Silica-Decorated NiAl-Layered Double Oxide for Enhanced CO/CO2 Methanation Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Catalytic Testing
2.3. Catalyst Characterization
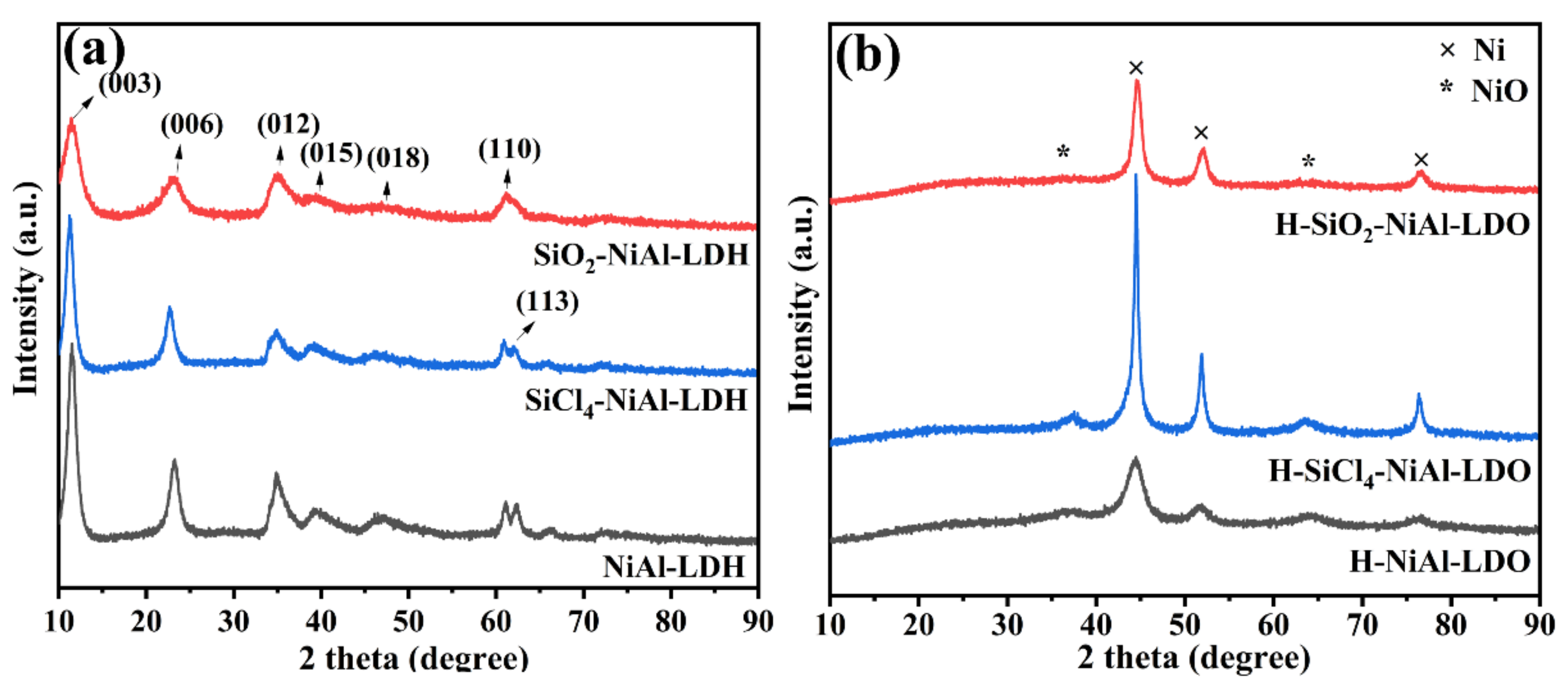
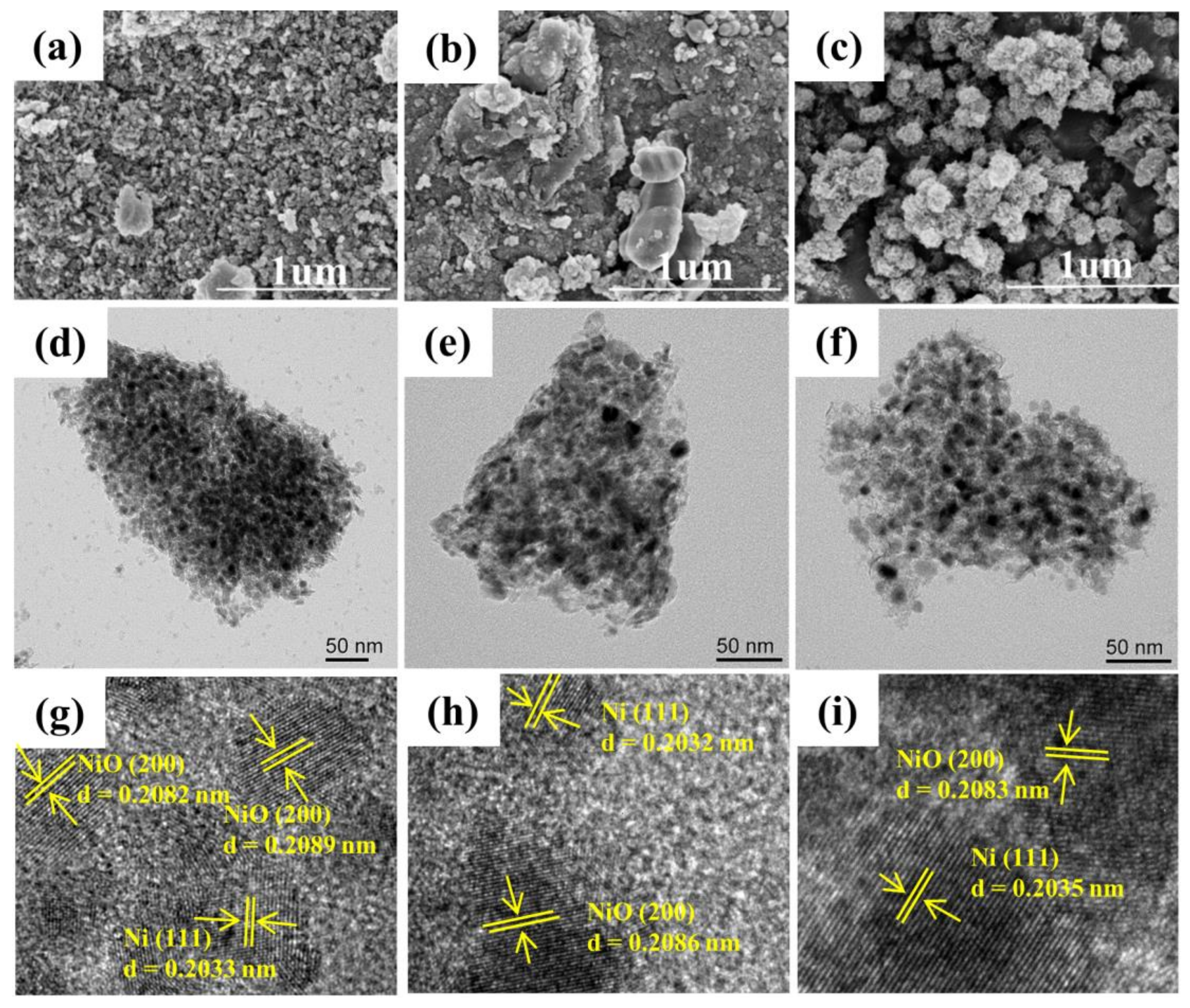
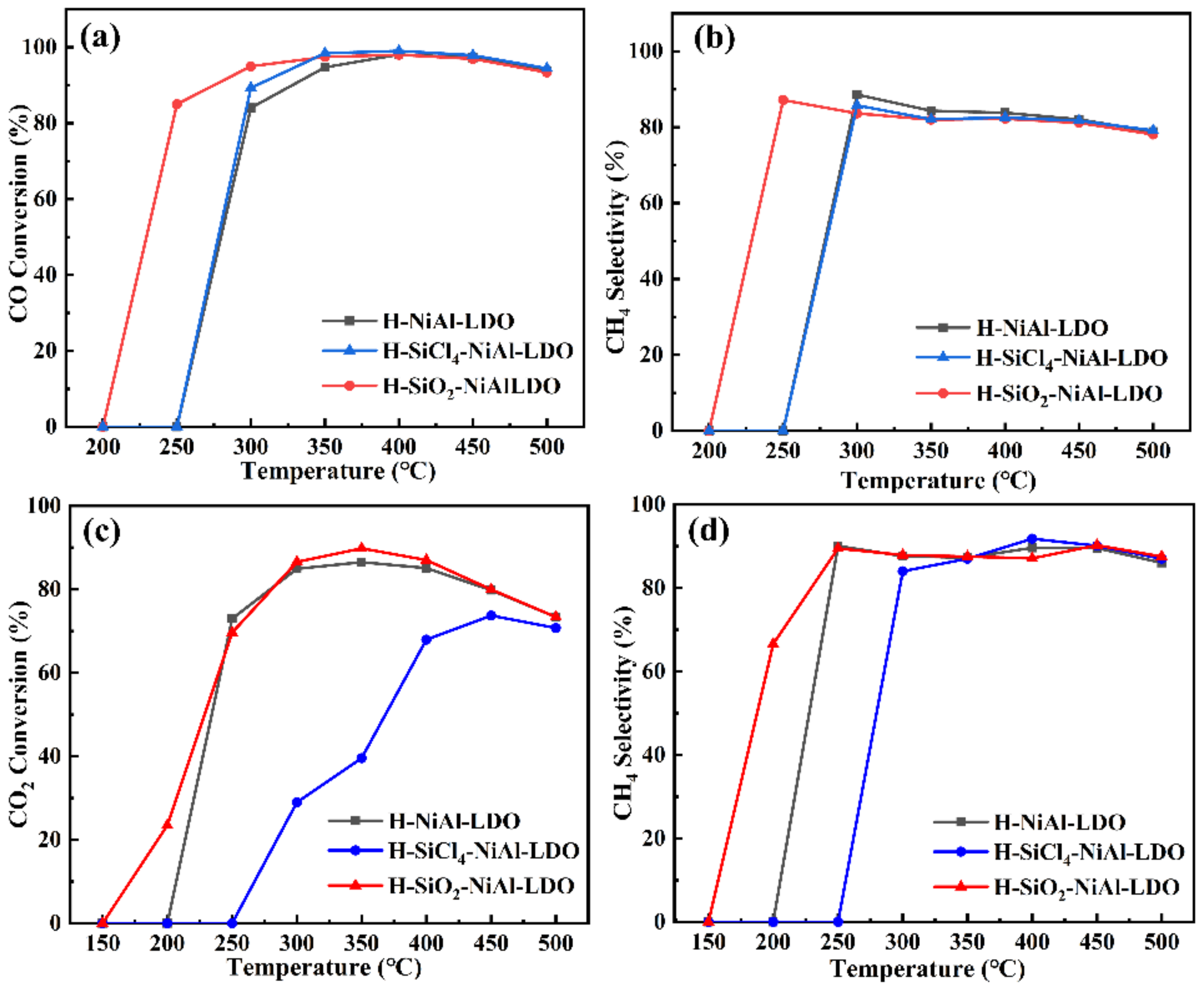
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rui, N.; Zhang, X.; Zhang, F.; Liu, Z.; Cao, X.; Xie, Z.; Zou, R.; Senanayake, S.D.; Yang, Y.; Rodriguez, J.A.; et al. Highly active Ni/CeO2 catalyst for CO2 methanation: Preparation and characterization. Appl. Catal. B Environ. 2021, 282, 119581. [Google Scholar] [CrossRef]
- Wolf, M.; Wong, L.H.; Schüler, C.; Hinrichsen, O. CO2 methanation on transition-metal-promoted Ni-Al catalysts: Sulfur poisoning and the role of CO2 adsorption capacity for catalyst activity. J. CO2 Util. 2020, 36, 276–287. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Ye, H.; Hu, R.; Yang, S.; Tang, G.; Li, K.; Yang, Y. Vacuum Thermal Treated Ni-CeO2/SBA-15 Catalyst for CO2 Methanation. Nanomaterials 2018, 8, 759. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Liu, Y.; Wang, S. CO2 methanation on the catalyst of Ni/MCM-41 promoted with CeO2. Sci. Total Environ. 2018, 625, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Tian, P.; Cao, X.; Chen, J.; Pu, T.; Shi, B.; Xu, J.; Moon, J.; Wu, Z.; Han, Y.-F. Vacancy engineering of the nickel-based catalysts for enhanced CO2 methanation. Appl. Catal. B Environ. 2021, 282, 119561. [Google Scholar] [CrossRef]
- Dewangan, N.; Hui, W.M.; Jayaprakash, S.; Bawah, A.-R.; Poerjoto, A.J.; Jie, T.; Jangam, A.; Hidajat, K.; Kawi, S. Recent progress on layered double hydroxide (LDH) derived metal-based catalysts for CO2 conversion to valuable chemicals. Catal. Today 2020, 356, 490–513. [Google Scholar] [CrossRef]
- Ulmer, U.; Dingle, T.; Duchesne, P.N.; Morris, R.H.; Tavasoli, A.; Wood, T.; Ozin, G.A. Fundamentals and applications of photocatalytic CO2 methanation. Nat. Commun. 2019, 10, 3169. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-T.; van Muyden, A.P.; Bobbink, F.D.; Huang, Z.; Dyson, P.J. Indirect CO2 Methanation: Hydrogenolysis of Cyclic Carbonates Catalyzed by Ru-Modified Zeolite Produces Methane and Diols. Angew. Chem. Int. Ed. 2019, 58, 557–560. [Google Scholar] [CrossRef]
