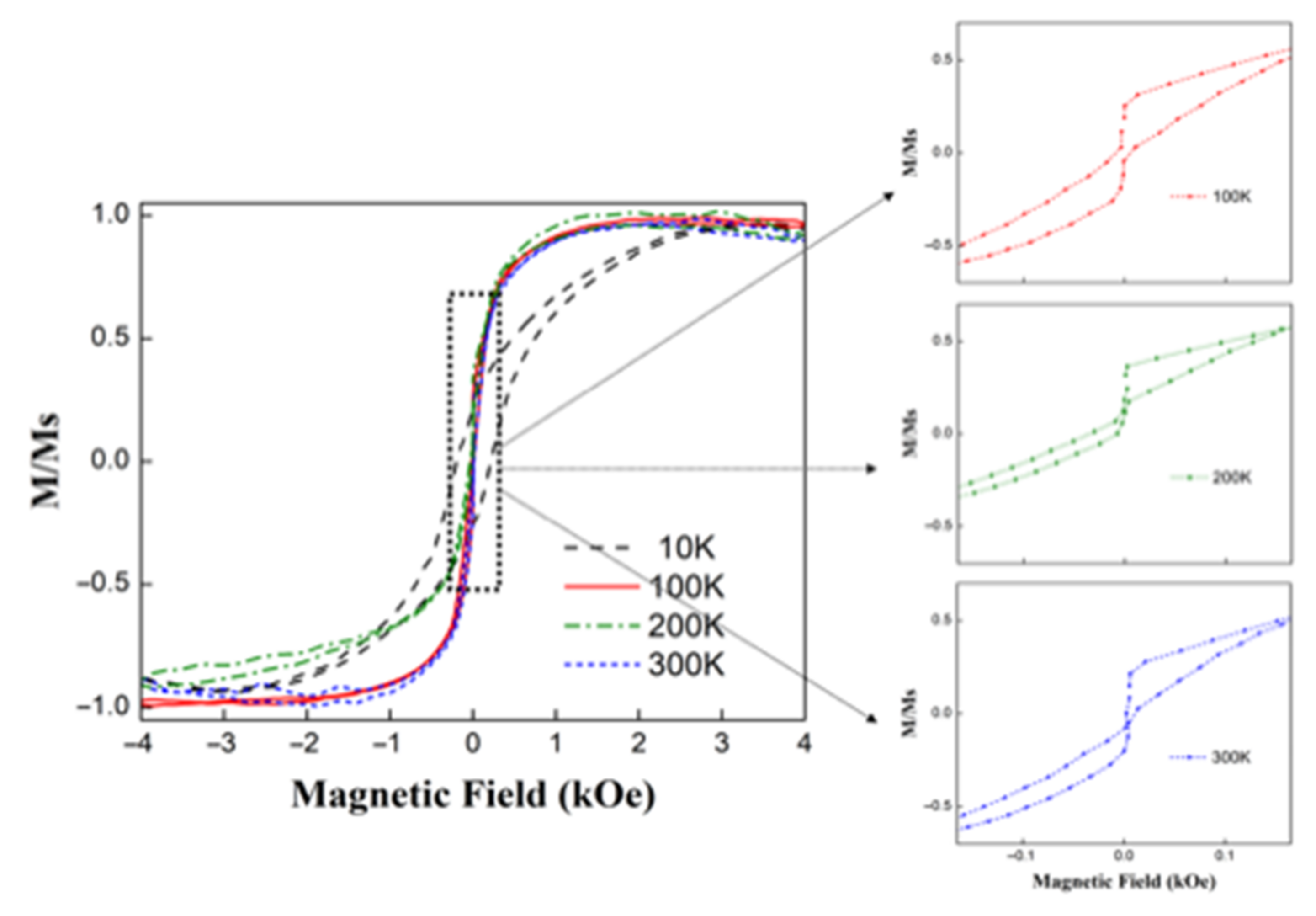
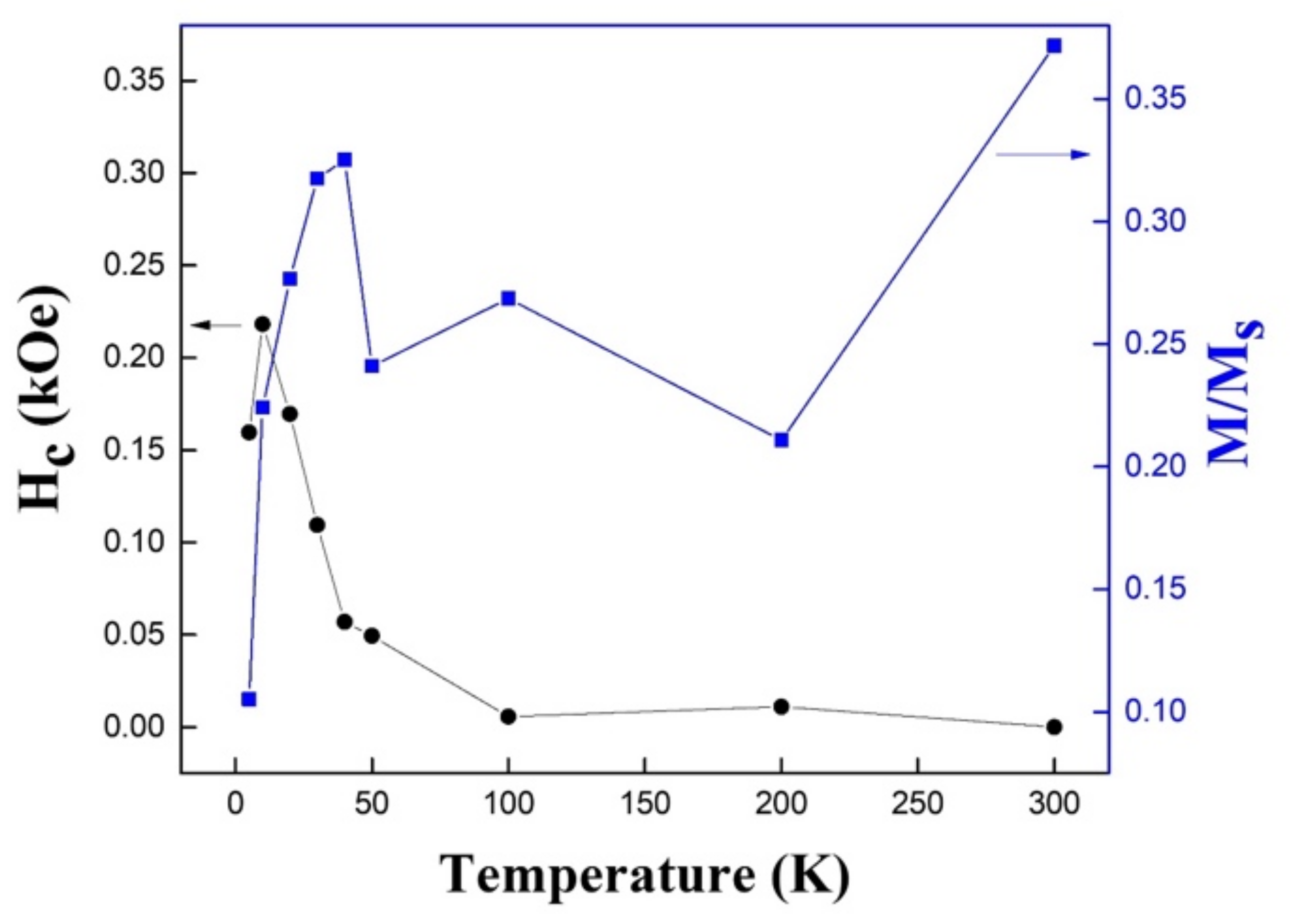
3.4. Magnetic Characterization of Hollow Fe3O4 Nanospheres
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goswami, M.M. Synthesis of micelles guided magnetite (Fe3O4) hollow spheres and their application for AC magnetic field responsive drug delivery. Sci. Rep. 2016, 6, 35721. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, T.; Xu, W.; Li, Y. Preparation and photocatalytic activity of magnetically separable Fe3O4@ZnO nanospheres. J. Mater. Sci. Mater. Electron. 2016, 27, 12155. [Google Scholar] [CrossRef]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 14813. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwon, S.G.; Park, J.-G.; Hyeon, T. Size dependence of metal-insulator transition in stoichiometric Fe3O4 nanocrystals. Nano Lett. 2015, 15, 4337. [Google Scholar] [CrossRef] [PubMed]
- Su, C. Environmental implications and applications of engineered nanoscale magnetite and its hybrid nanocomposites: A review of recent literature. J. Hazard. Mater. 2017, 322, 48. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.H.; Park, H.J.; Kang, S.W.; Park, J.C.; Nam, K.M. Synthesis of hollow iron oxide nanospheres and their application to gas sensors. J. Nanosci. Nanotech. 2018, 18, 1356. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Yakushkin, S.S.; Balaev, D.A.; Dubrovskiy, A.A.; Semenov, S.V.; Shaikhutdinov, K.A.; Kazakova, M.A.; Bukhtiyarova, G.A.; Martyanov, O.N.; Bayukov, O.A. Evolution of the Fe3+ ion local environment during the phase transition ε-Fe2O3 -> α-Fe2O3. J. Supercond. Nov. Magn. 2017, 31, 1209. [Google Scholar] [CrossRef]
- Lee, C.-W.; Jung, S.-S.; Lee, J.-S. Phase transformation of β-Fe2O3 hollow nanoparticles. Mater. Lett. 2008, 62, 561. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, Z.; Lyu, M.; Luo, B.; Wang, S.; Liu, G.; Cheng, H.M.; Wang, L. Hollow nanostructures for photocatalysis: Advantages and challenges. Adv. Mater. 2019, 31, 1801369. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Wang, D. Design of hollow nanostructures for energy storage, conversion and production. Adv. Mater. 2019, 31, 1801993. [Google Scholar] [CrossRef]
