Binder-Free MnO2/MWCNT/Al Electrodes for Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
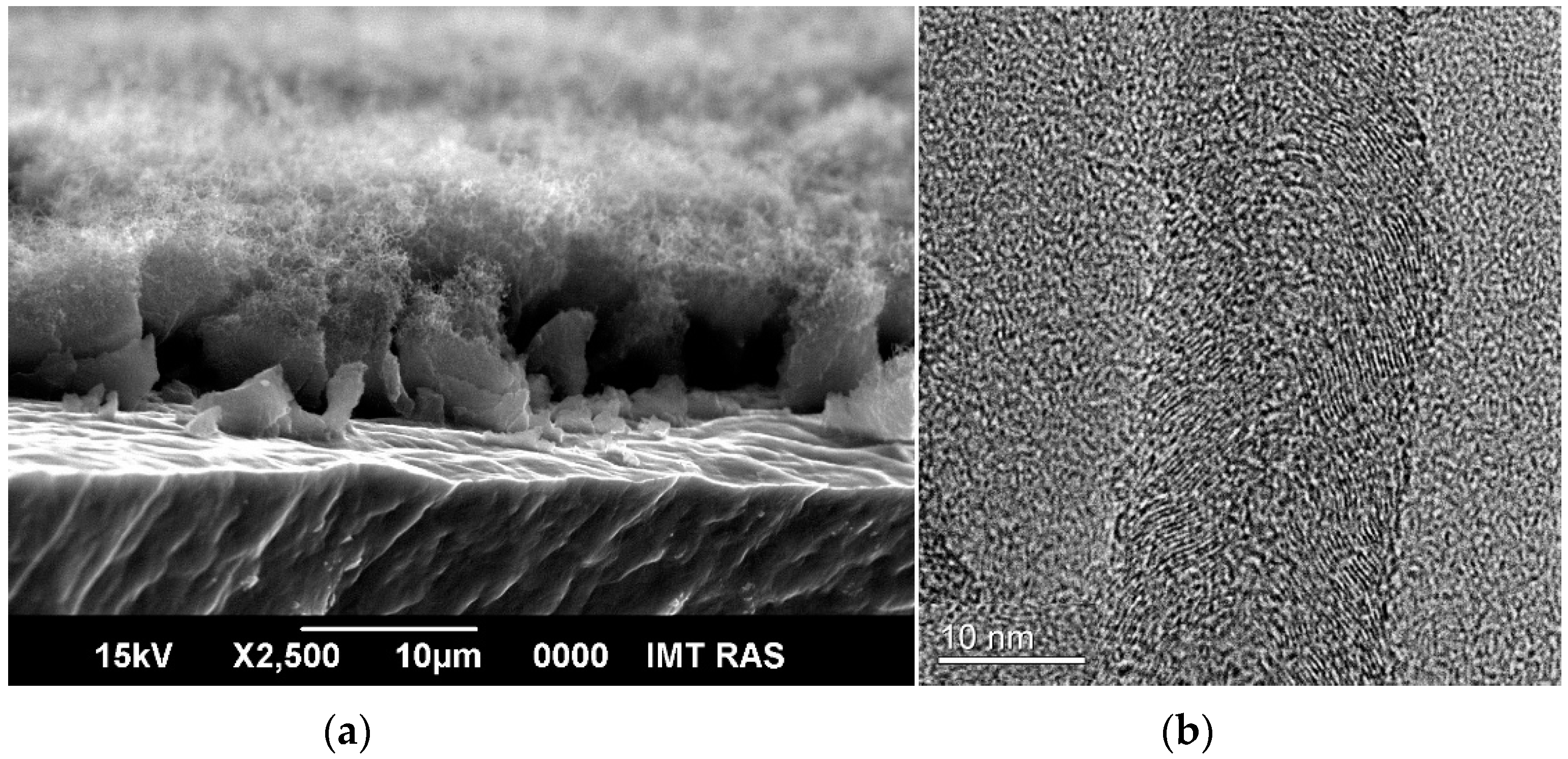
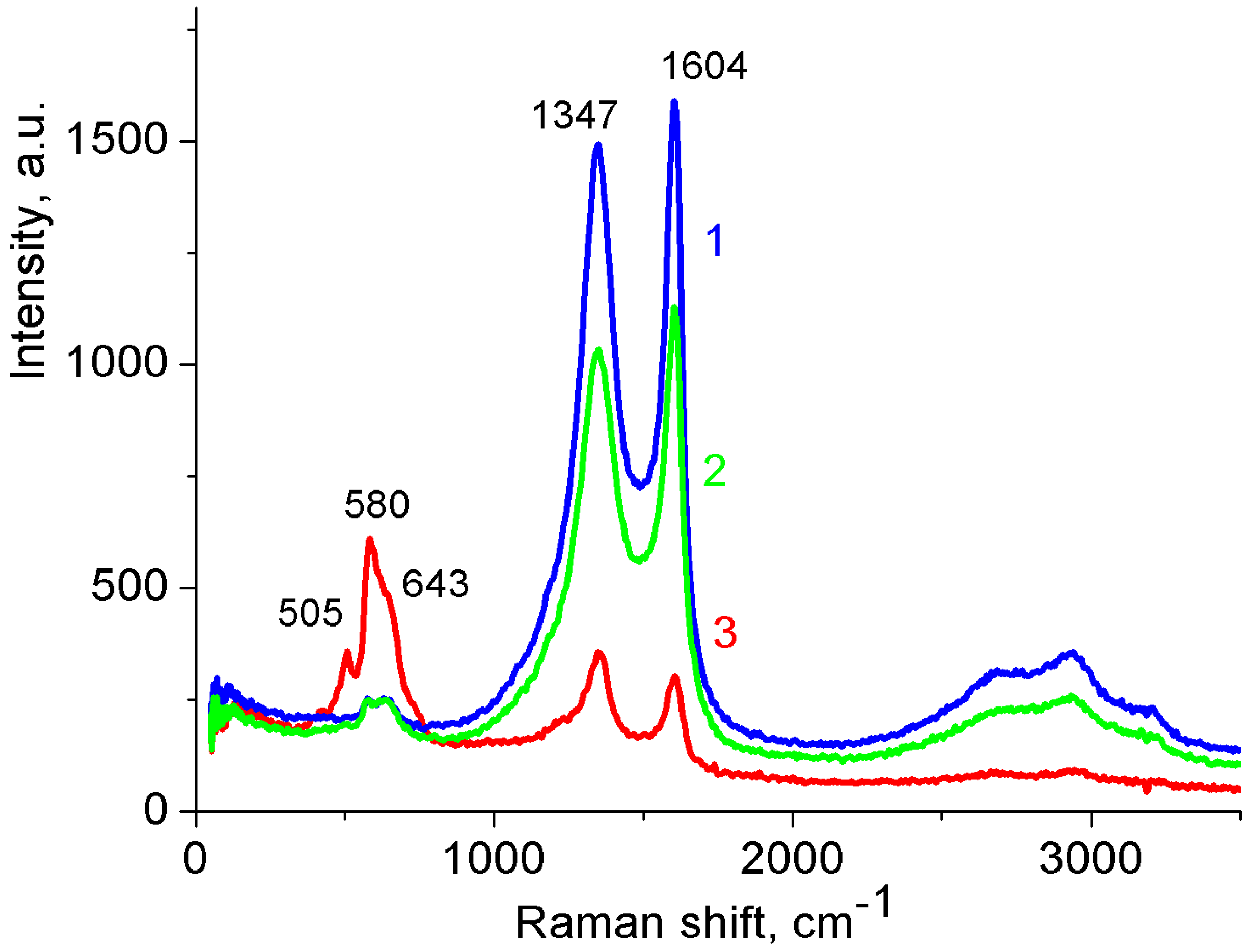
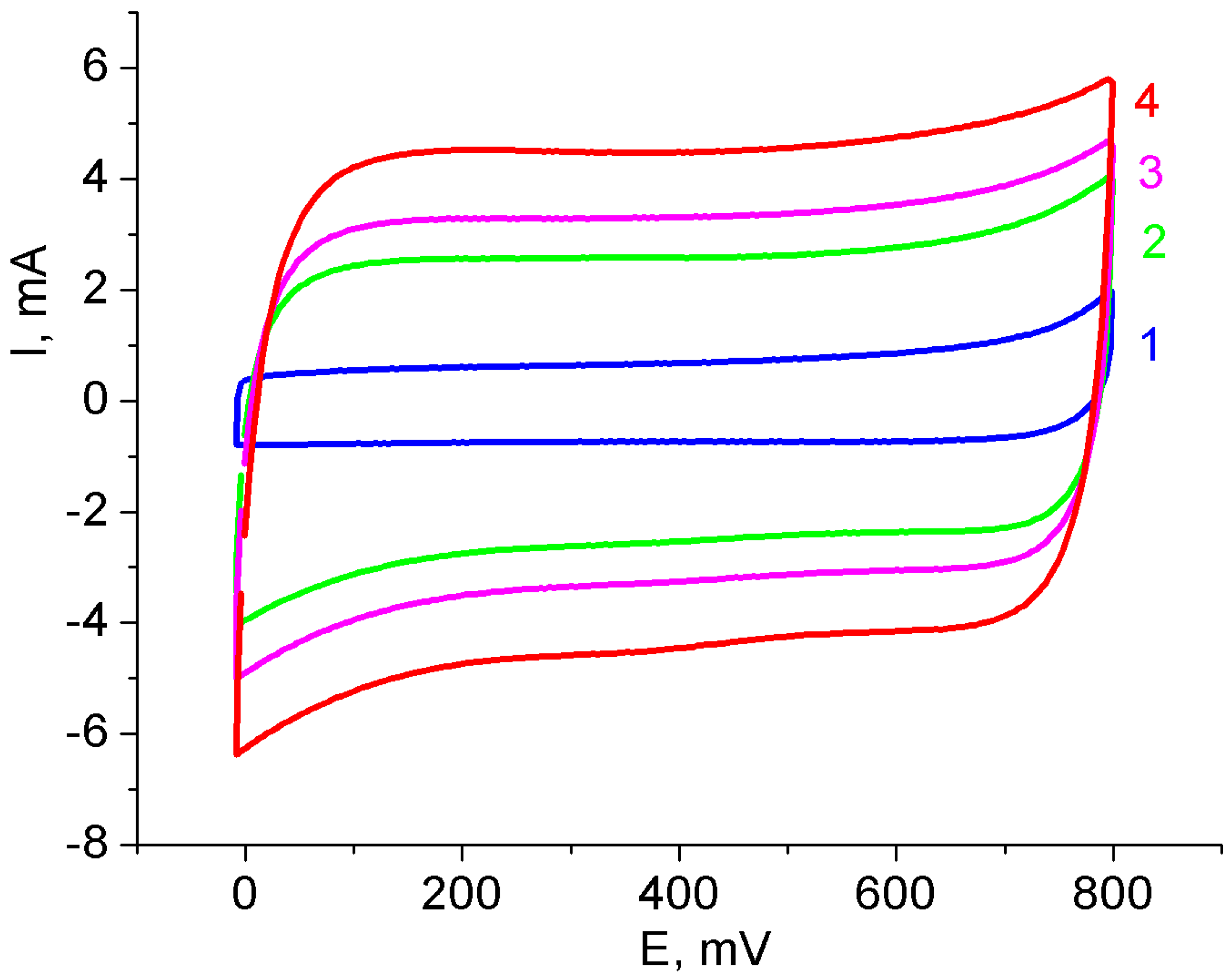
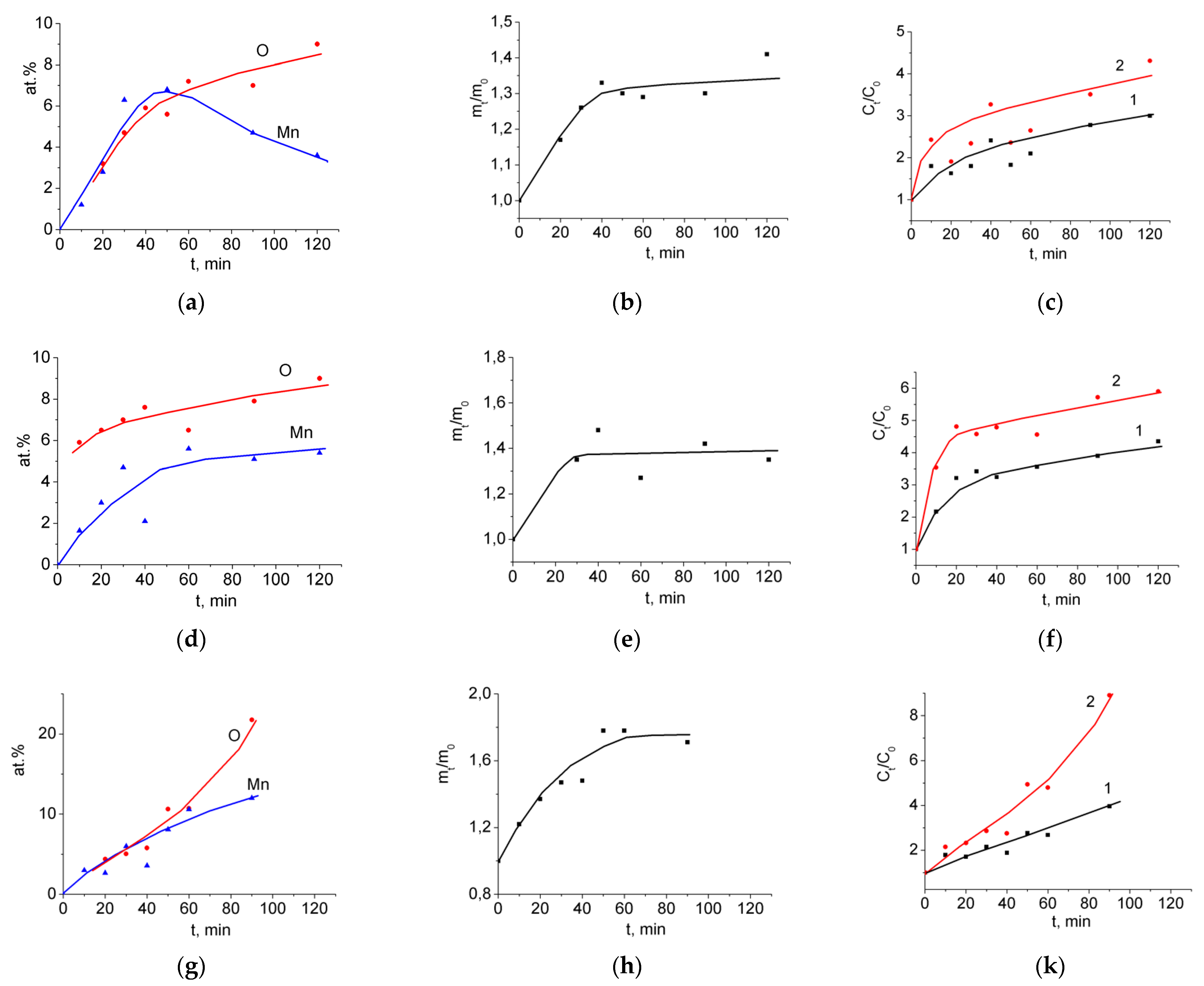
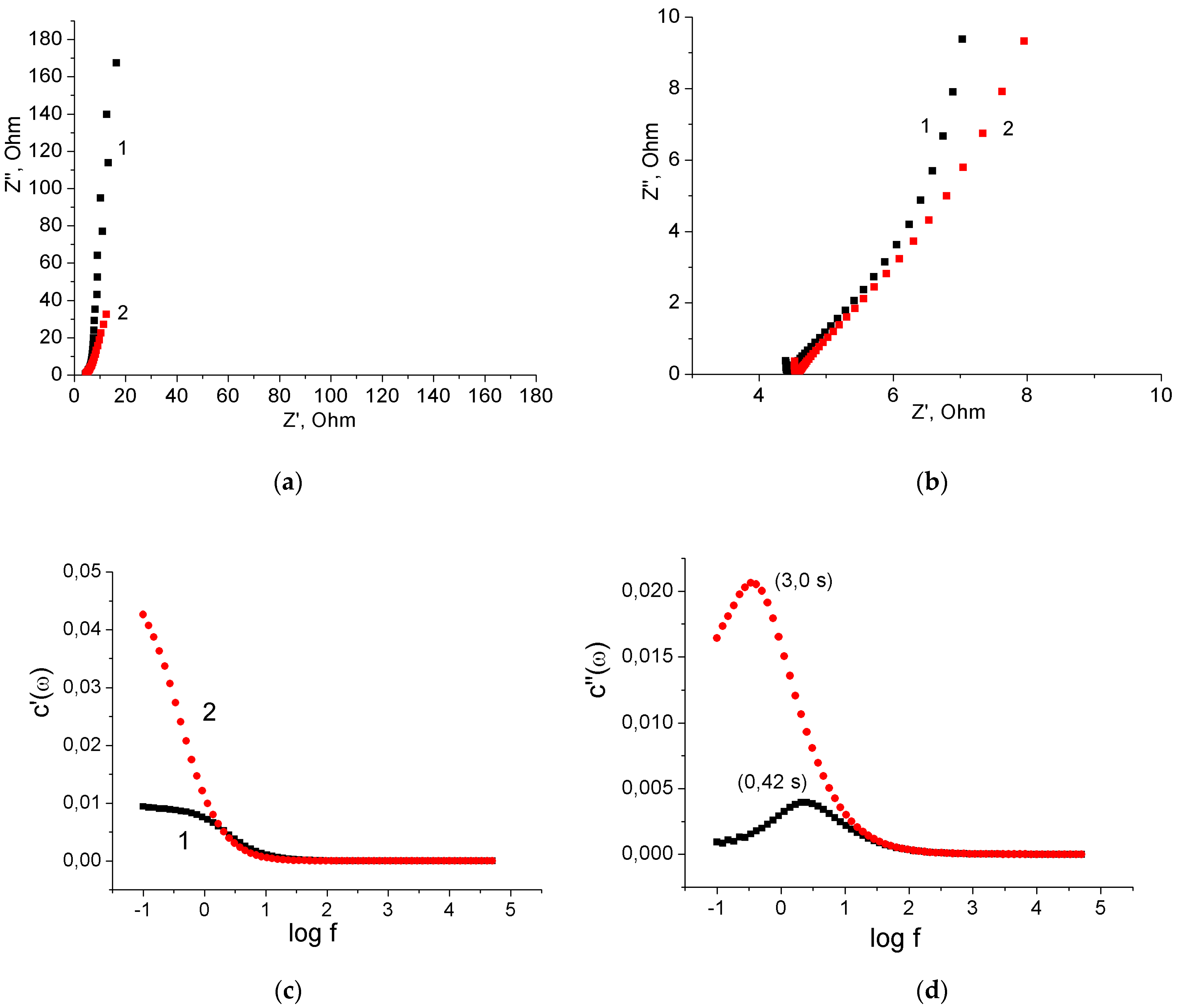
3.1. Binder-Free MnO2/MWCNT/Al Electrodes from As-Prepared MWCNT/Al Samples
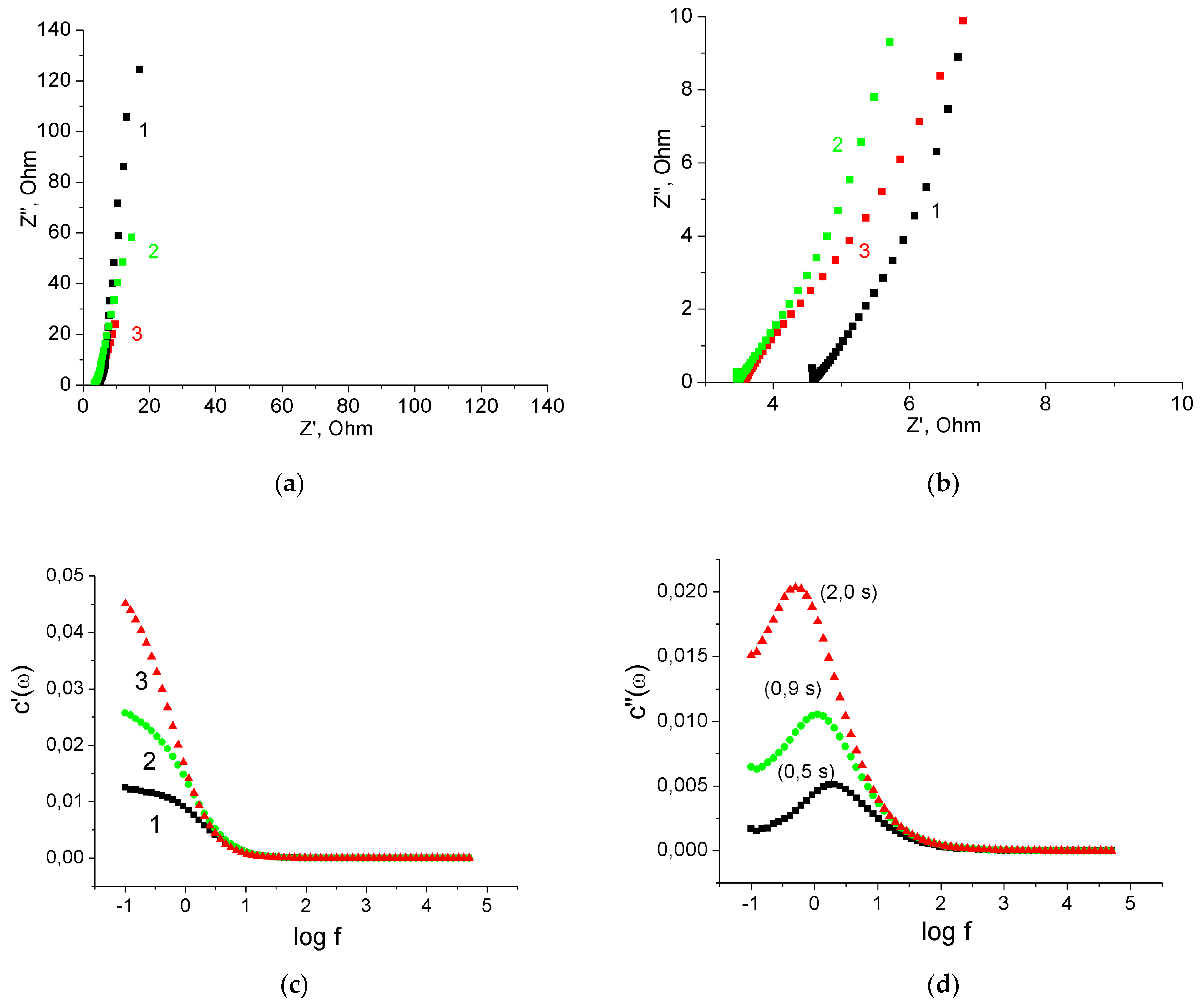
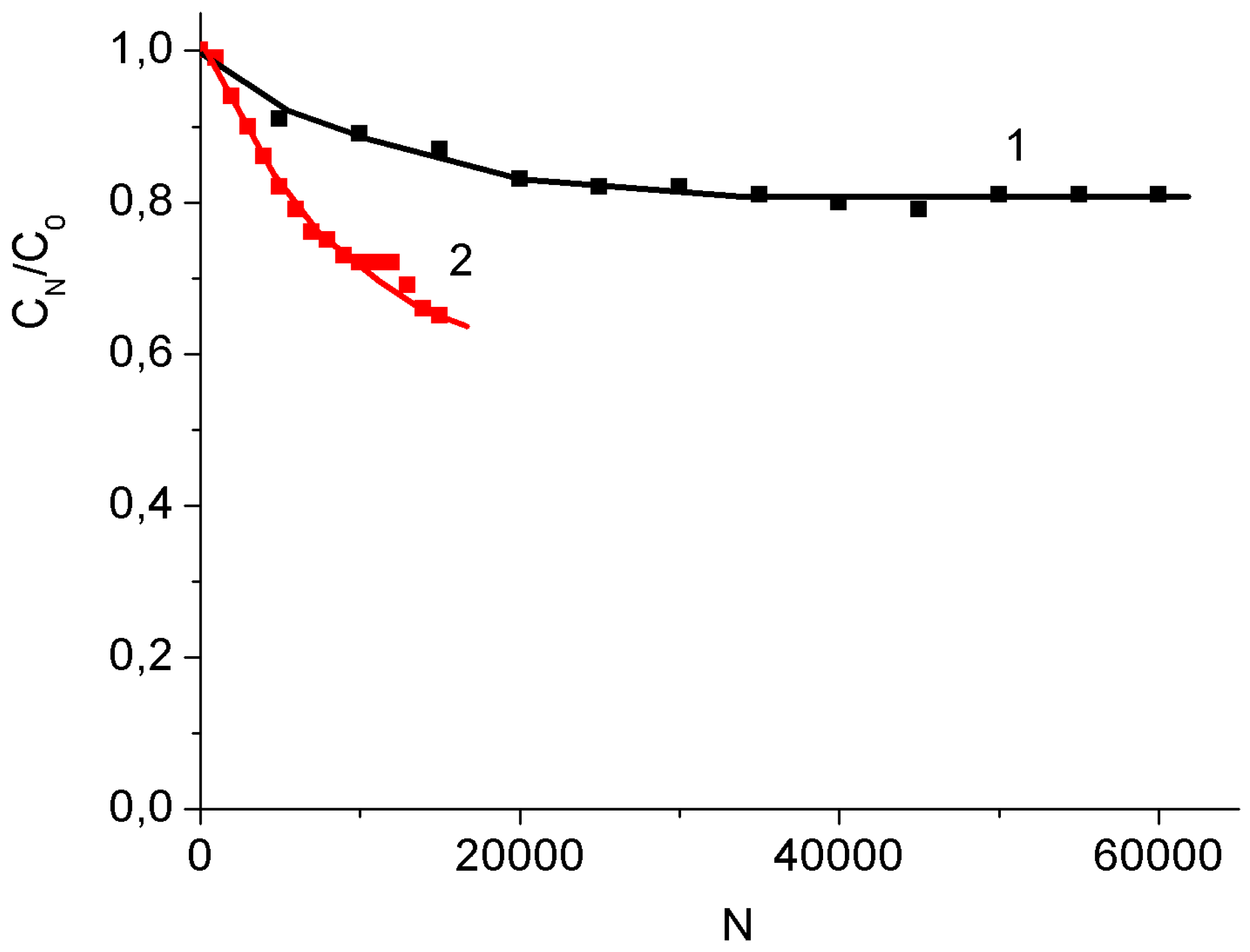
3.2. Binder-Free MnO2/MWCNT/Al Electrodes from Oxidized MWCNT/Al Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kebede, A.A.; Kalogiannis, T.; Mierlo, J.V.; Berecibar, M. A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for energy storage: Review of electrode materials and methods of increasing capacitance for supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- Briscoe, J.; Dunn, S. Piezoelectric nanogenerators—A review of nanostructured piezoelectric energy harvesters. Nano Energy 2015, 14, 15–29. [Google Scholar] [CrossRef]
- Maddukuri, S.; Malka, D.; Chae, M.S.; Elias, Y.; Luski, S.; Aurbach, D. On the challenge of large energy storage by electrochemical devices. Electrochim. Acta 2020, 354, 136771. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.H.; Azad, A.T.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Satpathy, S.; Das, S.; Bhattacharyya, B.K. How and where to use super-capacitors effectively, an integration of review of past and new characterization works on super-capacitors. J. Energy Storage 2020, 27, 101044. [Google Scholar] [CrossRef]
- Chandu, V.V.; Gopi, M.; Sambasivam, R.V.S.; Obaidat, I.M.; Kim, H.-J. Recent progress of advanced energy storage materials for flexible and wearable supercapacitor: From design and development to applications. J. Energy Storage 2020, 27, 101035. [Google Scholar]