- Li, X.; Han, Y.; Huang, Y.; Lin, J.; Pan, X.; Zhao, Z.; Zhou, Y.; Wang, H.; Yang, X.; Wang, A.; et al. Hydrogenated TiO2 supported Ru for selective methanation of CO in practical conditions. Appl. Catal. B Environ. 2021, 298, 120597. [Google Scholar] [CrossRef]
- Sakpal, T.; Lefferts, L. Structure-dependent activity of CeO2 supported Ru catalysts for CO2 methanation. J. Catal. 2018, 367, 171–180. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.K.; Zhao, Y.X.; Wei, G.P.; Ren, Y.; Asmis, K.R.; He, S.G. Catalytic Co-Conversion of CH4 and CO2 Mediated by Rhodium-Titanium Oxide Anions RhTiO2−. Angew. Chem. Int. Ed. 2021, 60, 13788–13792. [Google Scholar] [CrossRef]
- Su, H.-Y.; Yu, C.; Liu, J.-X.; Zhao, Y.; Ma, X.; Luo, J.; Sun, C.; Li, W.-X.; Sun, K. CO activation and methanation mechanism on hexagonal close-packed Co catalysts: Effect of functionals, carbon deposition and surface structure. Catal. Sci. Technol. 2020, 10, 3387–3398. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Mo, X.; Campos, A.; Goodwin, J.G.; Spivey, J.J. Hydrotalcite supported Co catalysts for CO hydrogenation. Appl. Catal. A Gen. 2011, 396, 91–100. [Google Scholar] [CrossRef]
- Luo, L.; Wang, M.; Cui, Y.; Chen, Z.; Wu, J.; Cao, Y.; Luo, J.; Dai, Y.; Li, W.X.; Bao, J.; et al. Surface Iron Species in Palladium-Iron Intermetallic Nanocrystals that Promote and Stabilize CO2 Methanation. Angew. Chem. Int. Ed. 2020, 59, 14434–14442. [Google Scholar] [CrossRef]
- Williamson, D.L.; Jones, M.D.; Mattia, D. Highly Selective, Iron-Driven CO2 Methanation. Energy Technol. 2019, 7, 294–306. [Google Scholar] [CrossRef]
- Yan, X.; Sun, W.; Fan, L.; Duchesne, P.N.; Wang, W.; Kubel, C.; Wang, D.; Kumar, S.G.H.; Li, Y.F.; Tavasoli, A.; et al. Nickel@ Siloxene catalytic nanosheets for high-performance CO2 methanation. Nat. Commun. 2019, 10, 2608. [Google Scholar] [CrossRef]
