Binder-Free Zinc–Iron Oxide as a High-Performance Negative Electrode Material for Pseudocapacitors
Abstract
:1. Introduction
2. Experimental
2.1. Materials
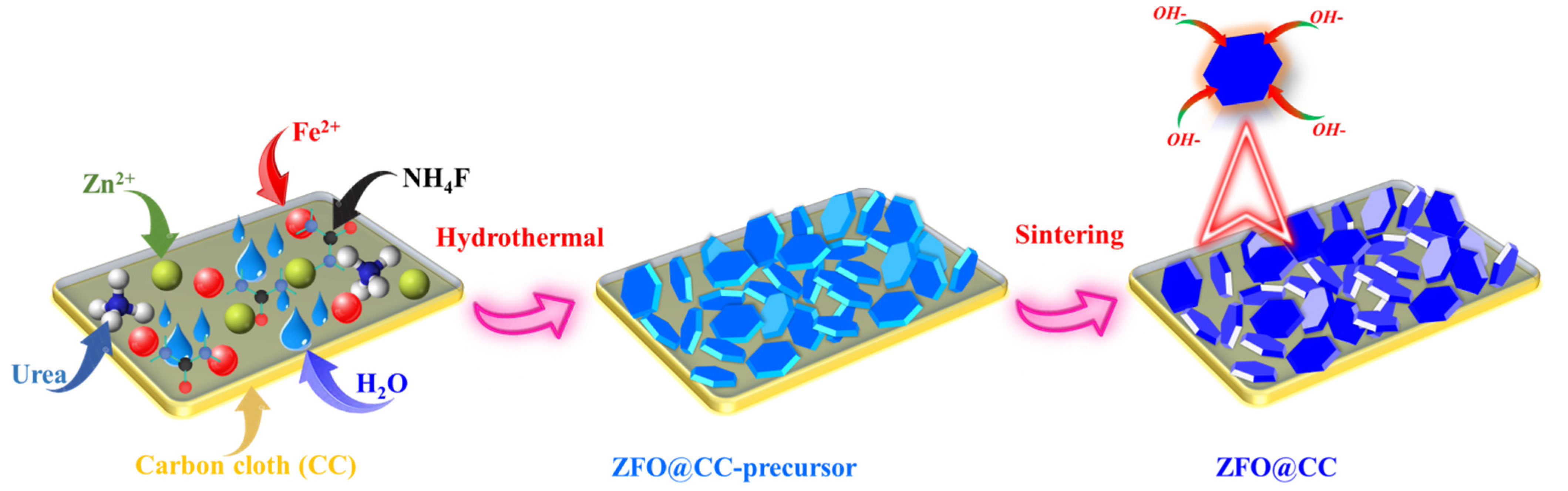
2.2. Synthesis of ZnFe2O4 Nanoflakes
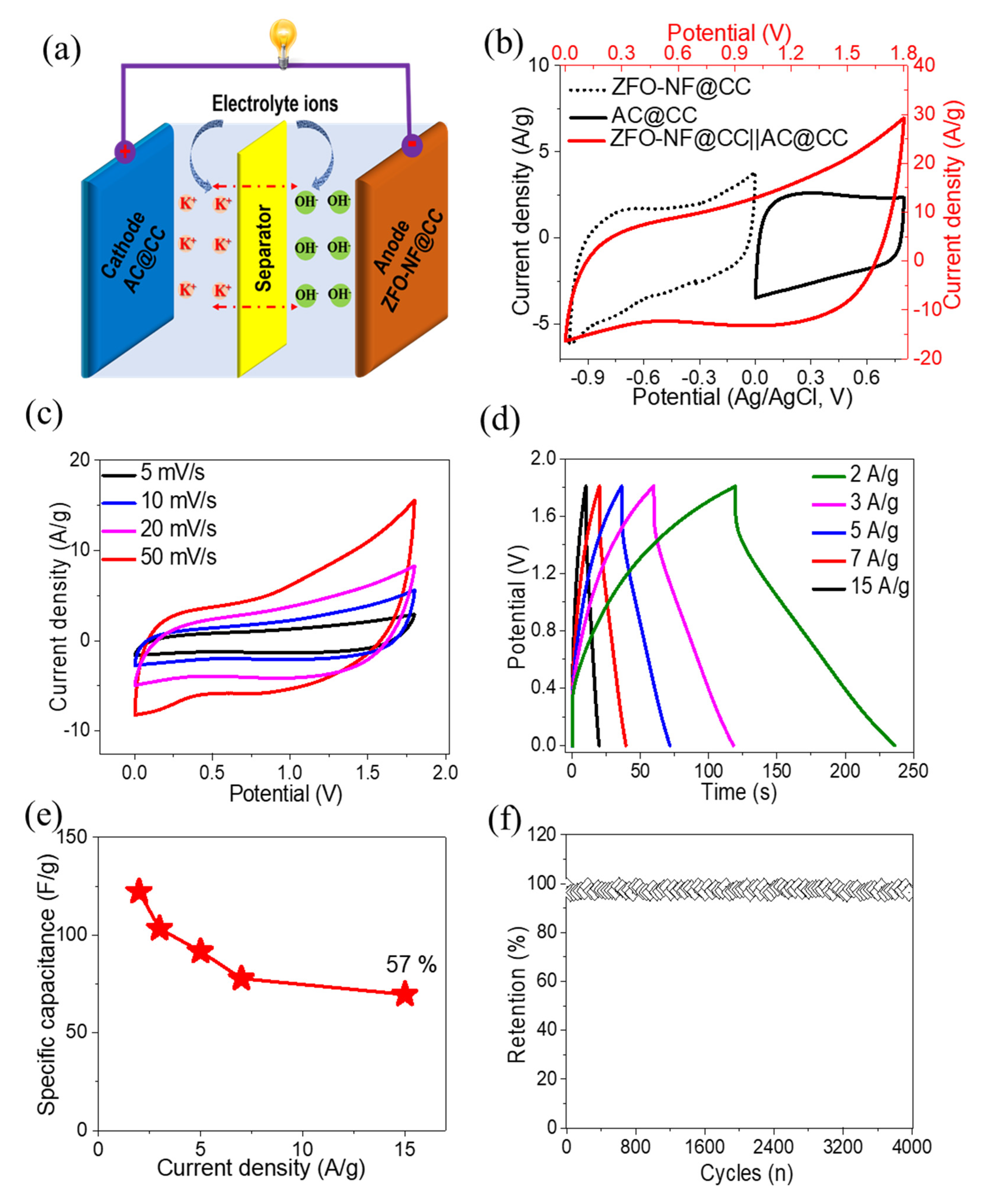
2.3. Fabrication of Single Electrode and Asymmetric Supercapacitor Device
2.4. Morphological Characterization of ZnFe2O4 Nanoflakes
2.5. Electrochemical Characterization
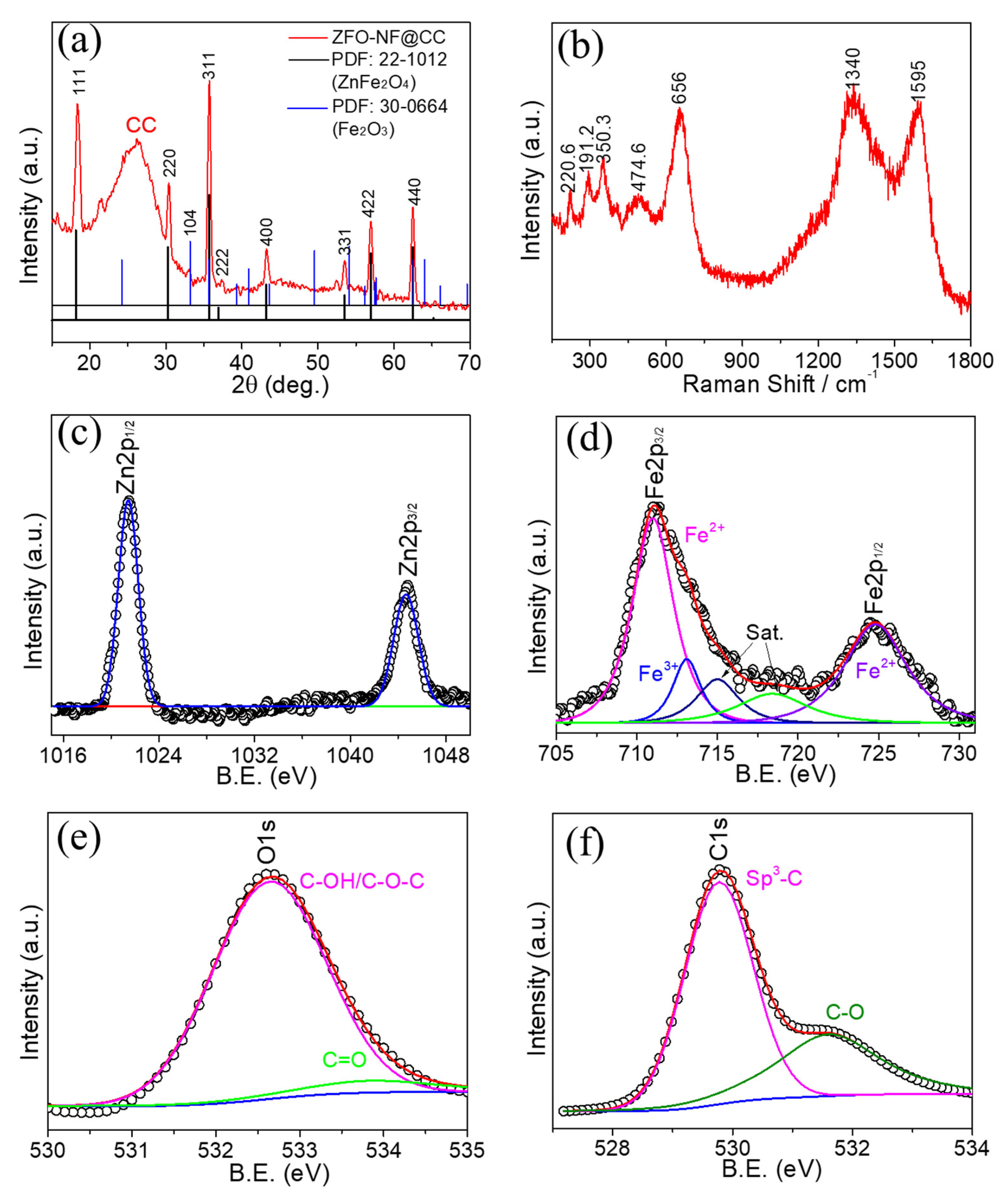
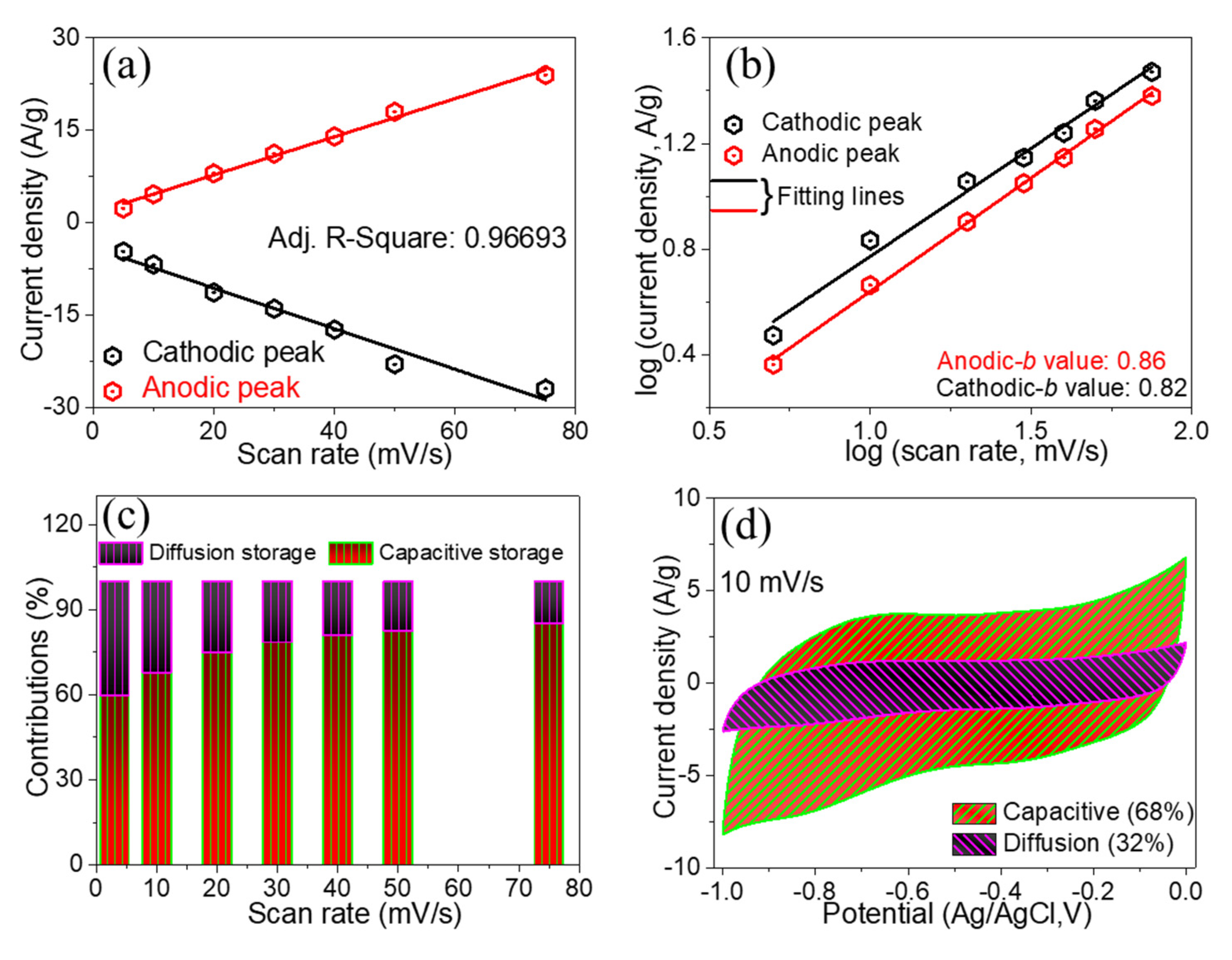
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyeremateng, N.A.; Brousse, T.; Pech, D. Microsupercapacitors as miniaturized energy-storage components for on-chip electronics. Nat. Nanotechnol. 2017, 12, 7–15. [Google Scholar] [CrossRef]
- Javed, M.S.; Shah, S.S.A.; Najam, T.; Siyal, S.H.; Hussain, S.; Saleem, M.; Zhao, Z.; Mai, W.J.N.E. Achieving high-energy density and superior cyclic stability in flexible and lightweight pseudocapacitor through synergic effects of binder-free CoGa2O4 2D-hexagonal nanoplates. Nano Energy 2020, 77, 105276. [Google Scholar] [CrossRef]
- Ji, X.; Long, X. A review of the ecological and socioeconomic effects of biofuel and energy policy recommendations. Renew. Sustain. Energy Rev. 2016, 61, 41–52. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar]
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Sci. Mag. 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.S.; Zhang, X.; Ali, S.; Mateen, A.; Idrees, M.; Sajjad, M.; Batool, S.; Ahmad, A.; Imran, M.; Najam, T.J.N.E. Heterostructured bimetallic–sulfide@ layered Ti3C2Tx–MXene as a synergistic electrode to realize high-energy-density aqueous hybrid-supercapacitors. Nano Energy 2022, 101, 107624. [Google Scholar] [CrossRef]
