An Overview of Recent Progress in Nanofiber Membranes for Oily Wastewater Treatment
Abstract
:1. Introduction
2. An Overview of Nanofiber Production Techniques
3. Parameters of Nanofiber Membrane Affecting the Oil/Water Separation Performance
4. Thin-Film Composite Nanofiber Membrane for Oil/Water Separation
5. Nanomaterials in Nanofiber Membrane for Oil/Water Separation
6. Sustainable Development of Nanofiber Membrane for Oil/Water Separation
7. Conclusions and Remarks for Future Directions
- Most of the oil/water emulsions tested in laboratories comprise two components. However, the real oily wastewaters discharged from numerous industries may contain abundant organic and inorganic compounds. These compounds may induce the nanofiber membranes to perform differently than the findings obtained from the binary mixtures. Some the organic compounds can swell the polymeric nanofibers and, eventually, may alter the nanofiber membrane properties. Investigation of using the real oily wastewaters in fouling and swelling could be an attractive topic in future research.
- Membrane surface modification to produce super wetting properties can improve the oil removal efficiencies. However, the preparation of the modified nanofiber membranes involves sophisticated procedures. Significant types of chemicals are expensive. Natural and sustainable resources with simple techniques for modified nanofiber membrane preparation are recommended in future studies.
- Most current oil/water separation studies use simple gravity-driven filtration systems and the membrane sizes are approximately 40–50 cm in diameter. To manage the large volumes of the oily wastewaters discharged from industries, a large-scale filtration system that can run for long-term operation is required.
- Modelling studies on oily wastewater and even oil/water separation using nanofiber membranes are hardly found in the literature. A vigorous model, which can accurately predict the nanofiber membrane performance, is required when scaling up the filtration system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheryan, M. Ultrafiltration and Microfiltration Handbook, 2nd ed.; Taylor & Francis: New York, NY, USA, 1998; p. 376. [Google Scholar]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, E.S.; Anokhina, T.S.; Novitsky, E.G.; Volkov, V.V.; Borisov, I.L.; Volkov, A.V. Polymeric membranes for oil-water separation: A review. Polymers 2022, 14, 980. [Google Scholar] [CrossRef] [PubMed]
- Choong, L.T.; Lin, Y.M.; Rutledge, G.C. Separation of oil-in-water emulsions using electrospun fibre membranes and modelling of the fouling mechanism. J. Membr. Sci. 2015, 15, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Bilad, M.R.; Azizo, A.S.; Wirzal, M.D.H.; Jia, L.J.; Putra, Z.A.; Nordin, N.A.H.M.; Mavukkandy, M.O.; Jasni, M.J.F.; Yusoff, A.R.M. Tackling membrane fouling in microalgae filtration using nylon 6,6 nanofiber membrane. J. Environ. Manag. 2018, 223, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Foong, C.Y.; Wirzal, M.D.H.; Bustam, M.A. A review on nanofibers membrane with amino-based ionic liquid for heavy metal removal. J. Mol. Liq. 2020, 297, 111793. [Google Scholar] [CrossRef]
- Saleem, H.; Trabzon, L.; Kilic, A.; Zaidi, S.J. Recent advances in nanofibrous membranes: Production and applications in water treatment and desalination. Desalination 2020, 478, 114178. [Google Scholar] [CrossRef]
- Pan, T.; Liu, J.; Deng, N.; Li, Z.; Wang, L.; Xia, Z.; Fan, J.; Liu, Y. ZnO nanowires@PVDF nanofiber membrane with superhydrophobicity for enhanced anti-wetting and anti-scaling properties in membrane distillation. J. Membr. Sci. 2021, 612, 118877. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Wei, L.; Sun, R.; Liu, C.; Xiong, J.; Qin, X. Mass production of nanofibers from needleless electrospinning by a novel annular spinneret. Mater. Des. 2019, 179, 107885. [Google Scholar] [CrossRef]
- Ellison, C.J.; Phatak, A.; Giles, D.W.; Macosko, C.W.; Bates, F.S. Melt blown nanofibers: Fiber diameter distributions and onset of fiber breakup. Polymer 2007, 48, 3306–3316. [Google Scholar] [CrossRef]
- Ma, H.; Zeng, J.; Realff, M.L.; Kumar, S.; Schiraldi, D.A. Processing, structure, and properties of fibers from polyester/carbon nanofiber composites. Compos. Sci. Technol. 2003, 63, 1617–1628. [Google Scholar] [CrossRef]
- Xing, X.; Wang, Y.; Li, B. Nanofiber drawing and nanodevice assembly in poly(trimethylene terephthalate). Opt. Express 2008, 16, 10815–10822. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Yagi, S.; Meng, J.; Dong, Y.; Qian, C.; Zhao, D.; Kumar, A.; Xu, T.; Lucchetti, A.; Xu, H. High-efficiency production of core-sheath nanofiber membrane via co-axial electro-centrifugal spinning for controlled drug release. J. Membr. Sci. 2022, 654, 120571. [Google Scholar] [CrossRef]
- Ji, X.; Li, R.; Liu, G.; Jia, W.; Sun, M.; Liu, Y.; Luo, Y.; Cheng, Z. Phase separation-based electrospun Janus nanofibers loaded with Rana chensinensis skin peptides/silver nanoparticles for wound healing. Mate. Des. 2021, 207, 109864. [Google Scholar] [CrossRef]
- Vakhrushev, A.Y.; Boitsova, T.B. TiO2 and TiO2/Ag nanofibers: Template synthesis, structure, and photocatalytic properties. J. Porous Mater. 2021, 28, 1023–1030. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Qu, R.; Geng, X.; Kong, X.; Sun, C.; Ji, C.; Wang, Y. Ag-coordinated self-assembly of aramid nanofiber-silver nanoparticle composite beads for selective mercury removal. Sep. Purif. Technol. 2022, 282, 120147. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Q.; Hua, D.; Xiong, R.; Zhao, J.; Rao, W.; Huang, S.; Zhan, X.; Chen, F.; Huang, C. Electrospun fibers for oil-water separation. RSC Adv. 2016, 6, 12868. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, D.; Xu, X.; Li, H.; Mao, T.; Zheng, C.; Huang, J.; Yang, H.; Niu, Z.; Wu, X. A polypropylene melt-blown strategy for the facile and efficient membrane separation of oil-water mixtures. Chin. J. Chem. Eng. 2021, 29, 383–390. [Google Scholar] [CrossRef]
