Enhancing the Thermal Conductivity of Amorphous Carbon with Nanowires and Nanotubes
Abstract
1. Introduction
2. Methods
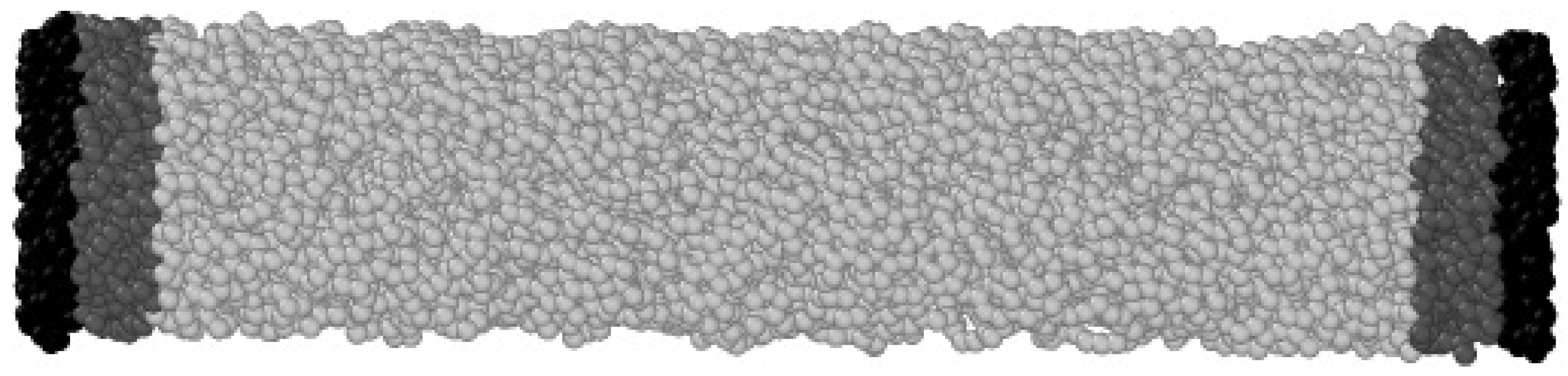
2.1. Non-Equilibrium MD (NEMD) Setup
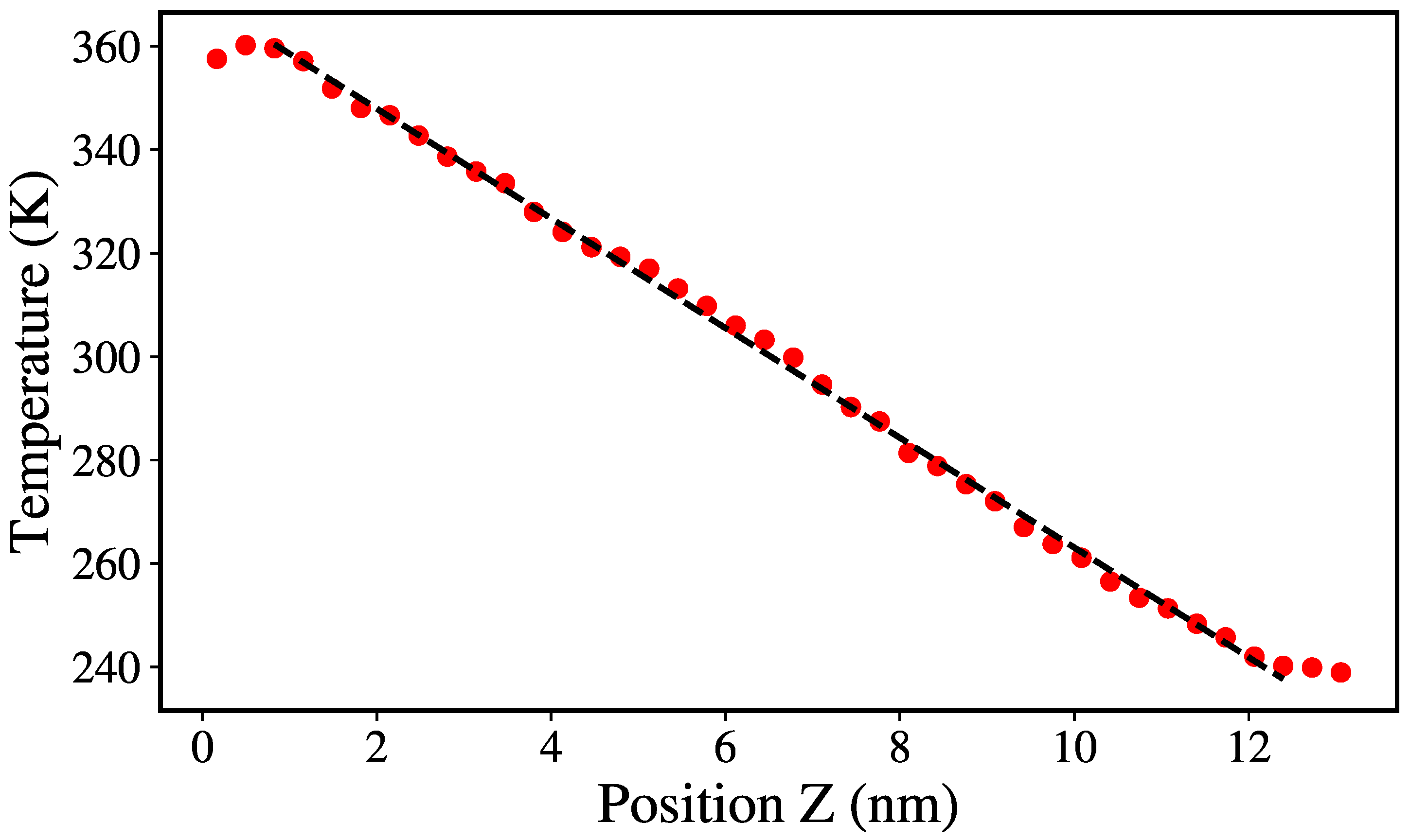
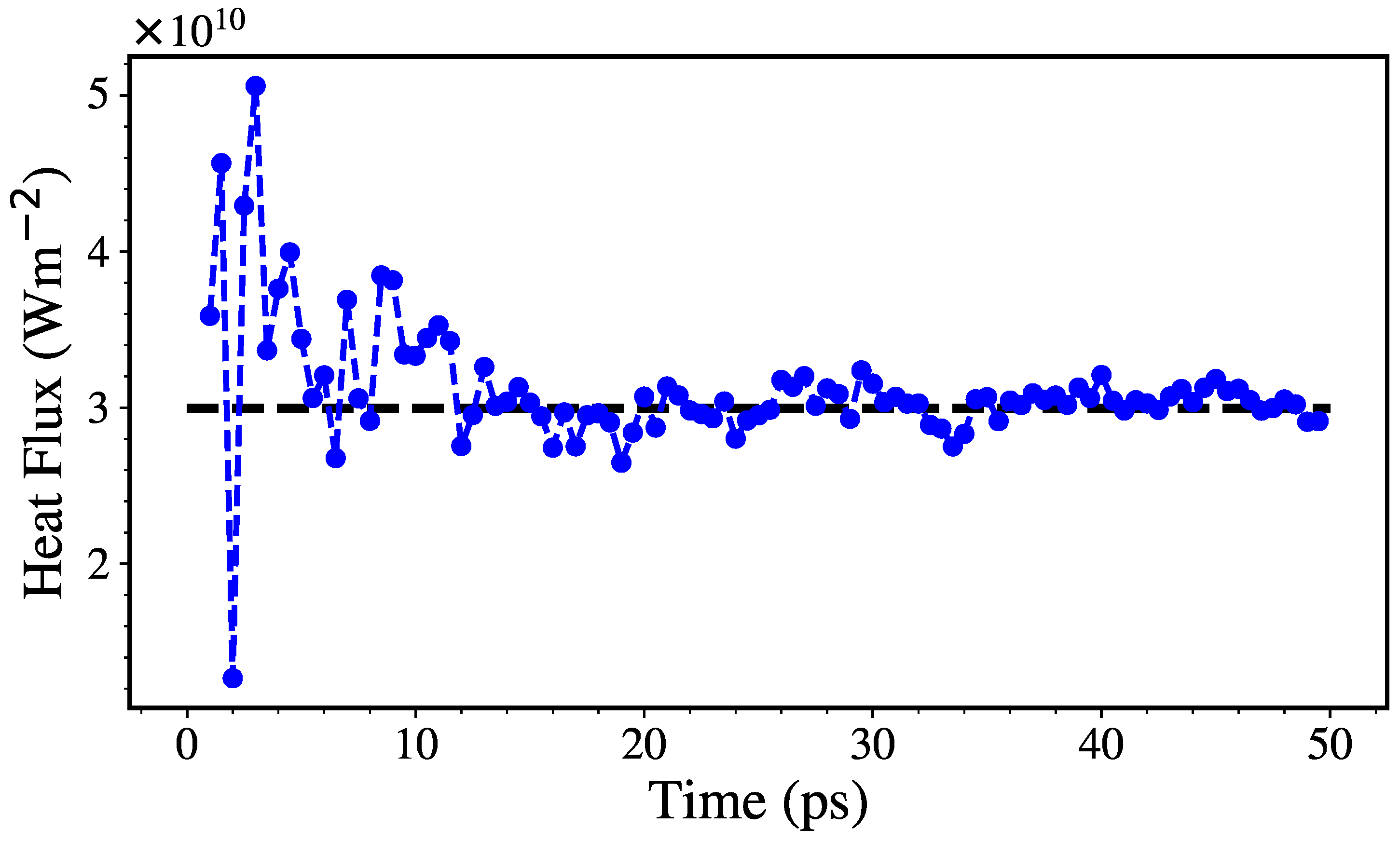
2.2. Thermal Conductivity Calculation
2.3. The Einstein Thermal Conductivity Limit
2.4. Quantum Corrections
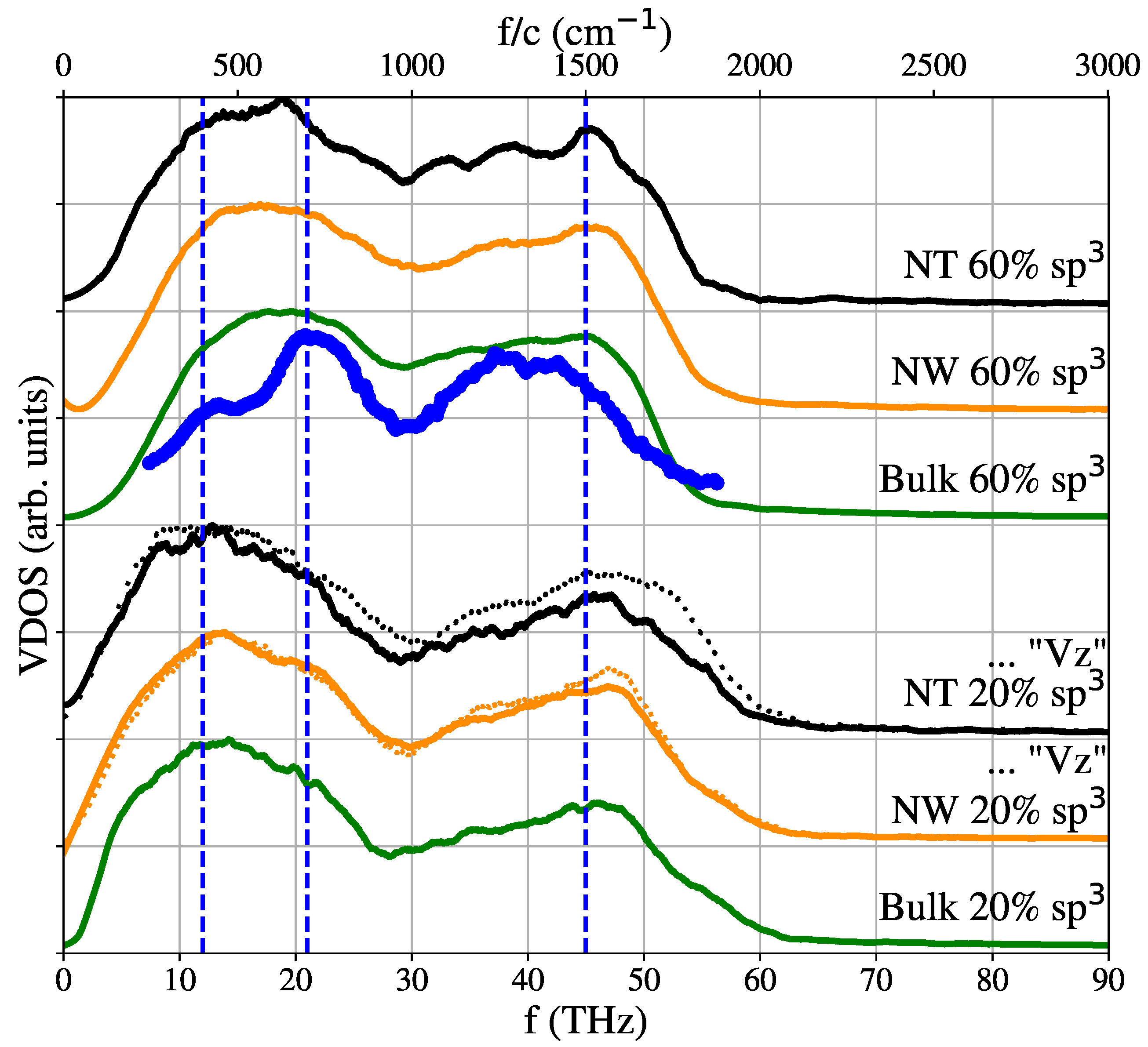
2.5. Vibrational Density of States (VDOS)
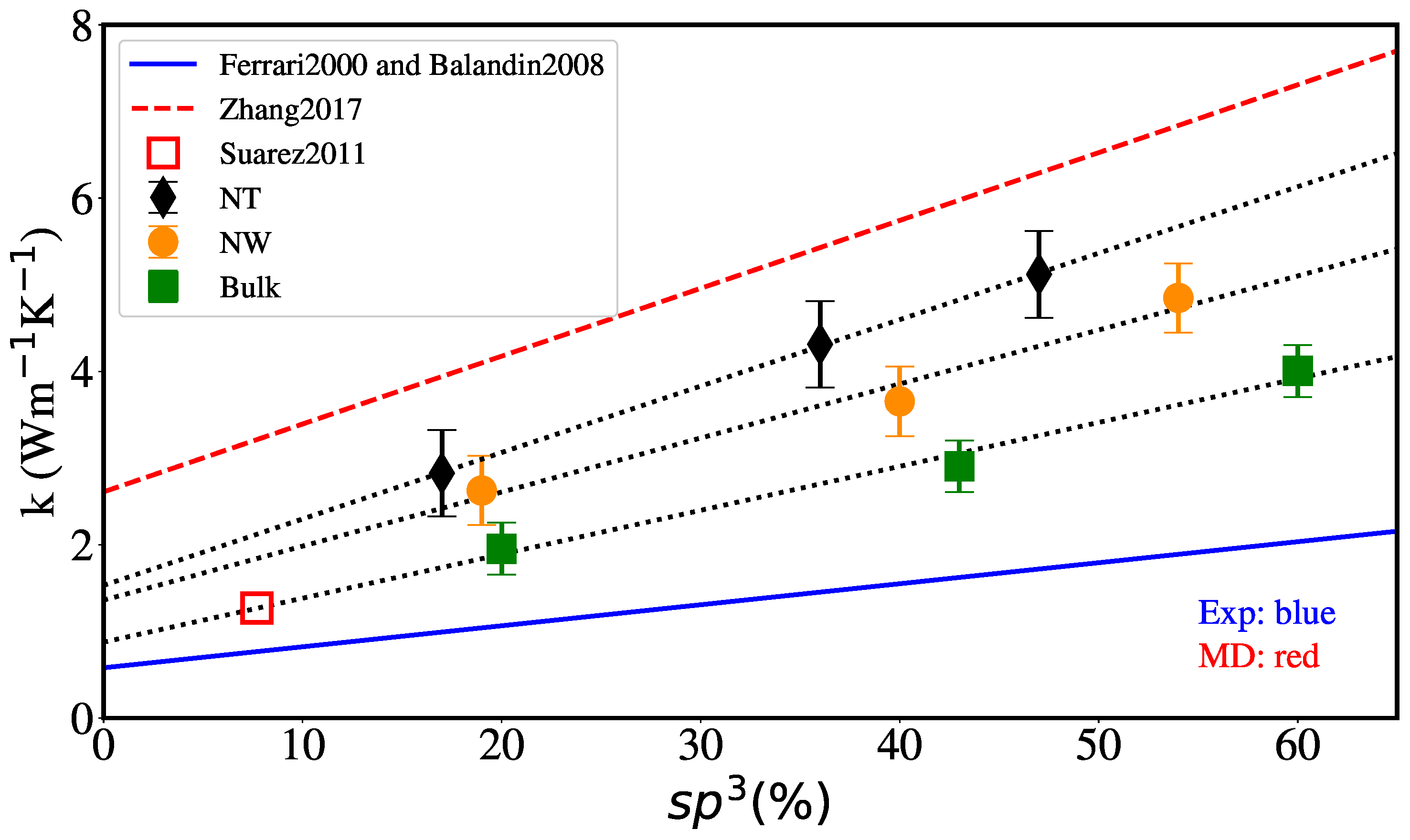
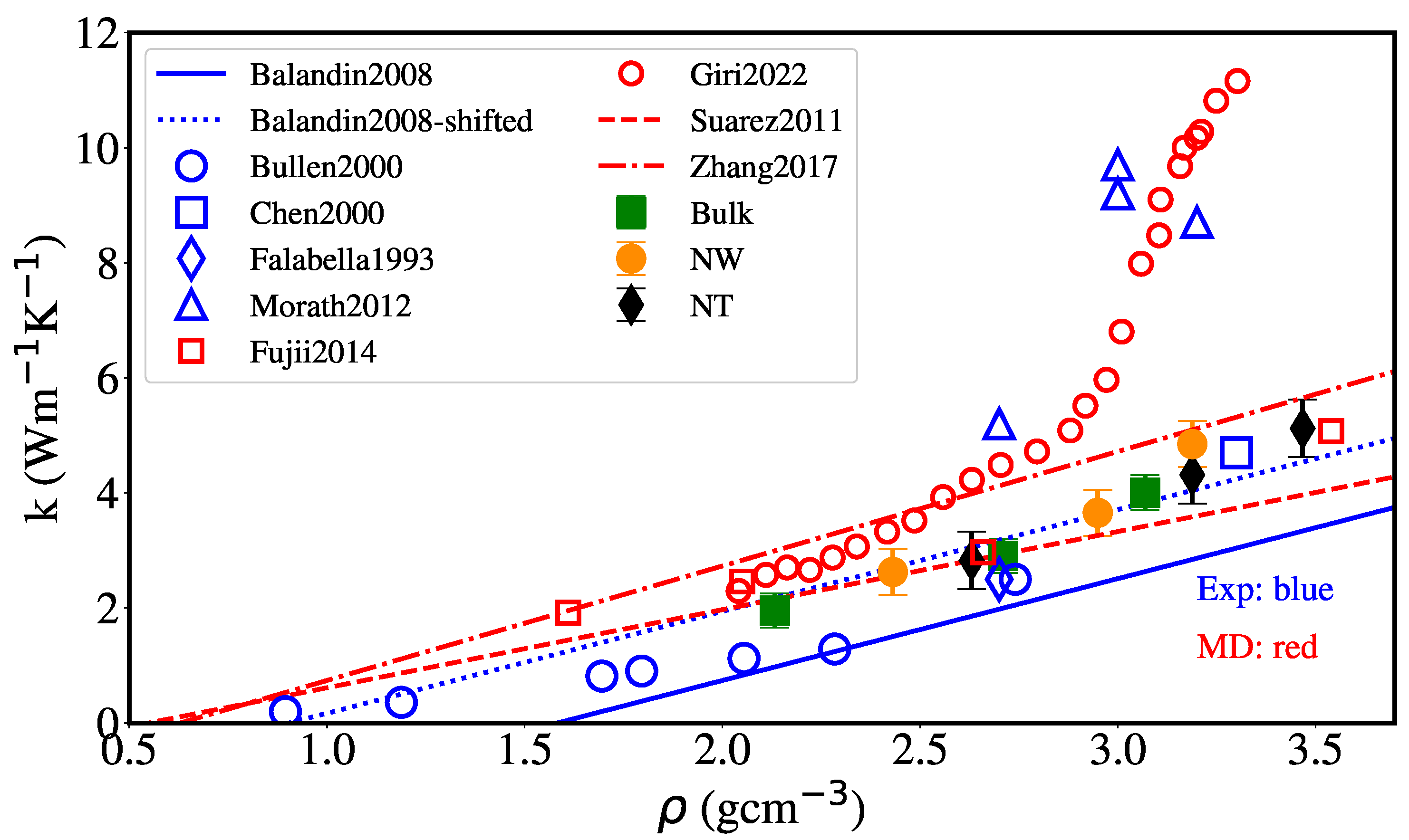
3. Results and Discussion
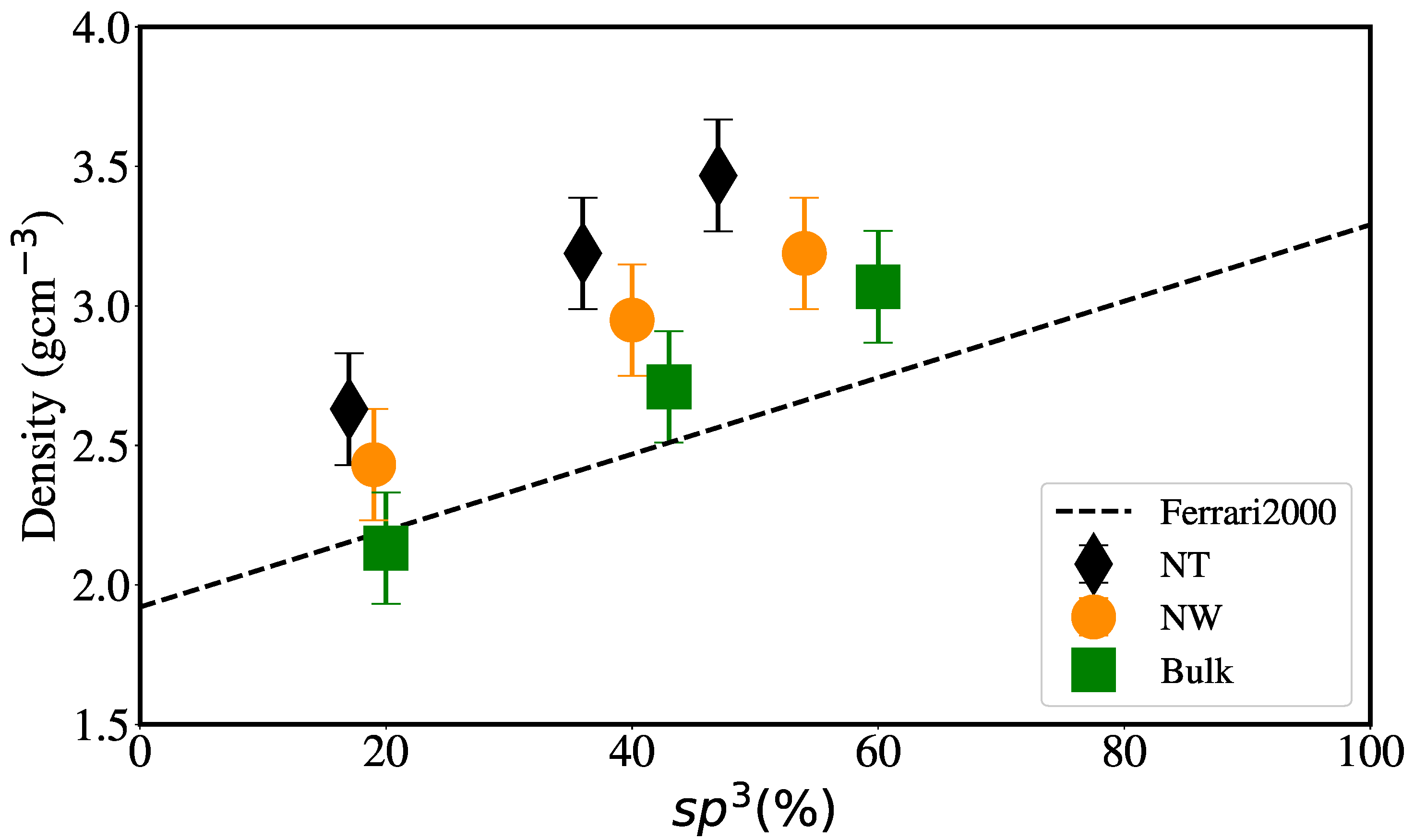
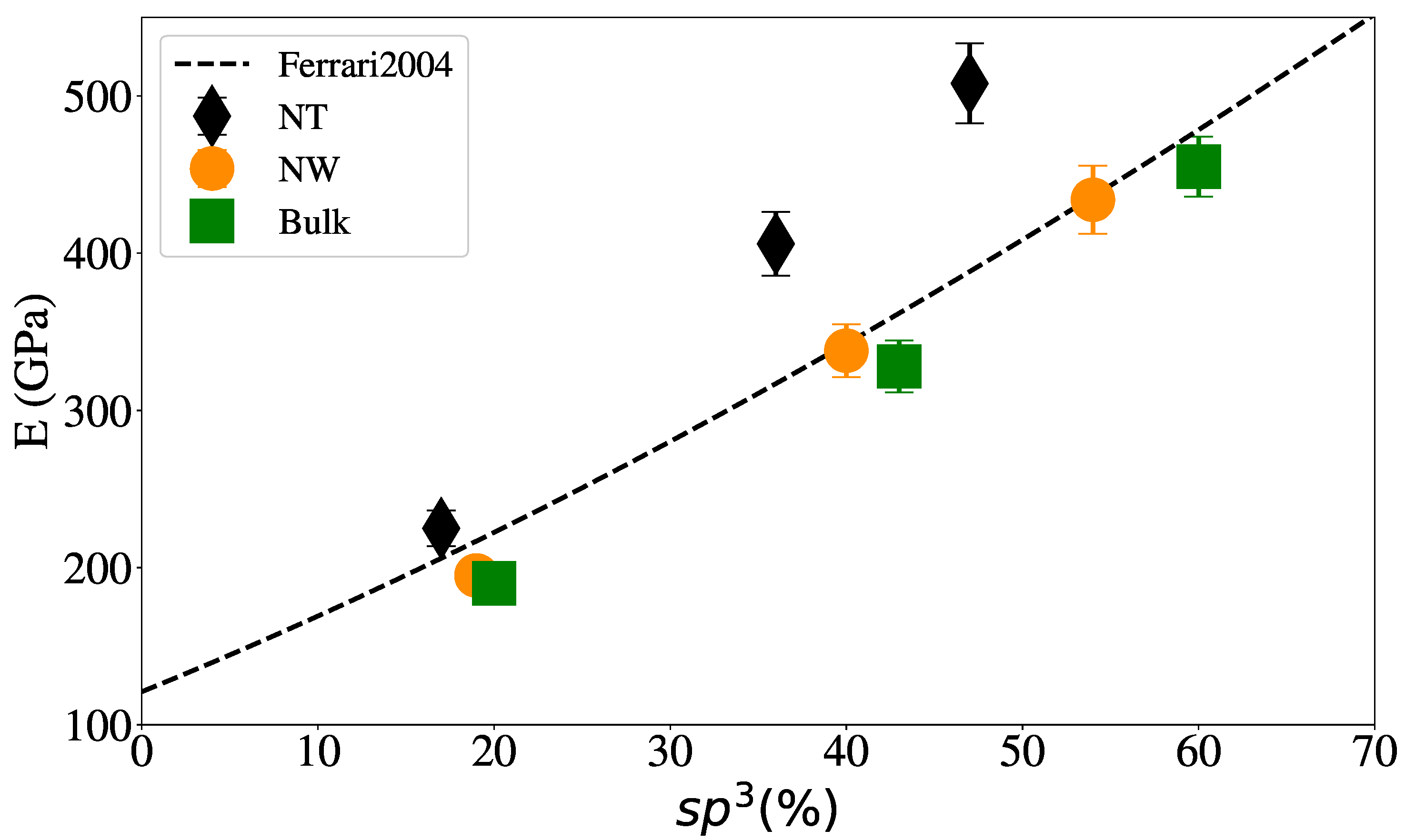
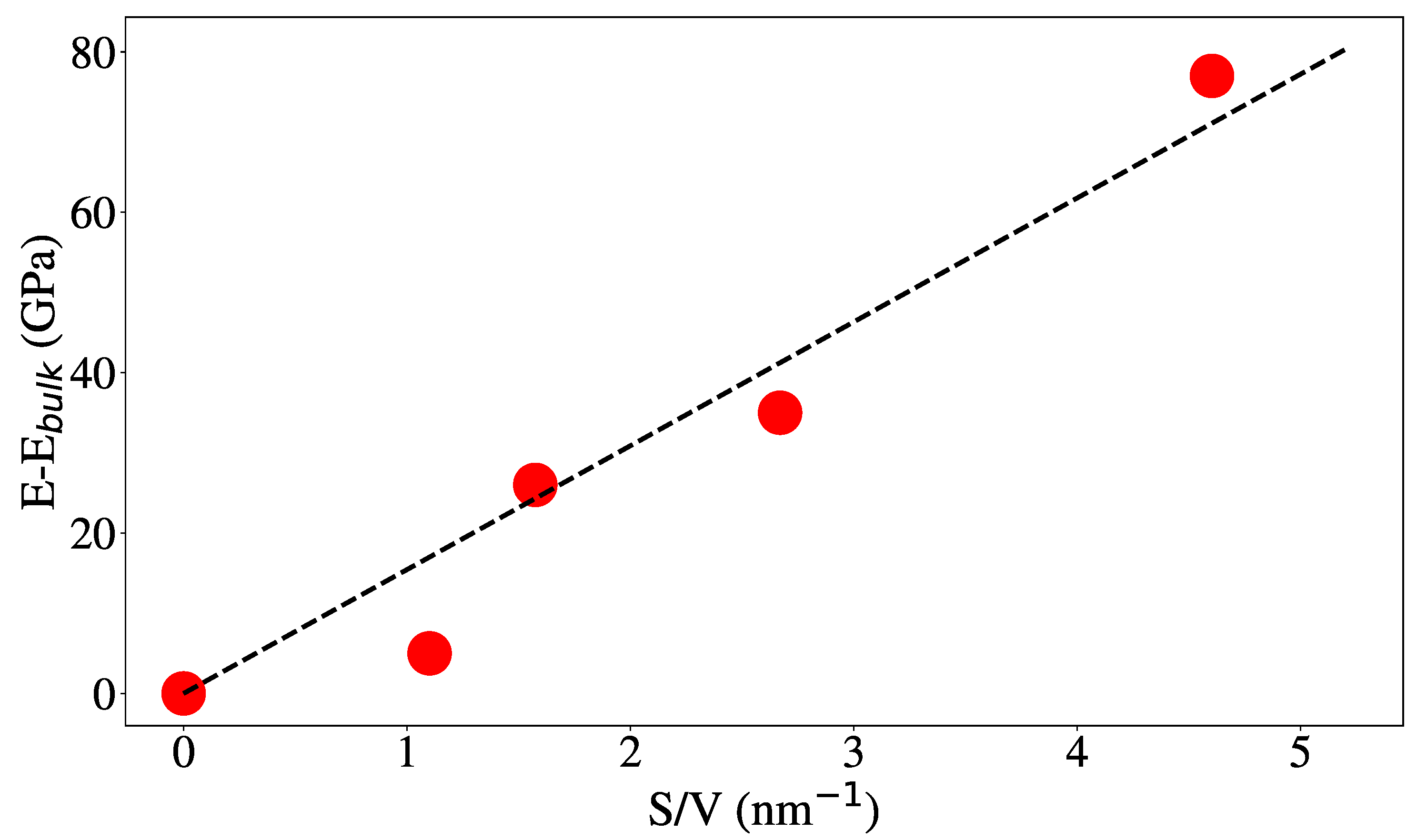
3.1. Structural Properties
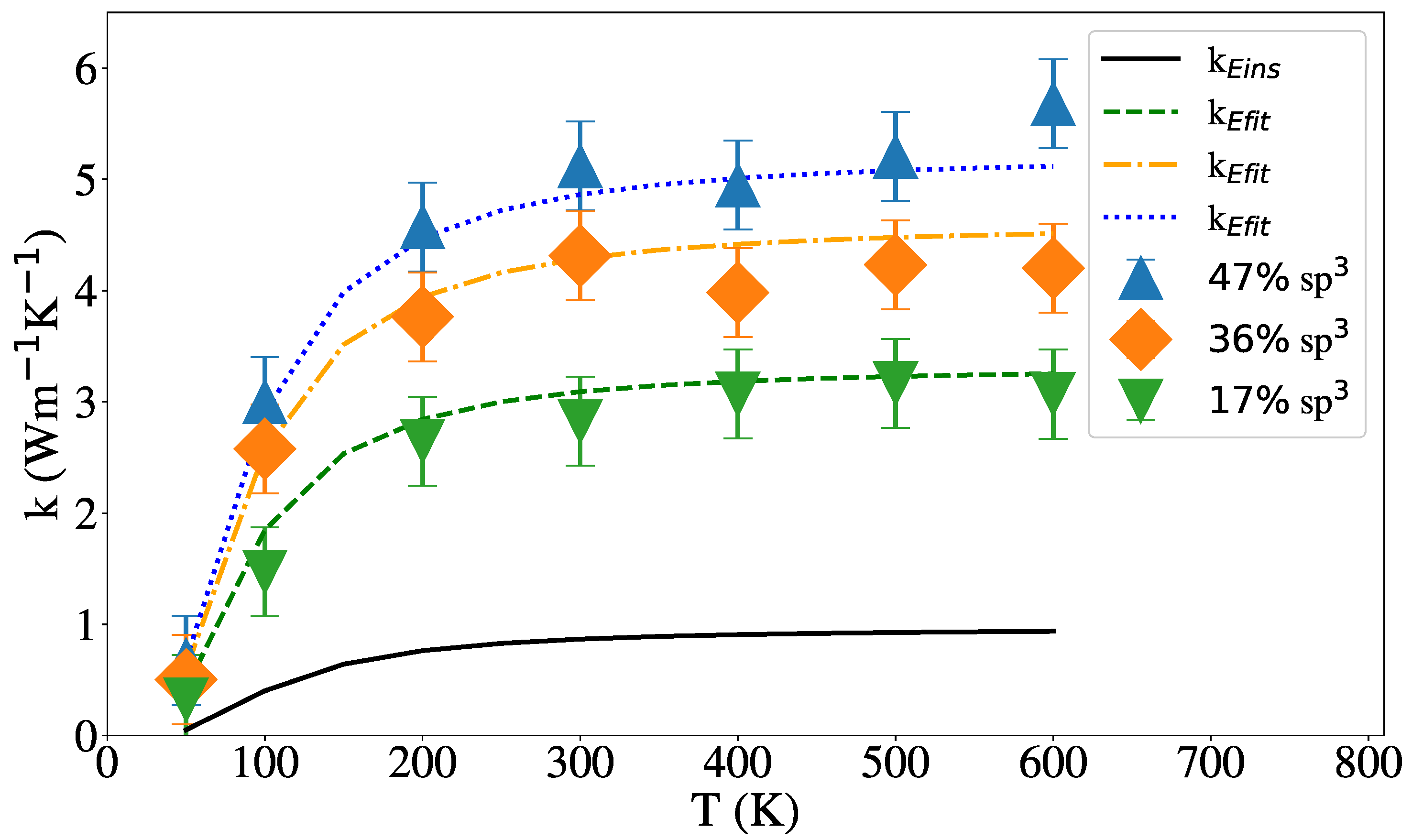
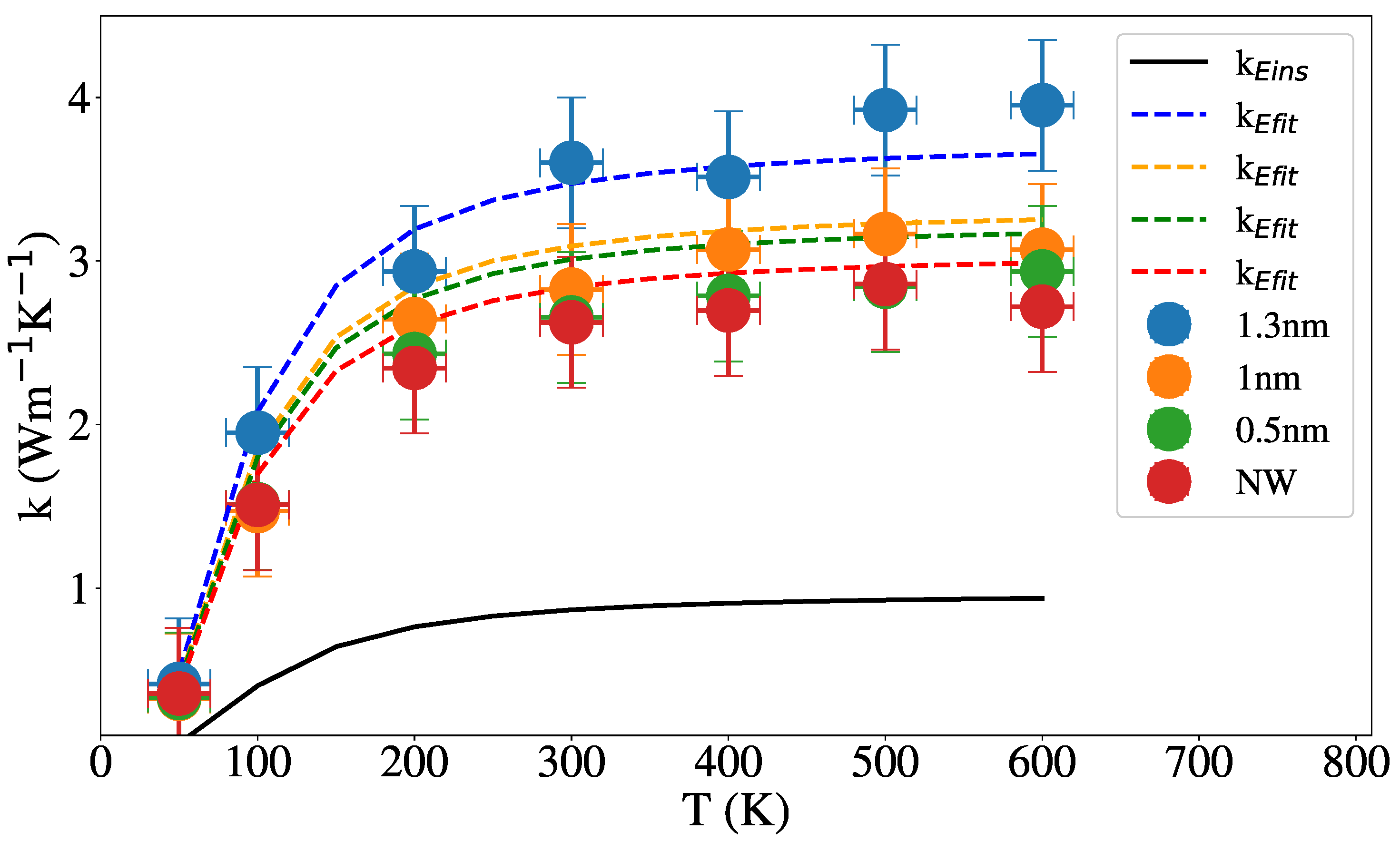
3.2. Thermal Conductivity
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cahill, D.G.; Ford, W.K.; Goodson, K.E.; Mahan, G.D.; Majumdar, A.; Maris, H.J.; Merlin, R.; Phillpot, S.R. Nanoscale thermal transport. J. Appl. Phys. 2003, 93, 793–818. [Google Scholar] [CrossRef]
- Robertson, J. Amorphous carbon. Adv. Phys. 1986, 35, 317–374. [Google Scholar] [CrossRef]
- Gao, Y.; Bao, W.; Meng, Q.; Jing, Y.; Song, X. The thermal transport properties of single-crystalline nanowires covered with amorphous shell: A molecular dynamics study. J. Non-Cryst. Solids 2014, 387, 132–138. [Google Scholar] [CrossRef]
- Cahill, D.G.; Braun, P.V.; Chen, G.; Clarke, D.R.; Fan, S.; Goodson, K.E.; Keblinski, P.; King, W.P.; Mahan, G.D.; Majumdar, A.; et al. Nanoscale thermal transport. II. 2003–2012. Appl. Phys. Rev. 2014, 1, 011305. [Google Scholar] [CrossRef]
- Zhu, W.; Zheng, G.; Cao, S.; He, H. Thermal conductivity of amorphous SiO2 thin film: A molecular dynamics study. Sci. Rep. 2018, 8, 10537. [Google Scholar] [CrossRef]
- DeAngelis, F.; Muraleedharan, M.G.; Moon, J.; Seyf, H.R.; Minnich, A.J.; McGaughey, A.J.H.; Henry, A. Thermal Transport in Disordered Materials. Nanoscale Microscale Thermophys. Eng. 2019, 23, 81–116. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, Y.; Chen, K.; Xie, G.; Wang, T.; Zhang, G. Thermal Conductivity of Amorphous Materials. Adv. Funct. Mater. 2019, 30, 1903829. [Google Scholar] [CrossRef]