- Javed, M.S.; Lei, H.; Li, J.; Wang, Z.; Mai, W. Construction of highly dispersed mesoporous bimetallic-sulfide nanoparticles locked in N-doped graphitic carbon nanosheets for high energy density hybrid flexible pseudocapacitors. J. Mater. Chem. A 2019, 7, 17435–17445. [Google Scholar] [CrossRef]
- Venkatesh, K.; Muthukutty, B.; Chen, S.-M.; Karuppiah, C.; Amanulla, B.; Yang, C.-C.; Ramaraj, S.K. Nanomolar level detection of non-steroidal antiandrogen drug flutamide based on ZnMn2O4 nanoparticles decorated porous reduced graphene oxide nanocomposite electrode. J. Hazard. Mater. 2021, 405, 124096. [Google Scholar] [CrossRef]
- Sen, P.; De, A. Electrochemical performances of poly (3,4-ethylenedioxythiophene)–NiFe2O4 nanocomposite as electrode for supercapacitor. Electrochim. Acta 2010, 55, 4677–4684. [Google Scholar] [CrossRef]
- Patil, S.J.; Dubal, D.P.; Lee, D.-W. Gold nanoparticles decorated rGO-ZnCo2O4 nanocomposite: A promising positive electrode for high performance hybrid supercapacitors. Chem. Eng. J. 2020, 379, 122211. [Google Scholar] [CrossRef]
- Shi, Z.; Sun, G.; Yuan, R.; Chen, W.; Wang, Z.; Zhang, L.; Zhan, K.; Zhu, M.; Yang, J.; Zhao, B. Scalable fabrication of NiCo2O4/reduced graphene oxide composites by ultrasonic spray as binder-free electrodes for supercapacitors with ultralong lifetime. J. Mater. Sci. Technol. 2022, 99, 260–269. [Google Scholar] [CrossRef]
- Javed, M.S.; Jiang, Z.; Yang, Q.; Wang, X.; Han, X.; Zhang, C.; Gu, X.; Hu, C. Exploring Li-ion hopping behavior in zinc ferrite and promoting performance for flexible solid-state supercapacitor. Electrochim. Acta 2019, 295, 558–568. [Google Scholar] [CrossRef]
