Experimental Investigation of Rheological Properties and Thermal Conductivity of SiO2–TiO2 Composite Nanofluids Prepared by Atomic Layer Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Nanofluid
2.3. Characterisation Methods
3. Results and Discussion
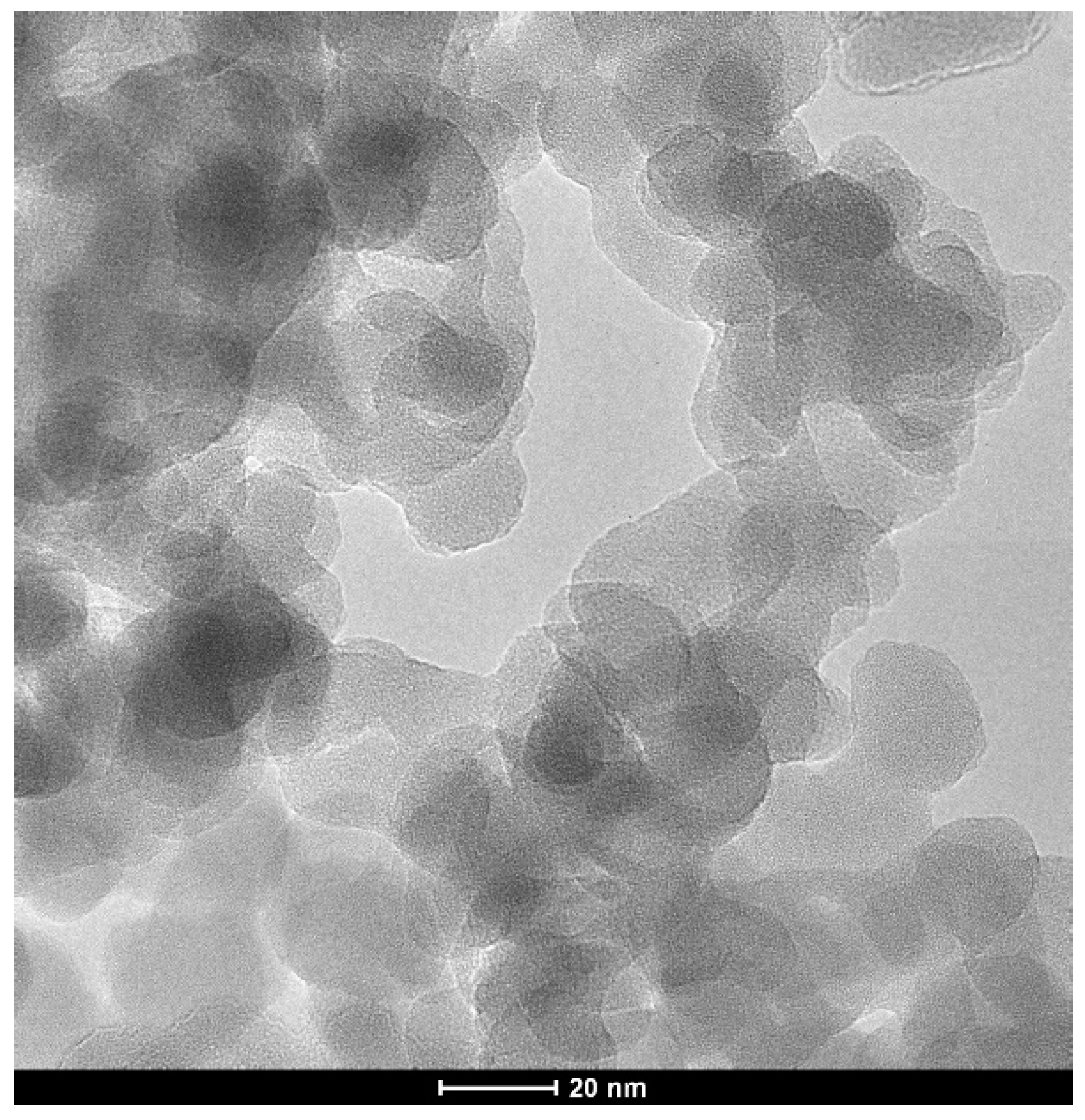
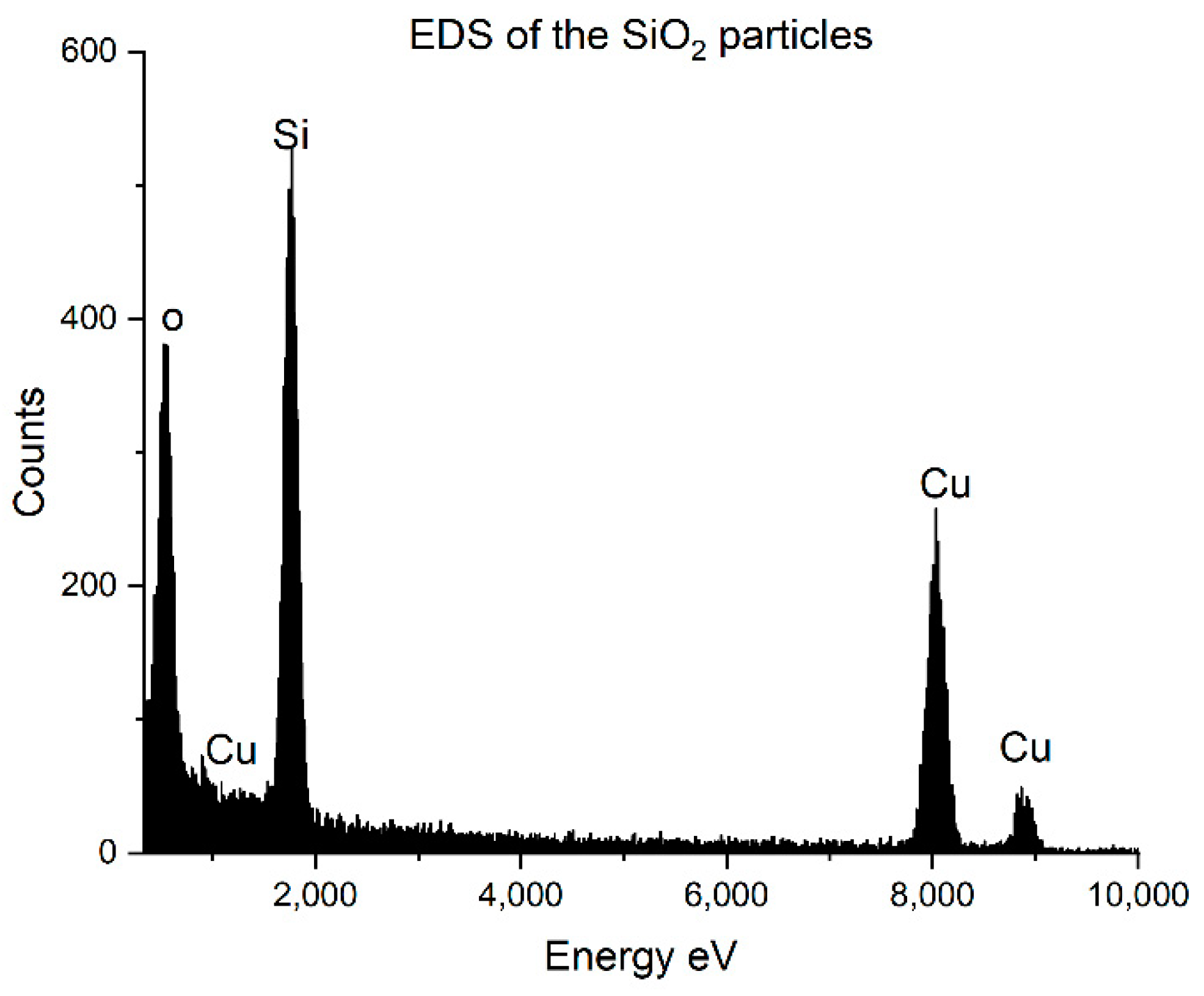
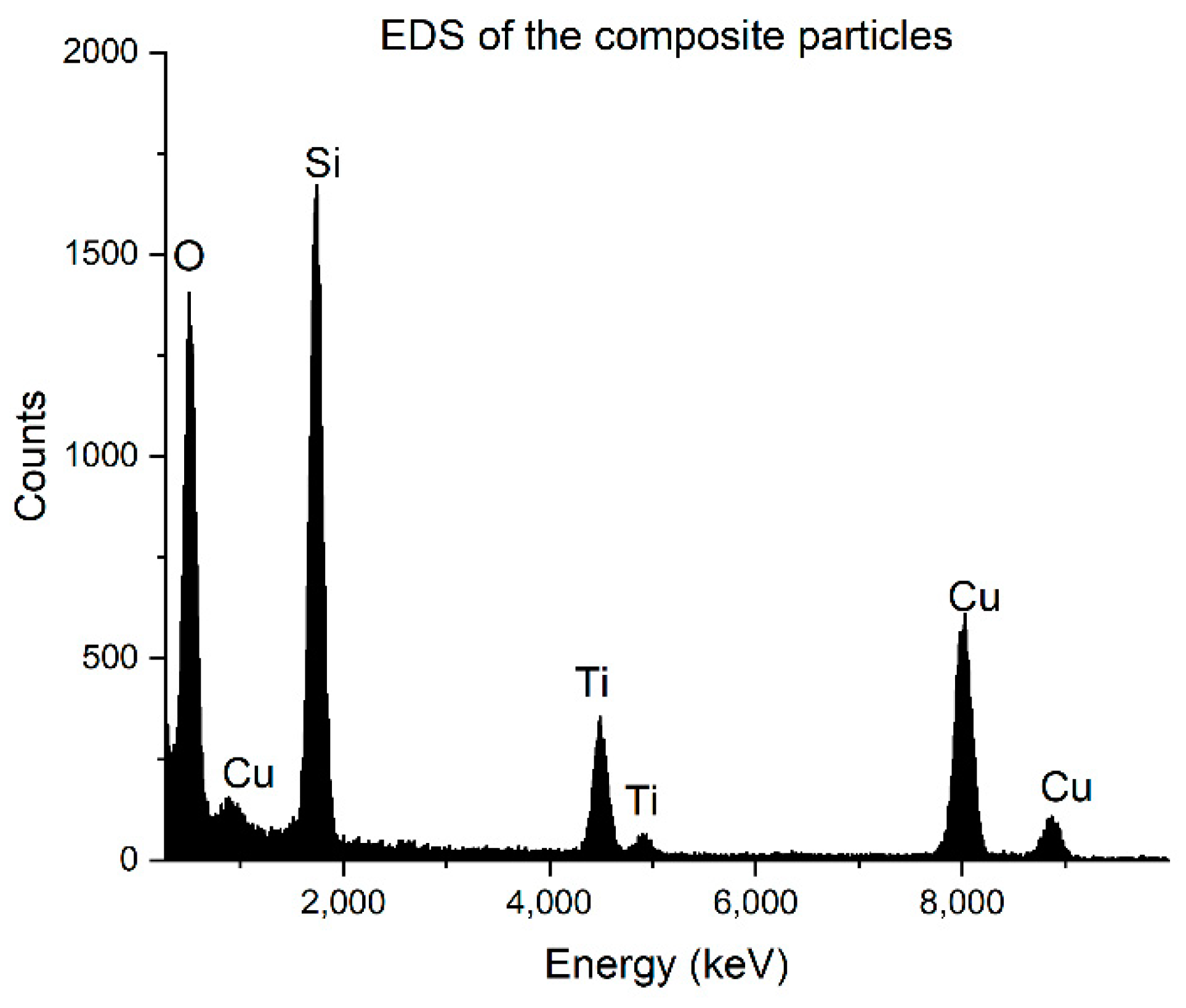
3.1. SiO2 and the Composite Particles
3.2. Zeta Potential Measurements
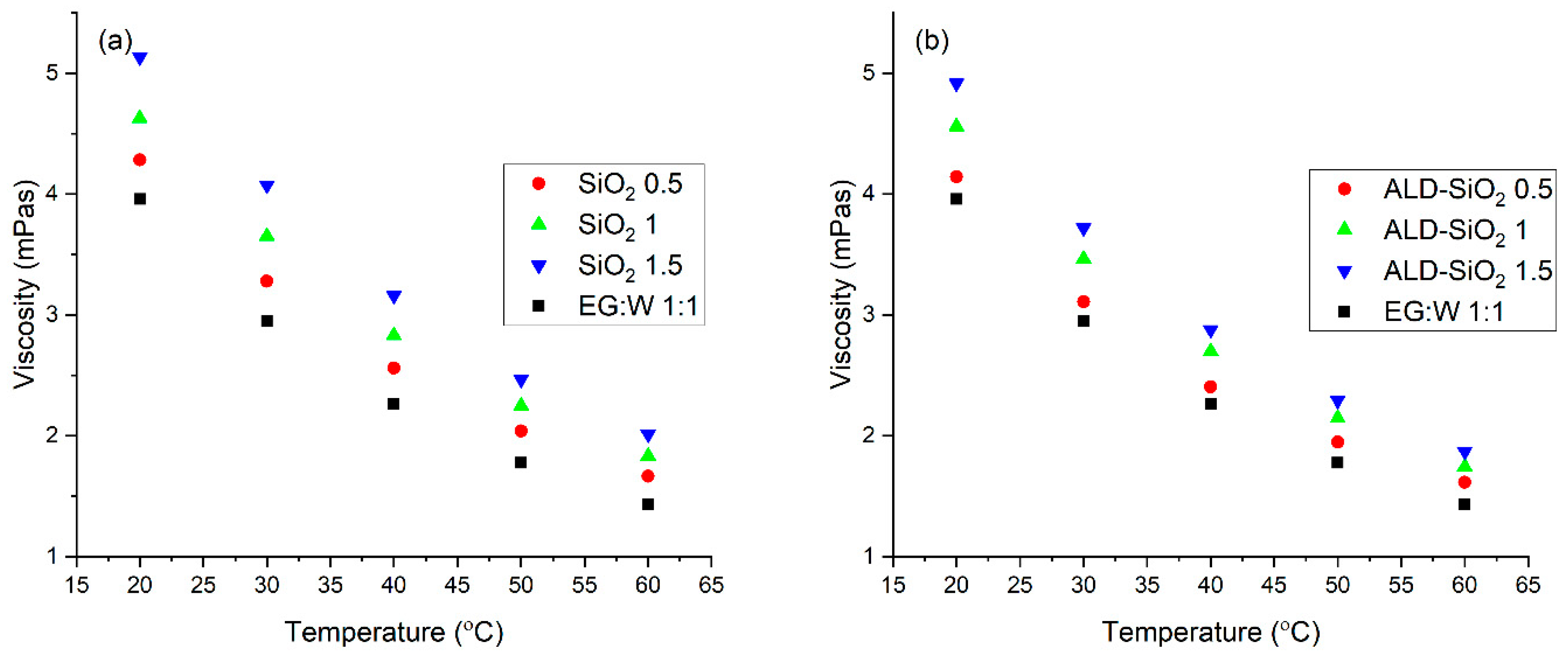
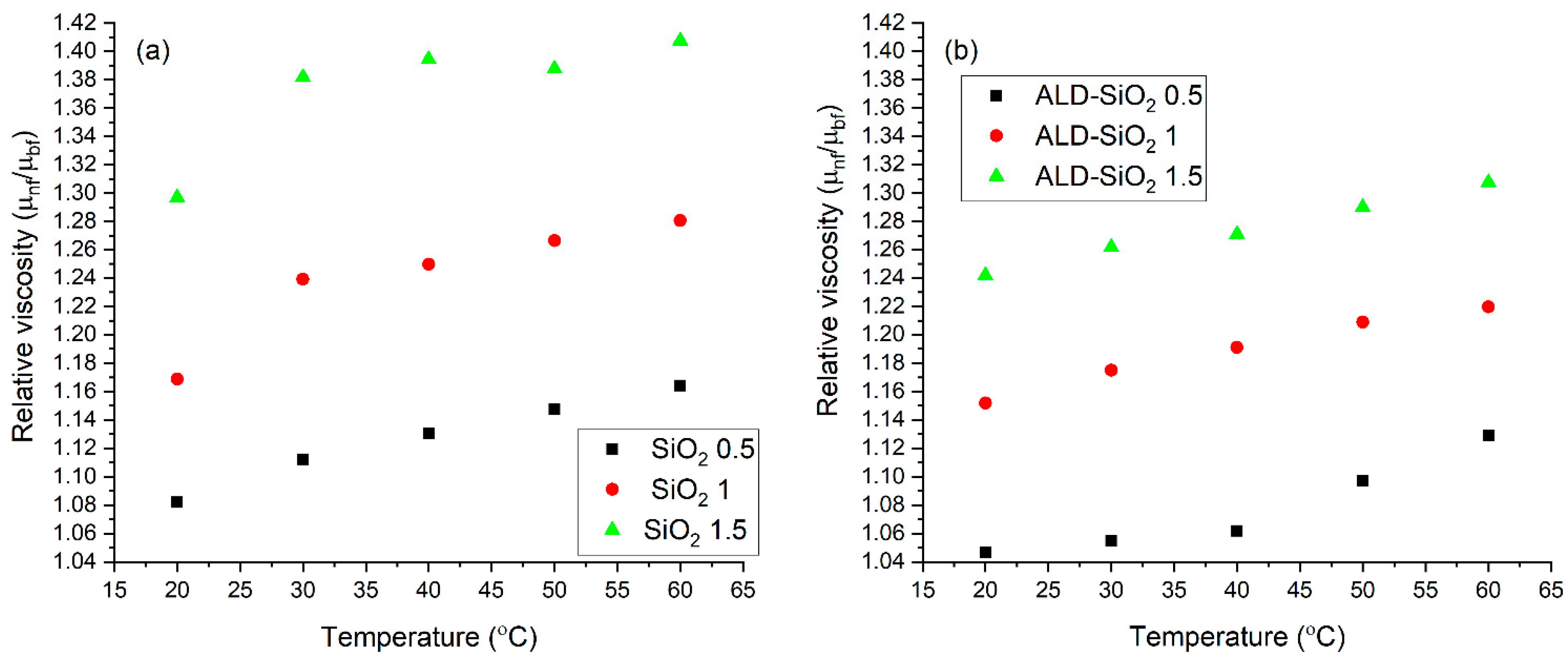
3.3. Rheological Properties
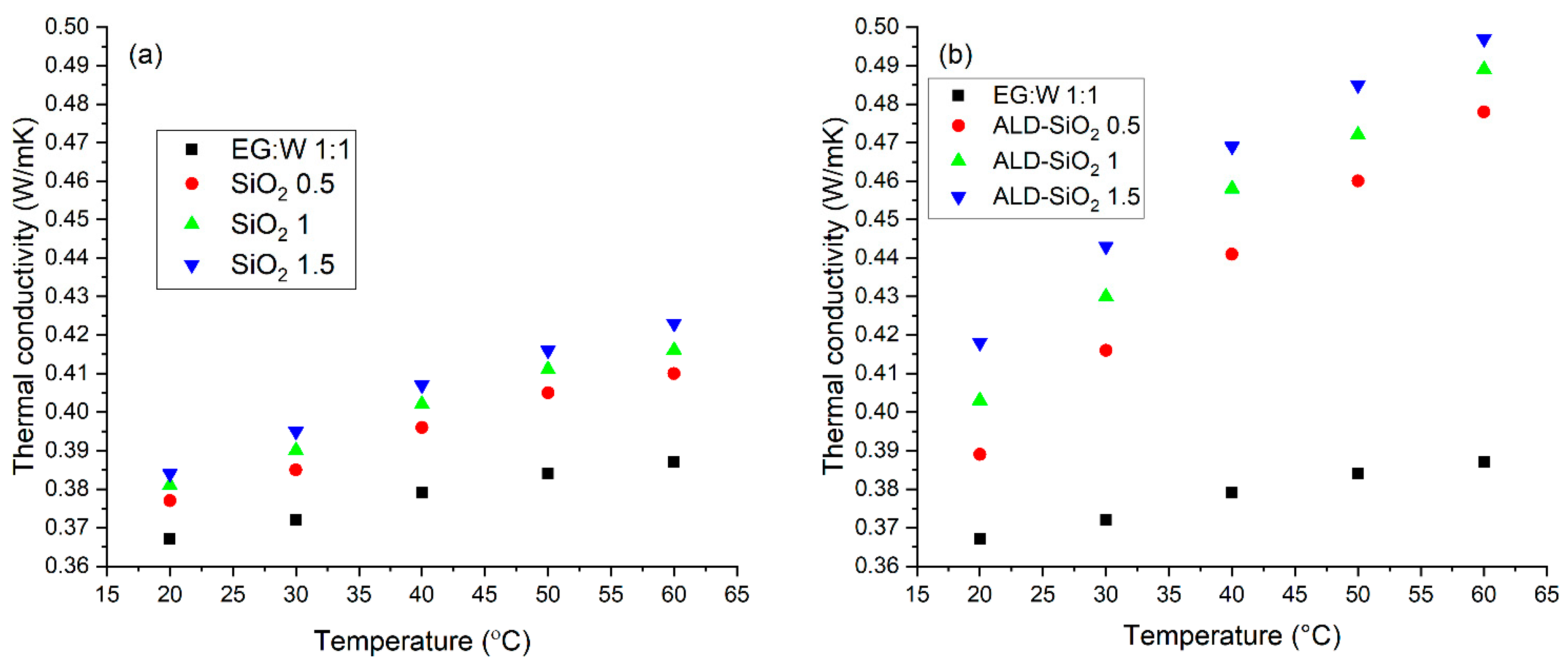
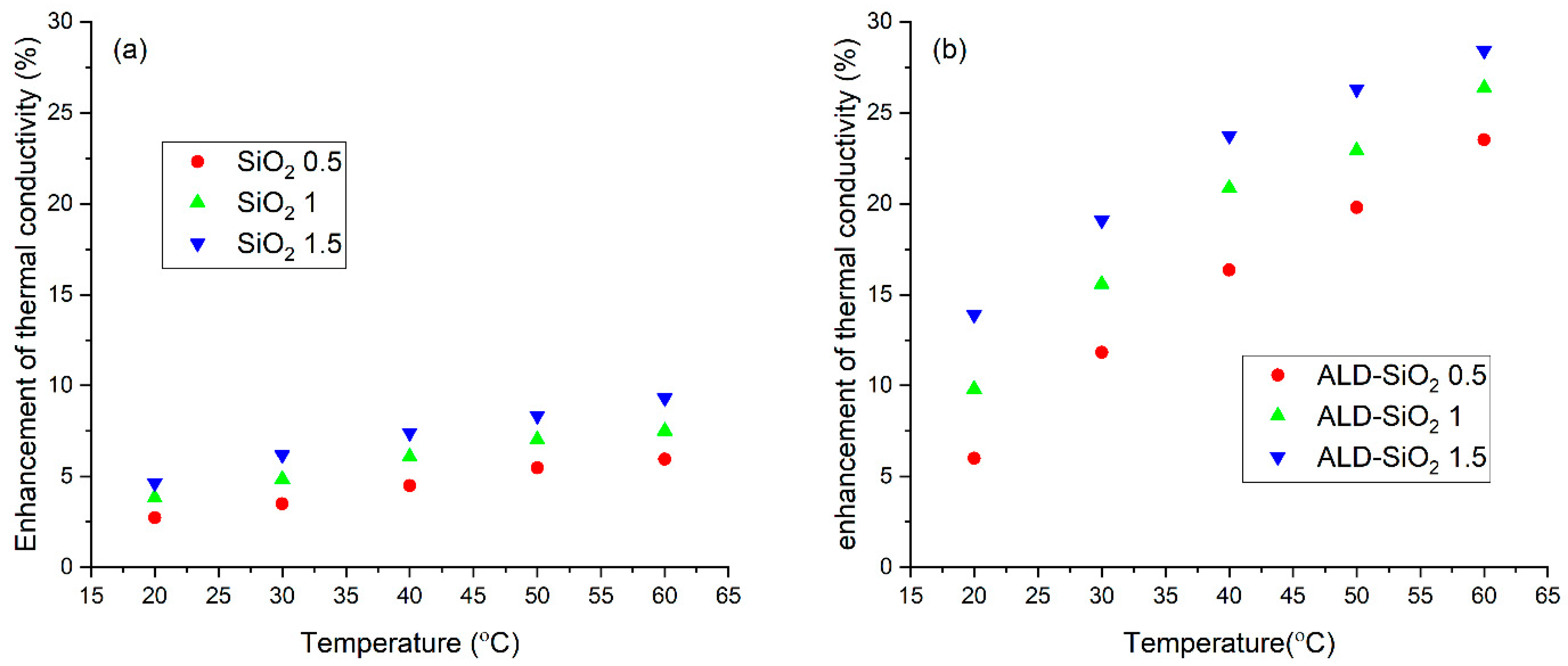
3.4. Thermal Conductivity
3.5. Regression
3.6. Balancing of the Viscosity and Thermal Conductivity
3.7. Future Research Directions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Choi, S.U.S.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; Argonne National Lab: Argonne, IL, USA, 1995. [Google Scholar]
- Taylor, R.; Coulombe, S.; Otanicar, T.; Phelan, P.; Gunawan, A.; Lv, W.; Rosengarten, G.; Prasher, R.; Tyagi, H. Small Particles, Big Impacts: A Review of the Diverse Applications of Nanofluids. J. Appl. Phys. 2013, 113, 011301. [Google Scholar] [CrossRef]
- Sundar, L.S.; Sharma, K.V.; Singh, M.K.; Sousa, A.C.M. Hybrid Nanofluids Preparation, Thermal Properties, Heat Transfer and Friction Factor—A Review. Renew. Sustain. Energy Rev. 2017, 68, 185–198. [Google Scholar] [CrossRef]
