Nanostructuring Effect of Nano-CeO2 Particles Reinforcing Cobalt Matrix during Electrocodeposition Process
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Layer Systems Obtained by Electrocodeposition
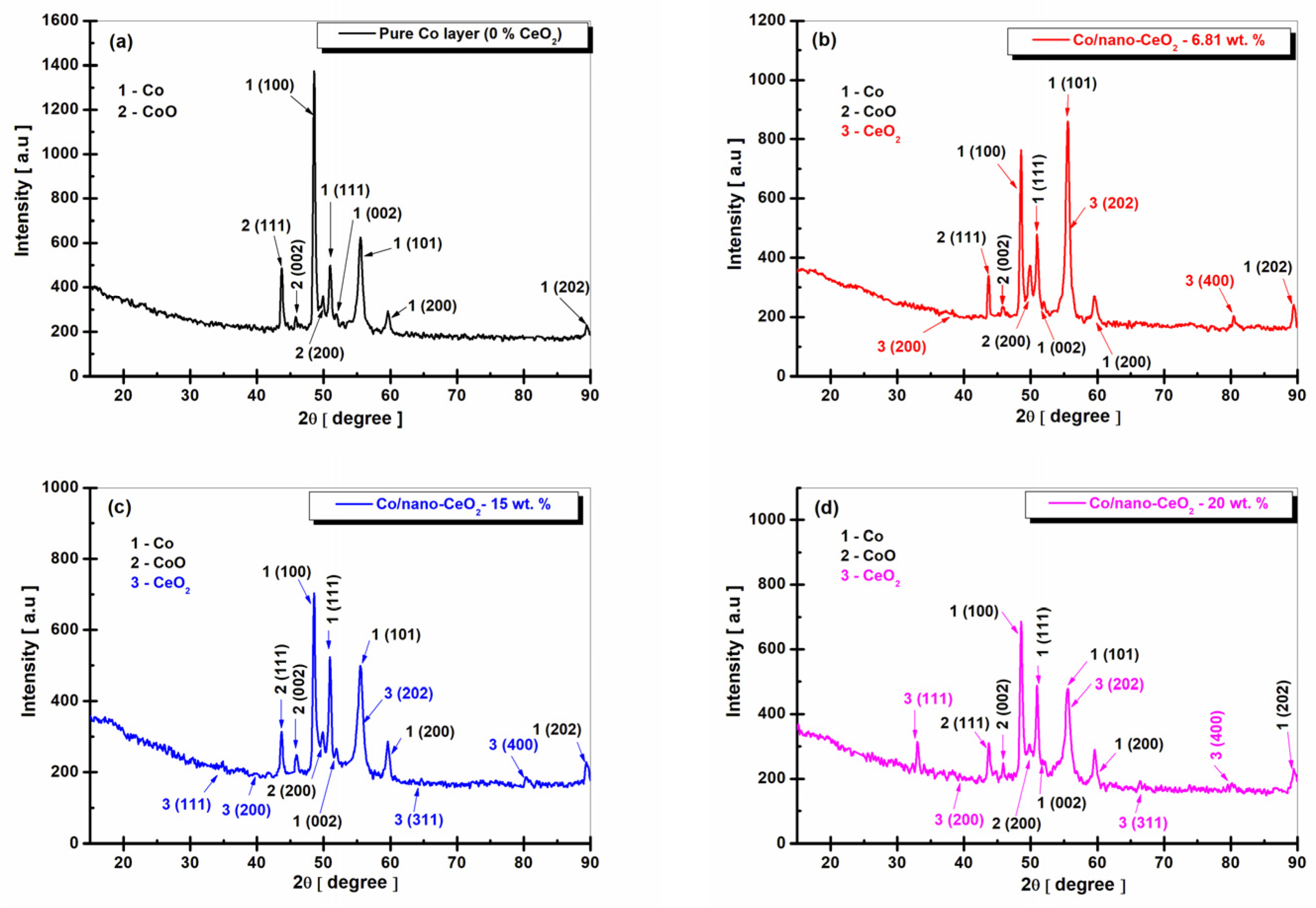
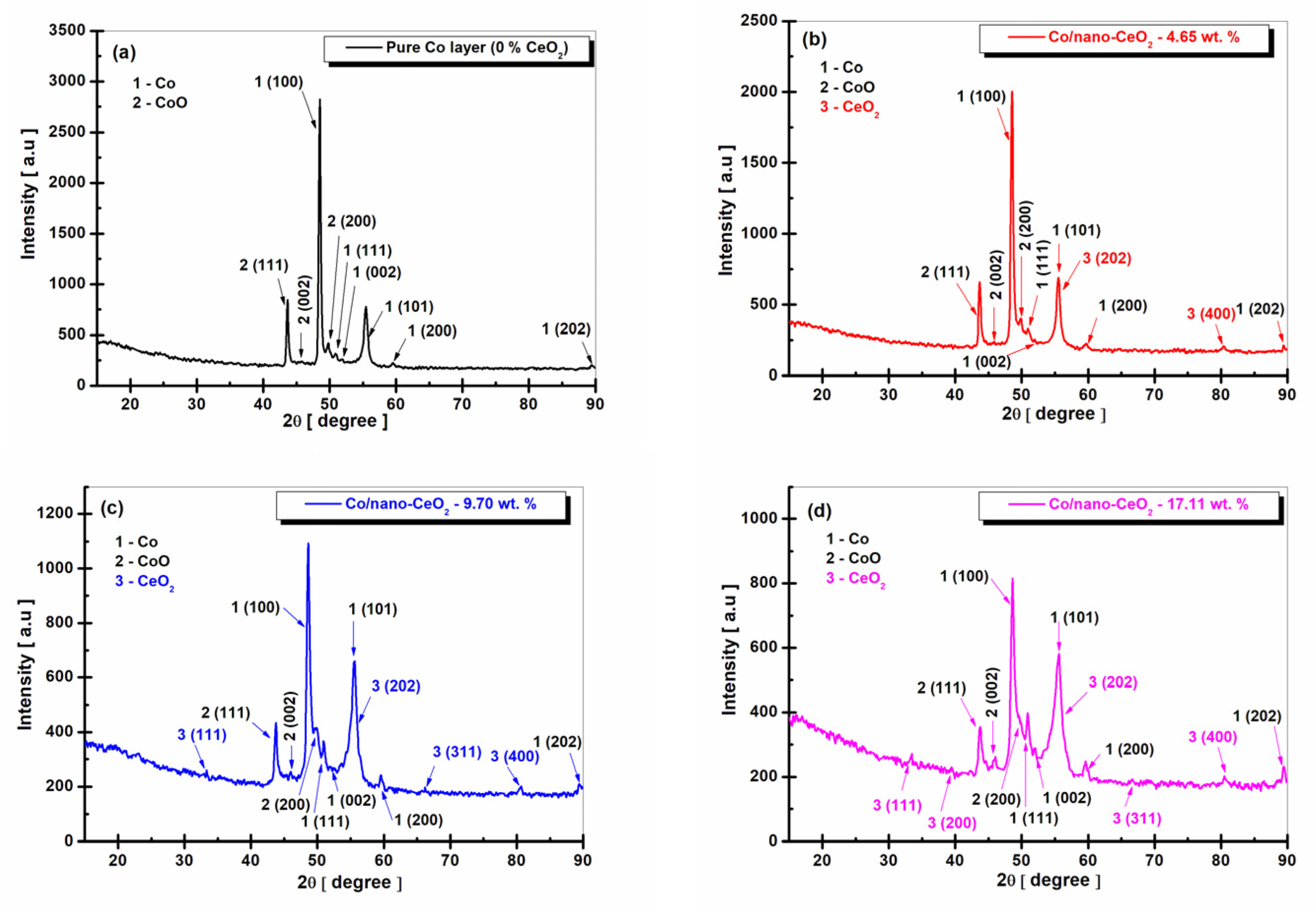
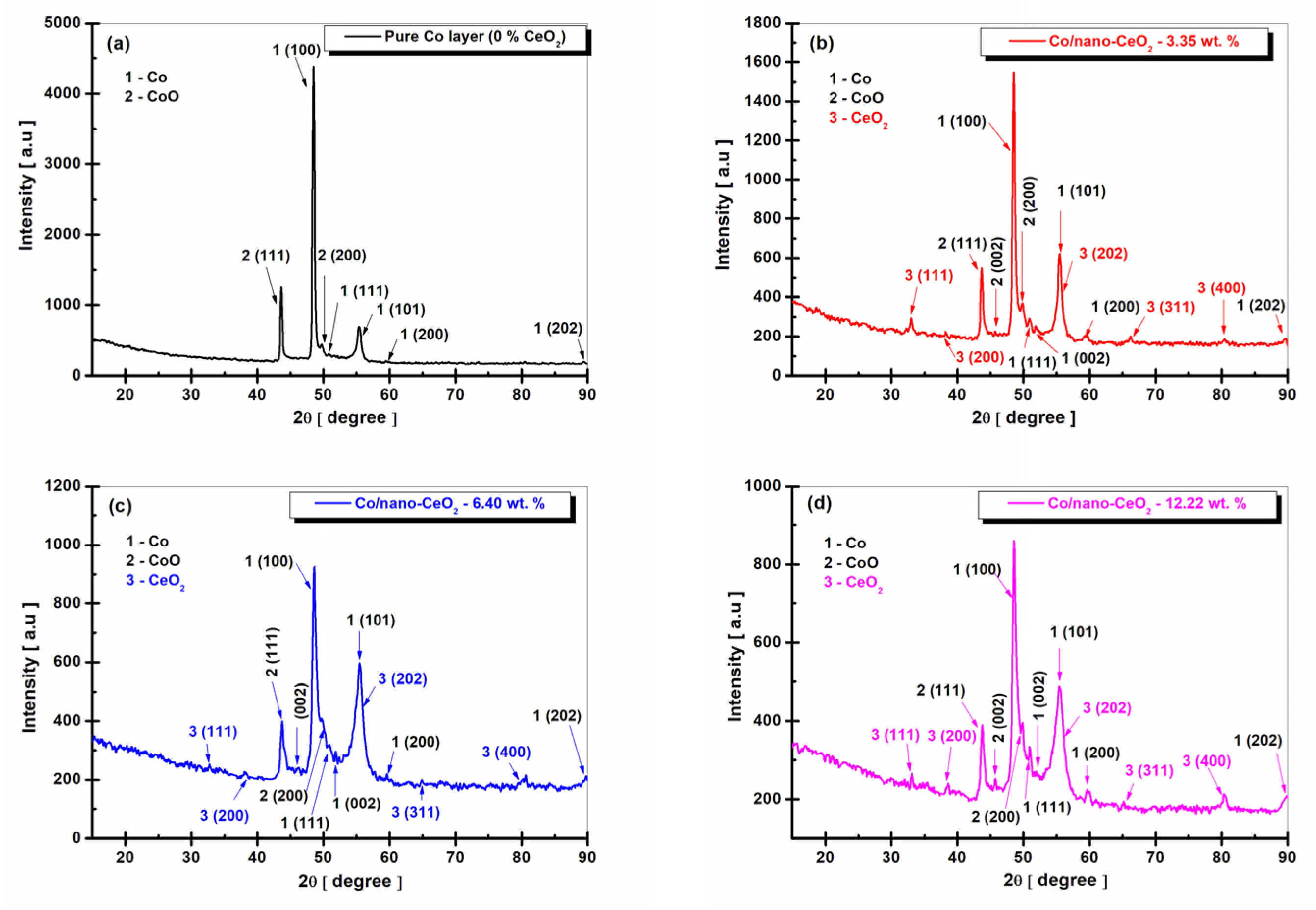
3.2. Structural Characterization of Nanocomposite Layers by XRD
3.3. Nanostructuring Effect of Nano-CeO2 Particles Embedded into the Cobalt Matrix
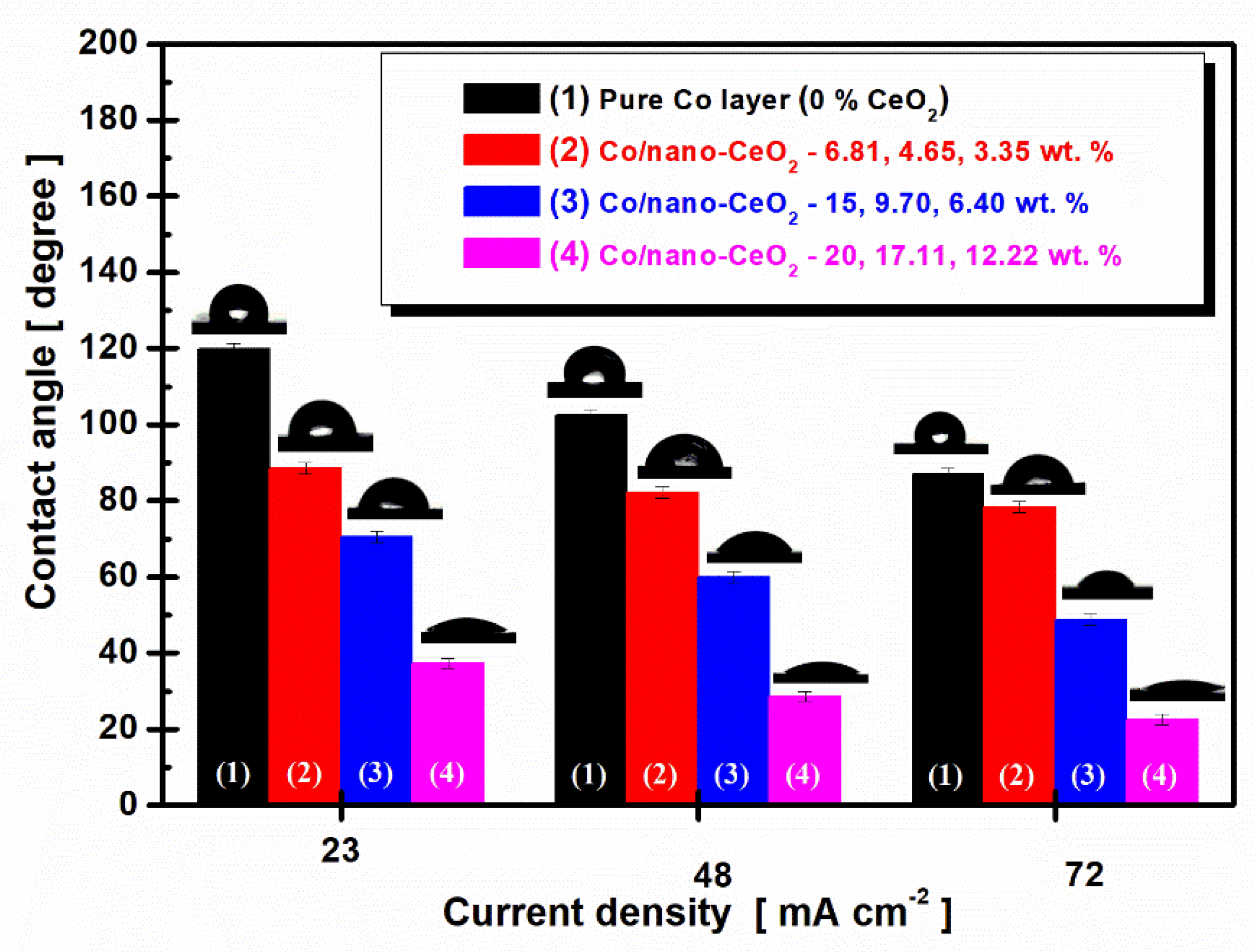
3.4. Surface Wettability and Roughness Characterization
3.5. Correlation between Crystallite Size Dimensions of Nanocomposite Layers and Wettability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, V.B.; Singh, D.K. An overview on the preparation, characterization and properties of electrodeposited-metal, matrix nanocomposites. Nanosci. Technol. 2014, 1, 1–20. [Google Scholar]
- Kaviyarasu, K.; Murmu, P.P.; Kennedy, J.; Thema, F.T.; Letsholathebe, D.; Kotsedi, L.; Maaza, M. Structural, optical and magnetic investigation of Gd implanted CeO2 nanocrystals. Nucl. Instrum. Methods Phys. Res. B 2017, 409, 147–152. [Google Scholar] [CrossRef]
- Kennedy, J.; Fang, F.; Futter, J.; Leveneur, J.; Murmu, P.P.; Panin, G.N.; Kang, T.W.; Manikandan, E. Synthesis and enhanced field emission of zinc oxide incorporated carbon nanotubes. Diam. Relat. Mater. 2017, 71, 79–84. [Google Scholar] [CrossRef]
- Abdel-Karim, R. Electrochemical synthesis of nanocomposites. In Electrodeposition of Composite Materials; Adel, M.A.M., Golden, T.D., Eds.; IntechOpen: London, UK, 2016; pp. 1–25. [Google Scholar]
- Nazari, M.H.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Thurber, C.R.; Ahmad, Y.H.; Calhoun, M.C.; Al-Shenawa, A.; D’Souza, N.; Mohamed, A.M.A.; Golden, T.D. Metal matrix composite coatings of cupronickel embedded with nanoplatelets for improved corrosion resistant properties. Int. J. Corros. 2018, 2018, 5250713. [Google Scholar] [CrossRef]
