Ag3PO4-Deposited TiO2@Ti3C2 Petals for Highly Efficient Photodecomposition of Various Organic Dyes under Solar Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Materials
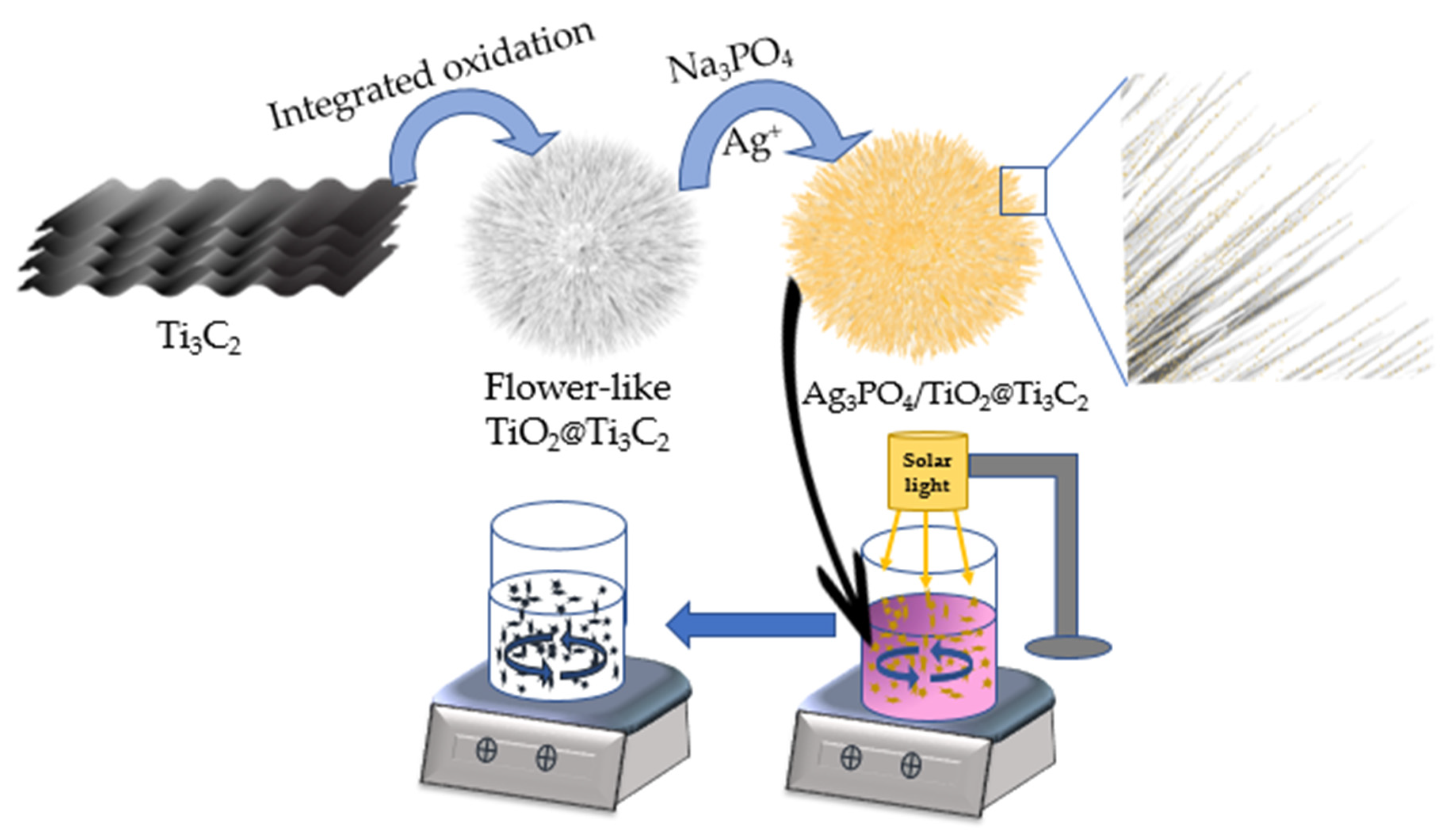
