Effect of Texturing Environment on Wetting of Biomimetic Superhydrophobic Surfaces Designed by Femtosecond Laser Texturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Femtosecond Laser Texturing Process
2.2. Sample Characterization
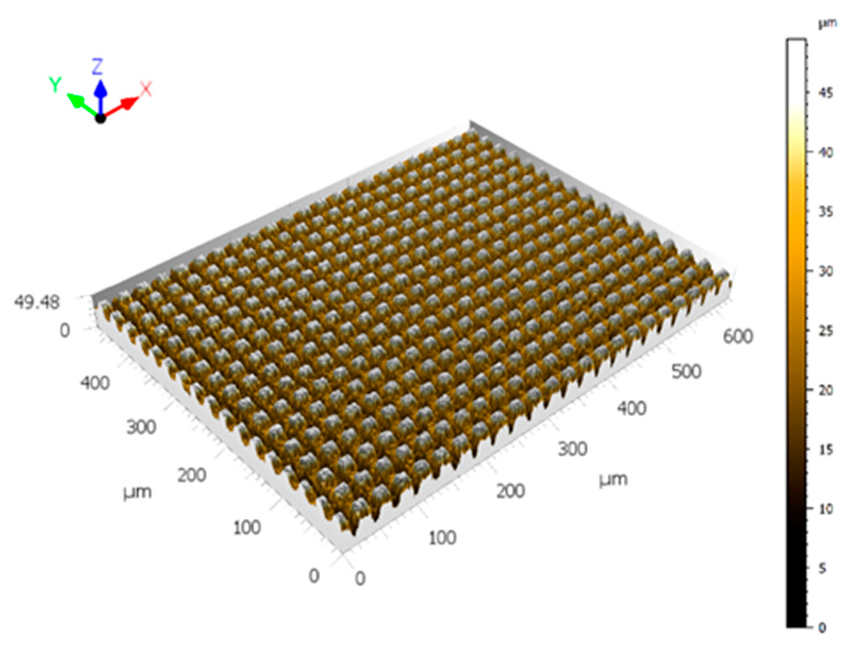
2.2.1. Topography Analysis
2.2.2. Surface Chemistry Analysis by XPS
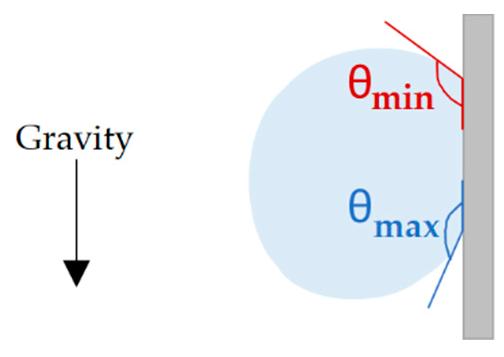
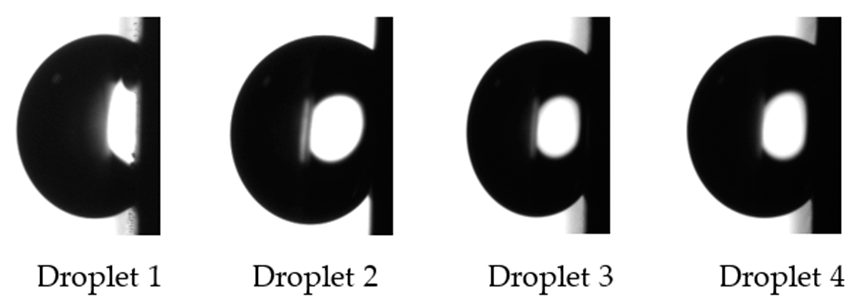
2.2.3. Contact Angle Measurements
3. Results and Discussion
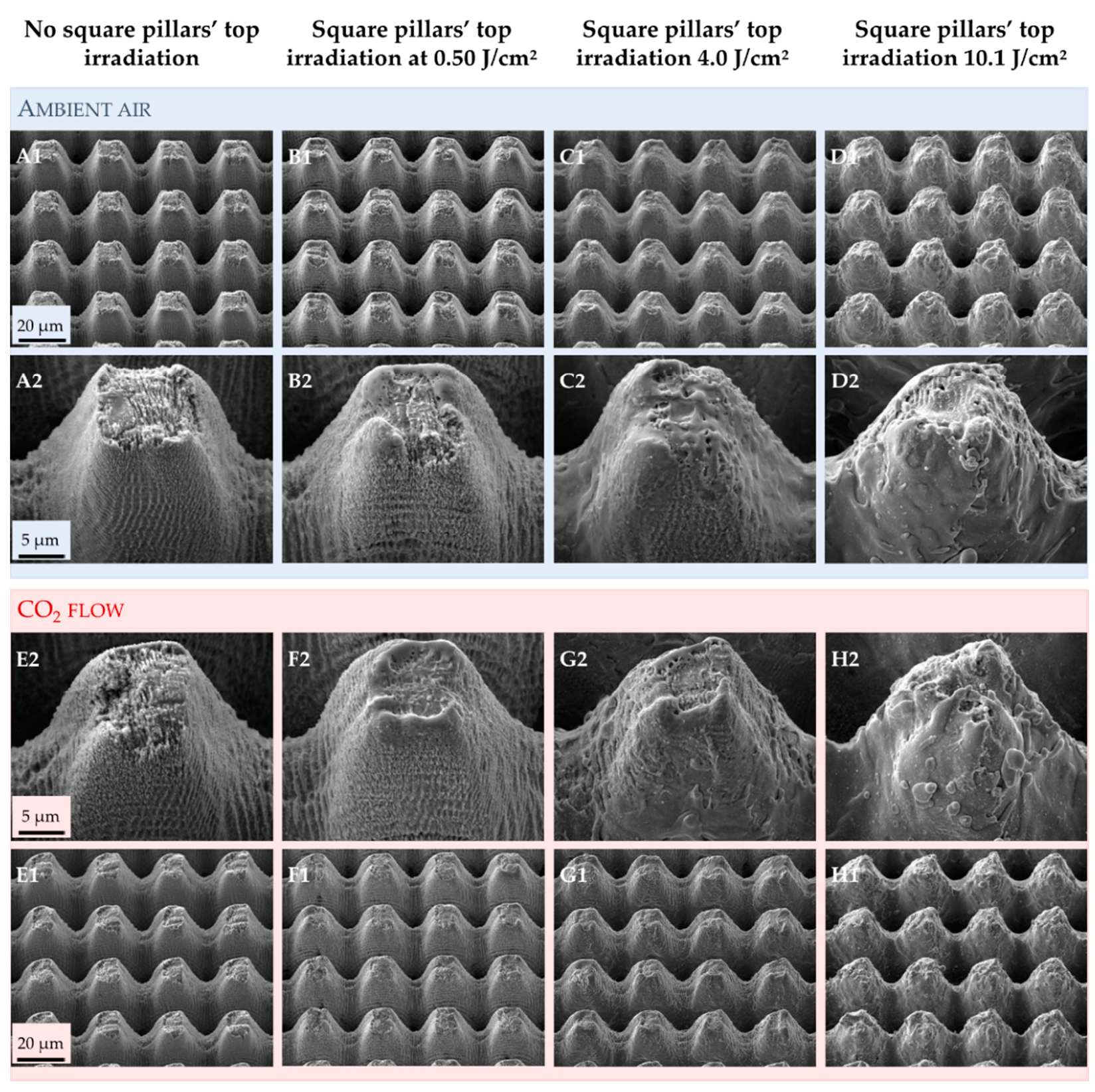
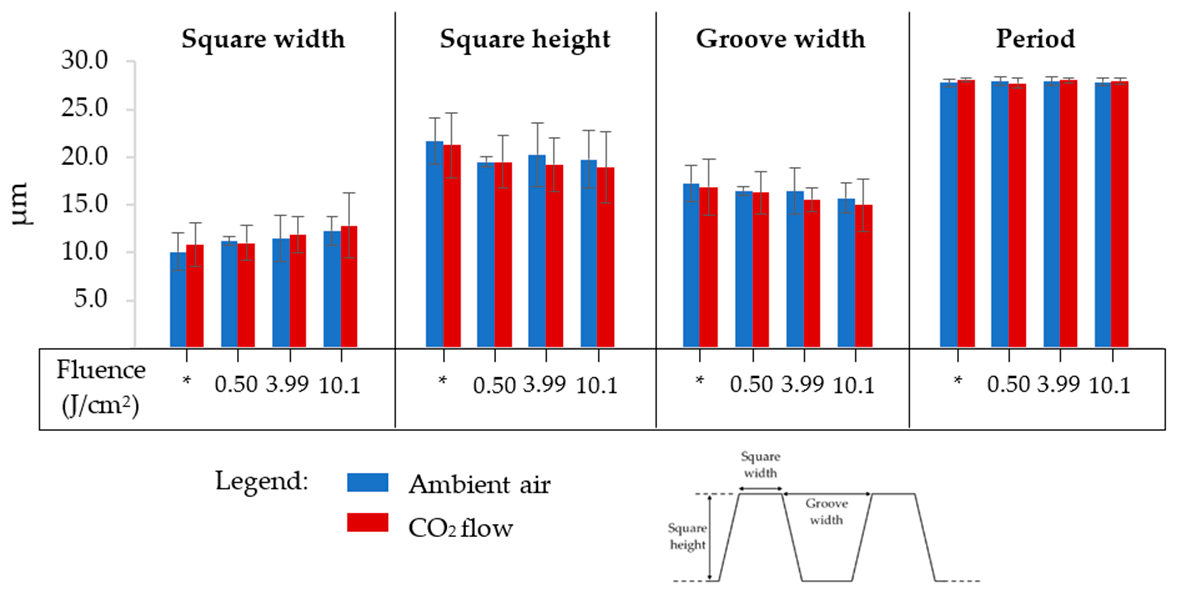
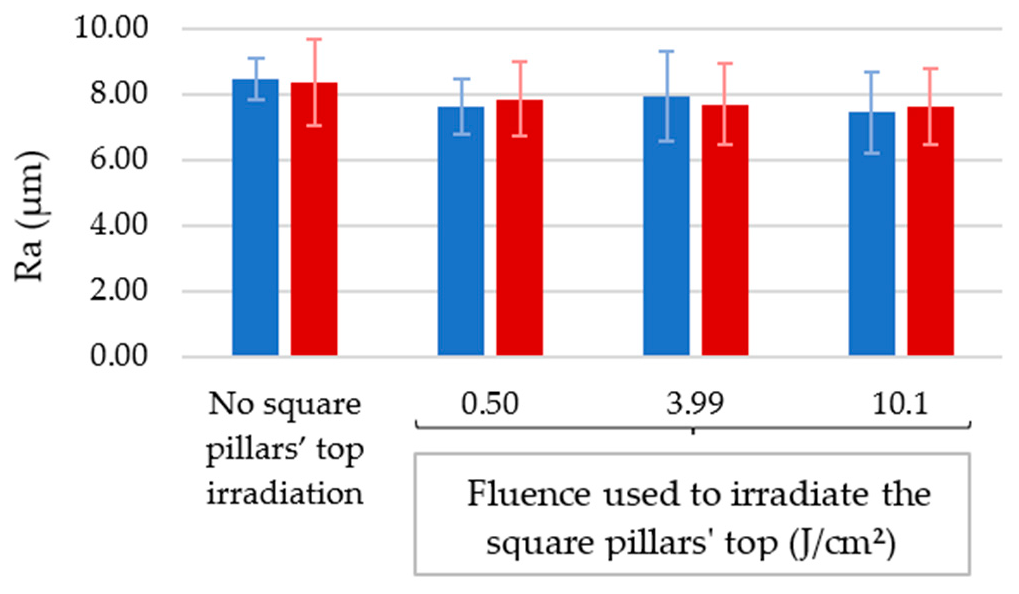
3.1. Effect of Texturing Process Environment on Topography
3.2. Effect of Texturing Process Environment on Wetting
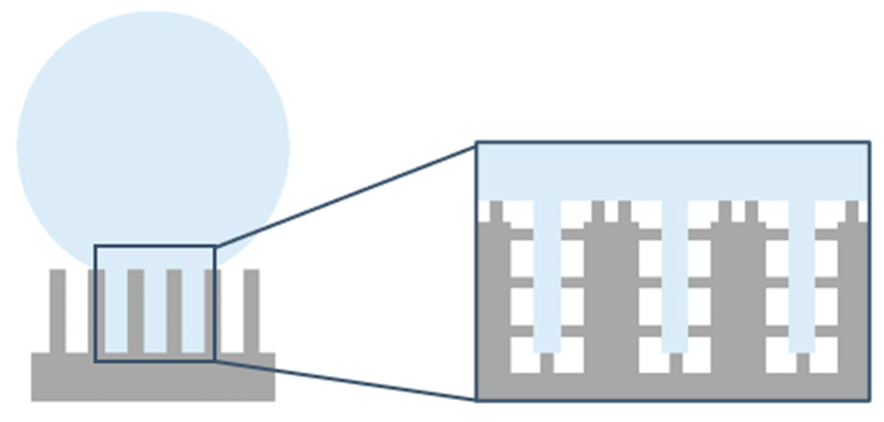
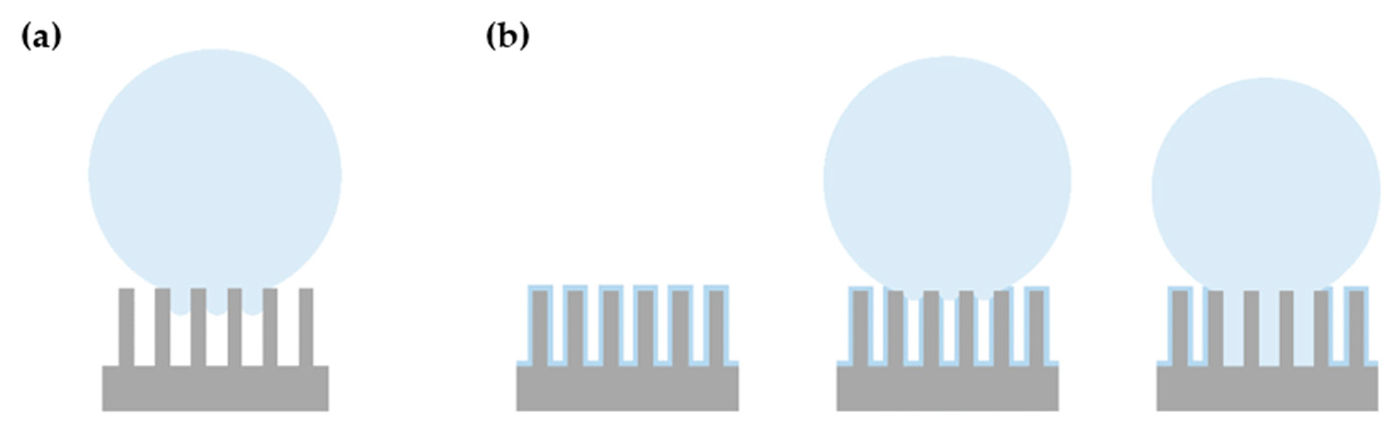
3.2.1. Samples for Which the Square Pillars’ Top Was Not Irradiated
3.2.2. Samples for Which the Square Pillars’ Top Was Irradiated at Different Fluences
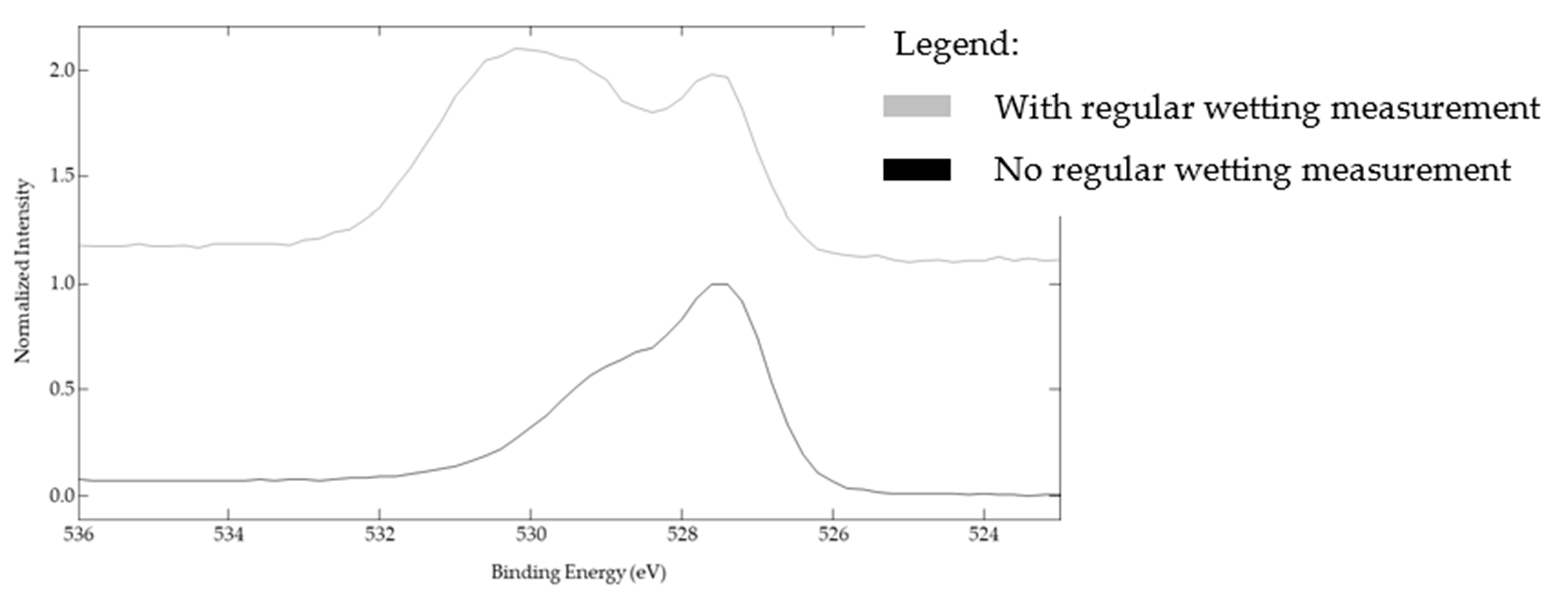
3.3. Wetting Regime and the Effect of Regular Wetting Measurements on Final Wettability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Lamb, R.N. Superhydrophobic Treatment for Textiles via Engineering Nanotextured Silica/Polysiloxane Hybrid Material onto Fibres. Surf. Eng. 2009, 25, 21–24. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Cassie, A.B.D. Contact Angles. Discuss. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Bhushan, B.; Nosonovsky, M. The Rose Petal Effect and the Modes of Superhydrophobicity. Phil. Trans. R. Soc. A 2010, 368, 4713–4728. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and Distribution of Water-Repellent, Self-Cleaning Plant Surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Agarwalla, S.V.; Ellepola, K.; Costa, M.C.F.; Fechine, G.J.M.; Morin, J.L.P.; Neto, A.H.C.; Seneviratne, C.J.; Rosa, V. Hydrophobicity of Graphene as a Driving Force for Inhibiting Biofilm Formation of Pathogenic Bacteria and Fungi. Dent. Mater. 2019, 35, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Epperlein, N.; Menzel, F.; Schwibbert, K.; Koter, R.; Bonse, J.; Sameith, J.; Krüger, J.; Toepel, J. Influence of Femtosecond Laser Produced Nanostructures on Biofilm Growth on Steel. Appl. Surf. Sci. 2017, 418, 420–424. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic Surfaces for Corrosion Protection: A Review of Recent Progresses and Future Directions. J. Coat. Technol. Res. 2015, 13, 11–29. [Google Scholar] [CrossRef]
- Zheng, H.; Chang, S.; Ma, G.; Wang, S. Anti-Icing Performance of Superhydrophobic Surface Fabricated by Femtosecond Laser Composited Dual-Layers Coating. Energy Build. 2020, 223, 110175. [Google Scholar] [CrossRef]