- Pop, E. Energy dissipation and transport in nanoscale devices. Nano Res. 2010, 3, 147–169. [Google Scholar] [CrossRef]
- Renteria, J.; Nika, D.; Balandin, A. Graphene Thermal Properties: Applications in Thermal Management and Energy Storage. Appl. Sci. 2014, 4, 525–547. [Google Scholar] [CrossRef]
- Zhan, H.J.; Wu, K.J.; Hu, Y.L.; Liu, J.W.; Li, H.; Guo, X.; Xu, J.; Yang, Y.; Yu, Z.L.; Gao, H.L.; et al. Biomimetic Carbon Tube Aerogel Enables Super-Elasticity and Thermal Insulation. Chem 2019, 5, 1871–1882. [Google Scholar] [CrossRef]
- Chen, C.; Hu, L. Super Elastic and Thermally Insulating Carbon Aerogel: Go Tubular Like Polar Bear Hair. Matter 2019, 1, 36–38. [Google Scholar] [CrossRef]
- Wang, Q.; Xiang, L.; Mei, D.; Xie, Y. Graphene Aerogels: Structure Control, Thermal Characterization and Thermal Transport. Int. J. Thermophys. 2020, 41, 155. [Google Scholar] [CrossRef]
- Vandevelde, T.; Vandierendonck, K.; Van Stappen, M.; Du Mong, W.; Perremans, P. Cutting applications of DLC, hard carbon and diamond films. Surf. Coat. Technol. 1999, 113, 80–85. [Google Scholar] [CrossRef]
- Liao, M.; Koide, Y. Carbon-based materials: Growth, properties, MEMS/NEMS technologies, and MEM/NEM switches. Crit. Rev. Solid State Mater. Sci. 2011, 36, 66–101. [Google Scholar] [CrossRef]
- Qian, X.; Zhou, J.; Chen, G. Phonon-engineered extreme thermal conductivity materials. Nat. Mater. 2021, 20, 1188–1202. [Google Scholar] [CrossRef]
- Frese, N.; Taylor Mitchell, S.; Bowers, A.; Gölzhäuser, A.; Sattler, K. Diamond-Like Carbon Nanofoam from Low-Temperature Hydrothermal Carbonization of a Sucrose/Naphthalene Precursor Solution. C 2017, 3, 23. [Google Scholar] [CrossRef]
- Yousefi, F.; Khoeini, F.; Rajabpour, A. Thermal conductivity and thermal rectification of nanoporous graphene: A molecular dynamics simulation. Int. J. Heat Mass Transf. 2020, 146, 118884. [Google Scholar] [CrossRef]