2.2. Preparation of TiO2@Ti3C2 Heterostructure
2.3. Preparation of Ag3PO4 Particles
2.4. Preparation of Ag3PO4/TiO2@Ti3C2
2.5. Photocatalysis of Dye Degradation
2.6. Characterization
3. Results and Discussion
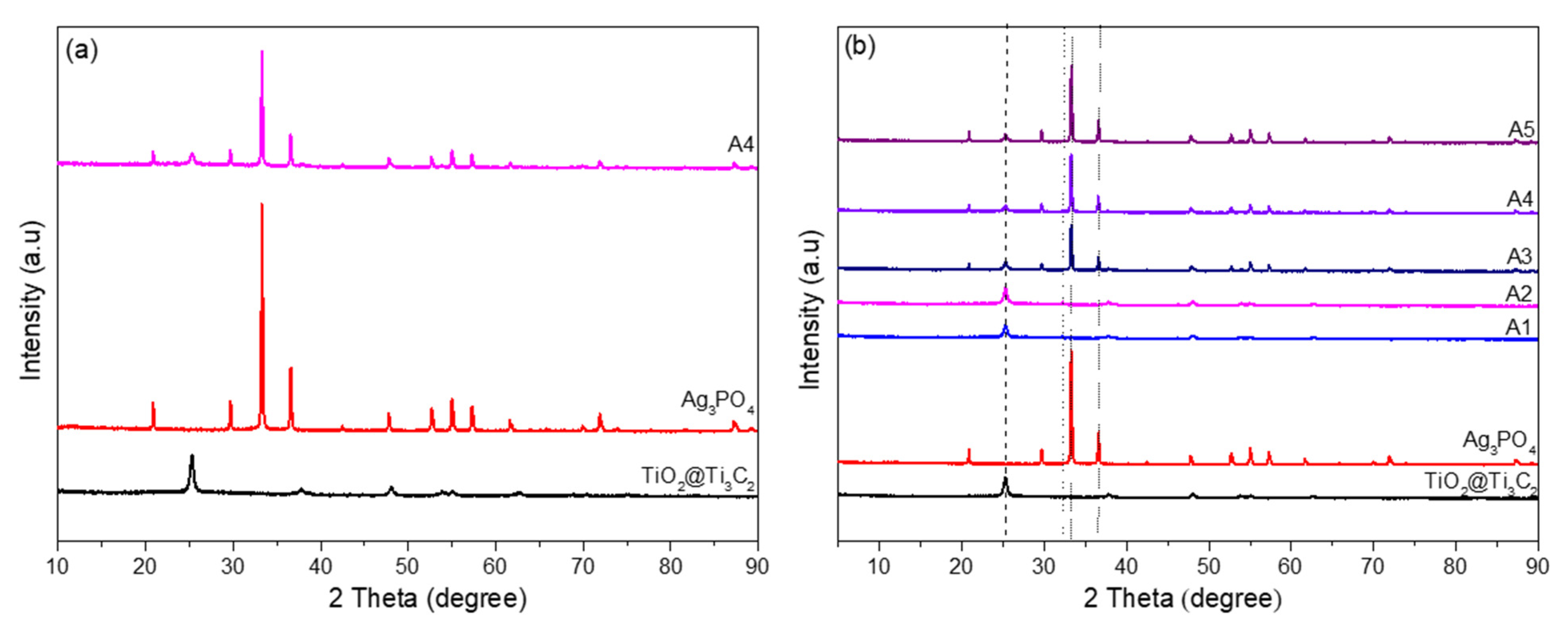