- Yin, B.; Fang, L.; Hu, J.; Tang, A.; Wei, W.; He, J. Preparation and Properties of Super-Hydrophobic Coating on Magnesium Alloy. Appl. Surf. Sci. 2010, 257, 1666–1671. [Google Scholar] [CrossRef]
- Cao, Z.; Lu, F.; Qiu, P.; Yang, F.; Liu, G.; Wang, S.; Zhong, H. Formation of a Hydrophobic and Corrosion Resistant Coating on Manganese Surface via Stearic Acid and Oleic Acid Diethanolamide. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 372–380. [Google Scholar] [CrossRef]
- Liu, F.; Ma, M.; Zang, D.; Gao, Z.; Wang, C. Fabrication of Superhydrophobic/Superoleophilic Cotton for Application in the Field of Water/Oil Separation. Carbohydr. Polym. 2014, 103, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Liu, G.; Hong, L.; Scott, E.J. Duncan Superamphiphobic Diblock Copolymer Coatings. Chem. Mater. 2011, 23, 4357–4366. [Google Scholar] [CrossRef]
- Yokoi, N.; Manabe, K.; Tenjimbayashi, M.; Shiratori, S. Optically Transparent Superhydrophobic Surfaces with Enhanced Mechanical Abrasion Resistance Enabled by Mesh Structure. ACS Appl. Mater. Interfaces 2015, 7, 4809–4816. [Google Scholar] [CrossRef] [PubMed]
- Beyler-Cigil, A. Designing Superhydrophobic and Flame Retardant Photo-Cured Hybrid Coatings. Prog. Org. Coat. 2020, 148, 105850. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Shi, Z.; Lei, L.; Mao, J.; Qu, L. Study of Water Repellency and Corrosion of STA-PFOA Modified Mortar. Constr. Build. Mater. 2022, 322, 126363. [Google Scholar] [CrossRef]
- Chen, F.; Song, J.; Lu, Y.; Huang, S.; Liu, X.; Sun, J.; Carmalt, C.J.; Parkin, I.P.; Wu, W. Creating Robust Superamphiphobic Coatings for Both Hard and Soft Materials. J. Mater. Chem. A 2015, 3, 20999–21008. [Google Scholar] [CrossRef]
- Li, H.; Yu, S.; Han, X. Fabrication of CuO Hierarchical Flower-like Structures with Biomimetic Superamphiphobic, Self-Cleaning and Corrosion Resistance Properties. Chem. Eng. J. 2016, 283, 1443–1454. [Google Scholar] [CrossRef]
- Hekster, F.M.; Laane, R.W.P.M.; de Voogt, P. Environmental and Toxicity Effects of Perfluoroalkylated Substances. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2003; pp. 99–121. ISBN 978-0-387-21731-4. [Google Scholar]
- Li, K.; Gao, P.; Xiang, P.; Zhang, X.; Cui, X.; Ma, L.Q. Molecular Mechanisms of PFOA-Induced Toxicity in Animals and Humans: Implications for Health Risks. Environ. Int. 2017, 99, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Ai, J.; Du, Q.; Liu, J.; Zeng, X. Superhydrophobic Polytetrafluoroethylene Surfaces with Accurately and Continuously Tunable Water Adhesion Fabricated by Picosecond Laser Direct Ablation. Mater. Des. 2019, 173, 107782. [Google Scholar] [CrossRef]
