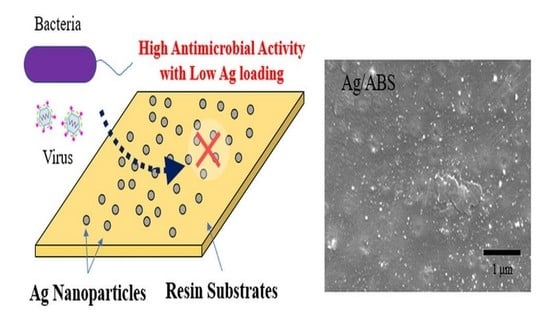
Development of Direct Immobilization Technique of Ag Nanoparticles on Resin Substrates Imparting High Antibacterial and Antiviral Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Characterization of Ag on ABS
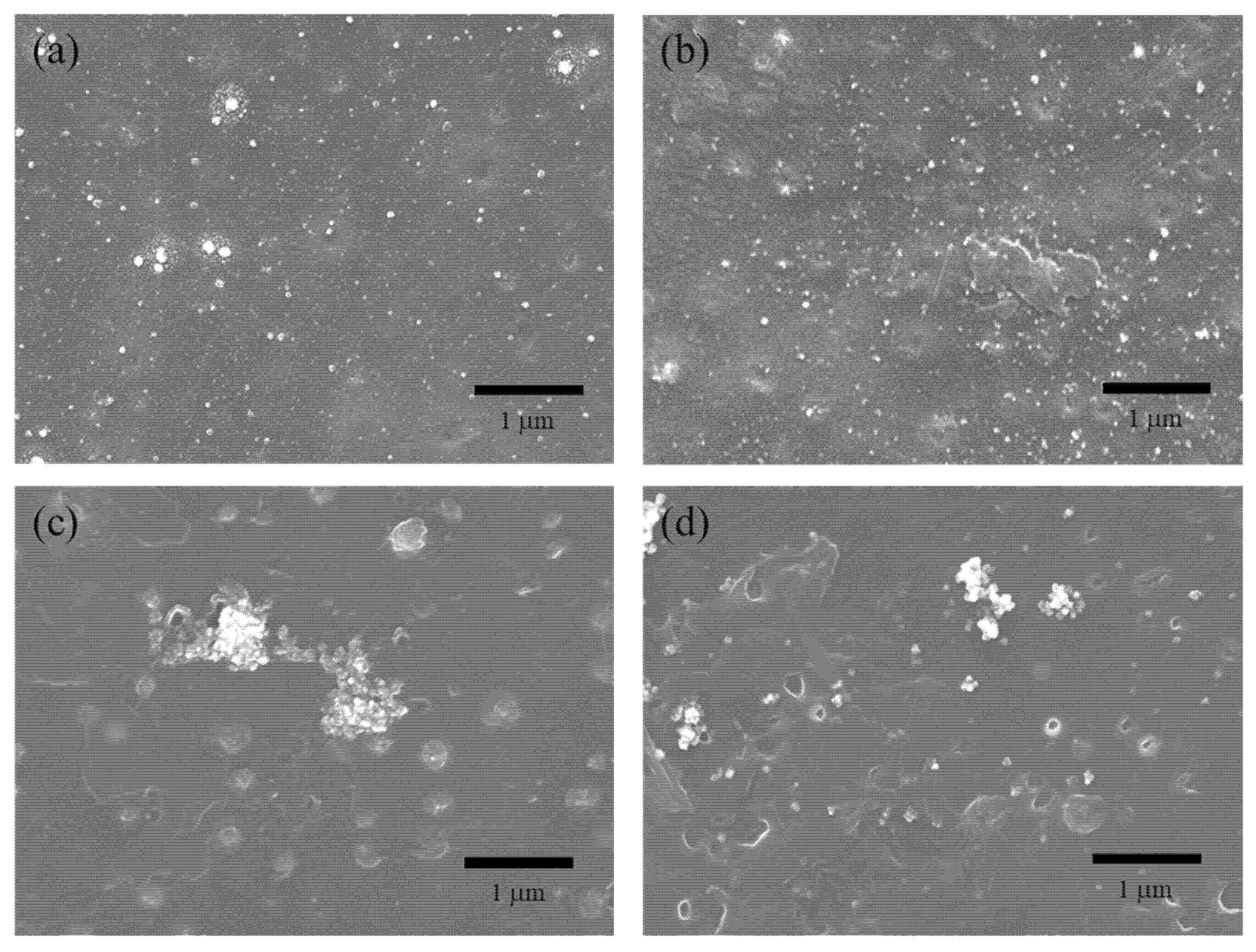
2.2. Radiochemical Synthesis of Silver Nanoparticles on Resin Substrates
2.3. Material Characterization
2.4. Antibacterial Test
- Before the test, the surface of the resin plate was wiped with gauze that had absorbed ethanol at a purity greater than 99%, and then air-dried at room temperature.