- Xu, D.; Zheng, X.; Xiao, R. Hydrophilic nanofibrous composite membrane prepared by melt-blending extrusion for effective separation of oil/water emulsion. RSC Adv. 2017, 7, 7108–7115. [Google Scholar] [CrossRef] [Green Version]
- Kerwald, J.; de Moura Junior, C.F.; Freitas, E.D.; de Moraes Segundo, J.D.P.; Vieira, R.S.; Beppu, M.M. Cellulose-based electrospun nanofibers: A review. Cellulose 2022, 29, 25–54. [Google Scholar] [CrossRef]
- Patil, N.A.; Gore, P.M.; Prakash, N.J.; Govindaraj, P.; Yadav, R.; Shanmugarajan, D.; Patil, S.; Kore, A.; Kandasubramanian, B. Needleless electrospun phytochemicals encapsulated nanofiber based 3-ply biodegradble mask for combating COVID-19 pandemic. Chem. Eng. J. 2021, 416, 129152. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Yeom, B.Y.; Wilkie, A.; Pourdeyhimi, B.; Khan, S.A. Fabrication of nanofiber meltblown membranes and their filtration properties. J. Membr. Sci. 2013, 427, 336–344. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Pypeć, K.; Irska, I.; Piesowicz, E. Functional polymer hybrid nanocomposites based on polyolefins: A review. Processes 2020, 8, 1475. [Google Scholar] [CrossRef]
- Garg, T.; Rath, G.; Goyal, A.K. Biomaterials-based nanofiber scaffold: Targeted and controlled carrier for cell and drug delivery. J. Drug Target. 2015, 23, 202–221. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Lu, B.; Mei, S.; Xu, Q.; Liu, F. Research on parametric model for polycaprolactone nanofiber produced by centrifugal spinning. J. Braz. Soc. Mech. Sci. 2018, 40, 196. [Google Scholar] [CrossRef]
- Kuchi, C.; Harish, G.S.; Reddy, P.S. Effect of polymer concentration, needle diameter and annealing temperature on TiO2-PVP composite nanofibers synthesized by electrospinning technique. Ceram. Int. 2018, 44, 5266–5272. [Google Scholar] [CrossRef]
- Wu, S.H.; Qin, X.H. Uniaxially aligned polyacrylonitrile nanofiber yarns prepared by a novel modified electrospinning method. Mater. Lett. 2013, 106, 204–207. [Google Scholar] [CrossRef]
- Mansouri, S.; Sheikholeslami, T.F.; Behzadmehr, A. Investigation on the electrospun PVDF/NP-ZnO nanofibers for application in environmental energy harvesting. J. Mater. Res. Technol. 2019, 8, 1608–1615. [Google Scholar] [CrossRef]
- Jatoi, A.W. Polyurethane nanofibers incorporated with ZnAg composite nanoparticles for antibacterial wound dressing applications. Compos. Commun. 2020, 19, 103–107. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.; Garcia-Cela, E.; Magan, N.; Rahatekar, S.S. Electrospinning alginate/polyethylene oxide and curcumin composite nanofibers. Mater. Lett. 2020, 270, 127662. [Google Scholar] [CrossRef]
- Song, X.; Gao, J.; Zheng, N.; Zhou, N.; Mai, Y.W. Interlaminar toughening in carbon fiber/epoxy composites interleaved with CNT-decorated polycaprolactone nanofibers. Compos. Commun. 2021, 24, 100622. [Google Scholar] [CrossRef]
- Mohandesnezhad, S.; Pilehvar-Soltanahmadi, Y.; Alizadeh, E.; Goodarzi, A.; Davaran, S.; Khatamian, M.; Zarghami, N.; Samiei, M.; Aghazadeh, M.; Akbarzadeh, A. In vitro evaluation of Zeolite-nHA blended PCL/PLA nanofibers for dental tissue engineering. Mater. Chem. Phys. 2020, 252, 123152. [Google Scholar] [CrossRef]
- Homaeigohar, S.S.; Buhr, K.; Ebert, K. Polyethersulfone electrospun nanofibrous composite membrane for liquid filtration. J. Membr. Sci. 2010, 365, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Sayed, M.M.; Mousa, H.M.; El-Aassar, M.R.; El-Deeb, N.M.; Ghazaly, N.M.; Dewidar, M.M.; Abdal-hay, A. Enhancing mechanical and biodegradable properties of polyvinyl alcohol/silk fibroin nanofibers composite patches for Cardiac Tissue Engineering. Mater. Lett. 2019, 255, 126510. [Google Scholar] [CrossRef]
- Šišková, A.O.; Frajová, J.; Nosko, M. Recycling of poly(ethylene terephthalate) by electrospinning to enhanced the filtration efficiency. Mater. Lett. 2020, 278, 128426. [Google Scholar] [CrossRef]
- Neibolts, N.; Platnieks, O.; Gaidukovs, S.; Barkane, A.; Thakur, V.K.; Filipova, I.; Mihai, G.; Zelca, Z.; Yamaguchi, K.; Enachescu, M. Needle-free electrospinning of nanofibrillated vellulose and graphene nanoplatelets based sustainable poly(butylene succinate) nanofibers. Mater. Today Chem. 2020, 17, 100301. [Google Scholar] [CrossRef]
- Kundrat, V.; Vykoukal, V.; Moravec, Z.; Simonikova, L.; Novotny, K.; Pinkas, J. Preparation of polycrystalline tungsten nanofibers by needleless electrospinning. J. Alloys Compd. 2022, 900, 163542. [Google Scholar] [CrossRef]
- Partheniadis, I.; Athanasiou, K.; Laidmäe, I.; Heinämäki, J.; Nikolakakis, I. Physicomechanical characterization and tablet compression of theophylline nanofibrous mats prepared by conventional and ultrasound enhanced electrospinning. Int. J. Pharm. 2022, 616, 121558. [Google Scholar] [CrossRef]
- Wei, L.; Liu, C.; Dong, L.; Fan, X.; Zhi, C.; Sun, R. Process investigation of nanofiber diameter based on linear needleless spinneret by response surface methodology. Polym. Test. 2022, 110, 107577. [Google Scholar] [CrossRef]
- Erben, J.; Klicova, M.; Klapstova, A.; Háková, M.; Lhotská, I.; Zatrochová, S.; Šatínský, D.; Chvojka, J. New polyamide 6 nanofibrous sorbents produced via alternating current electrospinning for the on-line solid phase extraction of small molecules in chromatography systems. Microchem. J. 2022, 174, 107084. [Google Scholar] [CrossRef]
- Klicova, M.; Oulehlova, Z.; Klapstova, A.; Hejda, M.; Krejcik, M.; Noval, O.; Mullerova, J.; Erben, J.; Rosendorf, J.; Palek, R.; et al. Biomimetic hierarchical nanofibrous surfaces inspired by superhydrophobic lotus leaf structure for preventing tissue adhesions. Mater. Des. 2022, 217, 110661. [Google Scholar] [CrossRef]