- Han, X.; Xiao, G.; Wang, Y.; Chen, X.; Duan, G.; Wu, Y.; Gong, X.; Wang, H. Design and fabrication of conductive polymer hydrogels and their applications in flexible supercapacitors. J. Mater. Chem. A 2020, 8, 23059–23095. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Wang, F.; Zhao, L.; Zhang, Q.; Xu, W.; He, S. Review on porous carbon materials engineered by ZnO templates: Design, synthesis and capacitance performance. Mater. Des. 2021, 201, 109518. [Google Scholar] [CrossRef]
- Wang, J.; Dong, S.; Ding, B.; Wang, Y.; Hao, X.; Dou, H.; Xia, Y.; Zhang, X. Pseudocapacitive materials for electrochemical capacitors: From rational synthesis to capacitance optimization. Natl. Sci. Rev. 2017, 4, 71–90. [Google Scholar] [CrossRef]
- Shi, Z.; Sun, G.; Yuan, R.; Chen, W.; Wang, Z.; Zhang, L.; Zhan, K.; Zhu, M.; Yang, J.; Zhao, B. Scalable fabrication of NiCo2O4/reduced graphene oxide composites by ultrasonic spray as binder-free electrodes for supercapacitors with ultralong lifetime. J. Mater. Sci. Technol. 2022, 99, 260–269. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Yuan, M.; Luo, F.; Rao, Y.; Yu, J.; Wang, Z.; Li, H.; Chen, X. SWCNT-bridged laser-induced graphene fibers decorated with MnO2 nanoparticles for high-performance flexible micro-supercapacitors. Carbon 2021, 183, 128–137. [Google Scholar] [CrossRef]
- Sun, G.; Ren, H.; Shi, Z.; Zhang, L.; Wang, Z.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B. V2O5/vertically-aligned carbon nanotubes as negative electrode for asymmetric supercapacitor in neutral aqueous electrolyte. J. Colloid Interface Sci. 2021, 588, 847–856. [Google Scholar] [CrossRef]