- Li, T.; Song, J.; Zhao, X.; Yang, Z.; Pastel, G.; Xu, S.; Jia, C.; Dai, J.; Chen, C.; Gong, A.; et al. Anisotropic, lightweight, strong, and super thermally insulating nanowood with naturally aligned nanocellulose. Sci. Adv. 2018, 4, eaar3724. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Q.; Hao, M.; Hu, Y.; Lin, Z.; Peng, L.; Wang, T.; Ren, X.; Wang, C.; Zhao, Z.; et al. Double-negative-index ceramic aerogels for thermal superinsulation. Science 2019, 363, 723–727. [Google Scholar] [CrossRef]
- Kang, J.; Jang, H.; Ma, J.; Yang, Q.; Hattar, K.; Diao, Z.; Yuan, R.; Zuo, J.; Sinha, S.; Cahill, D.G.; et al. Ultralow Thermal Conductivity in Nanoporous Crystalline Fe3O4. J. Phys. Chem. C 2021, 125, 6897–6908. [Google Scholar] [CrossRef]
- Wang, M.; Pan, N. Modeling and prediction of the effective thermal conductivity of random open-cell porous foams. Int. J. Heat Mass Transf. 2008, 51, 1325–1331. [Google Scholar] [CrossRef]
- Ding, B.; Li, X.; Zhou, W.; Zhang, G.; Gao, H. Anomalous strain effect on the thermal conductivity of low-buckled two-dimensional silicene. Natl. Sci. Rev. 2020, 8, nwaa220. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Xu, K.; Han, M.; Yao, Y.; Zhang, Z.; Zeng, X.; Xu, J.; Wu, J. Abnormally high thermal conductivity in fivefold twinned diamond nanowires. Mater. Today Phys. 2022, 25, 100705. [Google Scholar] [CrossRef]
- Yang, H.S.; Cahill, D.G.; Liu, X.; Feldman, J.; Crandall, R.; Sperling, B.; Abelson, J. Anomalously high thermal conductivity of amorphous Si deposited by hot-wire chemical vapor deposition. Phys. Rev. B 2010, 81, 104203. [Google Scholar] [CrossRef]
- Kim, Y.; Kamio, S.; Tajiri, T.; Hayashi, T.; Song, S.; Endo, M.; Terrones, M.; Dresselhaus, M. Enhanced thermal conductivity of carbon fiber/phenolic resin composites by the introduction of carbon nanotubes. Appl. Phys. Lett. 2007, 90, 093125. [Google Scholar] [CrossRef]
- Wang, S.; Liang, R.; Wang, B.; Zhang, C. Dispersion and thermal conductivity of carbon nanotube composites. Carbon 2009, 47, 53–57. [Google Scholar] [CrossRef]