- Morel, A.; Domaschke, S.; Kumaran, V.U.; Alexeev, D.; Sadeghpour, A.; Ramakrishna, S.N.; Ferguson, S.J.; Rossi, R.M.; Mazza, E.; Ehret, A.E.; et al. Correlating diameter, mechanical and structural properties of poly(L-lactide) fibres from needless electrospinning. Acta Biomater. 2018, 81, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Li, T.T.; Zhang, X.; Shiu, B.C.; Zhang, Y.; Ren, H.T.; Peng, H.K.; Lin, J.H.; Lou, C.W. In situ growth polydopamine decorated polypropylene melt-blown membrane for highly efficient oil/water separation. Chemosphere 2020, 254, 126873. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.O.; Im, J.N.; Park, W.H. Morphological and permeable properties of antibacterial double-layered composite nonwovens consisting of microfibers and nanofibers. Compos. B. Eng. 2015, 75, 256–263. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Macosko, C.W.; Bates, F.S. Nanofibers from water-extractable melt-blown immiscible polymer blends. Polymer 2016, 101, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Yesil, Y.; Bhat, G.S. Strcuture and mechanical properties of polyethylene melt blown nonwovens. Int. J. Cloth. Sci. Technol. 2016, 28, 780–793. [Google Scholar] [CrossRef]
- Deng, N.; He, H.; Yan, J.; Zhao, Y.; Ticha, E.B.; Liu, Y.; Kang, W.; Cheng, B. One-step melt-blowing of multi-scale micro/nano fabric membrane for advanced air-filtration. Polymer 2019, 165, 174–179. [Google Scholar] [CrossRef]
- Yu, Y.; Xiong, S.; Huang, H.; Zhao, L.; Nie, K.; Chen, S.; Xu, J.; Yin, X.; Wang, H.; Wang, L. Fabrication and application of poly(phenylene sulfide) ultrafine fiber. React. Funct. Polym. 2020, 150, 104539. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.; Andrade-Guel, M.; Medellin-Banda, D.I.; Ávila-Orta, C.A.; Cadenas-Pliego, G.; Sáenz-Galindo, A.; Radillo-Radillo, R.; Lara-Sánchez, J.F.; Melo-Lopez, L. Non-woven fabrics based on Nylon 6/carbon black-graphene nanoplatelets obtained by melt-blowing for adsorption of urea, uric acid and creatinine. Mater. Lett. 2022, 320, 132382. [Google Scholar] [CrossRef]
- Feng, J. Preparation and properties of poly(lactic acid) fiber melt blown non-woven disordered mats. Mater. Lett. 2017, 189, 180–183. [Google Scholar] [CrossRef]
- Lin, C.A.; Ku, T.H. Shear and elongational flow properties of thermoplastic polyvinyl alcohol melts with different plasticizer contents and degrees of polymerization. J. Mater. Process. Technol. 2008, 200, 331–338. [Google Scholar] [CrossRef]
- Zeng, J.; Saltysiak, B.; Johnson, W.S.; Schiradi, D.A.; Kumar, S. Processing and properties of poly(methyl methacrylate)/carbon nanofiber composites. Compos. B. Eng. 2004, 35, 245–249. [Google Scholar] [CrossRef]
- Li, M.; Xue, X.; Wang, D.; Lu, Y.; Wu, Z.; Zou, H. High performance filtration nanofibrous membranes based on hydrophilic poly(vinyl alcohol-co-ethylene) copolymer. Desalination 2013, 329, 50–56. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, R. Preparation and characterization of CNTs/PE micro-nanofibers. Polym. Adv. Technol. 2012, 23, 508–515. [Google Scholar] [CrossRef]
- Li, M.; Xiao, R.; Sun, G. Preparation of polyester nanofibers and nanofiber yarns from polyester/cellulose acetate butyrate immiscible polymer blends. J. Appl. Polym. Sci. 2012, 142, 28–36. [Google Scholar] [CrossRef]
- Xu, T.C.; Han, D.H.; Zhu, Y.M.; Duan, G.G.; Liu, K.M.; Hou, H.Q. High strength electrospun single copolyacrylonitirle (coPAN) nanofibers with improved molecular orientation by drawing. Chinese J. Polym. Sci. 2021, 39, 174–180. [Google Scholar] [CrossRef]
- Wang, J.; Langhe, D.; Ponting, M.; Wnek, G.E.; Korley, L.T.J.; Baer, E. Manufacturing of polymer continuous nanofibers using a novel co-extrusion and multiplication technique. Polymer 2014, 55, 673–685. [Google Scholar] [CrossRef]
- Bajáková, J.; Chaloupek, J.; Lukáš, D.; Lacarin, M. Drawing–The Production of Individual Nanofibers by Experimental Method. In Proceedings of the 3rd International Conference on Nanotechnology-Smart Materials, Brno, Czech Republic, 21–23 September 2011. [Google Scholar]
- Akia, M.; Rodriguez, C.; Materon, L.; Gilkerson, R.; Lozano, K. Antibacterial activity of polymeric nanofiber membranes impregnated with Texas sour orange juice. Eur. Polym. J. 2019, 115, 1–5. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Garza, D.D.; Gallegos, L.; Pokhrel, M.; Alcoutlabi, M. Forcespinning: A new method for the mass production of Sn/C composite nanofiber anodes for lithium ion batteries. Solid State Ion. 2016, 286, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Upson, S.J.; O’Haire, T.; Russell, S.J.; Dalgarno, K.; Ferrerira, A.M. Centrifugally spun PHBV micro and nanofibers. Mater. Sci. Eng. C 2017, 76, 190–195. [Google Scholar] [CrossRef]
- Wang, L.; Shi, J.; Liu, L.; Secret, E.; Chen, Y. Fabrication of polymer fiber scaffolds by centrifugal spinning for cell culture studies. Microelectron. Eng. 2011, 88, 1718–1721. [Google Scholar] [CrossRef]
- Loordhuswamy, A.M.; Krishnaswamy, V.R.; Korrapati, P.S.; Thinakaran, S.; Rengaswami, G.D.V. Fabrication of highly aligned fibrous scaffolds for tissue regeneration by centrifugal spinning technology. Mater. Sci. Eng. C 2014, 42, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Papenburg, B.J.; Bolhuis-Versteeg, L.A.M.; Grijpma, D.W.; Feijen, J.; Wessling, M.; Stamatialis, D. A facile method to fabricate poly(L-lactide) nanofibrous morphologies by phase inversion. Acta Biomater. 2010, 6, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wu, Y.; Hu, J.; Wang, P.; Liu, G.; Lin, S.; Tu, Y. Hierarchical aramid nanofibrous membranes from a nanofiber-based solvent-induced phase inversion process. J. Membr. Sci. 2019, 578, 16–26. [Google Scholar] [CrossRef]
- Tao, S.L.; Desai, T.A. Aligned arrays of biodegradable poly(ε-caprolactone) nanowires and nanofibers by template sunthesis. Nano Lett. 2007, 7, 1463–1468. [Google Scholar] [CrossRef]
- Ikegame, M.; Tajima, K.; Aida, T. Template synthesis of polypyrrole nanofibers insulated within one-dimensional silicate channels: Hexagonal versus lamellar for recombination of polarons into bipolarons. Angew. Chem. Int. Ed. 2003, 42, 2154–2157. [Google Scholar] [CrossRef]
- Koh, E.; Lee, Y.T. Preparation of an omniphobic nanofiber membrane by the self-assembly of hydrophobic nanoparticles for membrane distillation. Sep. Purif. Technol. 2021, 259, 118134. [Google Scholar] [CrossRef]