- Owen, A.; Dufès, C.; Moscatelli, D.; Mayes, E.; Lovell, J.F.; Katti, K.V.; Sokolov, K.; Mazza, M.; Fontaine, O.; Rannard, S.; et al. The application of nanotechnology in medicine: Treatment and diagnostics. Nanomedicine 2014, 9, 1291–1294. [Google Scholar] [CrossRef]
- Payal, P.P. Role of nanotechnology in electronics: A review of recent developments and patents. Recent Pat. Nanotechnol. 2022, 16, 45–66. [Google Scholar] [CrossRef]
- Ahmad, M.U.; Soukhat, M.A.; Ahmad, I. Applications of nanotechnology in pharmaceutical development. In Lipids in Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 171–190. [Google Scholar] [CrossRef]
- Vázquez, C.I.; Iglesias, R.A. Engineered nanomaterials in energy production industry. In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 713–723. [Google Scholar]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in cosmetics: Recent updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of nanotechnology in food science: Perception and overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Piracha, S.; Saleem-Momil, S.; Basharat, G.; Anjum, A.; Yaseen, Z. Nanoparticle: Role in chemical industries, potential sources and chemical catalysis applications. Sch. Int. J. Chem. Mater. Sci. 2021, 4, 40–45. [Google Scholar]
- Frenzilli, G. Nanotechnology for environmental and biomedical research. Nanomaterials 2020, 10, 2220. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Wei, S.; Taylor, M.P.; Chen, J.J.J.; Kennedy, J.V. Hybrid anode materials for rechargeable batteries—A review of Sn/TiO2 based nanocomposites. Energy Rep. 2021, 7, 2836–2848. [Google Scholar] [CrossRef]
- Panimalar, S.; Subash, M.; Chandrasekar, M.; Uthrakumar, R.; Inmozhi, C.; Al-Onazi, W.A.; Al-Mohaimeed, A.M.; Chen, T.W.; Kennedy, J.; Maaza, M.; et al. Reproducibility and long-term stability of Sn doped MnO2 nanostructures: Practical photocatalytic systems and wastewater treatment applications. Chemosphere 2022, 293, 133646. [Google Scholar] [CrossRef]