- Berber, S.; Kwon, Y.K.; Tománek, D. Unusually high thermal conductivity of carbon nanotubes. Phys. Rev. Lett. 2000, 84, 4613. [Google Scholar] [CrossRef]
- Kumanek, B.; Janas, D. Thermal conductivity of carbon nanotube networks: A review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Bullen, A.J.; O’Hara, K.E.; Cahill, D.G.; Monteiro, O.; von Keudell, A. Thermal conductivity of amorphous carbon thin films. J. Appl. Phys. 2000, 88, 6317–6320. [Google Scholar] [CrossRef]
- Balandin, A.A.; Shamsa, M.; Liu, W.L.; Casiraghi, C.; Ferrari, A.C. Thermal conductivity of ultrathin tetrahedral amorphous carbon films. Appl. Phys. Lett. 2008, 93, 043115. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Libassi, A.; Tanner, B.K.; Stolojan, V.; Yuan, J.; Yuan, J.; Brown, L.M.; Rodil, S.E.; Kleinsorge, B.; Robertson, J. Density, sp3 fraction, and cross-sectional structure of amorphous carbon films determined by x-ray reflectivity and electron energy-loss spectroscopy. Phys. Rev. Condens. Matter Mater. Phys. 2000, 62, 11089–11103. [Google Scholar] [CrossRef]
- Chen, G.; Hui, P.; Xu, S. Thermal conduction in metalized tetrahedral amorphous carbon (ta–C) films on silicon. Thin Solid Films 2000, 366, 95–99. [Google Scholar] [CrossRef]
- Morath, C.J.; Maris, H.J.; Cuomo, J.J.; Pappas, D.L.; Grill, A.; Patel, V.V.; Doyle, J.P.; Saenger, K.L. Picosecond optical studies of amorphous diamond and diamondlike carbon: Thermal conductivity and longitudinal sound velocity. J. Appl. Phys. 1994, 76, 2636–2640. [Google Scholar] [CrossRef]
- Lv, W.; Henry, A. Direct calculation of modal contributions to thermal conductivity via Green–Kubo modal analysis. New J. Phys. 2016, 18. [Google Scholar] [CrossRef]
- Mahajan, S.S.; Subbarayan, G.; Sammakia, B.G. Estimating thermal conductivity of amorphous silica nanoparticles and nanowires using molecular dynamics simulations. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2007, 76, 056701. [Google Scholar] [CrossRef]