- The bacterial solution (0.4 mL) was added dropwise to a resin plate of 50 mm × 50 mm.
- The surface of the resin plate was covered with a 40 mm × 40 mm polyethylene film to spread the bacterial solution evenly over the resin plate.
- Samples were incubated at 35 ± 1 °C and a relative humidity of no lower than 90% for 24 h.
- After incubation, 10 mL of SCDLP medium was used to wash out the bacterial solution.
- A series of 10-fold dilutions of washed-out suspension was prepared by using phosphate-buffered physiological saline. Next, 1 mL of each dilution was placed into separate sterile Petri dishes, and 15 mL of plate count agar (E-KB07, EIKEN CHEMICAL Co., Ltd., Tokyo, Japan) was poured into each Petri dish and swirled gently to disperse the bacteria. Then, the Petri dishes were incubated at 35 ± 1 °C for 40–48 h.
- Viable bacteria count per 1.0 mL of washed-out suspensions was measured using the plate count method. The results describe the viable bacteria count per cm2 of resin plate.
2.5. Antiviral Test
- Before the test, the surface of the resin plate was wiped with gauze that had absorbed ethanol at a purity greater than 99% and then air-dried at room temperature.
- The viral solution (0.4 mL) was added dropwise to a resin plate of 50 mm × 50 mm.
- The surface of the resin plate was covered with a 40 mm × 40 mm polyethylene film to spread the viral solution evenly over the resin plate.
- Samples were incubated at 25 ± 1 °C and a relative humidity of no lower than 90% for 24 h.
- After incubation, 10 mL of SCDLP medium was used to wash out the viral solution.
- A series of 10-fold dilutions of washed-out virus solution was prepared by using EMEM (for Influenza A virus) or 2% FBS-containing DMEM (for SARS-CoV-2). The growth medium was removed from 6-well plastic plates with the host cell monolayer, and inoculated in 0.1 mL of each solution. Next, the plates were placed in the CO2 incubator at 34 °C (for Influenza A virus) or 37 °C (for SARS-CoV-2) for the chosen time. After incubating for chosen time, 3 mL of the agar medium was poured into the wells for plaque assay. Then, the plates were incubated at 34 °C (for Influenza A virus) or 37 °C (for SARS-CoV-2) in the CO2 incubator for 2 to 3 days.
- The viral infectivity titer per 0.1 mL of washed-out virus solution was measured by plaque assay and calculate the viral infectivity titer per cm2 of resin plate.