- Ahmed, S.; Ding, X.; Bao, N.; Bian, P.; Zheng, R.; Wang, Y.; Murmu, P.P.; Kennedy, J.V.; Liu, R.; Fan, H.; et al. Inducing high coercivity in MoS2 nanosheets by transition element doping. Chem. Mater. 2017, 29, 9066–9074. [Google Scholar] [CrossRef]
- Maria Magdalane, C.; Kaviyarasu, K.; Raja, A.; Arularasu, M.V.; Mola, G.T.; Abdulgalim, B.I.; Al-Dhabi, N.A.; Arasu, M.V.; Jeyaraj, B.; Kennedy, J.; et al. Photocatalytic decomposition effect of erbium doped cerium oxide nanostructures driven by visible light irradiation: Investigation of cytotoxicity, antibacterial growth inhibition using catalyst. J. Photochem. Photobiol. B Biol. 2018, 185, 275–282. [Google Scholar] [CrossRef]
- Inshakova, E.; Inshakov, O. World market for nanomaterials: Structure and trends. MATEC Web Conf. 2017, 129, 02013. [Google Scholar] [CrossRef]
- Benea, L.; Simionescu, N.; Celis, J.P. Electro-codeposition of CeO2 nanoparticles into cobalt matrix to improve the tribocorrosion performances of Co/Nano CeO2 composite layers in biological solution for medical applications. J. Mech. Behav. Biomed. Mater. 2020, 101, 103443. [Google Scholar] [CrossRef]
- Simionescu, N.; Benea, L.; Celis, J.P. Wear-corrosion response of cerium oxide reinforced cobalt hybrid composite layers in biological solution. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012003. [Google Scholar] [CrossRef]
- Firouzi-Nerbin, H.; Nasirpouri, F.; Moslehifard, E. Pulse electrodeposition and corrosion properties of nanocrystalline nickel-chromium alloy coatings on copper substrate. J. Alloys Compd. 2020, 822, 153712. [Google Scholar] [CrossRef]
- Gurrappa, I.; Binder, L. Electrodeposition of nanostructured coatings and their characterization—A review. Sci. Technol. Adv. Mater. 2008, 9, 043001. [Google Scholar] [CrossRef]
- Isil, B.; Azem, A.; Funda, N.; Mustafa, T.; Erdal, C.; Tulay, K.D.; Sidika, Y.; Onur, B.; Tuncay, D. Preparation and characterization of Ni–TiO2 nanocomposite produced by electrodeposition technique. Front. Mater. 2016, 3, 46. [Google Scholar]