- Devi, R.; Patra, J.; Tapadia, K.; Chang, J.-K.; Maharana, T. Arrangement of ZnFe2O4@PPy nanoparticles on carbon cloth for highly efficient symmetric supercapacitor. J. Taiwan Inst. Chem. Eng. 2022, 138, 104474. [Google Scholar] [CrossRef]
- Tiwari, N.; Kadam, S.; Ingole, R.; Kulkarni, S. Facile hydrothermal synthesis of ZnFe2O4 nanostructures for high-performance supercapacitor application. Ceram. Int. 2022, 48, 29478–29483. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, A.-M.; Wan, L.-J.; Guo, Y.-G. Spin-coated silicon nanoparticle/graphene electrode as a binder-free anode for high-performance lithium-ion batteries. Nano Res. 2012, 5, 845–853. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, B.; Yin, Y.; Yin, T.; Cheng, J.; Zhan, K.; Yan, Y.; Yang, J.; Li, J. Fe2O3-decorated millimeter-long vertically aligned carbon nanotube arrays as advanced anode materials for asymmetric supercapacitors with high energy and power densities. J. Mater. Chem. A 2016, 4, 19026–19036. [Google Scholar] [CrossRef]
- Sun, G.; Ren, H.; Shi, Z.; Zhang, L.; Wang, Z.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B. V2O5/vertically-aligned carbon nanotubes as negative electrode for asymmetric supercapacitor in neutral aqueous electrolyte. J. Colloid Interface Sci. 2021, 588, 847–856. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, L.; Ren, H.; Miao, T.; Wang, Z.; Zhan, K.; Yang, J.; Zhao, B. V2CTx MXene as novel anode for aqueous asymmetric supercapacitor with superb durability in ZnSO4 electrolyte. J. Colloid Interface Sci. 2022, 626, 59–67. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, X.; Zhou, Y.; Zhuo, C.; Huang, J.; Li, S. Facile solvothermal synthesis of porous ZnFe2O4 microspheres for capacitive pseudocapacitors. RSC Adv. 2015, 5, 39270–39277. [Google Scholar] [CrossRef]