- Shi, P.; Li, L.; Hua, L.; Qian, Q.; Wang, P.; Zhou, J.; Sun, G.; Huang, W. Design of Amorphous Manganese Oxide@Multiwalled Carbon Nanotube Fiber for Robust Solid-State Supercapacitor. ACS Nano 2017, 11, 444–452. [Google Scholar] [CrossRef]
- Yue, T.; Shen, B.; Gao, P. Carbon material/MnO2 as conductive skeleton for supercapacitor electrode material: A review. Renew. Sustain. Energy Rev. 2022, 158, 112131. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Cui, R.; Huang, H.; Liu, B.; Li, Y.; Wang, D.; Zhao, D.; Dong, J.; Li, S.; et al. MnO2/Porous Carbon Nanotube/MnO2 Nanocomposites for High-Performance Supercapacitor. ACS Appl. Nano Mater. 2020, 3, 11152–11159. [Google Scholar] [CrossRef]
- Xu, N.; Liu, J.; Qiao, J.; Huang, H.; Zhou, X.-D. Interweaving between MnO2 nanowires/ nanorods and carbon nanotubes as robust multifunctional electrode for both liquid and flexible electrochemical energy devices. J. Power Sources 2020, 455, 227992. [Google Scholar] [CrossRef]
- Kannan, R.R.; Lenin, N.; Banu, A.A.; Sivabharathy, M. Electrochemical behavior of MnO2/MWCNT nanocomposites for electrode material in supercapacitor. Mater. Lett. 2022, 314, 131887. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zang, J.; Xin, G.; Yuan, Y.; Qu, X. Electrophoretic deposition of MnO2-coated carbon nanotubes on a graphite sheet as a flexible electrode for supercapacitors. Carbon 2012, 50, 5196–5202. [Google Scholar] [CrossRef]
- Sun, P.; Yi, H.; Peng, T.; Jing, Y.; Wang, R.; Wang, H.; Wang, X. Ultrathin MnO2 nanoflakes deposited on carbon nanotube networks for symmetrical supercapacitors with enhanced performance. J. Power Sources 2017, 341, 27–35. [Google Scholar] [CrossRef]