- Benea, L.; Danaila, E.; Celis, J.P. Influence of electro-codeposition parameters on nano-TiO2 inclusion into nickel matrix and properties characterization of nanocomposite coatings obtained. Mater. Sci. Eng. A 2014, 10, 106–115. [Google Scholar] [CrossRef]
- Biswal, A.; Panda, P.K.; Acharya, A.N.; Tripathy, B.C.; Alenazey, F.; Jiang, Z.-T.; Sundaram, M.M. Tuning the morphology and redox behaviour by varying concentration of Fe in a CoNiFe ternary oxide heterostructure for hybrid devices. New J. Chem. 2020, 44, 9921–9932. [Google Scholar] [CrossRef]
- Biswal, A.; Panda, P.K.; Acharya, A.N.; Mohapatra, S.; Swain, N.; Tripathy, B.C.; Jiang, Z.T.; Minakshi Sundaram, M. Role of additives in electrochemical deposition of ternary metal oxide microspheres for supercapacitor applications. ACS Omega 2020, 5, 3405–3417. [Google Scholar] [CrossRef]
- Benea, L.; Danaila, E. Electrochemical codeposition of UHMWPE biopolymer into cobalt matrix for biomedical applications. Key Eng. Mater. 2016, 699, 57–62. [Google Scholar] [CrossRef]
- Benea, L.; Mardare-Pralea, M. Electrodeposition of UHMWPE particles with cobalt for biomedical applications. Dig. J. Nanomater. Biostructures 2011, 6, 1025–1034. [Google Scholar]
- Benea, L.; Ponthiaux, P.; Wenger, F. Co-ZrO2 electrodeposited composite coatings exhibiting improved micro hardness and corrosion behavior in simulating body fluid solution. Surf. Coat. Technol. 2011, 205, 5379–5386. [Google Scholar]
- Guglielmi, N. Kinetics of the deposition of inert particles from electrolytic baths. J. Electrochem. Soc. 1972, 119, 1009–1012. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal matrix composites reinforced by nano-particles—A Review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Mahdavi, S.; Allahkaram, S.R. Composition, characteristics and tribological behavior of Cr, Co–Cr and Co–Cr/TiO2 nanocomposite coatings electrodeposited from trivalent chromium based baths. J. Alloys Compd. 2015, 635, 150–157. [Google Scholar] [CrossRef]