3.1. XRD Analysis
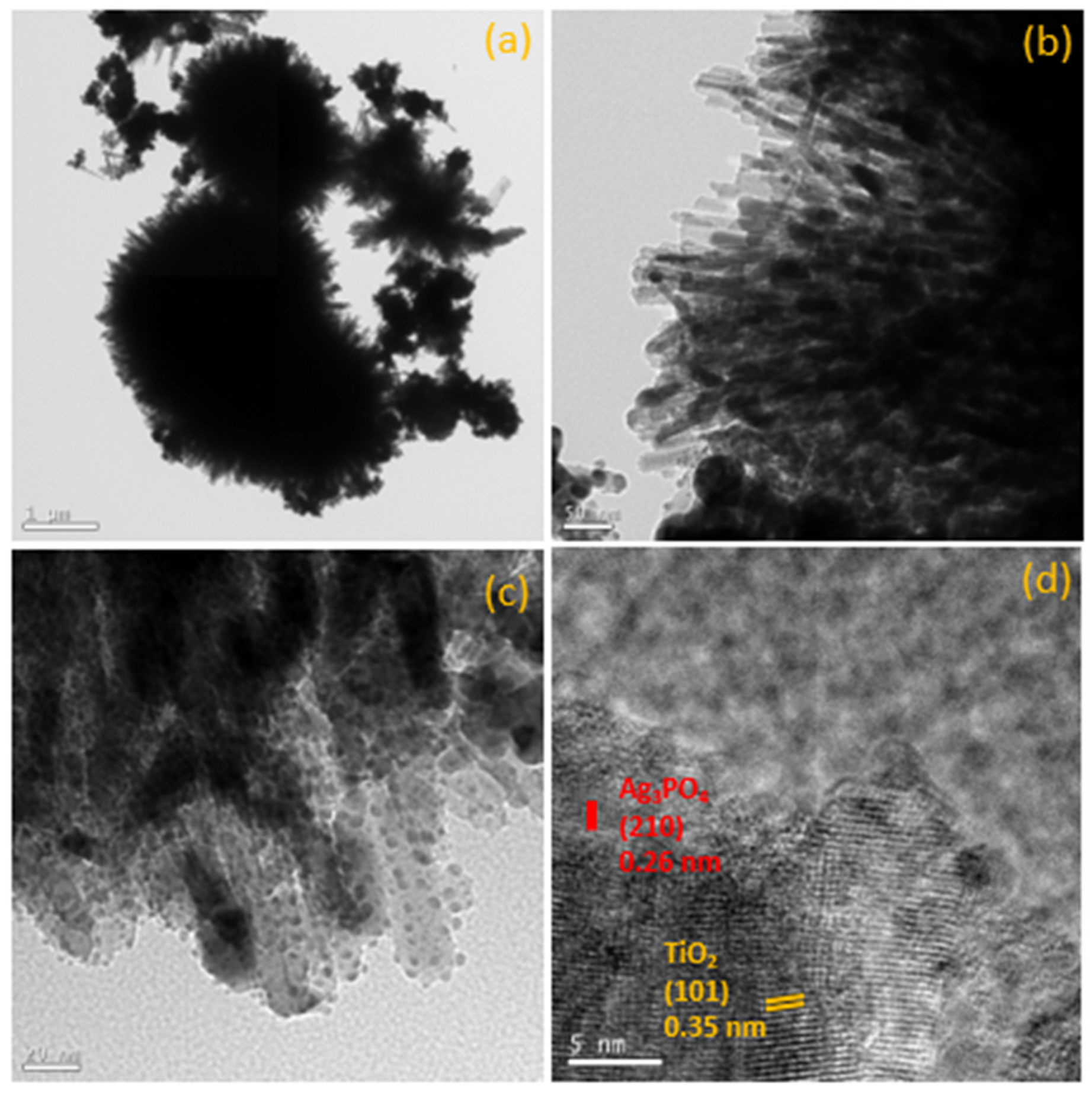
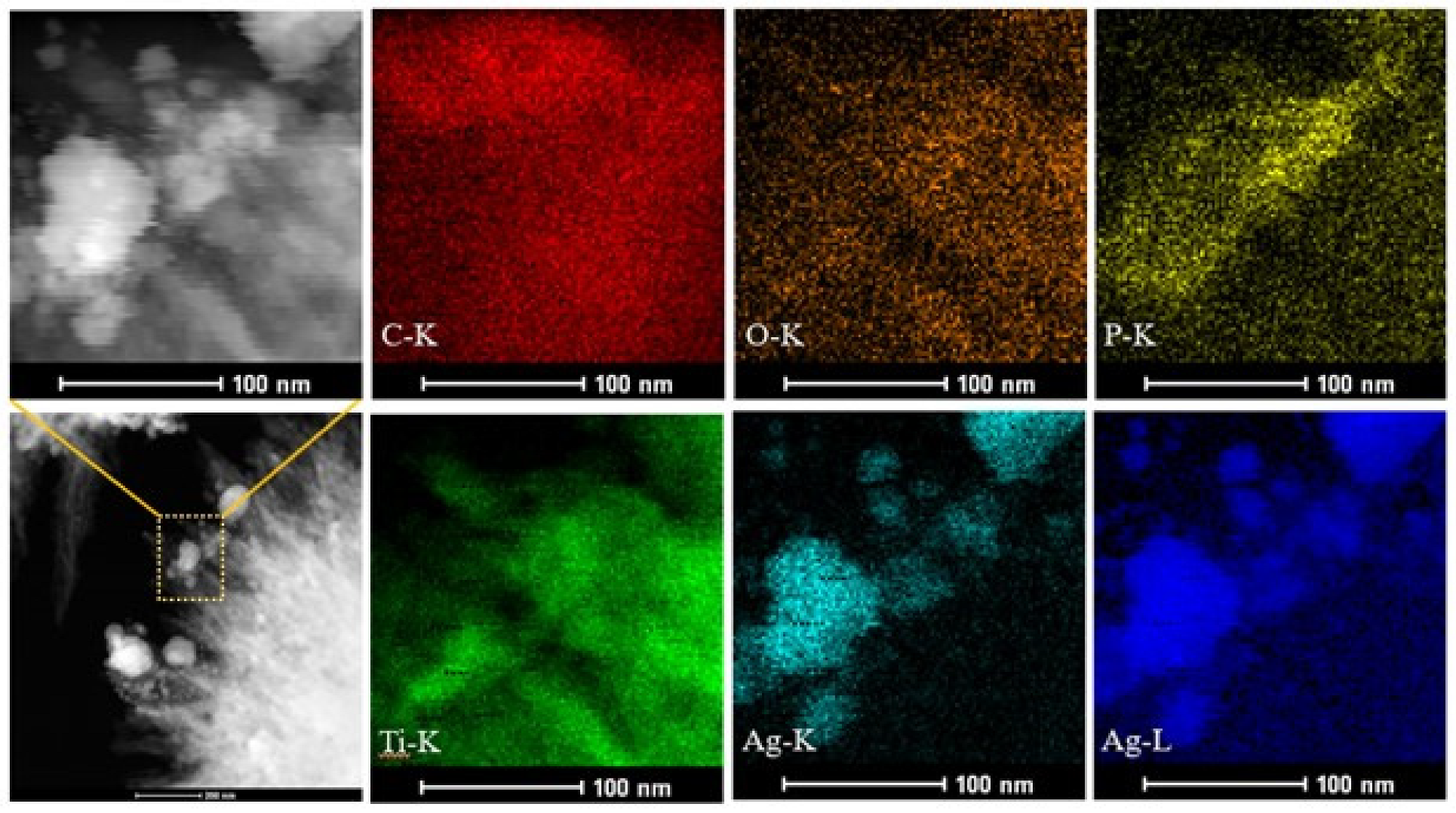
3.2. Morpholoy Analysis by SEM, TEM
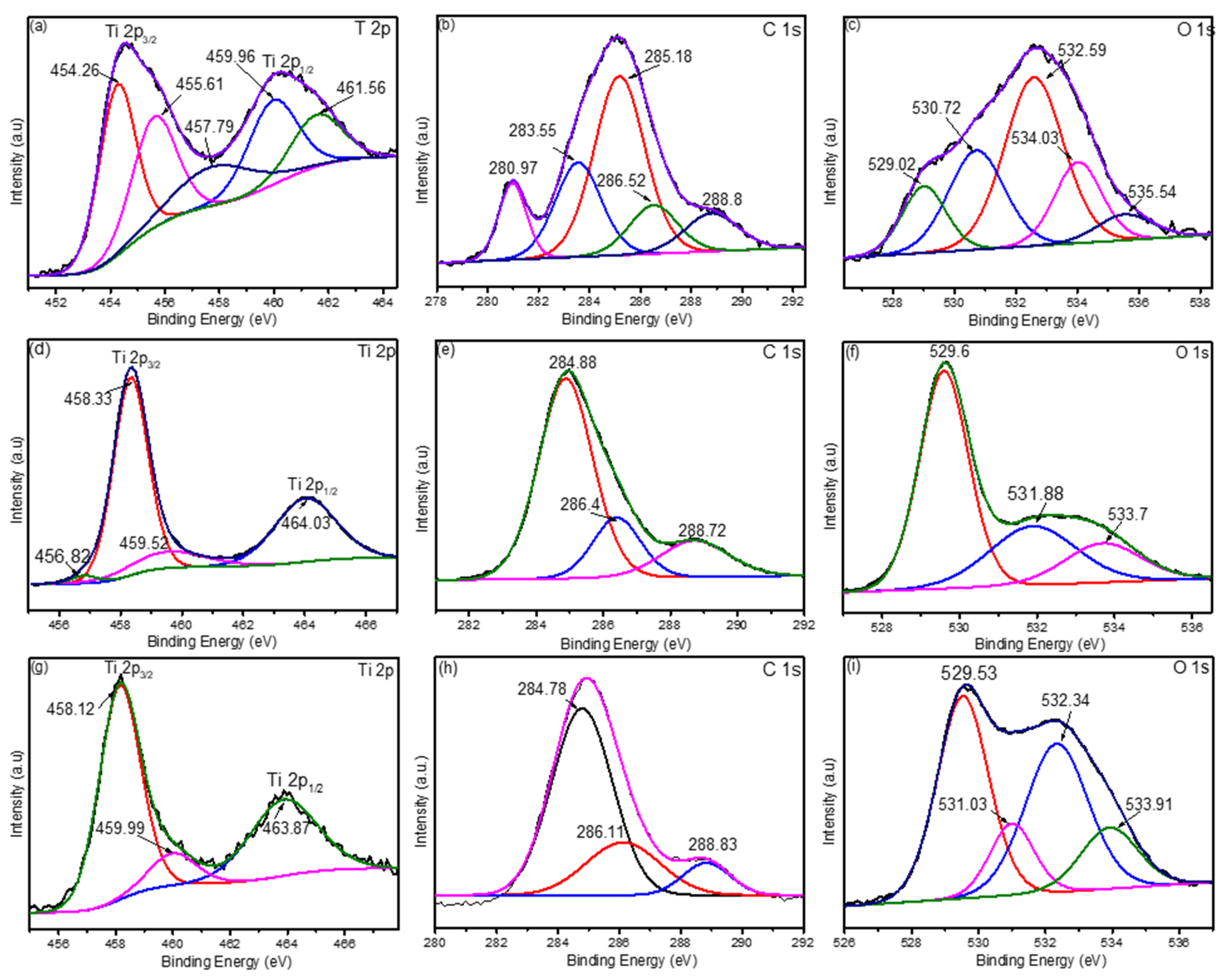
3.3. XPS Results
3.4. Surface Area and Optical Analysis