- Lu, Q.; Gao, F. Synthesis and property studies of hollow nanostructures. CrystEngComm 2016, 18, 7399. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Hou, Z.; Yu, Y.; Di, Q.; Wu, X.; Wei, G.; Quan, Z.; Zhang, J. Monodisperse tin nanoparticles and hollow tin oxide nanospheres as anode materials for hig performance lithium ion batteries. Inorg. Chem. Front. 2019, 6, 473. [Google Scholar] [CrossRef]
- Aguilera-Granja, F.; Montejano-Carrizales, J.M.; Vogel, E.E.; Escrig, J. Hollow structures of TinOm systems with m=2n: A density functional theoretical study. J. Phys. Chem. Sol. 2022, 164, 110646. [Google Scholar] [CrossRef]
- Adhikari, C.; Mishra, A.; Nayak, D.; Chakraborty, A. Drug delivery system composed of mesoporous silica and hollow mesoporous silica nanospheres for chemotherapeutic drug delivery. J. Drug. Deliv. Sci. Technol. 2018, 45, 303. [Google Scholar] [CrossRef]
- Hu, P.; Yu, L.; Zuo, A.; Guo, C.; Yuan, F. Fabrication of monodisperse magnetite hollow spheres. J. Phys. Chem. C 2008, 113, 900. [Google Scholar] [CrossRef]
- Xu, J.-S.; Zhu, Y.-J. γ-Fe2O3 and Fe3O4 magnetic hierarchically nanostructured hollow microspheres: Preparation, formation mechanism, magnetic property, and application in water treatment. J. Colloid Interface Sci. 2012, 385, 58. [Google Scholar] [CrossRef]
- Yadav, B.S.; Singh, R.; Vishwakarma, A.K.; Kumar, N. Facile synthesis of substantially magnetic hollow nanospheres of maghemite (γ-Fe2O3) originated from magnetite (Fe3O4) via solvothermal method. J. Supercond. Nov. Magn. 2020, 33, 2199. [Google Scholar] [CrossRef]
- Haghnegahdar, S.; Noroozifar, M. Deposition of PdPtAu nanoparticles on hollow nanospheres of Fe3O4 as a new catalyst for methanol electrooxidation: Application in direct methanol fuel cell. Electroanalysis 2017, 29, 2896. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.; Zhang, S.; Li, H.; Zhou, X.; Jiang, C. One-pot reaction and subsequent annealing to synthesis hollow spherical magnetite and maghemite nanocages. Nanoscale Res. Lett. 2009, 4, 926. [Google Scholar] [CrossRef]
- Cho, J.S.; Park, J.-S.; Kang, Y.C. Preparation of hollow Fe2O3 nanorods and nanospheres by nanoscale kirkendall difussion, and their electrochemical properties for use in lithium-ion batteries. Sci. Rep. 2016, 6, 38933. [Google Scholar] [CrossRef]