- Wang, J.-G.; Yang, Y.; Huang, Z.-H.; Kang, F. Synthesis and electrochemical performance of MnO2/CNTs–embedded carbon nanofibers nanocomposites for supercapacitors. Electrochim. Acta 2012, 75, 213–219. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Hu, J.; Wei, L.; Liu, J.; Zheng, M. Facile synthesis of MnO2 grown on nitrogen-doped carbon nanotubes for asymmetric supercapacitors with enhanced electrochemical performance. J. Power Sources 2018, 393, 135–144. [Google Scholar] [CrossRef]
- Kong, S.; Cheng, K.; Ouyang, T.; Ye, K.; Wang, G.; Cao, D. Freestanding MnO2 nanoflakes on carbon nanotube covered nickel foam as a 3D binder-free supercapacitor electrode with high performance. J. Electroanal. Chem. 2017, 786, 35–42. [Google Scholar] [CrossRef]
- Qi, W.; Li, X.; Wu, Y.; Zeng, H.; Kuang, C.; Zhou, S.; Huang, S.; Yang, Z. Flexible electrodes of MnO2/CNTs composite for enhanced performance on supercapacitors. Surf. Coat. Technol. 2017, 320, 624–629. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Zhang, X.; Chen, X.; Li, C. MnO2 grown in situ on graphene@CNTs as electrode materials for supercapacitors. Ceram. Int. 2015, 41, 12680–12685. [Google Scholar] [CrossRef]
- Wang, H.; Peng, C.; Peng, F.; Yu, H.; Yang, J. Facile synthesis of MnO2/CNT nanocomposite and its electrochemical performance for supercapacitors. Mater. Sci. Eng. B 2011, 176, 1073–1078. [Google Scholar] [CrossRef]
- Arkhipova, E.A.; Ivanov, A.S.; Isaikina, O.Y.; Novotortsev, R.Y.; Stolbov, D.N.; Xia, H.; Savilov, S.V. Application of MnO2/MWCNT composite in supercapacitors. Mater. Today Proc. 2022, 60, 1008–1011. [Google Scholar] [CrossRef]
- Ganguly, D.; Pahari, D.; Das, N.S.; Howli, P.; Das, B.; Banerjee, D.; Chattopadhyay, K.K. All-amorphous CNT-MnO2 nanoflaky hybrid for improved supercapacitor applications. J. Electroanal. Chem. 2016, 778, 12–22. [Google Scholar] [CrossRef]
- Wang, H.; Peng, C.; Zheng, J.; Peng, F.; Yu, H. Design, synthesis and the electrochemical performance of MnO2/C@CNT as supercapacitor material. Mater. Res. Bull. 2013, 48, 3389–3393. [Google Scholar] [CrossRef]