3.5. Photocatalytic Performance
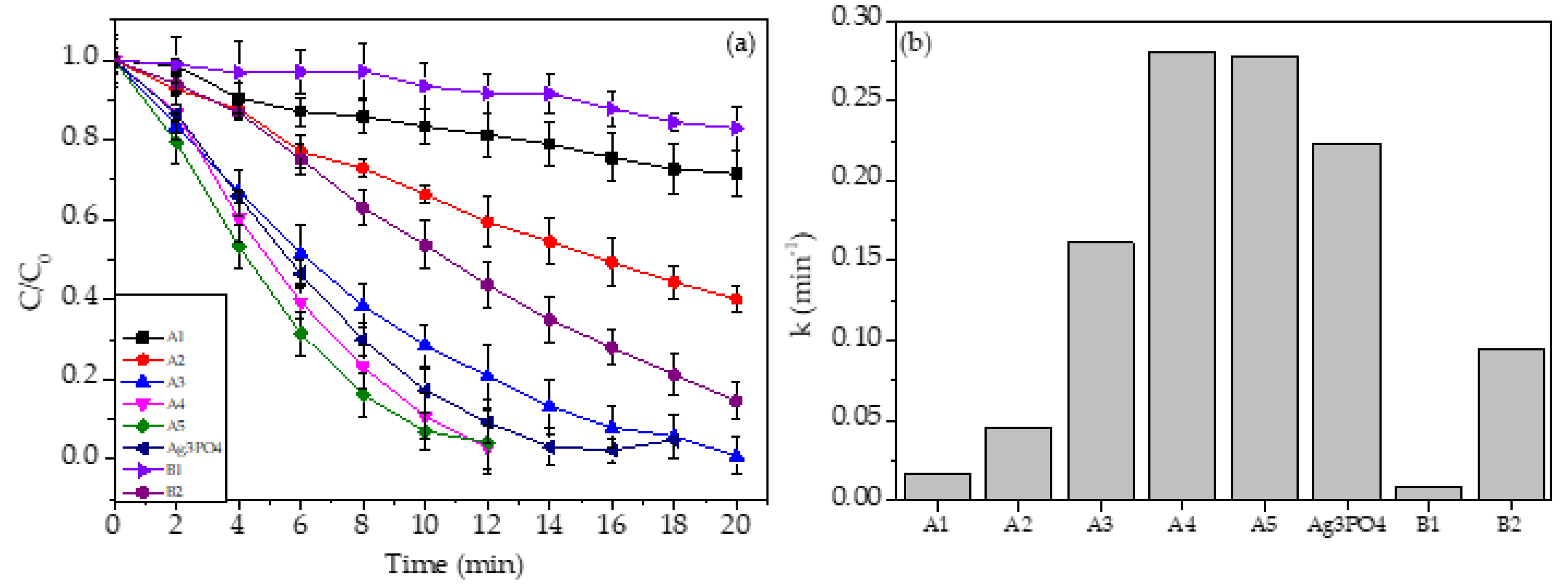
3.5.1. The Effects of Ag3PO4 Content on the Photodegradation of Rhodamine B
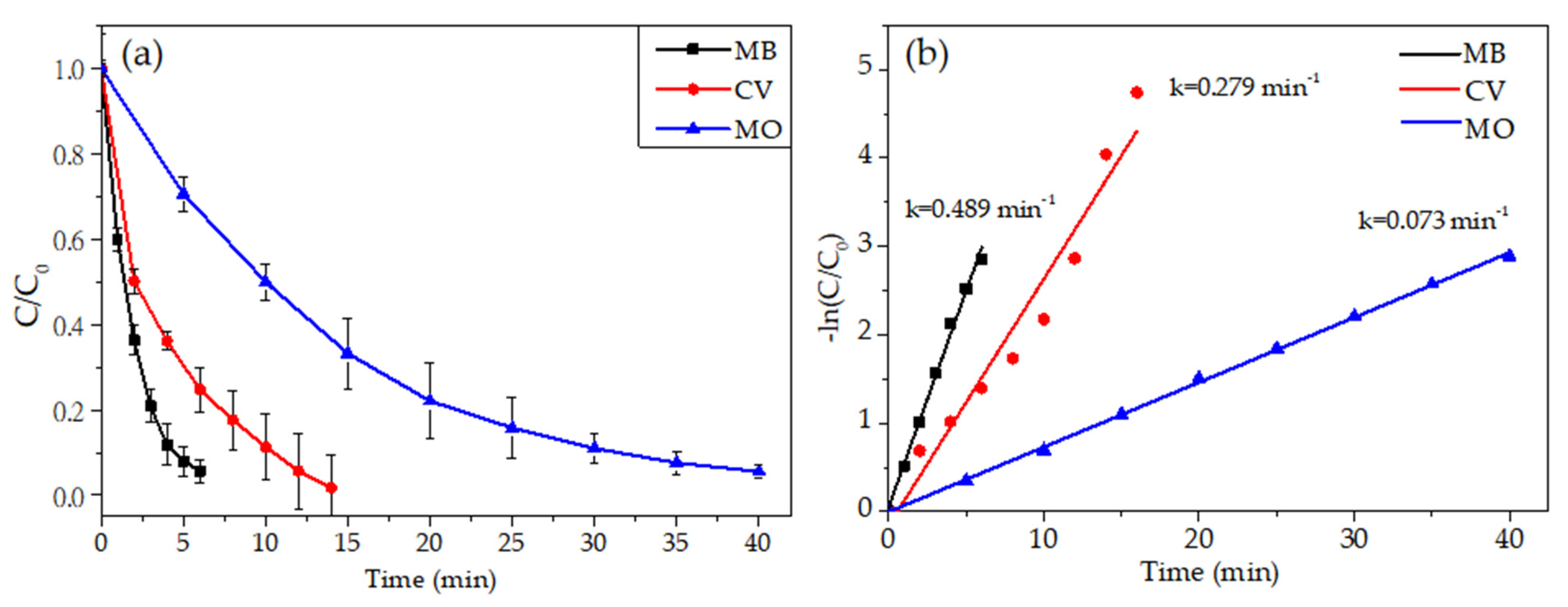
3.5.2. Photodegradation of Other Organic Dyes