- Liu, H.; Guo, Y.; Zhang, Y.; Wu, F.; Liu, Y.; Zhang, D. Synthesis and properties of ZnFe2O4 replica with biological hierarchical structure. Mater. Sci. Eng. B 2013, 178, 1057–1061. [Google Scholar] [CrossRef]
- Li, H.-H.; Wu, X.-L.; Zhang, L.-L.; Fan, C.-Y.; Wang, H.-F.; Li, X.-Y.; Sun, H.-Z.; Zhang, J.-P.; Yan, Q. Carbon-Free Porous Zn2GeO4 Nanofibers as Advanced Anode Materials for High-Performance Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 31722–31728. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Han, Z.; Zheng, F.; Sun, J.; Qiao, Z.; Yang, X.; Li, L.; Li, C.; Song, X.; Cao, B. ZnFe2O4 nanoparticles-cotton derived hierarchical porous active carbon fibers for high rate-capability supercapacitor electrodes. Carbon 2018, 134, 15–21. [Google Scholar] [CrossRef]
- Javed, M.S.; Chen, J.; Chen, L.; Xi, Y.; Zhang, C.; Wan, B.; Hu, C. Flexible full-solid state supercapacitors based on zinc sulfide spheres growing on carbon textile with superior charge storage. J. Mater. Chem. A 2016, 4, 667–674. [Google Scholar] [CrossRef]
- Pavese, A.; Levy, D.; Hoser, A.J.A.M. Cation distribution in synthetic zinc ferrite (Zn0.97Fe2.02O4) from in situ high-temperature neutron powder diffraction. J. Am. Mineral. 2000, 85, 1497–1502. [Google Scholar] [CrossRef]
- Agyemang, F.O.; Kim, H. Electrospun ZnFe2O4-based nanofiber composites with enhanced supercapacitive properties. Mater. Sci. Eng. B 2016, 211, 141–148. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Dong, W.; Zhang, L.; Kong, Q.; Wang, W. Efficient adsorption of sulfamethazine onto modified activated carbon: A plausible adsorption mechanism. Sci. Rep. 2017, 7, 12437. [Google Scholar] [CrossRef]