- Yan, X.; Yuan, C.; Bao, J.; Li, S.; Qi, D.; Wang, Q.; Zhao, B.; Hu, T.; Fan, L.; Fan, B.; et al. A Ni-based catalyst with enhanced Ni–support interaction for highly efficient CO methanation. Catal. Sci. Technol. 2018, 8, 3474–3483. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-Based Catalysts for CO2 Methanation: A Review. Nanomaterials 2021, 11, 28. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, B.; Liu, Y.; Zhang, Y. An active and stable nickel-based catalyst with embedment structure for CO2 methanation. Appl. Catal. B Environ. 2020, 269, 118801. [Google Scholar] [CrossRef]
- Ren, J.; Ouyang, S.; Xu, H.; Meng, X.; Wang, T.; Wang, D.; Ye, J. Targeting Activation of CO2 and H2 over Ru-Loaded Ultrathin Layered Double Hydroxides to Achieve Efficient Photothermal CO2 Methanation in Flow-Type System. Adv. Energy Mater. 2017, 7, 1601657. [Google Scholar] [CrossRef] [Green Version]
- Hatta, A.H.; Jalil, A.A.; Hassan, N.S.; Hamid, M.Y.; Rahman, A.F.; Teh, L.P.; Prasetyoko, D. A review on recent bimetallic catalyst development for synthetic natural gas production via CO methanation. Int. J. Hydrogen Energy 2021, in press. [Google Scholar] [CrossRef]
- Huynh, H.L.; Yu, Z.X. CO2 Methanation on Hydrotalcite-Derived Catalysts and Structured Reactors: A Review. Energy Technol. 2020, 8, 1901475. [Google Scholar] [CrossRef]
- Sikander, U.; Sufian, S.; Salam, M.A. A review of hydrotalcite based catalysts for hydrogen production systems. Int. J. Hydrogen Energy 2017, 42, 19851–19868. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Krebs, F.; Perathoner, S.; Abate, S.; Centi, G.; Palkovits, R. Hydrotalcite based Ni–Fe/(Mg, Al)Ox catalysts for CO2 methanation—tailoring Fe content for improved CO dissociation, basicity, and particle size. Catal. Sci. Technol. 2018, 8, 1016–1027. [Google Scholar] [CrossRef]
- Ren, J.; Mebrahtu, C.; Palkovits, R. Ni-based catalysts supported on Mg–Al hydrotalcites with different morphologies for CO2 methanation: Exploring the effect of metal–support interaction. Catal. Sci. Technol. 2020, 10, 1902–1913. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, R.; Liu, N.; Dai, C.; Yu, G.; Wang, N.; Chen, B. In situ Ce-doped catalyst derived from NiCeAl-LDHs with enhanced low-temperature performance for CO2 methanation. Appl. Surf. Sci. 2022, 579, 152204–152216. [Google Scholar] [CrossRef]