- Nor, Y.A.; Zhou, L.; Meka, A.K.; Xu, C.; Niu, Y.; Zhang, H.; Mitter, N.; Mahony, D.; Yu, C. Engineering ion oxide hollow nanospheres to enhance antimicrobial property: Understanding the cytotoxic origin in organic rich environment. Adv. Funct. Mater. 2016, 26, 5408. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Dong, G.; Xu, C.; Liu, D.; Lv, Y.; Zhong, B.; Wang, B. Temperature-controlled electrospinning of EVOH nanofibre mats encapsulated with Ag, CuO, and ZnO particles for skin wound dressing. Mater. Res. Express 2018, 6, 015007. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Yu, D.-G.; Zhu, M.-J.; Zhao, M.; Williams, G.R. Tunable zero-order drug delivery systems created by modified triaxial electrospinning. Chem. Eng. J. 2019, 356, 886. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Patiño, C.; Galotto, M.J.; Vásquez-Martínez, Y.; Torrent, C.; Alburquenque, D.; Pereira, A.; Escrig, J. Novel hollow titanium dioxide nanospheres with antimicrobial activity against resistant bacteria. Beilstein J. Nanotechnol. 2019, 10, 1716. [Google Scholar] [CrossRef]
- Márquez, P.; Alburquenque, D.; Celis, F.; Freire, R.M.; Escrig, J. Structural, morphological and magnetic properties of iron oxide thin films obtained by atomic layer deposition as a function of their thickness. J. Magn. Magn. Mater. 2021, 530, 167914. [Google Scholar] [CrossRef]
- Palma, J.L.; Pereira, A.; Alvaro, R.; García-Martín, J.M.; Escrig, J. Magnetic properties of Fe3O4 antidot arrays synthesized by AFIR: Atomic layer deposition, focused ion beam and thermal reduction. Beilstein J. Nanotechnol. 2018, 9, 1728. [Google Scholar] [CrossRef]
- Espejo, A.P.; Zierold, R.; Gooth, J.; Dendooven, J.; Detavernier, C.; Escrig, J.; Nielsch, K. Magnetic and electrical characterization of nickel-rich NiFe thin films synthesized by atomic layer deposition and subsequent thermal reduction. Nanotechnology 2016, 27, 345707. [Google Scholar] [CrossRef]