- Sahri, Z.; Allahkaram, S.R. Study on corrosion behaviour of cobalt nano composite coatings. Iran. J. Mater. Sci. Eng. 2013, 10, 49–57. [Google Scholar]
- Gomes, A.; Pereira, I.B.; Fernandez, B.; Pereiro, R. Advances in nanocomposites—Synthesis, characterization and industrial applications. In Electrodeposition of Metal Matrix Nanocomposites: Improvement of the Chemical Characterization Techniques; Reddy, B.S.R., Ed.; IntechOpen: London, UK, 2011; pp. 1–982. [Google Scholar]
- Chang, L.M.; An, M.Z.; Guo, H.F.; Shi, S.Y. Microstructure and properties of Ni Co/nano-Al2O3 composite coatings by pulse reversal current electrodeposition. Appl. Surf. Sci. 2006, 253, 2132–2137. [Google Scholar] [CrossRef]
- Mahdavi, S.; Allahkaram, S.R. Characteristics of electrodeposited cobalt and titania nano-reinforced cobalt composite coatings. Surf. Coat. Technol. 2013, 232, 198–203. [Google Scholar] [CrossRef]
- Premlatha, S.; Sivasakthi, P.; Ramesh Bapu, G.N.K. Synthesis of Co-CeO2 nanoflake arrays and their application to highly sensitive and selective electrochemical sensing of hydrazine. J. Electroanal. Chem. 2017, 788, 107–117. [Google Scholar] [CrossRef]
- Ebrahim-Ghajari, M.; Allahkaram, S.R.; Mahdavi, S. Corrosion behaviour of electrodeposited nanocrystalline Co and Co/ZrO2 nanocomposite coatings. Surf. Eng. 2014, 31, 251–257. [Google Scholar] [CrossRef]
- Cârâc, G.; Bund, A.; Thiemig, D. Electrocodeposition and characterization of cobalt lanthanide oxides composite coatings. Surf. Coat. Technol. 2007, 202, 403–411. [Google Scholar] [CrossRef]
- Shaigan, N.; Ivey, D.G.; Chen, W. Co/LaCrO3 composite coatings for AISI 430 stainless steel solid oxide fuel cell interconnects. J. Power Sources 2008, 185, 331–337. [Google Scholar] [CrossRef]
- Ravishankar, T.N.; Ramakrishnappa, T.; Nagaraju, G.; Rajanaika, H. Synthesis and characterization of CeO2 nanoparticles via solution combustion method for photocatalytic and antibacterial activity studies. ChemistryOpen 2015, 4, 146–154. [Google Scholar] [CrossRef]