- Tang, S.; Yao, Y.; Chen, T.; Kong, D.; Shen, W.; Lee, H.K. Recent advances in the application of layered double hydroxides in analytical chemistry: A review. Anal. Chim. Acta 2020, 1103, 32–48. [Google Scholar] [CrossRef]
- Yao, Y.; Yu, F.; Li, J.; Li, J.; Li, Y.; Wang, Z.; Zhu, M.; Shi, Y.; Dai, B.; Guo, X. Two-dimensional NiAl layered double oxides as non-noble metal catalysts for enhanced CO methanation performance at low temperature. Fuel 2019, 255, 115770. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Pino, L.; Frontera, P.; Ferraro, M.; Antonucci, V. Activity and stability of powder and monolith-coated Ni/GDC catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 226, 384–395. [Google Scholar] [CrossRef]
- Cisneros, S.; Chen, S.; Diemant, T.; Bansmann, J.; Abdel-Mageed, A.M.; Goepel, M.; Olesen, S.E.; Welter, E.S.; Parlinska-Wojtan, M.; Gläser, R.; et al. Effects of SiO2-doping on high-surface-area Ru/TiO2 catalysts for the selective CO methanation. Appl. Catal. B Environ. 2021, 282, 119483. [Google Scholar] [CrossRef]
- Li, P.; Zhu, M.; Dan, J.; Kang, L.; Lai, L.; Cai, X.; Zhang, J.; Yu, F.; Tian, Z.; Dai, B. Two-dimensional porous SiO2 nanomesh supported high dispersed Ni nanoparticles for CO methanation. Chem. Eng. J. 2017, 326, 774–780. [Google Scholar] [CrossRef]
- Moreno, Y.P.; Cardoso, M.B.; Ferrão, M.F.; Moncada, E.A.; dos Santos, J.H. Effect of SiCl4 on the preparation of functionalized mixed-structure silica from monodisperse sol–gel silica nanoparticles. Chem. Eng. J. 2016, 292, 233–245. [Google Scholar] [CrossRef]
- Tada, S.; Shoji, D.; Urasaki, K.; Shimoda, N.; Satokawa, S. Physical mixing of TiO2 with sponge nickel creates new active sites for selective CO methanation. Catal. Sci. Technol. 2016, 6, 3713–3717. [Google Scholar] [CrossRef]
- Yan, W.; Bao, W.; Tang, Y.; Li, Y.; Zhang, J.; Zhao, H.; Li, J.; Yu, F. Enhanced CO hydrogenation performance via two-dimensional NiAl-layered double oxide decorated by SiO2 nanoparticles. Int. J. Hydrogen Energy 2022, in press. [Google Scholar] [CrossRef]