- Weir, N.; Stevens, B.; Wagner, S.; Miles, A.; Ball, G.; Howard, C.; Chemmarappally, J.; McGinnity, M.; Hargreaves, A.J.; Tinsley, C. Aligned poly-L-lactic acid nanofibers induce self-assembly of primary cortical neurons into 3D cell clusters. ACS Biomater. Sci. Eng. 2022, 8, 765–776. [Google Scholar] [CrossRef]
- Yang, G.; Gong, J.; Yang, R.; Guo, H.; Wang, Y.; Liu, B.; Dong, S. Modification of electrode surface through electrospinning followed by self-assembly multilayer film of polyoxometalate and its photochromic. Electrochem. Commun. 2006, 8, 790–796. [Google Scholar] [CrossRef]
- Formhals, A. Process and Apparatus for Preparing Artificial Threads. US Patent 1975504, 2 October 1934. [Google Scholar]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibres. J. Electrostatic. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Grafe, T.; Graham, K. Polymeric nanofibers and nanofiber webs: A new class of nonwovens. Int. Nonwovens J. 2003, 12, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Banik, B.L.; Brown, J.L. Polymeric Biomaterials in Nanomedicine. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 387–395. [Google Scholar]
- Reneker, D.H.; Chun, I. Nanometer diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Fridrikh, S.V.; Yu, J.H.; Brenner, M.P.; Rutledge, G.C. Controlling the fibre diameter during electrospinning. Phys. Rev. Lett. 2003, 90, 144502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heikkilä, P.; Harlin, A. Parameter study of electrospinning of polyamide-6. Eur. Polym. J. 2008, 44, 3067–3079. [Google Scholar] [CrossRef]
- Tsou, S.Y.; Lin, H.S.; Wang, C. Studies on the electrospun Nylon 6 nanofibers from polyelectrolyte solutions: 1. Effects of solution concentration and temperature. Polymer 2011, 52, 3127–3136. [Google Scholar] [CrossRef]
- Filip, P.; Peer, P. Characterization of poly(ethylene oxide) nanofibers–Mutual relations between mean diameter of electrospun nanofibers and solution characteristics. Processes 2019, 7, 948. [Google Scholar] [CrossRef] [Green Version]
- Topuz, F.; Satilmis, B.; Uyar, T. Electrospinning of uniform nanofibers of polymers of intrinsic microporosity (PIM-1): The influence of solution conductivity and relative humidity. Polymer 2019, 178, 121610. [Google Scholar] [CrossRef]
- Cai, Y.; Gevelber, M. The effect of relative humidity and evaporation rate on electrospinning: Fiber diameter and measurement for control implications. J. Mater. Sci. 2013, 48, 7812–7826. [Google Scholar] [CrossRef]
- Cramariuc, B.; Cramariuc, R.; Scarlet, R.; Manea, L.R.; Lupu, I.G.; Cramariuc, O. Fiber diameter in electrospinning process. J. Electrostat. 2013, 71, 189–198. [Google Scholar] [CrossRef]
- McCulloch, J.G. The history of the development of melt blowing technology. Int. Nonwovens J. 1999, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wehmann, M.; McCulloh, W.J.G. Melt blowing technology. In Polypropylene; Karger-Kocsis, J., Ed.; Springer: Dordrecht, The Netherlands, 1999; Volume 2, pp. 415–420. [Google Scholar]
- Shah, A.A.; Yoo, Y.; Park, A.; Cho, Y.H.; Park, Y.I.; Park, H. Poly(ethylene-co-vinyl alcohol) electrospun nanofiber membranes for gravity-driven oil/water separation. Membranes 2022, 12, 382. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Guo, R.; Pei, H.; Wang, B.; Liu, N.; Mo, Z. Electrospinning PAN/PEI/MWCNT-COOH nanocomposite fiber membrane with excellent oil-in-water separation and heavy metal ion adsorption capacity. Colloids Surf. A. Physicochem. Eng. Asp. 2022, 641, 128557. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Luo, P.; Li, S.; Nie, Y.; Zhong, F.; Wang, Y.; Chen, L. Photocatalytic GO/M88A “interceptor plate” assembled nanofibrous membrane with photo-Feton self-cleaning performance for oil/water emulsion separation. Chem. Eng. J. 2022, 427, 130948. [Google Scholar] [CrossRef]
- Bae, J.; Baek, I.; Choi, H. Mechanically enhanced PES electrospun nanofiber membranes (ENMs) for microfiltration: The effects of ENM properties on membrane performance. Water Res. 2016, 105, 406–412. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.; Li, W.; Xing, J.; Xu, L.; He, J. Humidity-induced porous poly(lactic acid) membrane with enhanced flux for oil-water separation. Adsorp. Sci. Technol. 2019, 37, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Shahabadi, S.M.S.; Mousavi, S.A.; Bastani, D. High flux electrospun nanofiberous membrane: Preparation by statistical approach, characterization, and microfiltration assessment. J. Taiwan Inst. Chem. Eng. 2016, 50, 474–483. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Li, B.; Hsiao, B.S.; Chu, B. Electrospun nanofibrous membranes for high flux microfiltration. J. Membr. Sci. 2012, 392–393, 167–174. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.N.W.; Jamalludin, M.R.; Adam, M.R.; Ismail, N.H.; Jaafar, J.; Harun, Z.; Ismail, A.F. Electrospinning parameters evaluation of PVDF-ZnO/Ag2CO3/Ag2O composite nanofiber affect on porosity by using response surface methodology. Mater. Today: Proc. 2021, 46, 1824–1830. [Google Scholar] [CrossRef]
- Li, L.; Xu, Z.; Sun, W.; Chen, J.; Dai, C.; Yan, B.; Zeng, H. Bio-inspired membrane with adaptable wettability for smart oil/water separation. J. Membr. Sci. 2020, 598, 117661. [Google Scholar] [CrossRef]
- Xu, Z.; Li, L.; Liu, J.; Dai, C.; Sun, W.; Chen, J.; Zhu, Z.; Zhao, M.; Zeng, H. Mussel-inspired superhydrophilic membrane constructed on a hydrophilic polymer network for highly efficient oil/water separation. J. Colloid Interf. Sci. 2022, 608, 702–710. [Google Scholar] [CrossRef]
- Jiang, Y.; Hou, J.; Xu, J.; Shan, B. Switchable oil/water separation with efficient and robust Janus nanofiber membranes. Carbon 2017, 115, 477–485. [Google Scholar] [CrossRef]