- Wang, M.; Sun, L.; Cai, J.; Huang, P.; Su, Y.; Lin, C. A facile hydrothermal deposition of ZnFe2O4 nanoparticles on TiO2 nanotube arrays for enhanced visible light photocatalytic activity. J. Mater. Chem. A 2013, 1, 12082–12087. [Google Scholar] [CrossRef]
- Chen, M.; Shen, X.; Chen, K.; Wu, Q.; Zhang, P.; Zhang, X.; Diao, G. Nitrogen-doped mesoporous carbon-encapsulation urchin-like Fe3O4 as anode materials for high performance Li-ions batteries. Electrochim. Acta 2016, 195, 94–105. [Google Scholar] [CrossRef]
- Zheng, C.; Zhou, X.; Cao, H.; Wang, G.; Liu, Z. Synthesis of porous graphene/activated carbon composite with high packing density and large specific surface area for supercapacitor electrode material. J. Power Sources 2014, 258, 290–296. [Google Scholar] [CrossRef]
- Sarkar, S.; Akshaya, R.; Ghosh, S. Nitrogen doped graphene/CuCr2O4 nanocomposites for supercapacitors application: Effect of nitrogen doping on coulombic efficiency. Electrochim. Acta 2020, 332, 135368. [Google Scholar] [CrossRef]
- Zheng, Q.; Kvit, A.; Cai, Z.; Ma, Z.; Gong, S. A freestanding cellulose nanofibril–reduced graphene oxide–molybdenum oxynitride aerogel film electrode for all-solid-state supercapacitors with ultrahigh energy density. J. Mater. Chem. A 2017, 5, 12528–12541. [Google Scholar] [CrossRef]
- Javed, M.S.; Jiang, Z.; Zhang, C.; Chen, L.; Hu, C.; Gu, X. A high-performance flexible solid-state supercapacitor based on Li-ion intercalation into tunnel-structure iron sulfide. Electrochim. Acta 2016, 219, 742–750. [Google Scholar] [CrossRef]
- Javed, M.S.; Han, X.; Hu, C.; Zhou, M.; Huang, Z.; Tang, X.; Gu, X. Tracking Pseudocapacitive Contribution to Superior Energy Storage of MnS Nanoparticles Grown on Carbon Textile. ACS Appl. Mater. Interfaces 2016, 8, 24621–24628. [Google Scholar] [CrossRef]