- Mandoli, C.; Pagliari, F.; Pagliari, S.; Forte, G.; Di Nardo, P.; Licoccia, S.; Traversa, E. Stem cell aligned growth induced by CeO2 nanoparticles in PLGA scaffolds with improved bioactivity for regenerative medicine. Adv. Funct. Mater. 2010, 20, 1617–1624. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Monteiro-Riviere, N.A.; Aggarwal, R.; Davis, J.P.; Narayan, R.J.; Self, W.T.; McGinnis, J.; Seal, S. Nanoceria as antioxidant: Synthesis and biomedical applications. JOM 2008, 60, 33–37. [Google Scholar] [CrossRef]
- Benea, L. Electrochemical impedance spectroscopy and corrosion behavior of Co/CeO2 nanocomposite coatings in simulating body fluid solution. Metall. Mater. Trans. A 2013, 44, 1114–1122. [Google Scholar] [CrossRef]
- Lin, W.; Huang, Y.; Zhou, X.D.; Ma, Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int. J. Toxicol. 2006, 25, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Kumari, S.I.; Kamal, S.S.K.; Grover, P. Genotoxicity assessment of cerium oxide nanoparticles in female Wistar rats after acute oral exposure. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 775–776, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Simionescu-Bogatu, N.; Benea, L. Effect of the applied current density and deposition time on electro-codeposition process of cobalt matrix reinforced with nano-CeO2. Arch. Metall. Mater. 2022, 67, 615–622. [Google Scholar]
- Matveev, V.; Baranov, D.A.; Yurkov, G.Y.; Akatiev, N.G.; Dotsenko, I.P.; Gubin, S.P. Cobalt nanoparticles with preferential hcp structure: A confirmation by X-ray diffraction and NMR. Chem. Phys. Lett. 2006, 422, 402–405. [Google Scholar] [CrossRef]
- Heidari, F.; Irankhah, A. Effect of surfactants and digestion time on nanocrystalline cerium oxide characteristics synthesized by differential precipitation. Ceram. Int. 2014, 40, 12655–12660. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Zarinkamar, M.; Firoozabadi, T.P. Synthesis of Cerium Oxide (CeO2) nanoparticles using simple CO-precipitation method. Rev. Mex. Fis. 2016, 62, 496–499. [Google Scholar]