- Lu, Y.; Guan, Y.; Li, Y.; Yang, L.; Wang, M.; Wang, Y. Nanosecond Laser Fabrication of Superhydrophobic Surface on 316L Stainless Steel and Corrosion Protection Application. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125259. [Google Scholar] [CrossRef]
- Lei, Z.; Tian, Z.; Chen, X.; Chen, Y.; Bi, J.; Wu, S.; Sun, H. Large Spot Diameter Nanosecond Laser Treatment of Aluminum Alloy Sheets for High-Speed Superhydrophobic Hierarchical Micro- and Nanostructured Surface Preparation. Surf. Coat. Technol. 2019, 361, 249–254. [Google Scholar] [CrossRef]
- Divin-Mariotti, S.; Amieux, P.; Pascale-Hamri, A.; Auger, V.; Kermouche, G.; Valiorgue, F.; Valette, S. Effects of Micro-Knurling and Femtosecond Laser Micro Texturing on Aluminum Long-Term Surface Wettability. Appl. Surf. Sci. 2019, 479, 344–350. [Google Scholar] [CrossRef]
- Valette, S.; le Harzic, R.; Huot, N.; Audouard, E.; Fortunier, R. 2D Calculations of the Thermal Effects Due to Femtosecond Laser-Metal Interaction. Appl. Surf. Sci. 2005, 247, 238–242. [Google Scholar] [CrossRef]
- Hamad, A.H. Effects of Different Laser Pulse Regimes (Nanosecond, Picosecond and Femtosecond) on the Ablation of Materials for Production of Nanoparticles in Liquid Solution. In High Energy and Short Pulse Lasers; IntechOpen: London, UK, 2016; ISBN 978-953-51-2607-2. [Google Scholar]
- Gamaly, E.G.; Rode, A.V.; Luther-Davies, B.; Tikhonchuk, V.T. Ablation of Solids by Femtosecond Lasers: Ablation Mechanism and Ablation Thresholds for Metals and Dielectrics. Phys. Plasmas 2002, 9, 949–957. [Google Scholar] [CrossRef]
- Wang, S.Y.; Ren, Y.; Cheng, C.W.; Chen, J.K.; Tzou, D.Y. Micromachining of Copper by Femtosecond Laser Pulses. Appl. Surf. Sci. 2013, 265, 302–308. [Google Scholar]
- Zhang, F.; Dong, X.; Yin, K.; Song, Y.; Tian, Y.; Wang, C.; Duan, J. Temperature Effects on the Geometry during the Formation of Micro-Holes Fabricated by Femtosecond Laser in PMMA. Opt. Laser Technol. 2018, 100, 256–260. [Google Scholar] [CrossRef]
- Sohn, I.; Noh, Y.; Choi, S.; Ko, D.; Lee, J.; Choi, Y. Femtosecond Laser Ablation of Polypropylene for Breathable Film. Appl. Surf. Sci. 2008, 254, 4919–4924. [Google Scholar] [CrossRef]
- Oraevsky, A.A.; da Silva, L.B.; Rubenchik, A.M.; Feit, M.D.; Glinsky, M.E.; Perry, M.D.; Mammini, B.M.; Small, W.; Stuart, B.C. Plasma Mediated Ablation of Biological Tissues with Nanosecond-to-Femtosecond Laser Pulses: Relative Role of Linear and Nonlinear Absorption. IEEE J. Sel. Top. Quantum Electron. 1996, 2, 801–809. [Google Scholar] [CrossRef]