- Seong, J.-G.; Ko, T.H.; Lei, D.; Choi, W.-K.; Kuk, Y.-S.; Seo, M.-K.; Kim, B.-S. Engineered NiCo-LDH nanosheets-and ZnFe2O4 nanocubes-decorated carbon nanofiber bonded mats for high-rate asymmetric supercapacitors. Green Energy Environ. 2021. [Google Scholar] [CrossRef]
- Raut, S.S.; Sankapal, B.R. First report on synthesis of ZnFe2O4 thin film using successive ionic layer adsorption and reaction: Approach towards solid-state symmetric supercapacitor device. Electrochim. Acta 2016, 198, 203–211. [Google Scholar] [CrossRef]
- Yang, S.; Ai, J.; Han, Z.; Zhang, L.; Zhao, D.; Wang, J.; Yang, C.; Cao, B. Electrospun ZnFe2O4/carbon nanofibers as high-rate supercapacitor electrodes. J. Power Sources 2020, 469, 228416. [Google Scholar] [CrossRef]
- Li, L.; Bi, H.; Gai, S.; He, F.; Gao, P.; Dai, Y.; Zhang, X.; Yang, D.; Zhang, M.; Yang, P. Uniformly dispersed ZnFe2O4 nanoparticles on nitrogen-modified graphene for high-performance supercapacitor as electrode. Sci. Rep. 2017, 7, 43116. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Han, Z.; Sun, J.; Yang, X.; Hu, X.; Li, C.; Cao, B. Controllable ZnFe2O4/reduced graphene oxide hybrid for high-performance supercapacitor electrode. Electrochim. Acta 2018, 268, 20–26. [Google Scholar] [CrossRef]
- Yang, S.; Han, Z.; Sun, J.; Yang, X.; Li, C.; Wang, R.; Cao, B. Preparation of defective ZnFe2O4/graphene composites and their charge storage properties. Electrochem. Commun. 2018, 92, 19–23. [Google Scholar] [CrossRef]