- Teng, F.; Santhanagopalan, S.; Wang, Y.; Meng, D.D. In-situ hydrothermal synthesis of three-dimensional MnO2–CNT nanocomposites and their electrochemical properties. J. Alloys Compd. 2010, 499, 259–264. [Google Scholar] [CrossRef]
- Wang, K.; Gao, S.; Du, Z.; Yuan, A.; Lu, W.; Chen, L. MnO2-Carbon nanotube composite for high-areal-density supercapacitors with high rate performance. J. Power Sources 2016, 305, 30–36. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Chen, R.; Ni, H.; Zhan, W.; Zhang, B.; Zheng, F.; Dong, S. Defective carbon nanotube forest grown on stainless steel encapsulated in MnO2 nanosheets for supercapacitors. Electrochim. Acta 2018, 278, 61–71. [Google Scholar] [CrossRef]
- Lei, R.; Zhang, H.; Lei, W.; Li, D.; Fang, Q.; Ni, H.; Gu, H. MnO2 nanowires electrodeposited on freestanding graphenated carbon nanotubes as binder-free electrodes with enhanced supercapacitor performance. Mater. Lett. 2019, 249, 140–142. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Ji, S.-H.; Kim, D.-H. Low-cost superior symmetric solid-state supercapacitors based on MWCNTs/MnO2 nanocomposite thin film. J. Taiwan Inst. Chem. Eng. 2017, 80, 503–510. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, J.; Zhang, B.; Sun, F.; Liu, B.; Kuang, Y. Electrochemically induced deposition method to prepare g-MnO2/multi-walled carbon nanotube composites as electrode material in supercapacitors. Mater. Res. Bull. 2008, 43, 2085–2091. [Google Scholar] [CrossRef]