- Cao, H.-X.; Zhang, J.; Guo, C.-L.; Chen, J.G.; Ren, X.-K. Highly dispersed Ni nanoparticles on 3D-mesoporous KIT-6 for CO methanation: Effect of promoter species on catalytic performance. Chin. J. Catal. 2017, 38, 1127–1137. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Liu, Q. Highly Efficient Ni-Phyllosilicate Catalyst with Surface and Interface Confinement for CO2 and CO Methanation. Ind. Eng. Chem. Res. 2021, 60, 6981–6992. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Xu, Q.-Z.; Wang, J.-Y.; Li, N.; Guo, S.-H.; Su, Y.-Z.; Wang, H.-J.; Zhang, J.-H.; Chen, S. Facile hydrothermal synthesis of urchin-like NiCo2O4 spheres as efficient electrocatalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2013, 38, 6657–6662. [Google Scholar] [CrossRef]
- Li, Z.; Mo, L.; Kathiraser, Y.; Kawi, S. Yolk–Satellite–Shell Structured Ni–Yolk@Ni@SiO2 Nanocomposite: Superb Catalyst toward Methane CO2 Reforming Reaction. ACS Catal. 2014, 4, 1526–1536. [Google Scholar] [CrossRef]
- Chang, Z.; Liu, Z.; Wang, C.; Li, J.; Zeng, J.; Liu, Y.; Zhang, M.; Li, J.; Yu, F. Three-dimensional porous Mn–Ni/Al2O3 micro-spheres for enhanced low temperature CO hydrogenation to produce methane. Int. J. Hydrogen Energy 2021, 46, 7912–7925. [Google Scholar] [CrossRef]
- Lv, Y.; Xin, Z.; Meng, X.; Tao, M.; Bian, Z.; Gu, J.; Gao, W. Essential role of organic additives in preparation of efficient Ni/KIT-6 catalysts for CO methanation. Appl. Catal. A Gen. 2018, 558, 99–108. [Google Scholar] [CrossRef]
- Zhang, M.J.; Yu, F.; Li, J.B.; Chen, K.; Yao, Y.B.; Li, P.P.; Zhu, M.Y.; Shi, Y.L.; Wang, Q.; Guo, X.H. High CO Methanation Performance of Two-Dimensional Ni/MgAl Layered Double Oxide with Enhanced Oxygen Vacancies via Flash Nanoprecipitation. Catalysts 2018, 8, 363–376. [Google Scholar] [CrossRef]
- Zeng, J.; Lu, K.; Zhang, J.; Sun, Y.; Chang, Z.; Li, J.; Dai, B.; Yu, F.; Li, J.; Liu, J. Solution plasma-assisted preparation of highly dispersed NiMnAl-LDO catalyst to enhance low-temperature activity of CO2 methanation. Int. J. Hydrogen Energy 2022, 47, 2234–2244. [Google Scholar] [CrossRef]