- Xu, K.; Yang, J.; Hu, J. Synthesis of hollow NiCo2O4 nanospheres with large specific surface area for asymmetric supercapacitors. J. Colloid Interface Sci. 2018, 511, 456–462. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Sun, J.; Xu, C.; Chen, H. Simple Preparation of Porous FeCo2O4 Microspheres and Nanosheets for Advanced Asymmetric Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 11307–11317. [Google Scholar] [CrossRef]
- Le, K.; Gao, M.; Liu, W.; Liu, J.; Wang, Z.; Wang, F.; Murugadoss, V.; Wu, S.; Ding, T.; Guo, Z. MOF-derived hierarchical core-shell hollow iron-cobalt sulfides nanoarrays on Ni foam with enhanced electrochemical properties for high energy density asymmetric supercapacitors. Electrochim. Acta 2019, 323, 134826. [Google Scholar] [CrossRef]
- Yu, J.; Cui, Z.; Li, X.; Chen, D.; Ji, J.; Zhang, Q.; Sui, J.; Yu, L.; Dong, L. Facile fabrication of ZIF-derived graphene-based 2D Zn/Co oxide hybrid for high-performance supercapacitors. J. Energy Storage 2019, 27, 101165. [Google Scholar] [CrossRef]
- Hu, W.; Wei, H.; She, Y.; Tang, X.; Zhou, M.; Zang, Z.; Du, J.; Gao, C.; Guo, Y.; Bao, D. Flower-like nickel-zinc-cobalt mixed metal oxide nanowire arrays for electrochemical capacitor applications. J. Alloys Compd. 2017, 708, 146–153. [Google Scholar] [CrossRef]
- Hussain, I.; Mohamed, S.G.; Ali, A.; Abbas, N.; Ammar, S.M.; Al Zoubi, W. Uniform growth of Zn-Mn-Co ternary oxide nanoneedles for high-performance energy-storage applications. J. Electroanal. Chem. 2019, 837, 39–47. [Google Scholar] [CrossRef]




| Sr. No. | Electrode Materials | Electrolyte | Specific Capacitance (F g−1) | Current Density (A g−1) | No. of Cycles (n) | Retention Rate (%) | References |
|---|---|---|---|---|---|---|---|
| 1 | ZnFe2O4 nanoflakes | KOH | 509 | 1.5 | 10,000 | 93.5 | This work |
| 2 | ZnFe2O4 thin film | NaOH | 471 | 1 | 1000 | 72 | [35] |
| 3 | ZnFe2O4/carbon | KOH | 237 | 1 | 1000 | 88.2 | [36] |