- Nabil, M.F.; Azmi, W.H.; Hamid, K.A.; Mamat, R. Experimental Investigation of Heat Transfer and Friction Factor of TiO2-SiO2 Nanofluids in Water:Ethylene Glycol Mixture. Int. J. Heat Mass Transf. 2018, 124, 1361–1369. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Nabil, M.F.; Mamat, R. Experimental Investigation of Nanoparticle Mixture Ratios on TiO2-SiO2 Nanofluids Heat Transfer Performance under Turbulent Flow. Int. J. Heat Mass Transf. 2018, 118, 617–627. [Google Scholar] [CrossRef]
- Nabil, M.F.; Azmi, W.H.; Abdul Hamid, K.; Mamat, R.; Hagos, F.Y. An Experimental Study on the Thermal Conductivity and Dynamic Viscosity of TiO2-SiO2 Nanofluids in Water: Ethylene Glycol Mixture. Int. Commun. Heat Mass Transf. 2017, 86, 181–189. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Nabil, M.F.; Mamat, R.; Sharma, K.V. Experimental Investigation of Thermal Conductivity and Dynamic Viscosity on Nanoparticle Mixture Ratios of TiO2-SiO2 Nanofluids. Int. J. Heat Mass Transf. 2018, 116, 1143–1152. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Nabil, M.F.; Mamat, R. Improved Thermal Conductivity of TiO2-SiO2 Hybrid Nanofluid in Ethylene Glycol and Water Mixture. IOP Conf. Ser. Mater. Sci. Eng. 2017, 257, 012067. [Google Scholar] [CrossRef]
- Nabil, M.F.; Azmi, W.H.; Hamid, K.A.; Mamat, R. Heat Transfer and Friction Factor of Composite TiO2-SiO2 Nanofluids in Water-Ethylene Glycol (60:40) Mixture. IOP Conf. Ser. Mater. Sci. Eng. 2017, 257, 012066. [Google Scholar] [CrossRef]
- Mohamad, M.N.F.; Hamzah, W.A.W.; Hamid, K.A.; Mamat, R. Heat Transfer Performance of TiO2-SiO2 Nanofluid in Water-Ethylene Glycol Mixture. J. Mech. Eng. 2018, 5, 39–48. [Google Scholar]