- Hashimoto, H.; Kiyohara, J.; Isozaki, A.; Arakawa, Y.; Fujii, T.; Takada, J.; Inada, H.; Takaishi, T.; Asoh, H. Bright yellowish-red pigment based on hematite/alumina composites with a unique porous disk-like structure. ACS Omega 2020, 5, 4330. [Google Scholar] [CrossRef]
- Takada, T. On the effects of particle size and shape on the colour ferric oxide powders. J. Jpn. Soc. Powder Powder Metall. 1958, 4, 160. [Google Scholar] [CrossRef]
- Busquets, M.A.; Fernández-Pradas, J.M.; Serra, P.; Estelrich, J. Superparamagnetic nanoparticles with efficient near-infrared photothermal effect at the second biological window. Molecules 2020, 25, 5315. [Google Scholar] [CrossRef]
- Tang, J.; Myers, M.; Bosnick, K.A.; Brus, L.E. Magnetite Fe3O4 nanocrystals: Spectroscopic observation of aqueous oxidation kinetics. J. Phys. Chem. B 2003, 107, 7501. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhu, L.; Gao, M.Y.; Li, X.F.; Cao, Z.Y.; Zhai, H.F. Nonvolatile memory capacitors based on Al2O3 tunneling and HfO2 blocking layers with charge storage in atomic-layer-deposited Pt nanocrystals. Appl. Surf. Sci. 2014, 289, 332. [Google Scholar] [CrossRef]
- Zierold, R.; Le Lam, C.; Dendooven, J.; Gooth, J.; Bohnert, T.; Sergelius, P.; Munnik, F.; Montero Moreno, J.M.; Gorlitz, D.; Detavernier, C.; et al. Magnetic characterization and electrical field-induced switching of magnetite thin films synthesized by atomic layer deposition and subsequent thermal reduction. J. Phys. D Appl. Phys. 2014, 47, 485001. [Google Scholar] [CrossRef]
- Ramachandran, R.K.; Dendooven, J.; Detavernier, C. Plasma enhanced atomic layer deposition of Fe2O3 thin films. J. Mater. Chem. A 2014, 2, 10662. [Google Scholar] [CrossRef]
- Wang, H.X.; Pu, X.M.; Zhou, Y.Q.; Chen, X.C.; Liao, X.M.; Huang, Z.B.; Yin, G.F. Synthesis of macroporous magnetic Fe3O4 microparticles via a novel organic matter assisted open-cell hollow sphere aseembly method. Materials 2018, 11, 1508. [Google Scholar] [CrossRef]
- Thompson, C.V. Solid-state dewetting of thin films. Annu. Rev. Mater. Res. 2012, 42, 399. [Google Scholar] [CrossRef]
- Mangalam, R.; Thamilselvan, M.; Selvasekarapandian, S.; Jayakumar, S.; Manjuladevi, R. Polyvinyl pyrrolidone/Mg(ClO4)2 solid polymer electrolyte: Structural and electrical studies. Ionics 2017, 23, 2837. [Google Scholar] [CrossRef]