- Amade, R.; Jover, E.; Caglar, B.; Mutlu, T.; Bertran, E. Optimization of MnO2/vertically aligned carbon nanotube composite for supercapacitor application. J. Power Sources 2011, 196, 5779–5783. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, X.; Tynan, B.; Sha, Z.; Huang, F.; Islam, M.S.; Zhang, J.; Rider, A.N.; Dai, L.; Chu, D.; et al. High-performance hierarchical MnO2/CNT electrode for multifunctional supercapacitors. Carbon 2021, 184, 504–513. [Google Scholar] [CrossRef]
- Wang, J.-G.; Kang, F.; Wei, B. Engineering of MnO2-based nanocomposites for high-performance supercapacitors. Prog. Mater. Sci. 2015, 74, 51–124. [Google Scholar] [CrossRef]
- Wu, D.; Xie, X.; Zhang, Y.; Zhang, D.; Du, W.; Zhang, X.; Wang, B. MnO2/Carbon Composites for Supercapacitor: Synthesis and Electrochemical Performance. Front. Mater. 2020, 7, 2. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; He, S.; Zhang, Q.; Liu, K.; Han, X.; Duan, G.; Jiang, S. Design of wood-derived anisotropic structural carbon electrode for high-performance supercapacitor. Wood Sci. Technol. 2022, 56, 1191–1203. [Google Scholar] [CrossRef]
- Chen, L.; Wang, F.; Tian, Z.; Guo, H.; Cai, C.; Wu, Q.; Du, H.; Liu, K.; Hao, Z.; He, S.; et al. Wood-Derived High-Mass-Loading MnO2 Composite Carbon Electrode Enabling High Energy Density and High-Rate Supercapacitor. Small 2022, 18, 2201307. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zheng, J.; Feng, L.; Chen, W.; Yang, W.; Dong, Y.; Jiang, S.; Zhang, Q.; He, S. All-cellulose-based high-rate performance solid-state supercapacitor enabled by nitrogen doping and porosity tuning. Diam. Relat. Mater. 2022, 128, 109238. [Google Scholar] [CrossRef]
- How, Y.Y.; Numan, A.; Mustafa, M.N.; Walvekar, R.; Khalid, M.; Mubarak, N.M. A review on the binder-free electrode fabrication for electrochemical energy storage devices. J. Energy Storage 2022, 51, 104324. [Google Scholar] [CrossRef]