- Kuetemeyer, K.; Baumgart, J.; Lubatschowski, H.; Heisterkamp, A. Repetition Rate Dependency of Low-Density Plasma Effects during Femtosecond-Laser-Based Surgery of Biological Tissue. Appl. Phys. B 2009, 97, 695–699. [Google Scholar] [CrossRef]
- Ahmmed, K.M.T.; Kietzig, A.-M. Drag Reduction on Laser Patterned Hierarchical Superhydrophobic Surfaces. Soft Matter 2016, 12, 4912–4922. [Google Scholar] [CrossRef]
- Moradi, S.; Kamal, S.; Englezos, P.; Hatzikiriakos, S. Femtosecond Laser Irradiation of Metallic Surfaces: Effects of Laser Parameters on Superhydrophobicity. Nanotechnology 2013, 23, 415302. [Google Scholar] [CrossRef]
- Ha, N.S.; Lu, G. A Review of Recent Research on Bio-Inspired Structures and Materials for Energy Absorption Applications. Compos. Part B Eng. 2020, 181, 107496. [Google Scholar] [CrossRef]
- Zhiwu, H.; Junqiu, Z.; Chao, G.; Li, W.; Ren, L. Erosion Resistance of Bionic Functional Surfaces Inspired from Desert Scorpions. Langmuir 2012, 28, 2914–2921. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Schlangen, E. Development of a Bacteria-Based Self Healing Concrete. In Tailor Made Concrete Structures; Walraven, J.C., Stoelhorst, D., Eds.; Taylor & Francis: London, UK, 2008. [Google Scholar] [CrossRef]
- Lu, L.; Yao, W.; Xie, Y.; Li, K.; Wan, Z. Study on the Wettability of Biomimetic Stainless Steel Surfaces Inspired by Bauhinia Linn. Leaf. Surf. Coat. Technol. 2021, 405, 126721. [Google Scholar] [CrossRef]
- He, X.; Li, G.; Zhang, Y.; Lai, X.; Zhou, M.; Xiao, L.; Tang, X.; Hu, Y.; Liu, H.; Yang, Y.; et al. Bioinspired Functional Glass Integrated with Multiplex Repellency Ability from Laser-Patterned Hexagonal Texturing. Chem. Eng. J. 2021, 416, 129113. [Google Scholar] [CrossRef]
- Legrand, Q.; Benayoun, S.; Valette, S. Biomimetic Approach for the Elaboration of Highly Hydrophobic Surfaces: Study of the Links between Morphology and Wettability. Biomimetics 2021, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, E.; Bonse, J.; Heitz, J.; Siegel, J.; Tsibidis, G.D.; Skoulas, E.; Papadopoulos, A.; Mimidis, A.; Joel, A.-C.; Comanns, P.; et al. Laser Engineering of Biomimetic Surfaces. Mater. Sci. Eng. R Rep. 2020, 141, 100562. [Google Scholar] [CrossRef]
- Barberoglou, M.; Zorba, V.; Stratakis, E.; Spanakis, E.; Anastasiadis, S.H.; Fotakis, C.; Tzanetakis, P. Bio-Inspired Water Repellent Surfaces Produced by Ultrafast Laser Structuring of Silicon. Appl. Surf. Sci. 2009, 255, 5425–5429. [Google Scholar] [CrossRef]