- Desmarchelier, P.; Beardo, A.; Alvarez, F.X.; Tanguy, A.; Termentzidis, K. Atomistic evidence of hydrodynamic heat transfer in nanowires. Int. J. Heat Mass Transf. 2022, 194, 123003. [Google Scholar] [CrossRef]
- Wingert, M.C.; Kwon, S.; Hu, M.; Poulikakos, D.; Xiang, J.; Chen, R. Sub-amorphous Thermal Conductivity in Ultrathin Crystalline Silicon Nanotubes. Nano Lett. 2015, 15, 2605–2611. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Li, B. Remarkable Reduction of Thermal Conductivity in Silicon Nanotubes. Nano Lett. 2010, 10, 3978–3983. [Google Scholar] [CrossRef]
- Lv, W.; Henry, A. Phonon transport in amorphous carbon using Green–Kubo modal analysis. Appl. Phys. Lett. 2016, 108, 181905. [Google Scholar] [CrossRef]
- Seyf, H.R.; Lv, W.; Rohskopf, A.; Henry, A. The Importance of Phonons with Negative Phase Quotient in Disordered Solids. Sci. Rep. 2018, 8, 2627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Ai, L.Q.; Chen, M.; Xiong, D. Thermal conductive performance of deposited amorphous carbon materials by molecular dynamics simulation. Mol. Phys. 2017, 115, 831–838. [Google Scholar] [CrossRef]
- Tersoff, J. New empirical approach for the structure and energyof covalent systems. Phys. Rev. B 1988, 37, 6991–7000. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Martinez, I.; Marks, N. Effect of microstructure on the thermal conductivity of disordered carbon. Appl. Phys. Lett. 2011, 99, 033101. [Google Scholar] [CrossRef]
- Marks, N. Modelling diamond-like carbon with the environment-dependent interaction potential. J. Phys. Condens. Matter 2002, 14, 2901–2927. [Google Scholar] [CrossRef]
- Müller-Plathe, F. A simple nonequilibrium molecular dynamics method for calculating the thermal conductivity. J. Chem. Phys. 1997, 106, 6082–6085. [Google Scholar] [CrossRef]
- Shamsa, M.; Liu, W.L.; Balandin, A.A.; Casiraghi, C.; Milne, W.I.; Ferrari, A.C. Thermal conductivity of diamond-like carbon films. Appl. Phys. Lett. 2006, 89, 161921. [Google Scholar] [CrossRef]