- Alexander, M.R.; Short, R.D.; Jones, F.R.; Stollenwerk, M.; Zabold, J.; Michaeli, W. An X-ray photoelectron spectroscopic investigation into the chemical structure of deposits formed from hexamethyldisiloxane/oxygen plasmas. J. Mater. Sci. 1996, 31, 1879–1885. [Google Scholar] [CrossRef]
- Alfonsetti, R.; Lozzi, L.; Passacantando, M.; Picozzi, P.; Santucci, S. XPS studies on SiOx thin films. Appl. Surf. Sci. 1993, 70–71, 222–225. [Google Scholar] [CrossRef]
- Ayame, A.; Kitagawa, T. X-Ray photoelectron spectroscopic measurement and chemical characteristics of silica, alumina and silica-alumina. Bunseki Kagaku 1991, 40, 673–678. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Ma, H.; Fang, D. Ni–Al2O3 catalysts prepared by solution combustion method for syngas methanation. Catal. Commun. 2012, 17, 34–38. [Google Scholar] [CrossRef]
- Lin, X.; Lin, L.; Huang, K.; Chen, X.; Dai, W.; Fu, X. CO methanation promoted by UV irradiation over Ni/TiO2. Appl. Catal. B Environ. 2015, 168–169, 416–422. [Google Scholar] [CrossRef]
- Guo, C.L.; Wu, Y.Y.; Qin, H.Y.; Zhang, J.L. CO methanation over ZrO2/Al2O3 supported Ni catalysts: A comprehensive study. Fuel Process. Technol. 2014, 124, 61–69. [Google Scholar] [CrossRef]
- Zhang, X.; Rui, N.; Jia, X.; Hu, X.; Liu, C.-J. Effect of decomposition of catalyst precursor on Ni/CeO2 activity for CO methanation. Chin. J. Catal. 2019, 40, 495–503. [Google Scholar] [CrossRef]
- Jomjaree, T.; Sintuya, P.; Srifa, A.; Koo-Amornpattana, W.; Kiatphuengporn, S.; Assabumrungrat, S.; Sudoh, M.; Watanabe, R.; Fukuhara, C.; Ratchahat, S. Catalytic performance of Ni catalysts supported on CeO2 with different morphologies for low-temperature CO2 methanation. Catal. Today 2021, 375, 234–244. [Google Scholar] [CrossRef]
- Li, S.; Gong, D.; Tang, H.; Ma, Z.; Liu, Z.-T.; Liu, Y. Preparation of bimetallic Ni@Ru nanoparticles supported on SiO2 and their catalytic performance for CO methanation. Chem. Eng. J. 2018, 334, 2167–2178. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Zhang, S.; An, K.; Ma, Z.; Wang, L.; Liu, Y. Cerium-modified Ni-La2O3/ZrO2 for CO2 methanation. J. Energy. Chem. 2020, 43, 155–164. [Google Scholar] [CrossRef]