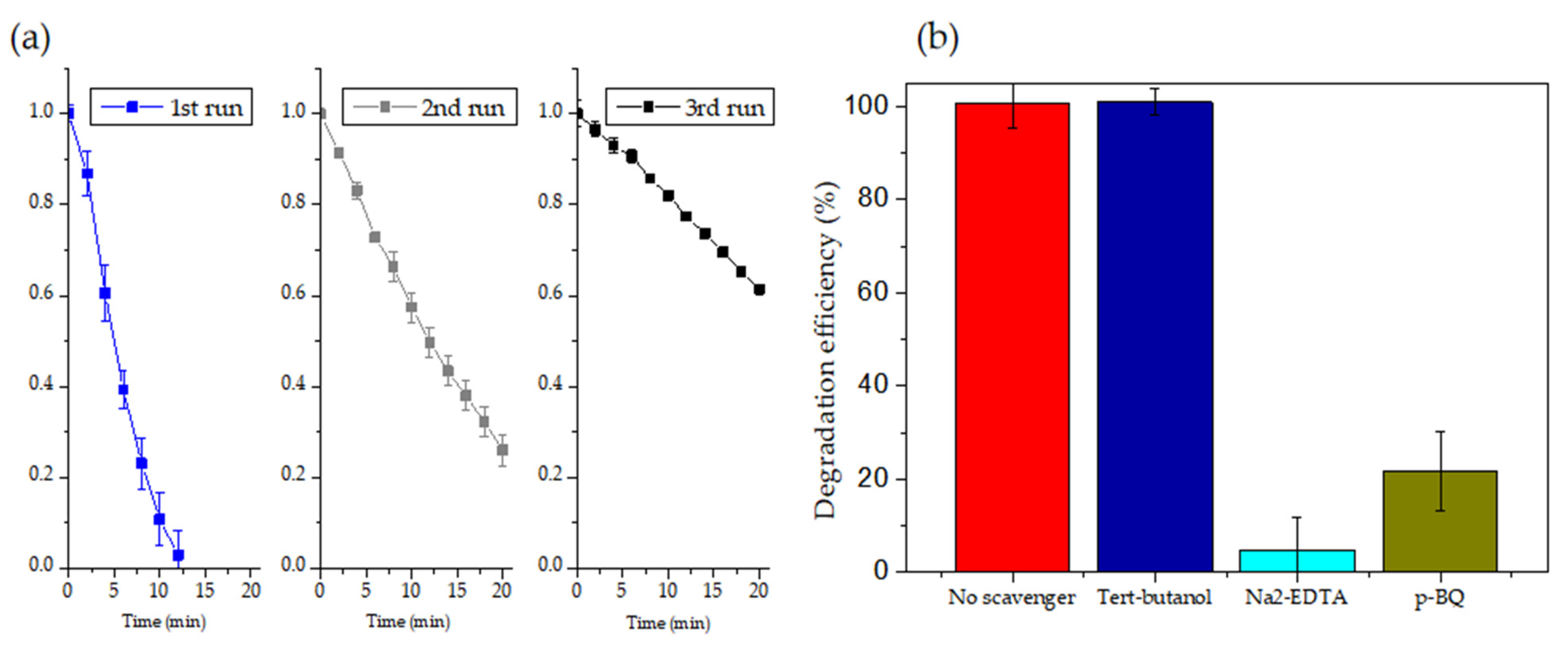
3.6. Scavenger Trapping and Recycling Tests of RhB Degradation
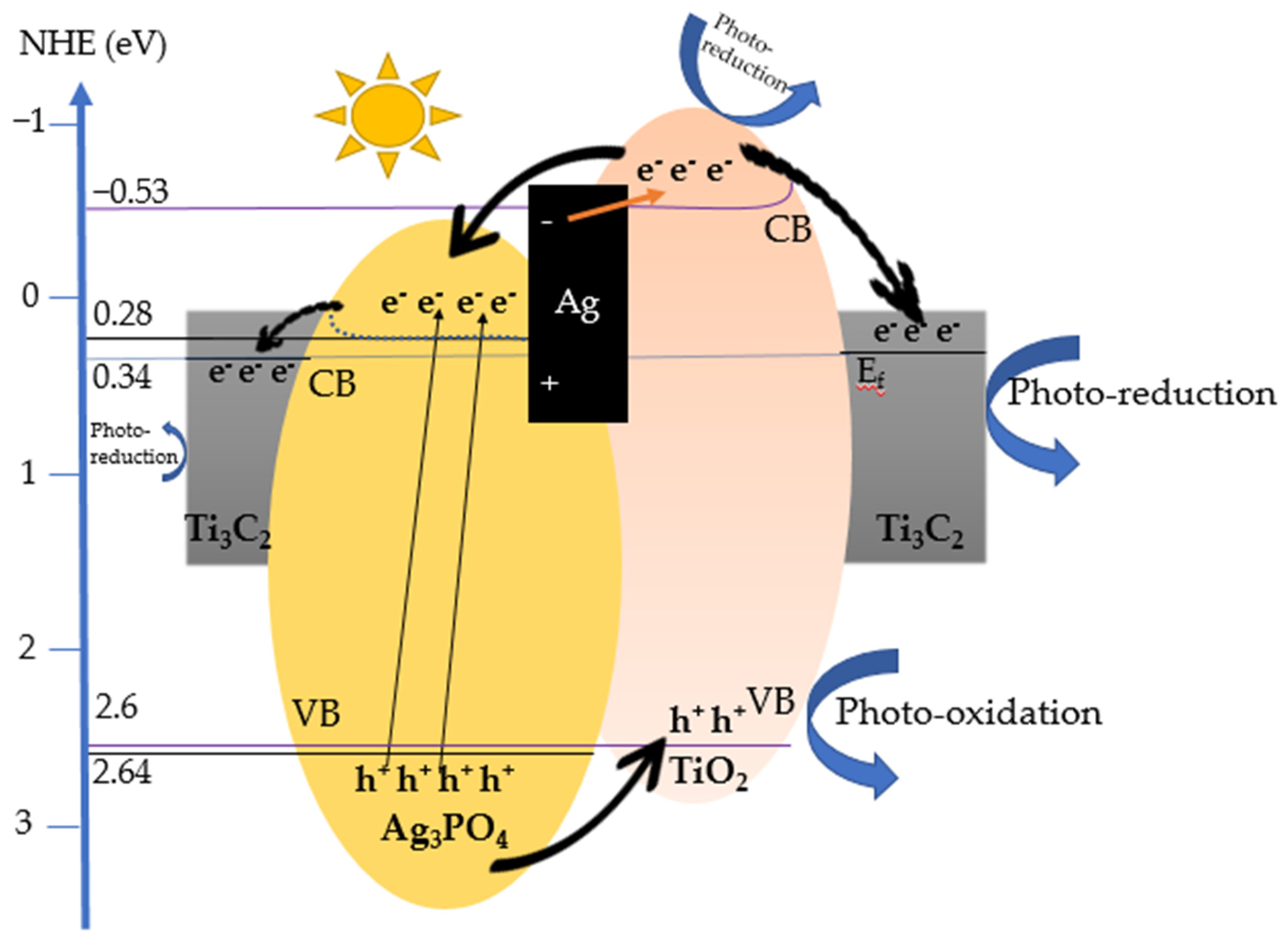
3.7. Proposed Photocatalytic Mechanism
- Photo-oxidation reaction:
- 2.