- Su, Y.; Wang, S.; Yao, D.; Fu, Y.; Zang, H.; Xu, H.; Polynkin, P. Stand-off Fabrication of Irregularly Shaped, Multi-Functional Hydrophobic and Antireflective Metal Surfaces Using Femtosecond Laser Filaments in Air. Appl. Surf. Sci. 2019, 494, 1007–1012. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Huang, L.; Ren, N.; Kong, X. Femtosecond Pulsed Laser Textured Titanium Surfaces with Stable Superhydrophilicity and Superhydrophobicity. Appl. Surf. Sci. 2016, 389, 585–593. [Google Scholar] [CrossRef]
- Lehr, J.; Kietzig, A.-M. Production of Homogenous Micro-Structures by Femtosecond Laser Micro-Machining. Opt. Lasers Eng. 2014, 57, 121–129. [Google Scholar] [CrossRef]
- Kietzig, A.; Lehr, J.; Matus, L.; Liang, F. Laser-Induced Patterns on Metals and Polymers for Biomimetic Surface Engineering. Laser Appl. Microelectron. Optoelectron. Manuf. 2014, 8967, 896705. [Google Scholar] [CrossRef]
- Lin, C.Y.; Cheng, C.W.; Ou, K.L. Micro/Nano-Structuring of Medical Stainless Steel Using Femtosecond Laser Pulses. Phys. Procedia 2012, 39, 661–668. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W. Superhydrophobic and Superhydrophilic Plant Surfaces: An Inspiration for Biomimetic Materials. Phil. Trans. R. Soc. A 2009, 367, 1487–1509. [Google Scholar] [CrossRef]
- Gou, X.; Guo, Z. The Superhydrophobic Plant Leaves: The Variation in Surface Morphologies and Wettability during the Vegetation Period. Langmuir 2019, 35, 1047–1053. [Google Scholar] [CrossRef]
- Bizi-Bandoki, P.; Valette, S.; Benayoun, S.; Audouard, E. Time Dependency of the Hydrophilicity and Hydrophobicity of Metallic Alloys Subjected to Femtosecond Laser Irradiations. Appl. Surf. Sci. 2013, 273, 399–407. [Google Scholar] [CrossRef]
- Kietzig, A.; Hatzikiriakos, S.; Englezos, P. Patterned Superhydrophobic Metallic Surfaces. Langmuir 2009, 25, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Zhong, M.; Zhang, H.; Fan, P. Superhydrophilicity to Superhydrophobicity Transition of Picosecond Laser Microstructured Aluminum in Ambient Air. J. Colloid Interface Sci. 2015, 441, 1–9. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Zhang, Z.; Jiang, Z.; Lian, J.; Ren, L. Facile and Environmentally-Friendly Fabrication of Underwater Superaerophobic and Superaerophilic Metallic Surfaces through Laser Ablation and Heat Treatment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126547. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, M.; Li, J.; Ye, X.; Li, G.; Cai, L. Superhydrophobic Surfaces Fabricated by Microstructuring of Stainless Steel Using a Femtosecond Laser. Appl. Surf. Sci. 2009, 256, 61–66. [Google Scholar] [CrossRef]