- Saroj, A.L.; Singh, R.K.; Chandra, S. Studies on polymer electrolyte poly(vinyl) pyrrolidone (PVP) complexed with ionic liquid: Effect of complexation on thermal stability, conductivity and relaxation behavior. Mater. Sci. Eng. B 2013, 178, 231. [Google Scholar] [CrossRef]
- Rooth, M.; Johansson, A.; Kukli, K.; Aarik, J.; Boman, M.; Harsta, A. Atomic layer deposition of iron oxide thin films and nanotubes using ferrocene and oxygen as precursors. Chem. Vap. Depos. 2008, 14, 67. [Google Scholar] [CrossRef]
- Alburquenque, D.; Bracamonte, V.; Del Canto, M.; Pereira, A.; Escrig, J. Dewetting of Co thin films obtained by atomic layer deposition due to the thermal reduction process. MRS Commun. 2017, 7, 848. [Google Scholar] [CrossRef]
- Dudchenko, N.O. Synthetic analogues of biogenic magnetite: Synthesis and characterization of magnetite nanoparticles. Mater. Und Werkst. 2011, 42, 89. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez, P.; Patiño Vidal, C.; Pereira, A.; Vivas, L.; Palma, J.L.; López de Dicastillo, C.; Escrig, J. Hollow Iron Oxide Nanospheres Obtained through a Combination of Atomic Layer Deposition and Electrospraying Technologies. Nanomaterials 2022, 12, 3180. https://doi.org/10.3390/nano12183180
Márquez P, Patiño Vidal C, Pereira A, Vivas L, Palma JL, López de Dicastillo C, Escrig J. Hollow Iron Oxide Nanospheres Obtained through a Combination of Atomic Layer Deposition and Electrospraying Technologies. Nanomaterials. 2022; 12(18):3180. https://doi.org/10.3390/nano12183180
Chicago/Turabian StyleMárquez, Paulina, Cristian Patiño Vidal, Alejandro Pereira, Leonardo Vivas, Juan Luis Palma, Carol López de Dicastillo, and Juan Escrig. 2022. "Hollow Iron Oxide Nanospheres Obtained through a Combination of Atomic Layer Deposition and Electrospraying Technologies" Nanomaterials 12, no. 18: 3180. https://doi.org/10.3390/nano12183180
APA StyleMárquez, P., Patiño Vidal, C., Pereira, A., Vivas, L., Palma, J. L., López de Dicastillo, C., & Escrig, J. (2022). Hollow Iron Oxide Nanospheres Obtained through a Combination of Atomic Layer Deposition and Electrospraying Technologies. Nanomaterials, 12(18), 3180. https://doi.org/10.3390/nano12183180