- Photo-reduction reaction:
- Photoexcited electrons and separation of e−/h+:
- Transfer routes and formation of O2●−:
- Degradation of pollutants:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and Consequences of Groundwater Contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, L.; Noblet, J. Water Contamination and Pollution. In Green Chemistry—An Inclusive Approach; Elsevier: Amsterdam, The Netherlands, 2018; pp. 261–290. [Google Scholar] [CrossRef]
- My Tran, N.; Thanh Hoai Ta, Q.; Sreedhar, A.; Noh, J.S. Ti3C2Tx MXene playing as a strong methylene blue adsorbent in wastewater. Appl. Surf. Sci. 2021, 537, 148006. [Google Scholar] [CrossRef]
- My Tran, N.; Thanh Hoai Ta, Q.; Noh, J.S. Unusual synthesis of safflower-shaped TiO2/Ti3C2 heterostructures initiated from two-dimensional Ti3C2 MXene. Appl. Surf. Sci. 2021, 538, 148023. [Google Scholar] [CrossRef]
- Muneer, I.; Farrukh, M.A.; Ali, D.; Bashir, F. Heterogeneous photocatalytic degradation of organic dyes by highly efficient GdCoSnO3, Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2021, 265, 115028. [Google Scholar] [CrossRef]
- Ton, N.N.T.; Dao, A.T.N.; Kato, K.; Ikenaga, T.; Trinh, D.X.; Taniike, T. One-pot synthesis of TiO2/graphene nanocomposites for excellent visible light photocatalysis based on chemical exfoliation method. Carbon 2018, 133, 109–117. [Google Scholar] [CrossRef]
- Kadam, R.L.; Kim, Y.; Gailkwad, S.; Chang, M.; Tarte, N.H.; Han, S. Catalytic decolorization of rhodamine B, Congo red, and crystal violet dyes, with a novel niobium oxide anchored molybdenum (Nb–O–Mo). Catalysts 2020, 10, 491. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Ghaffar, A.; Zhang, L.; Zhu, X.; Chen, B. Porous PVdF/GO Nanofibrous Membranes for Selective Separation and Recycling of Charged Organic Dyes from Water. Environ. Sci. Technol. 2018, 52, 4265–4274. [Google Scholar] [CrossRef]
- Pal, B.; Kaur, R.; Grover, I.S. Superior adsorption and photodegradation of eriochrome black-T dye by Fe3+ and Pt4+ impregnated TiO2 nanostructures of different shapes. J. Ind. Eng. Chem. 2016, 33, 178–184. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Rizzo, L.; Sannino, D. Photocatalytic activity of a visible light active structured photocatalyst developed for municipal wastewater treatment. J. Clean. Prod. 2018, 175, 38–49. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Lai, Q.; Fan, M. Review of the progress in preparing nano TiO2: An important environmental engineering material. J. Environ. Sci. 2014, 26, 2139–2177. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Cho, M.H. Highly Visible Light Responsive, Narrow Band gap TiO2 Nanoparticles Modified by Elemental Red Phosphorus for Photocatalysis and Photoelectrochemical Applications. Sci. Rep. 2016, 6, 25405. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Tian, J.; Liang, Z.; Cui, H. Ti3C2 MXene-derived Ti3C2/TiO2 nanoflowers for noble-metal-free photocatalytic overall water splitting. Appl. Mater. Today 2018, 13, 217–227. [Google Scholar] [CrossRef]
- Quyen, V.T.; Ha, L.T.T.; Thanh, D.M.; van Le, Q.; Viet, N.M.; Nham, N.T.; Thang, P.Q. Advanced synthesis of MXene-derived nanoflower-shaped TiO2@Ti3C2 heterojunction to enhance photocatalytic degradation of Rhodamine B. Environ. Technol. Innov. 2021, 21, 101286. [Google Scholar] [CrossRef]
- Frontistis, Z.; Antonopoulou, M.; Petala, A.; Venieri, D.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Photodegradation of ethyl paraben using simulated solar radiation and Ag3PO4 photocatalyst. J. Hazard. Mater. 2017, 323, 478–488. [Google Scholar] [CrossRef]