- Mousa, H.M.; Fahmy, H.S.; Abouzeid, R.; Abdel-Jaber, G.T.; Ali, W.Y. Polyvinylidene fluoride-cellulose nanocrystals hybrid nanofiber membrane for energy harvesting and oil-water separation applications. Mater. Lett. 2022, 306, 130965. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, R.; Li, Y.; Sun, B.; Li, Y.; Zhang, N.; Qiu, J.; Li, X.; Wang, C. Robust and durable superhydrophobic electrospun nanofibrous mats via a simple Cu nanocluster immobilization for oil-water contamination. Colloids Surf. A 2018, 538, 173–183. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Li, C.; Liu, R. Porous nanofibrous superhydrophobic membrane with embedded Au nanoparticles for the integration of oil/water separation and catalytic degradation. J. Membr. Sci. 2019, 582, 350–357. [Google Scholar] [CrossRef]
- Lin, Y.M.; Rutledge, G.C. Separation of oil-in-water emulsions stabilized by different types of surfactants using electrospun fiber membranes. J. Membr. Sci. 2018, 563, 247–258. [Google Scholar] [CrossRef]
- You, X.; Liao, Y.; Tian, M.; Chew, J.W.; Wang, R. Engineering highly effective nanofibrous membranes to demulsify surfactant-stabilized oil-in-water emulsions. J. Membr. Sci. 2020, 611, 118398. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Li, H.; Zhang, C.; Xue, J.; Wang, R.; Qiao, X.; Xue, Q. Enhancing oil-in-water emulsion separation performance of polyvinyl alcohol hydrogel nanofibrous membrane by squeezing coalescence demulsification. J. Membr. Sci. 2021, 630, 119324. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, R.; Pu, W.; Liu, R.; Fang, S. Coalescence and separation of surfactant-stabilized water-in-oil emulsion via membrane coalesce functionalized by demulsifier. J. Clean. Prod. 2022, 330, 129945. [Google Scholar] [CrossRef]
- Nunes, S.P.; Sforça, M.L.; Peinemann, K.L. Dense hydrophilic composite membranes for ultrafiltration. J. Membr. Sci. 1995, 106, 49–56. [Google Scholar] [CrossRef]
- Ju, H.; McCloskey, B.D.; Sagle, A.C.; Wu, Y.H.; Kusuma, V.A.; Freeman, B.D. Crosslinked poly(ethylene oxide) fouling resistant coating materials for oil/water separation. J. Membr. Sci. 2008, 307, 260–267. [Google Scholar] [CrossRef]
- Yoon, K.; Kim, K.; Wang, X.; Fang, D.; Hsiao, B.S.; Chu, B. High flux ultrafiltration membranes based on electrospun nanofibrous PAN scaffolds and chitosan coating. Polymer 2006, 47, 2434–2441. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. High flux ultrafiltration nanofibrous membranes based on polyacrylonitrile electrospun scaffolds and crosslinked polyvinyl alcohol coating. J. Membr. Sci. 2009, 338, 145–152. [Google Scholar] [CrossRef]
- Mulyati, S.; Takagi, R.; Fujii, A.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Improvement of the antifouling potential of an anion exchange membrane by surface modification with a polyelectrolyte for an electrodialysis process. J. Membr. Sci. 2012, 417–418, 137–143. [Google Scholar] [CrossRef]
- Wu, J.; Wei, W.; Li, S.; Zhong, Q.; Liu, F.; Zheng, J.; Wang, J. The effect of membrane surface charges on demulsification and fouling resistance during emulsion separation. J. Membr. Sci. 2018, 563, 126–133. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Li, H.; Xue, J.; Ma, C.; Yin, Y.; Qiao, X.; Sun, D.; Xue, Q. Multifunctional charged hydrogel nanofibrous membranes for metal ions contained emulsified oily wastewater purification. J. Membr. Sci. 2021, 621, 118950. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, Y.J.; Yang, H.; Xu, Z.L. Super-wetting, photoactive TiO2 coating on amino-silane modified PAN nanofiber membranes for high efficient oil-water emulsion separation application. J. Membr. Sci. 2019, 580, 40–48. [Google Scholar] [CrossRef]
- Moatmed, S.M.; Khedr, M.H.; El-dek, S.I.; Kim, H.Y.; El-Deen, A.G. Highly efficient and reusable superhydrophobic/superoleophilic polystyrene@ Fe3O4 nanofiber membrane for high-performance oil/water separation. J. Environ. Chem. Eng. 2019, 7, 103508. [Google Scholar] [CrossRef]
- Jiang, S.; Meng, X.; Chen, B.; Wang, N.; Chen, G. Electrospinning superhydrophobic-superoleophilic PVDF-SiO2 nanofibers membrane for oil-water separation. J. Appl. Polym. Sci. 2020, 137, 49546. [Google Scholar] [CrossRef]
- He, B.; Ding, Y.; Wang, J.; Yao, Z.; Qing, W.; Zhang, Y.; Liu, F.; Tang, C.Y. Sustaining fouling resistant membranes: Membrane fabrication, characterization and mechanism understanding of demulsification and fouling-resistance. J. Membr. Sci. 2019, 581, 105–113. [Google Scholar] [CrossRef]
- Obaid, M.; Tolba, G.M.K.; Motlak, M.; Fadali, O.A.; Khalil, K.A.; Almajid, A.A.; Kim, B.; Barakat, N.A.M. Effective polysulfone-amorphous SiO2 NPs electrospun nanofiber membrane for high flux oil/water separation. Chem. Eng. J. 2015, 279, 631–638. [Google Scholar] [CrossRef]
- Liang, Y.; Kim, S.; Kallem, P.; Choi, H. Capillary effect in Janus electrospun nanofiber membrane for oil/water emulsion separation. Chemosphere 2019, 221, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Tian, M.; Wang, R. A high-performance and robust membrane with switchable superwettability for oil/water separation under ultralow pressure. J. Membr. Sci. 2017, 543, 123–132. [Google Scholar] [CrossRef]
- Zhao, H.; He, Y.; Wang, Z.; Zhao, Y.; Sun, L. Mussel-inspired fabrication of PDA@PAN electrospun nanofibrous membrane for oil-in-water emulsion separation. Nanomaterials 2021, 11, 3434. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Ma, W.; Lu, T.; Xiong, R.; Huang, C. Multifunctional nanofibrous membranes with sunlight-driven self-cleaning performance for complex oily wastewater remediation. J. Colloid Interface Sci. 2022, 608, 164–174. [Google Scholar] [CrossRef]
- Huang, L.; Arena, J.T.; Manickam, S.S.; Jiang, X.; Willis, B.G.; McCutcheon, J.R. Improved mechanical properties and hydrophilicity of electrospun nanofiber membranes for filtration applications by dopamine modification. J. Membr. Sci. 2014, 460, 241–249. [Google Scholar] [CrossRef]
- Ding, Y.; Wu, J.; Wang, J.; Lin, H.; Wang, J.; Liu, G.; Pei, X.; Liu, F.; Tang, C.Y. Superhydrophilic and mechanical robust PVDF nanofibrous membrane through facile interfacial Span 80 welding for excellent oil/water separation. Appl. Surf. Sci. 2019, 485, 179–187. [Google Scholar] [CrossRef]