| 4 | ZnFe2O4/CNTs | KOH | 282 | 1 | 5000 | 56.73 | [34] |
| 5 | ZnFe2O4 powder/replica | KOH | 279.4 | 1 | 1000 | 80 | [20] |
| 6 | ZnFe2O4 nanoparticles | KOH | 244 | 0.5 | 5000 | 83.8 | [37] |
| 7 | ZnFe2O4 microspheres | KOH | 131 | 1 | 1000 | 92 | [19] |
| 8 | ZnFe2O4/rGO | KOH | 352.9 | 1 | 10,000 | 92.3 | [38] |
| 9 | ZnFe2O4/Nanofiber | KOH | 408 | 1 | 3000 | 93 | [25] |
| 10 | ZnFe2O4 nanoparticles | KOH | 474 | 1 | 20,000 | 90 | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, Q.; Mateen, A.; Khan, A.J.; Eldesoky, G.E.; Idrees, A.; Ahmad, A.; Eldin, E.T.; Das, H.T.; Sajjad, M.; Javed, M.S. Binder-Free Zinc–Iron Oxide as a High-Performance Negative Electrode Material for Pseudocapacitors. Nanomaterials 2022, 12, 3154. https://doi.org/10.3390/nano12183154
Abbas Q, Mateen A, Khan AJ, Eldesoky GE, Idrees A, Ahmad A, Eldin ET, Das HT, Sajjad M, Javed MS. Binder-Free Zinc–Iron Oxide as a High-Performance Negative Electrode Material for Pseudocapacitors. Nanomaterials. 2022; 12(18):3154. https://doi.org/10.3390/nano12183154
Chicago/Turabian StyleAbbas, Qasim, Abdul Mateen, Abdul Jabbar Khan, Gaber E. Eldesoky, Asim Idrees, Awais Ahmad, Elsayed Tag Eldin, Himadri Tanaya Das, Muhammad Sajjad, and Muhammad Sufyan Javed. 2022. "Binder-Free Zinc–Iron Oxide as a High-Performance Negative Electrode Material for Pseudocapacitors" Nanomaterials 12, no. 18: 3154. https://doi.org/10.3390/nano12183154
APA StyleAbbas, Q., Mateen, A., Khan, A. J., Eldesoky, G. E., Idrees, A., Ahmad, A., Eldin, E. T., Das, H. T., Sajjad, M., & Javed, M. S. (2022). Binder-Free Zinc–Iron Oxide as a High-Performance Negative Electrode Material for Pseudocapacitors. Nanomaterials, 12(18), 3154. https://doi.org/10.3390/nano12183154