- Efemov, V.A. Turnarea si Cristalizarea Otelurilor; Editura Tehnica: Bucuresti, Romania, 1976; pp. 1–552. [Google Scholar]
- Oniciu, L.; Constantinescu, E. Electrochimie si Coroziune; Editura Didactica si Pedagogica: Bucuresti, Romania, 1982; pp. 1–319. [Google Scholar]
- Yao, G.; Zhang, M.; Lv, J.; Xu, K.; Shi, S.; Gong, Z.; Tao, J.; Jiang, X.; Yang, L.; Cheng, Y. Effects of electrodeposition electrolyte concentration on microstructure, optical properties and wettability of ZnO nanorods. J. Electrochem. Soc. 2015, 162, D300–D304. [Google Scholar] [CrossRef]
- Sobolev, A.; Valkov, A.; Kossenko, A.; Wolicki, I.; Zinigrad, M.; Borodianskiy, K. Bioactive coating on Ti alloy with high osseointegration and antibacterial Ag nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39534–39544. [Google Scholar] [CrossRef]
- Eraković, S.; Janković, A.; Veljović, D.; Palcevskis, E.; Mitrić, M.; Stevanović, T.; Mišković-Stanković, V. Corrosion stability and bioactivity in simulated body fluid of silver/hydroxyapatite and silver/hydroxyapatite/lignin coatings on titanium obtained by electrophoretic deposition. J. Phys. Chem. B 2012, 117, 1633–1643. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Frank, K.; Alexander, G.; Max, H.; Ebker, T. Impact of dental implant surface modifications on osseointegration. Biomed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. A 2005, 74, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Wolansky, G.; Marmur, A. Apparent contact angles on rough surfaces: The Wenzel equation revisited. Colloids Surf. A Physicochem. Eng. Asp. 1999, 156, 381–388. [Google Scholar] [CrossRef]




| Characteristics | Technical Specifications |
|---|---|
| Molecular mass | 172.11 g/mol |
| Color | White-pale, yellow pal |
| Morphology | Nanopowder |
| The specific size of the surface | 35–55 m2/g |
| Particle size | <25 nm |
| Density at 20 °C | 7.22 g/cm2 |
| Melting point | 2340 °C |
| Boiling point | 3500 °C |
| Amount of Dispersed Nano-CeO2 Added to Plating Electrolyte | Current Density | Amount of Dispersed Nano-CeO2 Added to Plating Electrolyte |