- Huo, L.; Luo, J.; Huang, X.; Zhang, S.; Gao, S.; Long, B.; Gao, J. Superhydrophobic and anti-ultraviolet polymer nanofiber composite with excellent stretchability and durability for efficient oil/water separation. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 603, 125224. [Google Scholar] [CrossRef]
- Gao, J.; Li, B.; Wang, L.; Huang, X.; Xue, H. Flexible membranes with a hierarchical nanofiber/microsphere structure for oil adsorption and oil/water separation. J. Ind. Eng. Chem. 2018, 68, 416–424. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, M.; Liu, Z.; Kang, M.; Huang, C.; Fu. Fabrication of highly durable and robust superhydrophobic-superoleophilic nanofibrous membranes based on a fluorine-free system for efficient oil/water separation. J. Membr. Sci. 2019, 570–571, 303–313. [Google Scholar] [CrossRef]
- Sadeghi, I.; Govinna, N.; Cebe, P.; Asatekin, A. Superoleophilic, mechanically strong electrospun membranes for fast and efficient gravity-driven oil/water separation. ACS Appl. Polym. Mater. 2019, 1, 756–776. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Ma, W.; Wu, S.; Tang, G.; Cui, J.; Zhang, Q.; Chen, F.; Xiong, R.; Huang, C. Electrospun frogspawn structured membrane for gravity-driven oil-water separation. J. Colloid Interface Sci. 2019, 547, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Qing, W.; Shi, X.; Deng, Y.; Zhang, W.; Wang, J.; Tang, C.Y. Robust superhydrophobic-superoleophilic polytetrafluoroethylene nanofibrous membrane for oil/water separation. J. Membr. Sci. 2017, 540, 354–361. [Google Scholar] [CrossRef]
- Sun, F.; Li, T.T.; Ren, H.; Jiang, Q.; Peng, H.K.; Lin, Q.; Lou, C.W.; Lin, J.H. PP/TiO2 melt-blown membranes for oil/water separation and photocatalysis: Manufacturing techniques and property evaluations. Polymers 2019, 11, 775. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, L.; Zhang, H.; Long, X.; Zheng, Y.; Zuo, Y.; Jiao, F. Biomimetic modified polypropylene membranes based on tea polyphenols for efficient oil/water separation. Prog. Org. Coat. 2022, 164, 106723. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Cao, L.; Wang, Y.; Li, L.; Li, W. Electrospun PVDF-SiO2 nanofibrous membranes with enhanced surface roughness for oil-water coalescence separation. Sep. Purif. Technol. 2021, 269, 118726. [Google Scholar] [CrossRef]
- Islam, M.S.; McCutcheon, J.R.; Rahaman, M.S. A high flux polyvinyl acetate-coated electrospun nylon 6/SiO2 composite microfiltration membrane for the separation of oil-in-water emulsion with improved antifouling performance. J. Membr. Sci. 2017, 537, 297–309. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Pan, Y.; Di, H.; Zeng, G.; Zhang, L.; Zhang, C. A modified mussel-inspired method to fabricate TiO2 decorated superhydrophilic PVDF membrane for oil/water separation. J. Membr. Sci. 2016, 506, 60–70. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, Z.; Yang, H.; Wang, G.; Gu, L.; Xia, L.; Zeng, Z.; Zhu, L. Mussel-inspired hydrophilic modification of polypropylene membrane for oil-in-water emulsion separation. Surf. Coat. Technol. 2020, 403, 126375. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Zhang, Q.; Chen, W.; Lin, S.; Wang, Z.; Li, W. Enhanced coalescence separation of oil-in-water emulsions using electrospun PVDF nanofibers. Chin. J. Chem. Eng. 2021, 38, 76–83. [Google Scholar] [CrossRef]
- Sarkar, J.; Mridha, D.; Sarkar, J.; Orasugh, J.T.; Gangopadhyay, B.; Chattopadhyay, D.; Roychowdhury, T.; Acharya, K. Synthesis of nanosilica from agricultural wastes and its multifaceted applications: A review. Biocatal. Agric. Biotechnol. 2021, 37, 102175. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, J.H.; Lee, J.W.; Lee, H.S.; Chang, J.H.; Sang, B.I. Preparation of high purity silica originated from rice husks by chemically removing metallic impurities. J. Ind. Eng. Chem. 2017, 50, 79–85. [Google Scholar] [CrossRef]
- Chun, J.; Lee, J.H. Recent progress on the development of engineered silica particles derived from rice husk. Sustainability 2020, 12, 10683. [Google Scholar] [CrossRef]
- Falk, G.; Shinhe, G.P.; Teixeira, L.B.; Moraes, E.G.; Novaes de Oliveira, A.P. Synthesis of silica nanoparticles from sugarcane bagasse ash and nano-silica via magnesiothermic reactions. Ceram. Int. 2019, 45, 21618–21624. [Google Scholar] [CrossRef]
- Dirna, F.C.; Rahayu, I.; Maddu, A.; Darmawan, W.; Nandika, D.; Prihatini, E. Nanosilica synthesis from betung bamboo sticks and leaves by ultrasonication. Nanotechnol. Sci. Appl. 2020, 13, 131–136. [Google Scholar] [CrossRef]
- Imoisili, P.E.; Ukoba, K.O.; Jen, T.C. Green technology extraction and charaterisation of silica nanoparticles from palm kernel shell ash via sol-gel. J. Mater. Res. Technol. 2020, 9, 307–313. [Google Scholar] [CrossRef]
- Sajjadi, M.; Ahmadpoor, F.; Nasrollahzadeh, M.; Ghafuri, H. Lignin-derived (nano)materials for environmental pollution remediation: Current challenges and future perspectives. Int. J. Biol. Macromol. 2021, 178, 394–423. [Google Scholar] [CrossRef]
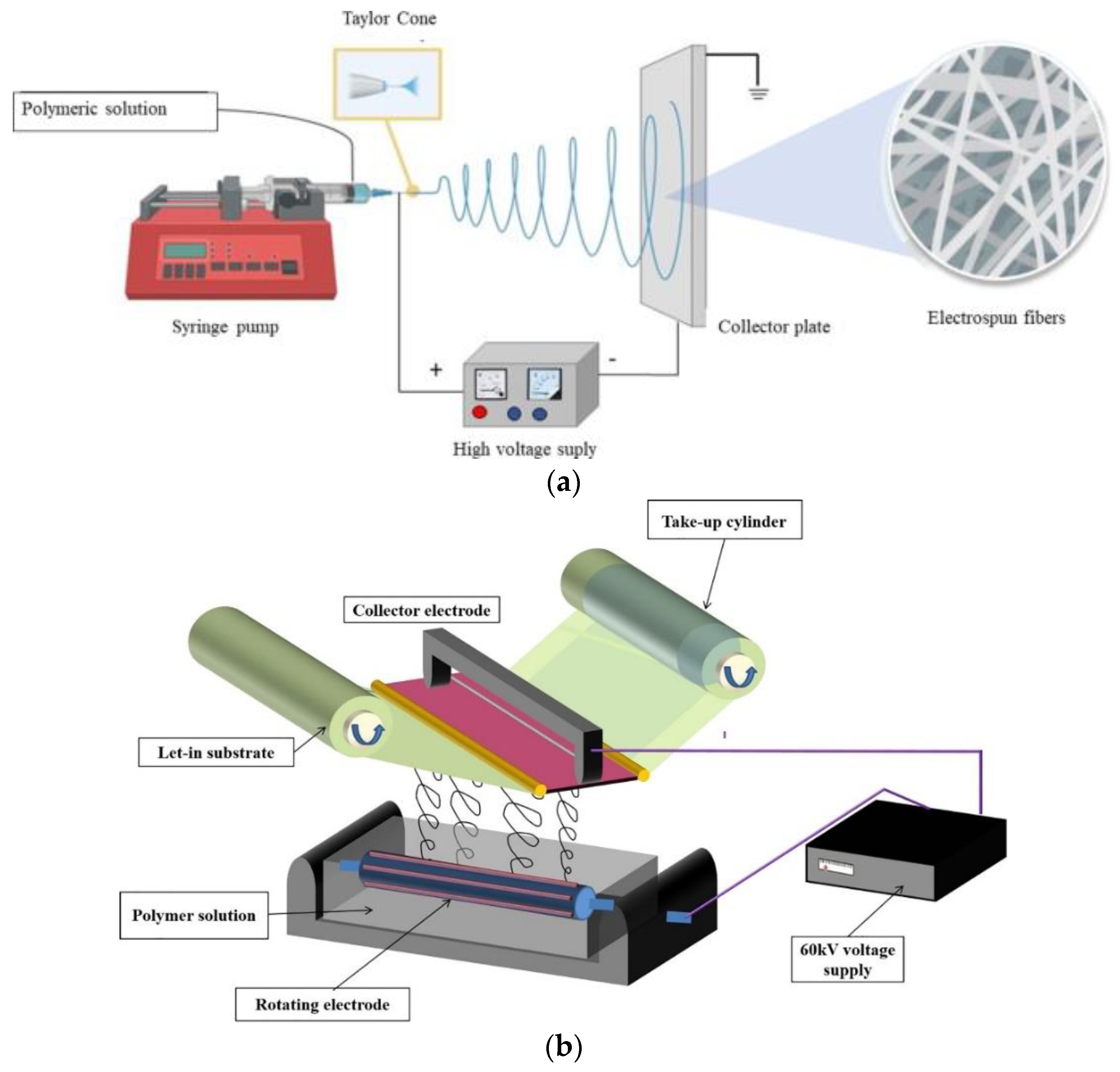
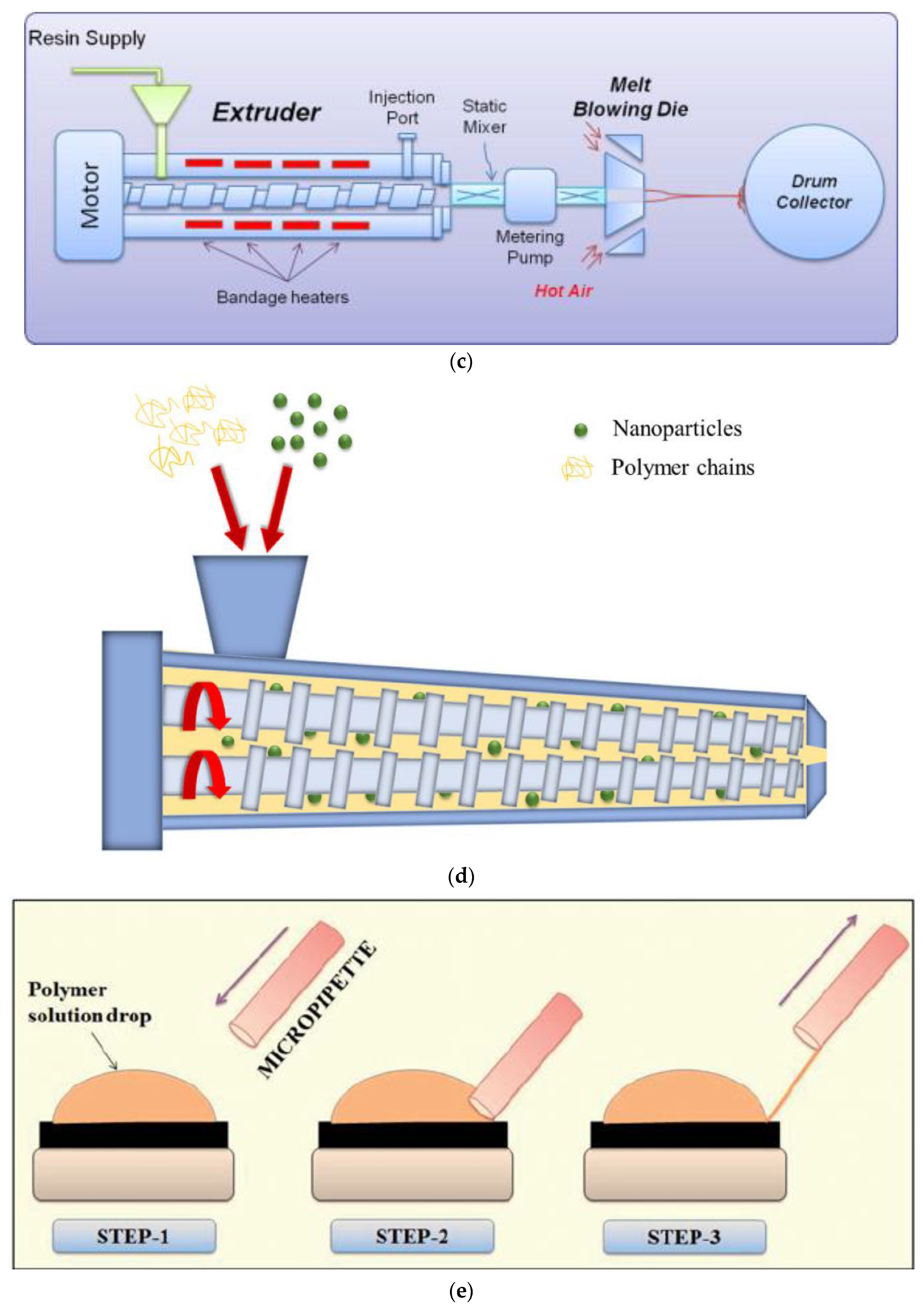
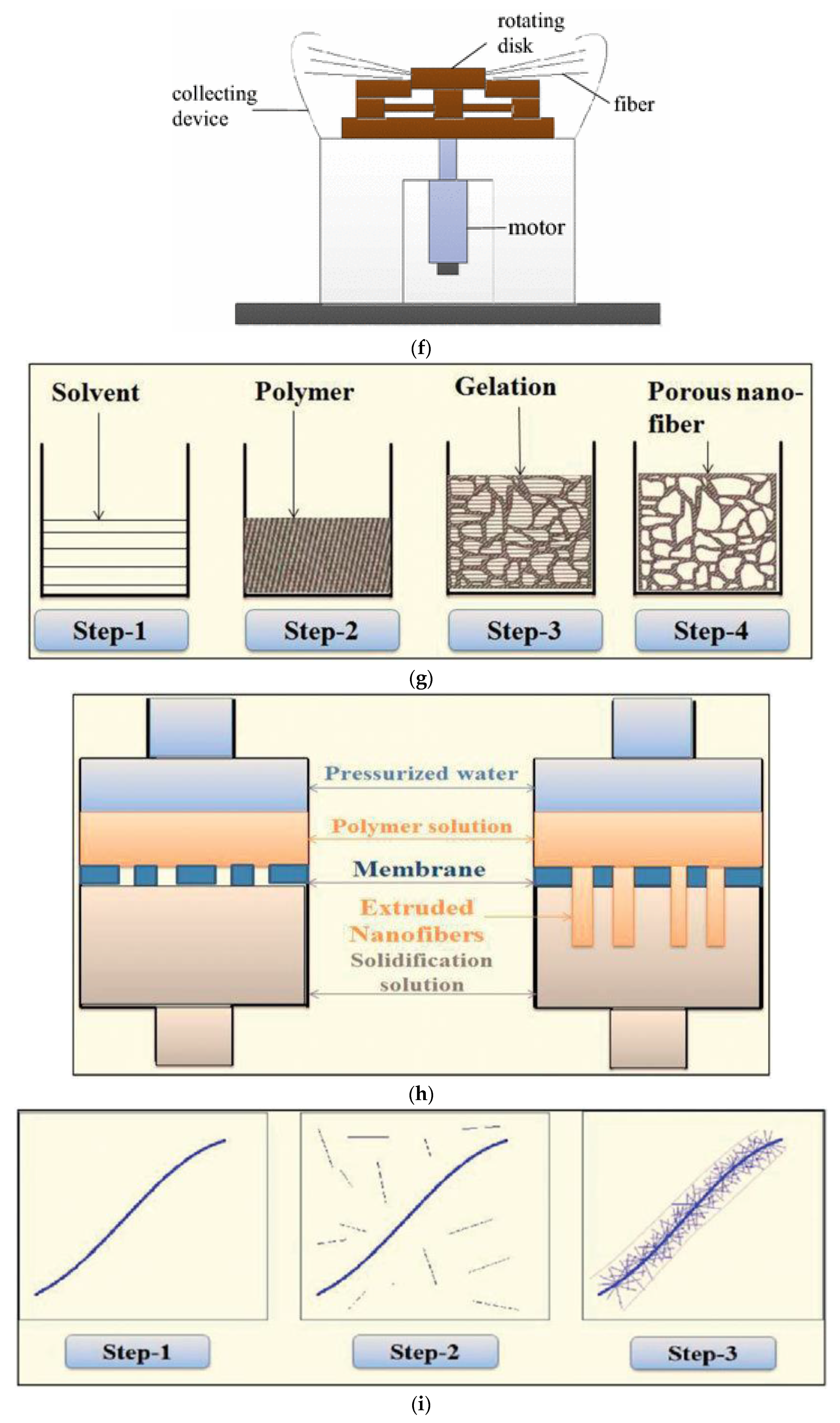
| Technique | Polymeric Material | Reference |
|---|---|---|
| Needle electrospinning | PVP, PAN, PVDF, PU, PEO, PLA, PCL, PES, Nylon 6, PSU, PVA, PET | [27,28,29,30,31,32,33,34,35,36] |
| Needleless electrospinning | PBS, PVA, EPS, PEO, PAN, PA, PCL, PLLA | [37,38,39,40,41,42,43] |
| Melt-blowing | PP, PU, PBT, PE, PS, PPS, Nylon 6, PLLA, TPVA | [44,45,46,47,48,49,50,51,52] |
| Melt-blending extrusion | PMMA, EVOH, PE, PET, PTT, PBT | [20,53,54,55,56] |
| Drawing | PAN, PCL, PEO, PET, PA, PVA, PVB, PMMA, HA, | [57,58,59] |
| Centrifugal force spinning | PVA, PLLA, Nylon 6, PAN, PHBV, PLGA, PS, PCL | [14,60,61,62,63,64] |
| Phase inversion | PLLA, PPTA | [65,66] |
| Template synthesis | PCL, PPy | [67,68] |
| Self-assembly | PA, PLLA, PAH, POM | [69,70,71] |
| Technique | Advantages | Disadvantages |