3. Results
3.1. Material Characterization of Ag on ABS
3.2. Antibacterial Activity
3.3. Antiviral Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Liu, J.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled Release of Biologically Active Silver from Nanosilver Surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [CrossRef]
- Seino, S.; Imoto, Y.; Kitagawa, D.; Kubo, Y.; Kosaka, T.; Kojima, T.; Nitani, H.; Nakagawa, T.; Yamamoto, T.A. Radiochemical synthesis of silver nanoparticles onto textile fabrics and their antibacterial activities. J. Nucl. Sci. Technol. 2015, 53, 1021–1027. [Google Scholar] [CrossRef]
- Imoto, Y.; Seino, S.; Nakagawa, T.; Yamamoto, T.A. Comparison of Quantitative Antifungal Testing Methods for Textile Fabrics. Biocontrol. Sci. 2017, 22, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Imoto, Y.; Seino, S.; Nakagawa, T.; Yamamoto, T.A. Quantitative Methods for Testing Antiviral Activities of Textile Fabrics. J. Antimicrobial. Agents 2017, 3, 1000146. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Aoki, T.; Seino, S.; Mori, O.; Ito, I.; Endo, K.; Yamamura, K. Radiolytic Synthesis of Pt-Particle/ABS Catalysts for H2O2 Decomposition in Contact Lens Cleaning. Nanomaterials 2017, 7, 235. [Google Scholar] [CrossRef] [PubMed]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, A.W.; Powell, C.J. NIST Standard Reference Database 20, Version 4.1; NIST: Gaithersburg, MD, USA, 2012. [Google Scholar] [CrossRef]
- Belloni, J. Nucleation, growth and properties of nanoclusters studied by radiation chemistry: Application to catalysis. Catal. Today 2006, 113, 141–156. [Google Scholar] [CrossRef]
- Morrissey, R.F.; Herring, C.M. Radiation sterilization: Past, present and future. Radiat. Phys. Chem. 2002, 63, 217–221. [Google Scholar] [CrossRef]
- Kasraei, S.; Sami, L.; Hendi, S.; AliKhani, M.Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kassaee, M.Z.; Akhavan, A.; Sheikh, N.; Sodagar, A. Antibacterial effects of a new dental acrylic resin containing silver nanoparticles. J. Appl. Polym. Sci. 2008, 110, 1699–1703. [Google Scholar] [CrossRef]
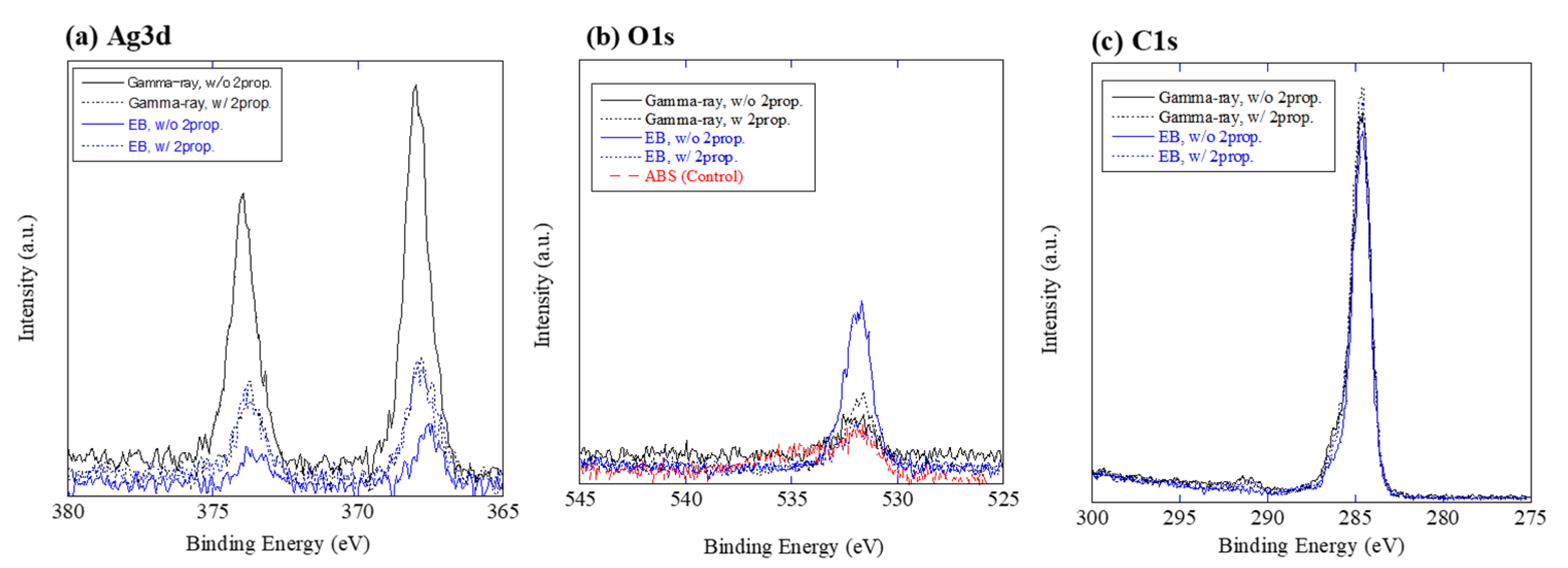
| Sample ID | XPS Peak Energy | ||
|---|---|---|---|
| Ag3d5/2 | O1s | C1s | |
| Gamma ray, without 2-prop. | 368.0 eV | 532.1 eV | 284.6 eV |
| Gamma ray, with 2-prop. (10% vol.) | 367.8 eV | 531.7 eV | 284.6 eV |
| Electron Beam, without 2-prop. | 367.6 eV | 531.9 eV | 284.6 eV |
| Electron Beam, with 2-prop. (10% vol.) | 367.8 eV | 531.9 eV | 284.6 eV |
| Samples | Viable Cell Count *1 | |
|---|---|---|
| PE Film (Control) | Immediately after inoculation | 4.05 |
| After 24 h | 6.08 | |
| ABS (Control) | After 24 h | 5.69 |
| Ag/ABS (Gamma ray, without 2-prop.) | After 24 h | <−0.20 |