- Le Ba, T.; Várady, Z.I.; Lukács, I.E.; Molnár, J.; Balczár, I.A.; Wongwises, S.; Szilágyi, I.M. Experimental Investigation of Rheological Properties and Thermal Conductivity of SiO2–P25 TiO2 Hybrid Nanofluids. J. Therm. Anal. Calorim. 2020, 146, 493–507. [Google Scholar] [CrossRef]
- Vidhya, R.; Balakrishnan, T.; Kumar, B.S.; Palanisamy, R.; Panchal, H.; Angulo-Cabanillas, L.; Shaik, S.; Saleh, B.; Alarifi, I.M. An Experimental Study of ZrO2-CeO2 Hybrid Nanofluid and Response Surface Methodology for the Prediction of Heat Transfer Performance: The New Correlations. J. Nanomater. 2022, 2022, 6596028. [Google Scholar] [CrossRef]
- Cingarapu, S.; Singh, D.; Timofeeva, E.V.; Moravek, M.R. Nanofluids with Encapsulated Tin Nanoparticles for Advanced Heat Transfer and Thermal Energy Storage. Int. J. Energy Res. 2014, 38, 51–59. [Google Scholar] [CrossRef]
- Navarrete, N.; La Zara, D.; Goulas, A.; Valdesueiro, D.; Hernández, L.; van Ommen, J.R.; Mondragón, R. Improved Thermal Energy Storage of Nanoencapsulated Phase Change Materials by Atomic Layer Deposition. Sol. Energy Mater. Sol. Cells 2019, 206, 110322. [Google Scholar] [CrossRef]
- Gil-Font, J.; Hatte, M.A.; Bailey, M.R.; Navarrete, N.; Ventura-Espinosa, J.; Goulas, A.; La Zara, D.; van Ommen, J.R.; Mondragón, R.; Hernández, L. Improving Heat Transfer of Stabilised Thermal Oil-Based Tin Nanofluids Using Biosurfactant and Molecular Layer Deposition. Appl. Therm. Eng. 2020, 178, 115559. [Google Scholar] [CrossRef]