- Lv, Y.; Xin, Z.; Meng, X.; Tao, M.; Bian, Z.; Gu, J.; Gao, W. Effect of La, Mg and Mo additives on dispersion and thermostability of Ni species on KIT-6 for CO methanation. Appl. Catal. A Gen. 2017, 543, 125–132. [Google Scholar] [CrossRef]
- Ahmad, F.; Lovell, E.C.; Masood, H.; Cullen, P.J.; Ostrikov, K.K.; Scott, J.A.; Amal, R. Low-Temperature CO2 Methanation: Synergistic Effects in Plasma-Ni Hybrid Catalytic System. ACS Sustain. Chem. Eng. 2020, 8, 1888–1898. [Google Scholar] [CrossRef]
- Bukhari, S.N.; Chong, C.C.; Teh, L.P.; Vo, D.V.N.; Ainirazali, N.; Triwahyono, S.; Jalil, A.A.; Setiabudi, H.D. Promising hydrothermal technique for efficient CO2 methanation over Ni/SBA-15. Int. J. Hydrogen Energy 2019, 44, 20792–20804. [Google Scholar] [CrossRef]
- Cerdá-Moreno, C.; Chica, A.; Keller, S.; Rautenberg, C.; Bentrup, U. Ni-sepiolite and Ni-todorokite as efficient CO2 methanation catalysts: Mechanistic insight by operando DRIFTS. Appl. Catal. B Environ. 2020, 264, 118546. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, M.; Wang, Q.; Huang, R.; Jin, Y.; Bian, B.; Tumurbaatar, C.; Ishtsog, B.; Bold, T.; Yang, Y. Enhanced activity and stability of Ni/La2O2CO3 catalyst for CO2 methanation by metal-carbonate interaction. Appl. Catal. B Environ. 2020, 277, 119271. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Moreno, M.V.; Ognier, S.; Motak, M.; Grzybek, T.; Da Costa, P.; Gálvez, M.E. Ni-Fe layered double hydroxide derived catalysts for non-plasma and DBD plasma-assisted CO2 methanation. Int. J. Hydrogen Energy 2020, 45, 10423–10432. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, B.; Sun, P. Highly stable Ni-based catalysts derived from LDHs supported on zeolite for CO2 methanation. Int. J. Hydrogen Energy 2020, 45, 16183–16192. [Google Scholar] [CrossRef]

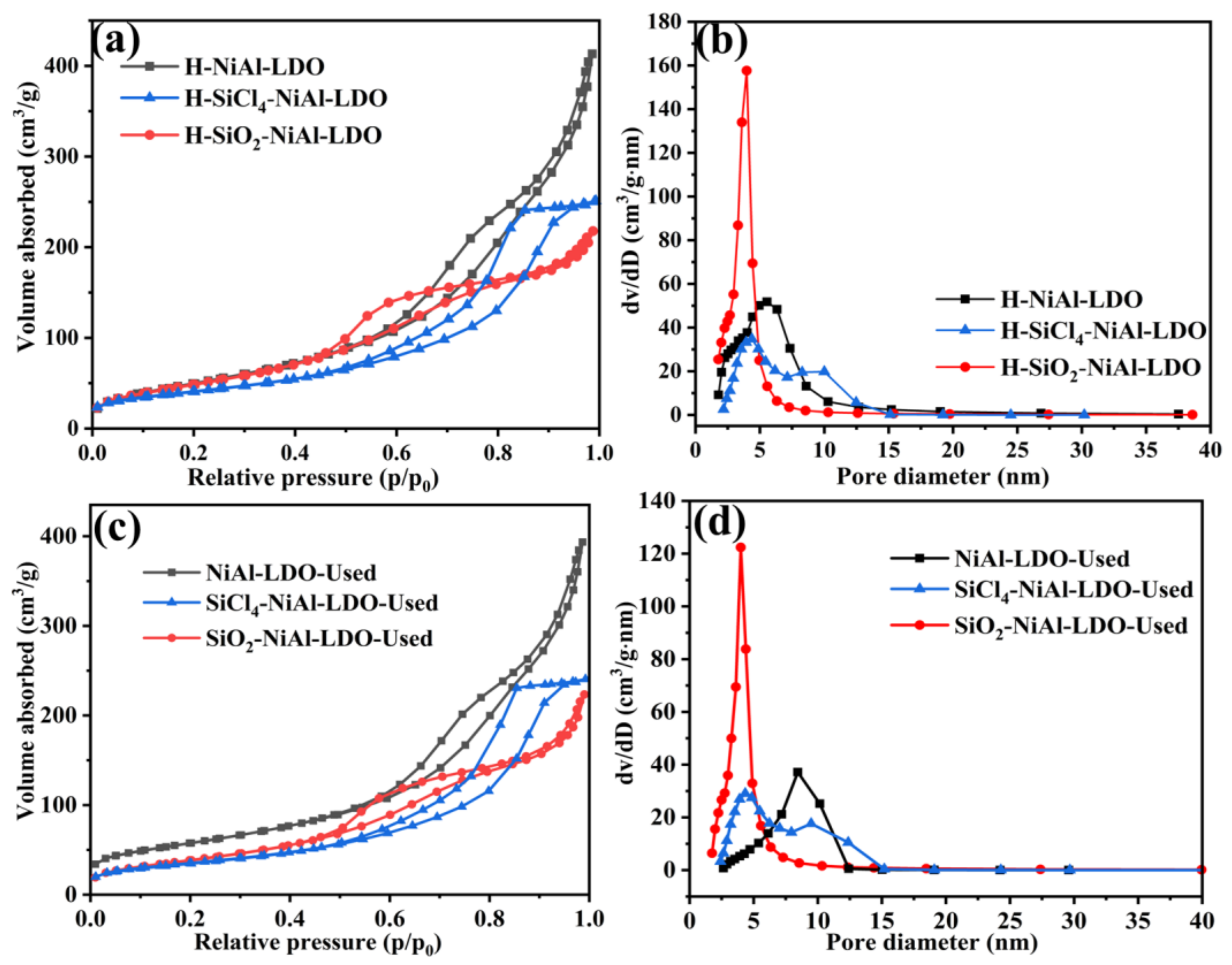


| Samples | SBET (m2·g−1) | DBJH (nm) | Vp (cm3·g−1) |
|---|---|---|---|