| Ag/ABS (Electron Beam, without 2-prop.) | After 24 h | <−0.20 |
| Samples | Viable Cell Count *1 | |
|---|---|---|
| PE Film (Control) | Immediately after inoculation | 4.05 |
| After 24 h | 5.86 | |
| PE (Control) | After 24 h | 4.80 |
| Ag/PE (without 2-prop.) | After 24 h | <−0.20 |
| Ag/PE (with 2-prop. 10% vol.) | After 24 h | <−0.20 |
| PP (Control) | After 24 h | 3.11 |
| Ag/PP (without 2-prop.) | After 24 h | <−0.20 |
| Ag/PP (with 2-prop. 10% vol.) | After 24 h | <−0.20 |
| PVC (Control) | After 24 h | 5.55 |
| Ag/PVC (without 2-prop.) | After 24 h | <−0.20 |
| Ag/PVC (with 2-prop. 10% vol.) | After 24 h | <−0.20 |
| PC (Control) | After 24 h | 5.64 |
| Ag/PC (without 2-prop.) | After 24 h | <−0.20 |
| Ag/PC (with 2-prop. 10% vol.) | After 24 h | <−0.20 |
| Samples | Common Logarithm of Infectivity Titer Value (PFU/cm2) *1 | |
|---|---|---|
| ABS (Control) | Immediately after inoculation | 5.46 |
| After 24 h | 4.63 | |
| Ag/ABS (EB, without 2-prop.) | After 24 h | 2.88 |
| Ag/ABS (EB, with 2-prop. 1% vol.) | After 24 h | 2.66 |
| Ag/ABS (EB, with 2-prop. 10% vol.) | After 24 h | 1.53 |
| Samples | Common Logarithm of Infectivity Titer Value (PFU/cm2) *1 | |
|---|---|---|
| ABS (Control) | Immediately after inoculation | 5.65 |
| After 24 h | 4.82 | |
| Ag/ABS (Gamma ray, without 2-prop.) | After 24 h | 1.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seino, S.; Ohkubo, Y.; Magara, T.; Enomoto, H.; Nakajima, E.; Nishida, T.; Imoto, Y.; Nakagawa, T. Development of Direct Immobilization Technique of Ag Nanoparticles on Resin Substrates Imparting High Antibacterial and Antiviral Activities. Nanomaterials 2022, 12, 3046. https://doi.org/10.3390/nano12173046
Seino S, Ohkubo Y, Magara T, Enomoto H, Nakajima E, Nishida T, Imoto Y, Nakagawa T. Development of Direct Immobilization Technique of Ag Nanoparticles on Resin Substrates Imparting High Antibacterial and Antiviral Activities. Nanomaterials. 2022; 12(17):3046. https://doi.org/10.3390/nano12173046
Chicago/Turabian StyleSeino, Satoshi, Yuji Ohkubo, Tomonari Magara, Hiroki Enomoto, Eri Nakajima, Tomoki Nishida, Yasuo Imoto, and Takashi Nakagawa. 2022. "Development of Direct Immobilization Technique of Ag Nanoparticles on Resin Substrates Imparting High Antibacterial and Antiviral Activities" Nanomaterials 12, no. 17: 3046. https://doi.org/10.3390/nano12173046
APA StyleSeino, S., Ohkubo, Y., Magara, T., Enomoto, H., Nakajima, E., Nishida, T., Imoto, Y., & Nakagawa, T. (2022). Development of Direct Immobilization Technique of Ag Nanoparticles on Resin Substrates Imparting High Antibacterial and Antiviral Activities. Nanomaterials, 12(17), 3046. https://doi.org/10.3390/nano12173046