- Wu, K.; Ye, Z.; Ding, Y.; Zhu, Z.; Peng, X.; Li, D.; Ma, G. Facile co-deposition of the carbon nanotube@MnO2 heterostructure for high-performance flexible supercapacitors. J. Power Sources 2020, 477, 229031. [Google Scholar] [CrossRef]
- Kavian, R.; Vicenzo, A.; Bestetti, M. Composite Supercapacitor Electrodes by Electrodeposition of MnO2 on MWCNT Felt Directly Grown on Aluminum. J. New Mater. Electrochem. Syst. 2015, 18, 043–048. [Google Scholar] [CrossRef]
- Dorfler, S.; Felhosi, I.; Marek, T.; Thieme, S.; Althues, H.; Nyikos, L.; Kaskel, S. High power supercap electrodes based on vertical aligned carbon nanotubes on aluminum. J. Power Sources 2013, 227, 218–228. [Google Scholar] [CrossRef]
- Arcila-Velez, M.R.; Zhu, J.; Childress, A.; Karakaya, M.; Podila, R.; Rao, A.M.; Roberts, M.E. Roll-to-roll synthesis of vertically aligned carbon nanotube electrodes for electrical double layer capacitors. Nano Energy 2014, 8, 9–16. [Google Scholar] [CrossRef]
- Dogru, I.B.; Durukan, M.B.; Turel, O.; Unalan, H.E. Flexible supercapacitor electrodes with vertically aligned carbon nanotubes grown on aluminum foils. Prog. Nat. Sci.–Mater. 2016, 26, 232–236. [Google Scholar] [CrossRef]
- Redkin, A.N.; Mitina, A.A.; Yakimov, E.E. Simple technique of multiwalled carbon nanotubes growth on aluminum foil for supercapacitors. Mater. Sci. Eng. B 2021, 272, 115342. [Google Scholar] [CrossRef]
- Redkin, A.N.; Mitina, A.A.; Yakimov, E.E.; Kabachkov, E.N. Electrochemical Improvement of the MWCNT/Al Electrodes for Supercapacitors. Materials 2021, 14, 7612. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Q.; Ma, Q.; Li, Y.; Zhu, Z. Chemical treatment of CNTs in acidic KMnO4 solution and promoting effects on the corresponding Pd–Pt/CNTs catalyst. J. Mol. Catal. A Chem. 2012, 356, 114–120. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, L.; Zhang, J.; Miao, T.; Yuan, R.; Chen, W.; Wang, Z.; Yang, J.; Zhao, B. Na+ pre-intercalated Na0.11MnO2 on three-dimensional graphene as cathode for aqueous zinc ion hybrid supercapacitor with high energy density. Carbon 2022, 198, 46–56. [Google Scholar] [CrossRef]

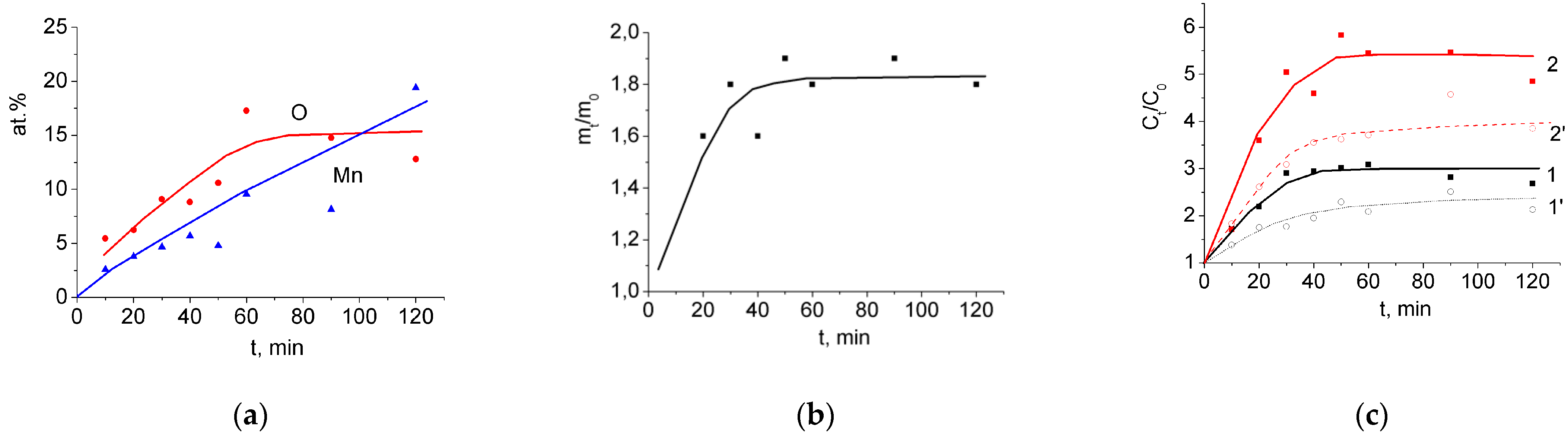

| Treatment Duration, min | 0.2% KMnO4 | 1% KMnO4 | 2% KMnO4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C, at.% | O, at.% | Mn, at.% | C, at.% | O, at.% | Mn, at.% | C, at.% | O, at.% | Mn, at.% | |
| 10 | 93 | 5.8 | 1.2 | 92.6 | 5.8 | 1.6 | - | - | - |
| 20 | 93.9 | 3.3 | 2.8 | 90.5 | 6.5 | 3.0 | 92.9 | 4.4 | 2.7 |
| 30 | 89 | 4.7 | 6.3 | 88.2 | 7.0 | 4.8 | 90.0 | 4.8 | 5.2 |
| 40 | 85.5 | 5.9 | 8.6 | - | - | - | 90.6 | 5.8 | 3,6 |
| 50 | 87.6 | 5.6 | 6.8 | - | - | - | 81.3 | 10.6 | 8.1 |
| 60 | - | - | - | 87.4 | 7.0 | 5.6 | 78.7 | 10.7 | 10.6 |
| 90 | 88.3 | 7 | 4.7 | 87.5 | 7.4 | 5.1 | 66.2 | 21.8 | 12.0 |
| 120 | 87.4 | 9 | 3.6 | 85.6 | 9.0 | 5.4 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redkin, A.N.; Mitina, A.A.; Yakimov, E.E. Binder-Free MnO2/MWCNT/Al Electrodes for Supercapacitors. Nanomaterials 2022, 12, 2922. https://doi.org/10.3390/nano12172922
Redkin AN, Mitina AA, Yakimov EE. Binder-Free MnO2/MWCNT/Al Electrodes for Supercapacitors. Nanomaterials. 2022; 12(17):2922. https://doi.org/10.3390/nano12172922
Chicago/Turabian StyleRedkin, Arkady N., Alena A. Mitina, and Eugene E. Yakimov. 2022. "Binder-Free MnO2/MWCNT/Al Electrodes for Supercapacitors" Nanomaterials 12, no. 17: 2922. https://doi.org/10.3390/nano12172922
APA StyleRedkin, A. N., Mitina, A. A., & Yakimov, E. E. (2022). Binder-Free MnO2/MWCNT/Al Electrodes for Supercapacitors. Nanomaterials, 12(17), 2922. https://doi.org/10.3390/nano12172922