- Zhao, F.M.; Pan, L.; Wang, S.; Deng, Q.; Zou, J.J.; Wang, L.; Zhang, X. Ag3PO4/TiO2 composite for efficient photodegradation of organic pollutants under visible light. Appl. Surf. Sci. 2014, 317, 833–838. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; He, H.; Cheng, D.; Mao, M.; Jiang, Q.; Song, L.; Xiong, J. Facile synthesis of hierarchical Ag3PO4/TiO2 nanofiber heterostructures with highly enhanced visible light photocatalytic properties. Appl. Surf. Sci. 2015, 355, 921–929. [Google Scholar] [CrossRef]
- Khalid, N.R.; Mazia, U.; Tahir, M.B.; Niaz, N.A.; Javid, M.A. Photocatalytic degradation of RhB from an aqueous solution using Ag3PO4/N-TiO2 heterostructure. J. Mol. Liq. 2020, 313, 113522. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Wang, Y.; Li, H.; Liu, B.; Yin, S.; Sato, T. Substantial change in phenomenon of “self-corrosion” on Ag3PO4/TiO2 compound photocatalyst. Appl. Catal. B Environ. 2014, 158–159, 314–320. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Y.; Wang, X. Methods and mechanism for improvement of photocatalytic activity and stability of Ag3PO4: A review. J. Alloys Compd. 2015, 649, 910–932. [Google Scholar] [CrossRef]
- Nekouei, F.; Nekouei, S.; Pouzesh, M.; Liu, Y. Porous-CdS/Cu2O/graphitic-C3N4 dual p-n junctions as highly efficient photo/catalysts for degrading ciprofloxacin and generating hydrogen using solar energy. Chem. Eng. J. 2020, 385, 123710. [Google Scholar] [CrossRef]
- Nyankson, E.; Efavi, J.K.; Agyei-Tuffour, B.; Manu, G. Synthesis of TiO2-Ag3PO4photocatalyst material with high adsorption capacity and photocatalytic activity: Application in the removal of dyes and pesticides. RSC Adv. 2021, 11, 17032–17045. [Google Scholar] [CrossRef]
- Zanella, R.; Giorgio, S.; Henry, C.R.; Louis, C. Alternative methods for the preparation of gold nanoparticles supported on TiO2. J. Phys. Chem. B 2002, 106, 7634–7642. [Google Scholar] [CrossRef]
- Kong, F.; He, X.; Liu, Q.; Qi, X.; Zheng, Y.; Wang, R.; Bai, Y. Improving the electrochemical properties of MXene Ti3C2 multilayer for Li-ion batteries by vacuum calcination. Electrochim. Acta 2018, 265, 140–150. [Google Scholar] [CrossRef]
- Cao, M.; Wang, F.; Wang, L.; Wu, W.; Lv, W.; Zhu, J. Room Temperature Oxidation of Ti3C2 MXene for Supercapacitor Electrodes. J. Electrochem. Soc. 2017, 164, A3933–A3942. [Google Scholar] [CrossRef]
- Shen, C.; Wang, L.; Zhou, A.; Wang, B.; Wang, X.; Lian, W.; Hu, Q.; Qin, G.; Liu, X. Synthesis and electrochemical properties of two-dimensional RGO/Ti3C2Tx nanocomposites. Nanomaterials 2018, 8, 80. [Google Scholar] [CrossRef]
- Xia, Q.X.; Fu, J.; Yun, J.M.; Mane, R.S.; Kim, K.H. High volumetric energy density annealed-MXene-nickel oxide/MXene asymmetric supercapacitor. RSC Adv. 2017, 7, 11000–11011. [Google Scholar] [CrossRef]
- Liu, W.; Liu, D.; Wang, K.; Yang, X.; Hu, S.; Hu, L. Fabrication of Z-scheme Ag3PO4/TiO2 Heterostructures for Enhancing Visible Photocatalytic Activity. Nanoscale Res. Lett. 2019, 14, 203. [Google Scholar] [CrossRef]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, L.; Li, N.; Yan, W.; Li, X. Heterostructures of Ag3PO4/TiO2 mesoporous spheres with highly efficient visible light photocatalytic activity. J. Colloid Interface Sci. 2015, 450, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Liu, E.; Fan, J.; Hu, X.; Kang, L.; Wan, J. Heterostructured Ag3PO4/TiO2 nano-sheet film with high efficiency for photodegradation of methylene blue. Ceram. Int. 2014, 40, 15447–15453. [Google Scholar] [CrossRef]
- Pant, B.; Prasad Ojha, G.; Acharya, J.; Park, M. Ag3PO4-TiO2-Carbon nanofiber Composite: An efficient Visible-light photocatalyst obtained from electrospinning and hydrothermal methods. Sep. Purif. Technol. 2021, 276, 119400. [Google Scholar] [CrossRef]