|---|---|---|
| Needle electrospinning | Scalable, feasible of fiber dimension control, fibers are long and continuous | Solvent recovery issues, low productivity, instable jetting, high voltage requirement |
| Needleless electrospinning | Scalable, feasible of fiber dimension control, fibers are long and continuous, high productivity | Solvent recovery issues, high voltage requirement |
| Melt-blowing | Scalable, feasible of fiber dimension control, fibers are long and continuous, high productivity, solvent recovery is not required | Number of suitable polymers is limited, high temperature requirement |
| Melt-blending extrusion | Scalable, feasible of fiber dimension control, fibers are long and continuous, high productivity, solvent recovery is not required | Number of suitable polymers is limited, high temperature requirement |
| Drawing | Simple process | Low scalability, incapable of fiber dimension control, discontinuous process |
| Centrifugal force spinning | Scalable, feasible of fiber dimension control, high voltage is not required | Require high temperature |
| Phase inversion | Simple equipment | Low scalability, incapable of fiber dimension control, limited to selective polymers |
| Template synthesis | Easy to modify the fiber diameter by using different size of template | Complex process |
| Self-assembly | Easy to obtain smaller nanofibers | Low scalability, incapable of fiber dimension control, complex process |
| Base Polymer | Nanomaterials | Wettability | Oil/Water System | Oil Content in Water | Filtration Mode | (L/m2 h) | (%) | Findings | Reference |
|---|---|---|---|---|---|---|---|---|---|
| PVDF | P(MMA-r-FDMA) | Highly hydrophobic and superoleophilic WCA: 140 ± 5° OCA: <1° UWOCA: ~0° in ~0.6 s | Dodecane/water Dichloromethane/water | 1:1 volume ratio | Gravity-driven | 2500–3000 a | - | Enhanced up to 7 times higher Young’s modulus; exhibited up to 17 times faster permeation of oil and organic solvent; highly stable and excellent fouling resistant during a 70 min continuous oil/water separation filtration; flux was 24 times higher than the pristine PVDF. | [125] |
| PI | SNPs (avg. size 7–40 nm) | Superhydrophobic and superoleophilic WCA: 155.75° OCA: <10° | Dichloromethane/water 1,2-dichloromethane/water Trichloromethane/water Carbon tetrachloride/water Bromobenzene/water | 50%, v/v | Gravity-driven | >4400 | 98.81 99.36 99.55 98.07 98.40 | Mimicked to a frogspawn structure; high resistance to damages due to high temperature (150 °C), acid/basic conditions and organic/inorganic solvents; the permeate flux greater than 4400 L/m2 h after 20 separation cycles. | [126] |
| PVDF | SNPs (avg, dia. 20 nm) | Superhydrophobic and superoleophilic WCA: 150.0 ± 1.5° OCA: 0° | Hexane/water Petroleum/water Vegetable oil/water Vacuum pump oil/water | 1:1 volume ratio | Gravity-driven | 1857 ± 101 | 99 | Excellent multi-cycle performance and stable chemical resistance. | [113] |
| PI | SNPs (avg. size 7–40 nm) | Superhydrophobic and superoleophilic WCA: >154° OCA: ~0° in 30 s UWOCA: <20° | Dichloromethane/water Trichloromethane/waterDichloroethane/water Bromobenzene/water Carbon tetrachloride/water | 1:1 volume ratio | Gravity-driven | 4798 | >99 | A fluorine-free membrane dip-coated and in situ crosslinked with PBZ; superhydrophobicity was maintained after immersing in either acidic or alkaline aqueous solutions for 24 h; superhydrophobicity was maintained within 350 °C; high salt tolerance; good recyclability after 20 separation cycles; oil content in the permeate below 5 ppm | [124] |
| PVA | PTFE NPs (size ~200 nm) | Superhydrophobic and superoleophilic WCA: 155° SA: 5.1° | Chloroform/water | 1:1 volume ratio | Gravity-driven | 1215 | - | Tensile strength was as high as 19.7 MPa compared with pristine PVA-PTFE at 7.5 MPa; superhydrophobicity was maintained after exposure to both acidic and alkaline solution for 2 h, and after 30 cycles of abrasion test. | [127] |
| PAN | Ag, Cu nanocluster | Superhydrophobic and superoleophilic WCA: 147.6–154.6° SA: 8.0° | Heavy oil mixture: Chloroform/water Light oil mixtures: Motor oil/water Diesel/water Toluene/water | 1:1 volume ratio | Gravity-driven | - | >99.40 b>98.50 c | The PAN-Cu-Sh-120 membrane exhibited WCA greater than 150° after immersed in different NaCl concentration solutions for up to 7 days; no change in weight before and after ultrasonic treatment which indicated the adhesion strength of copper nanocluster to PAN was strong; elongation at break decreased from 26.07 to 11.79% after electroless deposition Cu. | [98] |
| PP | PDA/APTES | Superhydrophilic and underwater superoleophobic WCA: 0° UWOCA: >150° | Petroleum ether/water Toluene/water | 50:50 volume ratio | Gravity-driven | 186,477.5 202,935.5 | >99 d | PDA created nano-scale roughness on the fiber; APTES improved the adhesion or interactions between the PDA coatings and PP; breaking elongation reduced from 52% to 36% when the basis weight of PP membrane increased. | [44] |
| PP | TiO2 | Hydrophobic and superlipophilic WCA: 130–140° OCA: 0° | Kerosene/water Hexane/water Petroleum ether/water Toluene/water | 1:1 volume ratio | Gravity-driven | 14,789–15,410 | 95–98 | TiO2 enhanced the thermostability of PP; thermal decomposition temperature was proportional to the content of TiO2 which the temperatures were 180–230 °C; remained stable after 6 h ultraviolet irradiation; retained the oil/water separation capability even after 100 repeated test. | [128] |
| PP | TP/APTES | Superhydrophilic and underwater superoleophobic WCA: 0° in few seconds UWOCA: >150° | n-hexane/water cyclohexane/water petroleum ether/water kerosene/water colza oil/water | 1:1 volume ratio | Gravity-driven | ~110,000 ~99,000 ~90,000 113,000 49,000 | >99.1 | The R was maintained at 99.8% after 30 cycles of separation; UWOCA kept at above 153° after immersed in ultrasonic water for a long time; UWOCA remained above 150° after immersed into various inorganic salt solutions and solutions pH 2 to pH10 for 24 h; TP/APTEST coating decomposed in the solution pH 12 and greater. | [129] |