- Ahsan, S.; Dewanda, F.; Lee, M.S.; Sekita, H.; Sumiyoshi, T. Formation of Superhydrophobic Soda-Lime Glass Surface Using Femtosecond Laser Pulses. Appl. Surf. Sci. 2013, 265, 784–789. [Google Scholar] [CrossRef]
- Yalishev, V.S.; Iqbal, M.; Kim, V.V.; Khan, S.A.; Ganeev, R.A.; Alnaser, A.S. Reversible Wettability Transition of Laser-Textured Metals after Vacuum Storing and Low-Temperature Annealing. Appl. Phys. A 2021, 127, 393. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Perkin-Elmer Corporation, Physical Electronics Division: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Trtica, M. Surface Behavior of 16Cr3Al ODS Steel—Effects of High Laser Intensity 1014 W/Cm2 in Ambiences of Air, Helium and Vacuum. Fusion Eng. Des. 2020, 150, 111360. [Google Scholar] [CrossRef]
- Bashir, S.; Rafique, M.S.; Nathala, C.S.; Ajami, A.A.; Husinsky, W.; Whitmore, K. Pulse Duration and Environmental Effects on the Surface Nanostructuring and Mechanical Properties of Zinc during Femtosecond Laser Irradiation. J. Opt. Soc. Am. B 2020, 37, 2878–2891. [Google Scholar] [CrossRef]
- Zhigilei, L.V.; Lin, Z.; Ivanov, D.S. Atomistic Modeling of Short Pulse Laser Ablation of Metals: Connections between Melting, Spallation, and Phase Explosion. J. Phys. Chem. C 2009, 113, 11892–11906. [Google Scholar] [CrossRef]
- Kietzig, A.; Mirvakili, M.N.; Kamal, S.; Englezos, P.; Hatzikiriakos, S. Laser Patterned Superhydrophobic Pure Metallic Substrates-Cassie to Wenzel Wetting Transitions. J. Adhes. Sci. Technol. 2011, 25, 2789–2809. [Google Scholar] [CrossRef]
- Giannuzzi, G.; Gaudiuso, C.; di Mundo, R.; Mirenghi, L.; Fraggelakis, F.; Kling, R.; Lugara, P.M.; Ancona, A. Short and Long Term Surface Chemistry and Wetting Behavior of Stainless Steel with 1D and 2D Periodic Structures Induced by Bursts of Femtosecond Laser Pulses. Appl. Surf. Sci. 2019, 494, 1055–1065. [Google Scholar] [CrossRef]
- Exir, H.; Weck, A. Mechanism of Superhydrophilic to Superhydrophobic Transition of Femtosecond Laser-Induced Periodic Surface Structures on Titanium. Surf. Coat. Technol. 2019, 378, 124931. [Google Scholar] [CrossRef]
- Raimbault, O.; Benayoun, S.; Anselme, K.; Mauclair, C.; Bourgade, T.; Kietzig, A.; Girard-Lauriault, P.; Valette, S.; Donnet, C. The Effects of Femtosecond Laser-Textured Ti-6Al-4V on Wettability and Cell Response. Mater. Sci. Eng. C 2016, 69, 311–320. [Google Scholar]
- Eral, H.B.; Mannetje, D.J.C.M.; Oh, J.M. Contact Angle Hysteresis: A Review of Fundamentals and Applications. Colloid Polym. Sci. 2013, 291, 247–260. [Google Scholar] [CrossRef]