- Saud, P.S.; Pant, B.; Twari, A.P.; Ghouri, Z.K.; Park, M.; Kim, H.Y. Effective photocatalytic efficacy of hydrothermally synthesized silver phosphate decorated titanium dioxide nanocomposite fibers. J. Colloid Interface Sci. 2016, 465, 225–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Duoerkun, G.; Shi, Z.; Cao, W.; Liu, T.; Liu, J.; Zhang, L.; Li, M.; Chen, Z. Construction of TiO2/Ag3PO4 nanojunctions on carbon fiber cloth for photocatalytically removing various organic pollutants in static or flowing wastewater. J. Colloid Interface Sci. 2020, 571, 213–221. [Google Scholar] [CrossRef]
- Sheu, F.J.; Cho, C.P.; Liao, Y.T.; Yu, C.T. Ag3PO4-TiO2-graphene oxide ternary composites with efficient photodegradation, hydrogen evolution, and antibacterial properties. Catalysts 2018, 8, 57. [Google Scholar] [CrossRef]
- Cai, T.; Wang, L.; Liu, Y.; Zhang, S.; Dong, W.; Chen, H.; Yi, X.; Yuan, J.; Xia, X.; Liu, C.; et al. Ag3PO4/Ti3C2 MXene interface materials as a Schottky catalyst with enhanced photocatalytic activities and anti-photocorrosion performance. Appl. Catal. B Environ. 2018, 239, 545–554. [Google Scholar] [CrossRef]

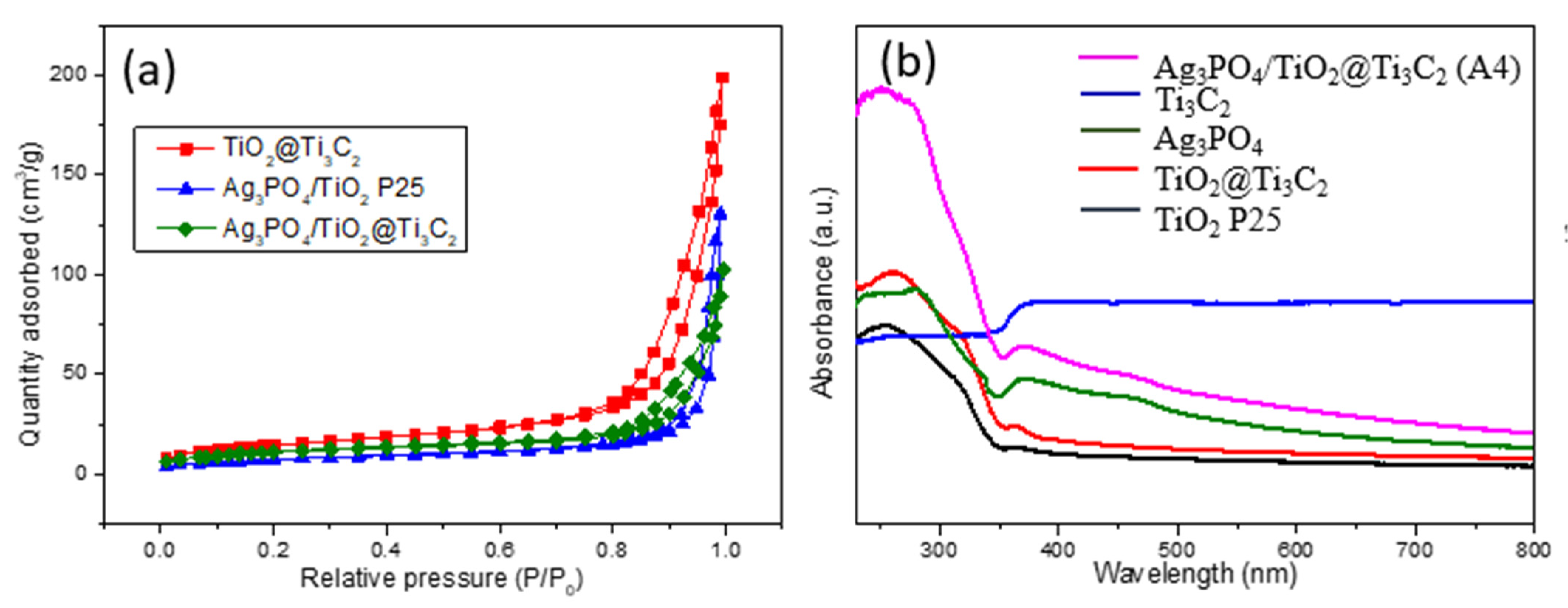
| Sample | BET Surface Area (m2g−1) | Pore Volume (cm3g−1) | Pore Size (nm) |
|---|---|---|---|
| TiO2@Ti3C2 | 53 | 0.309 | 21.91 |
| Ag3PO4/TiO2 P25 | 27 | 0.202 | 31.04 |
| Ag3PO4/TiO2@Ti3C2 (A4) | 40 | 0.156 | 20.12 |
| Pollutant | Pollutant conc. | Photocatalyst | Decoration Ag3PO4 Method | Ag3PO4 Diameter | Dosage (g L−1) | Irradiation Time (min) | Efficiency (%) | Light Source | [Ref.] |
|---|---|---|---|---|---|---|---|---|---|
| RhB MO MB CV | 9 ppm 10 ppm 10 ppm 10 ppm | Ag3PO4/TiO2@Ti3C2 | In situ precipitation | 4–10 nm | 0.5 | 12 40 6 14 | 97 92.4 94 99.2 | Solar light | Current study |
| RhB | 20 µm | Safflower-shaped TiO2/Ti3C2 | - | - | 0.8 | 60 | 95 | Visible light | [4] |
| RhB | 10 ppm | TiO2@Ti3C2 nanoflower | - | - | 0.43 | 40 | 97 | Visible light | [17] |
| RhB, MO, phenol | 0.02 mM 0.06 mM 0.2 mM | Ag3PO4/TiO2 | In situ precipitation | 300–500 nm | 0.5 | 15 80 90 | ~100 90 ~70 | Visible light | [19] |
| RhB | 10 ppm | Ag3PO4/TiO2 NFs | In situ precipitation | 300–360 nm | 0.4 | 10 (Vis) | ~100 | Visible light | [20] |
| RhB, MB, pesticides | 6 ppm 8 ppm - | Ag3PO4/TiO2 | One pot | 100–400 nm | 0.5 | ~ 6 4 | 98 99.1 | Tungsten halogen light | [25] |
| MB | 20 ppm | Ag3PO4/m-TiO2 | In situ precipitation | ~4 nm | 0.5 | 28 | 100 | Visible light | [33] |
| MB | 10 mM | Ag3PO4/TiO2 nanosheet film | Impregnating-deposition process | 100–300 nm | - | 70 | 98.1 | Visible light | [34] |
| MB | 10 ppm | Ag3PO4-TiO2-CNFs | Hydrothermal | 20–32 nm | 0.5 | 10 | 100 | Visible light | [35] |
| MB | 10 ppm | Ag3PO4/TiO2 NFs | Hydrothermal | - | 0.4 | 15 | 100 | Sunlight | [36] |
| RhB | 10 ppm | CFC/TiO2/Ag3PO4 | Hydrothermal | 20–100 nm | - | 100 | 98.4 | Visible light | [37] |
| MO | 20 ppm | Ag3PO4-TiO2-GO | Ion exchange | 250–600 nm | 1 | 120 | 80 | Solar light | [38] |
| RhB | 10 ppm | Ag3PO4/TiO2 | Hydrothermal and ultrasonication | 0.1–1.5 µm | 1 | 25 | 100 | Solar light | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.T.A.; Kim, H. Ag3PO4-Deposited TiO2@Ti3C2 Petals for Highly Efficient Photodecomposition of Various Organic Dyes under Solar Light. Nanomaterials 2022, 12, 2464. https://doi.org/10.3390/nano12142464
Nguyen NTA, Kim H. Ag3PO4-Deposited TiO2@Ti3C2 Petals for Highly Efficient Photodecomposition of Various Organic Dyes under Solar Light. Nanomaterials. 2022; 12(14):2464. https://doi.org/10.3390/nano12142464
Chicago/Turabian StyleNguyen, Ngoc Tuyet Anh, and Hansang Kim. 2022. "Ag3PO4-Deposited TiO2@Ti3C2 Petals for Highly Efficient Photodecomposition of Various Organic Dyes under Solar Light" Nanomaterials 12, no. 14: 2464. https://doi.org/10.3390/nano12142464
APA StyleNguyen, N. T. A., & Kim, H. (2022). Ag3PO4-Deposited TiO2@Ti3C2 Petals for Highly Efficient Photodecomposition of Various Organic Dyes under Solar Light. Nanomaterials, 12(14), 2464. https://doi.org/10.3390/nano12142464