- Shang, L.; Qu, J.; Wang, Z.; Zhang, M.; Li, C. Optical Absorption Property and Photo-Thermal Conversion Performance of Ag@Al2O3 Plasmonic Nanofluids with Al2O3 Nano-Shell Fabricated by Atomic Layer Deposition. J. Mol. Liq. 2021, 326, 115388. [Google Scholar] [CrossRef]
- Arsana, I.M.; Muhimmah, L.C.; Nugroho, G.; Wahyuono, R.A. Enhanced Heat Transfer Effectiveness Using Low Concentration SiO2–TiO2 Core–Shell Nanofluid in a Water/Ethylene Glycol Mixture. J. Eng. Phys. Thermophys. 2021, 94, 423–430. [Google Scholar] [CrossRef]
- Botha, S.S.; Ndungu, P.; Bladergroen, B.J. Physicochemical Properties of Oil-Based Nanofluids Containing Hybrid Structures of Silver Nanoparticles Supported on Silica. Ind. Eng. Chem. Res. 2011, 50, 3071–3077. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Kamath, S.D.; Patil, U.P.; Patil, H.A.; Pandit, A.B.; Sonawane, S.H. Intensification of Heat Transfer Using PANI Nanoparticles and PANI-CuO Nanocomposite Based Nanofluids. Chem. Eng. Process. Process Intensif. 2016, 104, 172–180. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sarkar, I.; Haldar, K.; Pal, S.K.; Chakraborty, S. Synthesis of Cu-Al Layered Double Hydroxide Nanofluid and Characterization of Its Thermal Properties. Appl. Clay Sci. 2015, 107, 98–108. [Google Scholar] [CrossRef]
- Bohus, M.; Le Ba, T.; Hernadi, K.; Gróf, G.; Kónya, Z.; Erdélyi, Z.; Parditka, B.; Tamás, I.; Miklós, S.I. Thermal Conductivity Enhancement of Atomic Layer Deposition Surface-Modified Carbon Nanosphere and Carbon Nanopowder Nanofluids. Nanomaterials 2022, 12, 2226. [Google Scholar] [CrossRef]