| Base Polymer | Nanomaterials | Wettability | Oil/Water System | Oil Content in Water | Filtration Mode | (L/m2 h) | (%) | Findings | Reference |
|---|---|---|---|---|---|---|---|---|---|
| PAN | Single-walled CNTs (OD: <2 nm, L: 5–30 μm) | Switchable hydrophobic and hydrophilic | Petroleum ether/water | 1:9 volume ratio | Vacuum driven at −0.07 MPa | ~55,000 | 99.96 | Hydrophobic CNT side and hydrophilic PAN side. | [96] |
| PVDF | SNPs (dia. 30 nm, 50 nm, 200 nm, 1 μm) | Hydrophobic and oleophilic WCA: 135° OCA: 0° in 2 s UWOCA: 87° | Octane/water Hexadecane/water Diesel oil/water Rapeseed oil/water | 500–2000 mg/L | Dead-end, 0–10 kPa | - | 97.95 98.60 92.70 90.80 | Exhibited excellent performances in oil-water separation for the flow velocities below 1.98 m/min; surface roughness and pores increased the probability of droplets capture by interception and collision. | [130] |
| N6 | SNPs | Superhydrophilic and underwater oleophobic WCA: 0° in 1 s UWOCA: 116° | Machine/water + SDS | 250–1000 mg/L | Dead-end stirred cell filtration, 4 psi | 4814 a | >98.80 | SNPs increased the surface roughness from 193 to 285 nm; incorporation of SNPs enhanced the tensile strength to 22.48 MPa due to the integrated network structure; strong interaction between the N6 nanofiber and PVAc coat maintained the stability after permeation with acidic and alkaline solutions for 3 h. | [131] |
| PVDF | PDA and TiO2 | Superhydrophilic and underwater superoleophobic WAC: 0° in 1–34 s UWOCA: 158.6° | Diesel oil/water n-hexadecane/water 1,3,5-trimethylbenzene/water Petroleum ether/water | 1:100 volume ratio + 0.2 mg/ml SDS | Vacuum filtration, ΔP at 0.09 MPa | 785 | 99.52 99.34 99.13 98.86 | The modified membrane exhibited excellent stability under acidic, salty and physical stress; PDA disintegrated in a strongly alkaline environment; superhydrophlicity maintained and no loss of NPs even after strong shear flow at 30°C for 30 days. | [132] |
| PAN | Electrospun PS | Hydrophobic WCA: 113–126° | Hexane/water | 1 mL hexane in 99 mL deionized water, 0.1 wt% SDS | Gravity-driven | 209–1841 b 227–430 c | - | Emulsion flux of J-ENMs was 1.7 times higher than that of single layer PAN NF; PS concentrations affected emulsion fluxes. | [116] |
| PAN | Ag, ZnO | Superhydrophilic and underwater superoleophobic WCA: 0° in 0.6 s UWOCA: 154.4° | Soybean oil/water | 1% soybean oil mixed with 20 mg/L cationic dye or anionic dye | Gravity-driven | 619 | >99.7 | Micro/nano sized hierarchical structure greatly increased the roughness; strong resistance to different pH solutions, organic solvents and salt solutions for 24 h with WCA and UWOCA maintained; | [119] |
| PAN | Au | Superhydrophobic and underwater superoleophilic WCA: ~155.5° OCA: ~0° UOWCA: ~158° | Chloroform/water | 6 ml chloroform in 0.54 g Tween 80 and 54 mL water | Gravity-driven | - | 97.8 d | Separation efficiency maintained at 85% after 16 cycles of separation; | [99] |
| PAN | TiO2 | Superhydrophilic and superoleophobic OCA: 166–162° | Petroleum ether/water Bump oil/water Soybean oil/water | 1:1000 weight ratio with 0.1 mg/mL Tween 80 in water 1:99 weight ratio without surfactant | Gravity-driven, 0.01 bar | 600–2000 | 99 | Emulsion property such as viscosity affected the separation efficiency; no obvious decline of permeation; robust recyclability; soybean emulsion flux decreased quickly with time because the oil drop size was smaller. | [111] |
| PAN | PDA | Superhydrophilic and underwater superoleophobic WCA: 0° in 0.12 s UWOCA: 165 ± 1° | Toluene/water | 3.0 ml in 0.03 g SLS and 297 mL deionized water | Gravity-driven | 11,666 ± 978 e | 99.9 | Micro/nano-spehres formed in the PAN-PDAc; permeability of PAN-PDAc NF was about 2.7 times of the pristine PAN; initial permeability of PAN-PDAc was 23.3% higher than PAN; the permeability after 2 h in PAN-PDAc was 174.8% higher than PAN. | [114] |
| PP | TA/DA/PEI | Superhydrophilic and underwater uperoleophobic WCA: 0° in 1 s UWOCA: >154° | 1,2-dichloroethane/water Toluene/water n-hexane/water Cyclohexane/water Petroleum ether/water | 10 mL in 990 mL deionized water with 20 mg Tween-80 | Gravity-driven | - 463 ± 30 489 ± 24 509 ± 35 513 ± 32 | 99.8 | Mussel-inspired hydrophilic structure; tannin-inspired coating used to improve the adhesion; oil droplets form filter cake and block the pores on the surface; R was greater than 95% even after 10 cycles for 1,2-dichloroethane/water; some TA/DA/PEI particles detached in alkaline solutions at pH12 and pH14; more stable for acidic, weak alkaline and organic solvents. | [133] |
| PET | Electrospun PVDF NF | Hydrophobic and lipophilic WCA: 130° OCA: 0° UWOCA: 65.7° | Hexadecane/water Octane/water Diesel/water Rapeseed oil/water | Concentration of oils ranged from 500 to 2000 mg/L | Dead-end filtration | - | ~99 ~98 ~95 ~92 | R increased from 73.0% to 99.5% when the number of electrospun PVDF NF layer increased from 1 to 4; R decreased to 95.8% for 5 layers of electrospun PVDF NF; R for the highly viscous oil (rapeseed oil) was slightly low due to the difficulty of the oil collided and coalesced. | [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarbatly, R.; Chiam, C.-K. An Overview of Recent Progress in Nanofiber Membranes for Oily Wastewater Treatment. Nanomaterials 2022, 12, 2919. https://doi.org/10.3390/nano12172919
Sarbatly R, Chiam C-K. An Overview of Recent Progress in Nanofiber Membranes for Oily Wastewater Treatment. Nanomaterials. 2022; 12(17):2919. https://doi.org/10.3390/nano12172919
Chicago/Turabian StyleSarbatly, Rosalam, and Chel-Ken Chiam. 2022. "An Overview of Recent Progress in Nanofiber Membranes for Oily Wastewater Treatment" Nanomaterials 12, no. 17: 2919. https://doi.org/10.3390/nano12172919
APA StyleSarbatly, R., & Chiam, C.-K. (2022). An Overview of Recent Progress in Nanofiber Membranes for Oily Wastewater Treatment. Nanomaterials, 12(17), 2919. https://doi.org/10.3390/nano12172919