- Giri, A.; Dionne, C.J.; Hopkins, P.E. Atomic coordination dictates vibrational characteristics and thermal conductivity in amorphous carbon. NPJ Comput. Mater. 2022, 8, 55. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comp. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Tomas, C.D.; Suarez-Martinez, I.; Marks, N.A.; de Tomas, C.; Suarez-Martinez, I.; Marks, N.A. Graphitization of amorphous carbons: A comparative study of interatomic potentials. Carbon 2016, 109, 681–693. [Google Scholar] [CrossRef]
- Valencia, F.J.; Santiago, J.; González, R.I.; González-Arrabal, R.; Ruestes, C.; Perez Díaz, M.; Monclus, M.A.; Molina-Aldareguia, J.; Nuñez, P.D.; Munoz, F.; et al. Nanoindentation of Amorphous Carbon: A combined experimental and simulation approach. Acta Mater. 2021, 203, 116485. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Verdier, M.; Lacroix, D.; Termentzidis, K. Roughness and amorphization impact on thermal conductivity of nanofilms and nanowires: Making atomistic modeling more realistic. J. Appl. Phys. 2019, 126, 164305. [Google Scholar] [CrossRef]
- Malhotra, A.; Maldovan, M. Thermal transport in semiconductor nanotubes. Int. J. Heat Mass Transf. 2019, 130, 368–374. [Google Scholar] [CrossRef]
- Cahill, D.G.; Watson, S.K.; Pohl, R.O. Lower limit to the thermal conductivity of disordered crystals. Phys. Rev. B 1992, 46, 6131–6140. [Google Scholar] [CrossRef]
- Seyf, H.R.; Gordiz, K.; Deangelis, F.; Henry, A. Using Green–Kubo modal analysis (GKMA) and interface conductance modal analysis (ICMA) to study phonon transport with molecular dynamics. J. Appl. Phys. 2019, 125, 081101. [Google Scholar] [CrossRef]
- Esfahani, M.N.; Jabbari, M.; Xu, Y.; Soutis, C. Effect of nanoscale defects on the thermal conductivity of graphene. Mater. Today Commun. 2021, 26, 101856. [Google Scholar] [CrossRef]
- Pathria, R.K.; Beale, P.D. Statistical Mechanics, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2011; p. 744. [Google Scholar] [CrossRef]
- Cahill, D.G.; Pohl, R.O. Heat flow and lattice vibrations in glasses. Solid State Commun. 1989, 70, 927–930. [Google Scholar] [CrossRef]