- Mehrali, M.; Ghatkesar, M.K.; Pecnik, R. Full-Spectrum Volumetric Solar Thermal Conversion via Graphene/Silver Hybrid Plasmonic Nanofluids. Appl. Energy 2018, 224, 103–115. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C.M. Enhanced Heat Transfer and Friction Factor of MWCNT-Fe3O4/Water Hybrid Nanofluids. Int. Commun. Heat Mass Transf. 2014, 52, 73–83. [Google Scholar] [CrossRef]
- Sundar, L.S.; Venkata Ramana, E.; Graça, M.P.F.; Singh, M.K.; Sousa, A.C.M. Nanodiamond-Fe3O4 Nanofluids: Preparation and Measurement of Viscosity, Electrical and Thermal Conductivities. Int. Commun. Heat Mass Transf. 2016, 73, 62–74. [Google Scholar] [CrossRef]
- Wu, X.; Li, L.; Pan, J.; Wang, X.; Song, S.; Zhang, H. CeO2 Modified Ni-MOF as an Efficient Catalyst for Electrocatalytic Urea Oxidation. Mater. Lab 2022, 1, 220009. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Bakos, L.P.; Nóra, J.; da Costa, U.C.M.d.S.B.; László, K.; Lábár, J.; Igricz, T.; Varga–Josepovits, K.; Pasierb, P.; Färm, E.; et al. Photocatalytic and Gas Sensitive Multiwalled Carbon Prepared by Atomic Layer Deposition. Nanomaterials 2020, 10, 252. [Google Scholar]
- Le Ba, T.; Bohus, M.; Lukács, I.E.; Wongwises, S.; Gróf, G.; Hernadi, K.; Szilágyi, I.M. Comparative Study of Carbon Nanosphere and Carbon Nanopowder on Viscosity and Thermal Conductivity of Nanofluids. Nanomaterials 2021, 11, 608. [Google Scholar] [CrossRef]
- Le Ba, T.; Mahian, O.; Wongwises, S.; Szilágyi, I.M. Review on the Recent Progress in the Preparation and Stability of Graphene-Based Nanofluids. J. Therm. Anal. Calorim. 2020, 142, 1145–1172. [Google Scholar] [CrossRef]