|---|---|---|
| 0 g/L CeO2 | 23 | 0 |
| 48 | 0 | |
| 72 | 0 | |
| 10 g/L CeO2 | 23 | 6.81 ± 0.2 |
| 48 | 4.65 ± 0.3 | |
| 72 | 3.35 ± 0.1 | |
| 20 g/L CeO2 | 23 | 15.0 ± 0.4 |
| 48 | 9.70 ± 0.1 | |
| 72 | 6.40 ± 0.2 | |
| 30 g/L CeO2 | 23 | 20.0 ± 0.1 |
| 48 | 17.11 ± 0.2 | |
| 72 | 12.22 ± 0.3 |
| Surface Studied | Current Density [mA/cm2] | Ra [µm] |
|---|---|---|
| Pure Co layer (0% CeO2) | 23 | 0.142 ± 0.002 |
| 48 | 0.207 ± 0.004 | |
| 72 | 0.297 ± 0.001 | |
| Co/nano-CeO2—10 g/L layer (6.81, 4.65, and 3.35 wt. %) | 23 | 0.346 ± 0.006 |
| 48 | 0.421 ± 0.003 | |
| 72 | 0.504 ± 0.005 | |
| Co/nano-CeO2—20 g/L layer (15, 9.70, and 6.40 wt. %) | 23 | 0.609 ± 0.001 |
| 48 | 0.693 ± 0.003 | |
| 72 | 0.755 ± 0.009 | |
| Co/nano-CeO2—30 g/L layer (20, 17.11, and 12.22 wt. %) | 23 | 0.849 ± 0.004 |
| 48 | 0.991 ± 0.007 | |
| 72 | 1.233 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoleta, B.; Lidia, B.; Daniela-Laura, B.; Vasile, B.; Jean-Pierre, C. Nanostructuring Effect of Nano-CeO2 Particles Reinforcing Cobalt Matrix during Electrocodeposition Process. Nanomaterials 2022, 12, 2923. https://doi.org/10.3390/nano12172923
Nicoleta B, Lidia B, Daniela-Laura B, Vasile B, Jean-Pierre C. Nanostructuring Effect of Nano-CeO2 Particles Reinforcing Cobalt Matrix during Electrocodeposition Process. Nanomaterials. 2022; 12(17):2923. https://doi.org/10.3390/nano12172923
Chicago/Turabian StyleNicoleta, Bogatu, Benea Lidia, Buruiană Daniela-Laura, Bașliu Vasile, and Celis Jean-Pierre. 2022. "Nanostructuring Effect of Nano-CeO2 Particles Reinforcing Cobalt Matrix during Electrocodeposition Process" Nanomaterials 12, no. 17: 2923. https://doi.org/10.3390/nano12172923
APA StyleNicoleta, B., Lidia, B., Daniela-Laura, B., Vasile, B., & Jean-Pierre, C. (2022). Nanostructuring Effect of Nano-CeO2 Particles Reinforcing Cobalt Matrix during Electrocodeposition Process. Nanomaterials, 12(17), 2923. https://doi.org/10.3390/nano12172923