- Wei, Y.X.; Wang, R.J.; Wang, W.H. Soft phonons and phase transition in amorphous carbon. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 72, 22–25. [Google Scholar] [CrossRef]
- Lv, W.; Henry, A. Examining the Validity of the Phonon Gas Model in Amorphous Materials. Sci. Rep. 2016, 6, 37675. [Google Scholar] [CrossRef]
- Bedoya-Martínez, O.N.; Barrat, J.L.; Rodney, D. Computation of the thermal conductivity using methods based on classical and quantum molecular dynamics. Phys. Rev. B 2014, 89, 014303. [Google Scholar] [CrossRef]
- Khalkhali, M.; Khoeini, F. Impact of torsion and disorder on the thermal conductivity of Si nanowires: A nonequilibrium molecular dynamics study. J. Phys. Chem. Solids 2018, 112, 216–221. [Google Scholar] [CrossRef]
- Mizuno, H.; Mossa, S.; Barrat, J.L. Elastic heterogeneity, vibrational states, and thermal conductivity across an amorphisation transition. EPL Europhys. Lett. 2013, 104, 56001. [Google Scholar] [CrossRef]
- Turney, J.E.; McGaughey, A.J.H.; Amon, C.H. Assessing the applicability of quantum corrections to classical thermal conductivity predictions. Phys. Rev. B 2009, 79, 224305. [Google Scholar] [CrossRef]
- Sääskilahti, K.; Oksanen, J.; Tulkki, J.; McGaughey, A.J.; Volz, S. Vibrational mean free paths and thermal conductivity of amorphous silicon from non-equilibrium molecular dynamics simulations. AIP Adv. 2016, 6, 121904. [Google Scholar] [CrossRef]
- Zeng, Y.J.; Liu, Y.Y.; Zhou, W.X.; Chen, K.Q. Nanoscale thermal transport: Theoretical method and application. Chin. Phys. B 2018, 27, 036304. [Google Scholar] [CrossRef]
- Soleimani, A.; Araghi, H.; Zabihi, Z.; Alibakhshi, A. A comparative study of molecular dynamics simulation methods for evaluation of the thermal conductivity and phonon transport in Si nanowires. Comput. Mater. Sci. 2018, 142, 346–354. [Google Scholar] [CrossRef]
- He, Y.; Savić, I.; Donadio, D.; Galli, G. Lattice thermal conductivity of semiconducting bulk materials: Atomistic simulations. Phys. Chem. Chem. Phys. 2012, 14, 16209. [Google Scholar] [CrossRef]