- Le Ba, T.; Alkurdi, A.Q.; Lukács, I.E.; Molnár, J.; Wongwises, S.; Gróf, G.; Szilágyi, I.M. A Novel Experimental Study on the Rheological Properties and Thermal Conductivity of Halloysite Nanofluids. Nanomaterials 2020, 10, 1834. [Google Scholar] [CrossRef]
- Cheng, H.-E.; Chen, C.-C. Morphological and Photoelectrochemical Properties of ALD TiO2 Films. J. Electrochem. Soc. 2008, 155, D604. [Google Scholar] [CrossRef]
- Musić, S.; Filipović-Vinceković, N.; Sekovanić, L. Precipitation of Amorphous SiO2 Particles and Their Properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]



| Authors | Material of the Base | Coating Material | Coating Method |
|---|---|---|---|
| Cingarapu et al. [13] | Sn | SiO2 | sol–gel silica encapsulation process |
| Navarrete et al. [14] | Sn | SiO2 or Al2O3 | ALD |
| Gil-Font et al. [15] | Sn | Polyethylene terepthalate | Molecular layer deposition |
| Shang et al. [16] | Ag | Al2O3 | ALD |
| Arsana et al. [17] | SiO2 | TiO2 | - |
| Botha et al. [18] | SiO2 | Ag | Chemical reaction |
| Bhanvase et al. [19] | CuO | polyaniline | In situ emulsion polymerization |
| Chakraborty [20] | Cu-Al layered double hydroxides | One pot chemical reaction | |
| Bohus et al. [21] | Carbon nanosphere or carbon nanopowder | TiO2 | ALD |
| Mehrali et al. [22] | Graphene oxide nanosheets | Ag | Chemical reaction |
| Sundar et al. [23] | MWCNT (multiwall carbon nanotube) | Fe3O4 | In situ chemical reaction. |