- Allahyari, E.; Nivas, J.J.J.; Oscurato, S.L.; Salvatore, M.; Ausanio, G.; Vecchione, A.; Fittipaldi, R.; Maddalena, P.; Bruzzese, R.; Amoruso, S. Laser Surface Texturing of Copper and Variation of the Wetting Response with the Laser Pulse Fluence. Appl. Surf. Sci. 2019, 470, 817–824. [Google Scholar] [CrossRef]
- Jalil, S.A.; Akram, M.; Hayes, J.J.; Singh, S.C.; ElKabbash, M.; Guo, C. Creating Superhydrophobic and Antibacterial Surfaces on Gold by Femtosecond Laser Pulses. Appl. Surf. Sci. 2020, 506, 144952. [Google Scholar] [CrossRef] [PubMed]
- Jagdheesh, R.; Pathiraj, B.; Karatay, E.; Römer, G.R.B.E.; in’t Veld Huis, A.J. Laser-Induced Nanoscale Superhydrophobic Structures on Metal Surfaces. Langmuir 2011, 27, 8464–8469. [Google Scholar] [CrossRef] [PubMed]
- Dettre, R.H.; Johnson, R.E. Contact Angle Hysteresis-Contact Angle Measurements on Rough Surfaces. Adv. Chem. 1964, 43, 136–144. [Google Scholar]
- Ghosh, U.U.; Nair, S.; Das, A.; Mukherjee, R.; DasGupta, S. Replicating and Resolving Wetting and Adhesion Characteristics of a Rose Petal. Colloids Surf. A 2019, 561, 9–17. [Google Scholar] [CrossRef]
- Bhushan, B.; Nosonovsky, M. Rose Petal Effect. In Encyclopedia of Nanotechnology; Springer: Dordrecht, The Netherlands, 2012; pp. 2265–2272. [Google Scholar] [CrossRef]
- Wood, M.J.; Servio, P.; Kietzig, A.-M. The Tuning of LIPSS Wettability during Laser Machining and through Post-Processing. Nanomaterials 2021, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, K.; Kodama, T.; Tabata, M.; Yoshida, T.; Tsuji, M.; Tamaura, Y. Adsorption of CO2 on Oxygen-Deficient Magnetite: Adsorption Enthalpy and Adsorption Isotherm. J. Chem. Soc. Faraday Trans. 1992, 88, 2771–2773. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Wang, L.; Wu, T.; Peng, S. Studies on the Decomposing Carbon Dioxide into Carbon with Oxygen-Deficient Magnetite: II. The Effects of Properties of Magnetite on Activity of Decomposition CO2 and Mechanism of the Reaction. Mater. Chem. Phys. 2000, 62, 52–61. [Google Scholar] [CrossRef]
- Xu, L.; Wu, Z.; Zhang, W.; Jin, Y.; Yuan, Q.; Ma, Y.; Huang, W. Oxygen Vacancy-Induced Novel Low-Temperature Water Splitting Reactions on FeO(111) Monolayer-Thick Film. J. Phys. Chem. C 2012, 116, 22921–22929. [Google Scholar] [CrossRef]
- Lu, G.; Linsebigler, A.; Yates, J.T., Jr. Ti3+ Defect Sites on TiO2(110): Production and Chemical Detection of Active Sites. J. Phys. Chem. 1994, 98, 11733–11738. [Google Scholar] [CrossRef]
- Schaub, R.; Thostrup, P.; Lopez, N.; Laegsgaard, E.; Stensgaard, I.; Norskov, J.K.; Besenbacher, F. Oxygen Vacancies as Active Sites for Water Dissociation on Rutile TiO2(110). Phys. Rev. Lett. 2001, 87, 266104. [Google Scholar] [CrossRef] [PubMed]