| NiAl-LDO | 157 | 6.72 | 0.38 |
| SiCl4-NiAl-LDO | 147 | 7.21 | 0.38 |
| SiO2-NiAl-LDO | 210 | 4.98 | 0.34 |
| NiAl-LDO-Used | 114 | 8.37 | 0.35 |
| SiCl4-NiAl-LDO-Used | 132 | 7.65 | 0.37 |
| SiO2-NiAl-LDO-Used | 163 | 6.30 | 0.34 |
| Catalysts | Ni0/(Ni0 + Ni2+ + Ni3+) | Osurf/(Osurf + Odef + Olatt) | Olatt/(Osurf + Odef + Olatt) |
|---|---|---|---|
| H-NiAl-LDO | 0.13 | 0.12 | 0.49 |
| H-SiCl4-NiAl-LDO | 0.19 | 0.22 | 0.44 |
| H-SiO2-NiAl-LDO | 0.21 | 0.37 | 0.15 |
| Samples | H-NiAl-LDO | H-SiCl4-NiAl-LDO | H-SiO2-NiAl-LDO |
|---|---|---|---|
| Ni (wt%) | 60.62 | 51.33 | 53.31 |
| Al (wt%) | 10.43 | 5.70 | 7.29 |
| Si (wt%) | 0 | 4.70 | 3.02 |
| Catalysts | WHSV (mL·g−1·h−1) | T (°C) | CO2 Conversion (%) | Ref |
|---|---|---|---|---|
| 15% Ni/Al2O3 (Plasma) | 8700 | 250 | 40 | [54] |
| Ni/SBA-15 | 15,000 | 250 | 82 | [55] |
| Ni-sepiolite | 36,000 | 250 | 10 | [56] |
| Ni/La2O2CO3 | 20,000 | 300 | 25 | [57] |
| Ni20Fe1.5-LDH | 12,000 | 250 | 76 | [58] |
| Ni/MgAl-LDH | 5017 | 250 | 20 | [25] |
| NaY-Ni5-LDHs | 30,000 | 250 | 13 | [59] |
| H-SiO2-NiAl-LDO | 52,000 | 200 | 23 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Li, Y.; Zeng, J.; Bao, W.; Zhao, H.; Li, J.; Gunawan, P.; Yu, F. Silica-Decorated NiAl-Layered Double Oxide for Enhanced CO/CO2 Methanation Performance. Nanomaterials 2022, 12, 3041. https://doi.org/10.3390/nano12173041
Yan W, Li Y, Zeng J, Bao W, Zhao H, Li J, Gunawan P, Yu F. Silica-Decorated NiAl-Layered Double Oxide for Enhanced CO/CO2 Methanation Performance. Nanomaterials. 2022; 12(17):3041. https://doi.org/10.3390/nano12173041
Chicago/Turabian StyleYan, Wenxia, Yangyang Li, Junming Zeng, Wentao Bao, Huanhuan Zhao, Jiangbing Li, Poernomo Gunawan, and Feng Yu. 2022. "Silica-Decorated NiAl-Layered Double Oxide for Enhanced CO/CO2 Methanation Performance" Nanomaterials 12, no. 17: 3041. https://doi.org/10.3390/nano12173041
APA StyleYan, W., Li, Y., Zeng, J., Bao, W., Zhao, H., Li, J., Gunawan, P., & Yu, F. (2022). Silica-Decorated NiAl-Layered Double Oxide for Enhanced CO/CO2 Methanation Performance. Nanomaterials, 12(17), 3041. https://doi.org/10.3390/nano12173041