| Sundar et al. [24] | C (nanodiamond) | Fe3O4 | Chemical reduction |
| Chamber pressure, mbar | 6.3 |
| Reactor pressure, mbar | 1.3 |
| TiCl4 pulse time, ms | 300 |
| H2O pulse time, ms | 300 |
| TiCl4 purge time, ms | 3000 |
| H2O purge time, ms | 3000 |
| Temperature, °C | 108 |
| Number of cycles | 410 |
| Properties | SiO2 Particles | Composite Particles | Ethylene Glycol | Water |
|---|---|---|---|---|
| color | white | white | limpid | limpid |
| Molecular mass, g/mol | 60.08 | - | 62.07 | 18.02 |
| Average particle diameter, nm | 10–20 | 11–21 | - | - |
| Density, at 20 °C, kg/m3 | 2138 ± 50 | 2150 ± 50 | 1113 | 997 |
| Melting point, °C | 2230 | - | −12.7 | 0 |
| Boiling point, at 101.3, kPa | - | - | 198 | 100 |
| Viscosity, at 20 °C, mPas | - | - | 20.9 | 1.00 |
| Thermal conductivity, W/mK | - | - | 0.258 | 0.609 |
| Specific heat, at 20 °C, J/kgK | - | - | 2347 | 4186 |
| Sample Name | Nanoparticle vol% | Base Fluid vol% |
|---|---|---|
| SiO2-ALD TiO2 0.5 | 0.5 | 99.5 |
| SiO2-ALD TiO2 1.0 | 1.0 | 99.0 |
| SiO2-ALD TiO2 1.5 | 1.5 | 98.5 |
| SiO2 0.5 | 0.5 | 99.5 |
| SiO2 1.0 | 1.0 | 99.0 |
| SiO2 1.5 | 1.5 | 98.5 |
| Wavenumber (cm−1) | Vibration |
|---|---|
| 3435 | O-H stretching (from water) |
| 3246 (appears in the shoulder) | Si-OH stretching |
| 1630 | H-O-H bending (from water) |
| 1384 | Si-O stretching |
| 1101 | O-Si-O asymmetrical stretching |
| 961 | Si-OH |
| 801 | O-Si-O symmetrical stretching |
| 475 | Si-O-Si stretching |
| Sample Name | Zeta Potential (mV) |
|---|---|