- Jagdheesh, R.; Diaz, M.; Ocana, J.L. Bio Inspired Self-Cleaning Ultrahydrophobic Aluminium Surface by Laser Processing. RSC Adv. 2016, 6, 72933–72941. [Google Scholar] [CrossRef]
- Wippermann, S.; Schmidt, W.G.; Thissen, P. Dissociative and Molecular Adsorption of Water on α-Al2O3(0001). Phys. Status Solidi C 2010, 7, 137–140. [Google Scholar] [CrossRef]










| Fluence Used to Irradiate the Top of the Square Pillars (J/cm2) | SSCA on Day 250 | Number of Days for Stabilized SSCA | θmax − θmin at 90° of Inclination |
|---|---|---|---|
| None | 122 ± 5° | 37 | 12 ± 7° |
| 0.50 | 138 ± 2° | 50 | 10 ± 4° |
| 3.99 | 131 ± 3° | 43 | 9 ± 5° |
| 10.1 | 130 ± 1° | 21 | 10 ± 5° |
| Fluence Used to Irradiate the Top of the Square Pillar (J/cm2) | SSCA on Day 250 | Number of Days for Stabilized SSCA | θmax − θmin at 90° of Inclination |
|---|---|---|---|
| None | 139 ± 2° | 43 | 10 ± 2° |
| 0.50 | 141 ± 2° | 43 | 12 ± 4° |
| 3.99 | 131 ± 2° | 36 | 11 ± 5° |
| 10.1 | 132 ± 2° | 16 | 9 ± 2° |
| Type of Bonding | Bonding Energy (eV) | At% with Regular Wetting Measurement | At% without Regular Wetting Measurement |
|---|---|---|---|
| Oxides | 529.5 | 22% | 39% |
| Hydroxides | 531.5 | 75% | 59% |
| Water | 533 | 3% | 2% |
| Type of Bonding | Bonding Energy (eV) | At% with Regular Wetting Measurement | At% without Regular Wetting Measurement |
|---|---|---|---|
| C–C/C–H | 284.8 | 70% | 76% |
| C–O–C/C–O–H | 286 | 21% | 14% |
| O–C=O/C=O | 288 | 9% | 10% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basset, S.; Heisbourg, G.; Pascale-Hamri, A.; Benayoun, S.; Valette, S. Effect of Texturing Environment on Wetting of Biomimetic Superhydrophobic Surfaces Designed by Femtosecond Laser Texturing. Nanomaterials 2022, 12, 3099. https://doi.org/10.3390/nano12183099
Basset S, Heisbourg G, Pascale-Hamri A, Benayoun S, Valette S. Effect of Texturing Environment on Wetting of Biomimetic Superhydrophobic Surfaces Designed by Femtosecond Laser Texturing. Nanomaterials. 2022; 12(18):3099. https://doi.org/10.3390/nano12183099
Chicago/Turabian StyleBasset, Salomé, Guillaume Heisbourg, Alina Pascale-Hamri, Stéphane Benayoun, and Stéphane Valette. 2022. "Effect of Texturing Environment on Wetting of Biomimetic Superhydrophobic Surfaces Designed by Femtosecond Laser Texturing" Nanomaterials 12, no. 18: 3099. https://doi.org/10.3390/nano12183099
APA StyleBasset, S., Heisbourg, G., Pascale-Hamri, A., Benayoun, S., & Valette, S. (2022). Effect of Texturing Environment on Wetting of Biomimetic Superhydrophobic Surfaces Designed by Femtosecond Laser Texturing. Nanomaterials, 12(18), 3099. https://doi.org/10.3390/nano12183099