| SiO2-ALD TiO2 0.5 | −30.25 |
| SiO2-ALD TiO2 1.0 | −33.62 |
| SiO2-ALD TiO2 1.5 | −32.86 |
| SiO2 0.5 | −32,82 |
| SiO2 1.0 | −33.03 |
| SiO2 1.5 | −44.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Várady, Z.I.; Ba, T.L.; Parditka, B.; Erdélyi, Z.; Hernadi, K.; Karacs, G.; Gróf, G.; Szilágyi, I.M. Experimental Investigation of Rheological Properties and Thermal Conductivity of SiO2–TiO2 Composite Nanofluids Prepared by Atomic Layer Deposition. Nanomaterials 2022, 12, 3014. https://doi.org/10.3390/nano12173014
Várady ZI, Ba TL, Parditka B, Erdélyi Z, Hernadi K, Karacs G, Gróf G, Szilágyi IM. Experimental Investigation of Rheological Properties and Thermal Conductivity of SiO2–TiO2 Composite Nanofluids Prepared by Atomic Layer Deposition. Nanomaterials. 2022; 12(17):3014. https://doi.org/10.3390/nano12173014
Chicago/Turabian StyleVárady, Zalán István, Thong Le Ba, Bence Parditka, Zoltán Erdélyi, Klara Hernadi, Gábor Karacs, Gyula Gróf, and Imre Miklós Szilágyi. 2022. "Experimental Investigation of Rheological Properties and Thermal Conductivity of SiO2–TiO2 Composite Nanofluids Prepared by Atomic Layer Deposition" Nanomaterials 12, no. 17: 3014. https://doi.org/10.3390/nano12173014
APA StyleVárady, Z. I., Ba, T. L., Parditka, B., Erdélyi, Z., Hernadi, K., Karacs, G., Gróf, G., & Szilágyi, I. M. (2022). Experimental Investigation of Rheological Properties and Thermal Conductivity of SiO2–TiO2 Composite Nanofluids Prepared by Atomic Layer Deposition. Nanomaterials, 12(17), 3014. https://doi.org/10.3390/nano12173014