- Einstein, A. Planck’s theory of radiation and the theory of specific heat. Ann. Phys. 1907, 22, 180–190. [Google Scholar] [CrossRef]
- Stevens, R.J.; Zhigilei, L.V.; Norris, P.M. Effects of temperature and disorder on thermal boundary conductance at solid–solid interfaces: Nonequilibrium molecular dynamics simulations. Int. J. Heat Mass Transf. 2007, 50, 3977–3989. [Google Scholar] [CrossRef]
- Gutiérrez, G.; Menéndez-Proupin, E.; Loyola, C.; Peralta, J.; Davis, S. Computer simulation study of amorphous compounds: Structural and vibrational properties. J. Mater. Sci. 2010, 45, 5124–5134. [Google Scholar] [CrossRef][Green Version]
- Shafai, G.; Ortigoza, M.A.; Rahman, T.S. Vibrations of Au13 and FeAu12 nanoparticles and the limits of the Debye temperature concept. J. Physics. Condens. Matter Inst. Phys. J. 2012, 24, 104026. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.M.; McGaughey, A.J. Thermal conductivity accumulation in amorphous silica and amorphous silicon. Phys. Rev. B Condens. Matter Mater. Phys. 2014, 89, 144303. [Google Scholar] [CrossRef]
- Li, M.; Deng, T.; Zheng, B.; Zhang, Y.; Liao, Y.; Zhou, H. Effect of defects on the mechanical and thermal properties of graphene. Nanomaterials 2019, 9, 347. [Google Scholar] [CrossRef]
- Di Pierro, A.; Bernal, M.M.; Martinez, D.; Mortazavi, B.; Saracco, G.; Fina, A. Aromatic molecular junctions between graphene sheets: A molecular dynamics screening for enhanced thermal conductance. RSC Adv. 2019, 9, 15573–15581. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tiwari, J.; Feng, T. Reduced anharmonic phonon scattering cross-section slows the decrease of thermal conductivity with temperature. Mater. Today Phys. 2022, 24, 100689. [Google Scholar] [CrossRef]
- Pereira, L.F.C. Investigating mechanical properties and thermal conductivity of 2D carbon-based materials by computational experiments. Comput. Mater. Sci. 2021, 196, 110493. [Google Scholar] [CrossRef]
- Chen, Y.; An, X.; Liao, X. Mechanical behaviors of nanowires. Appl. Phys. Rev. 2017, 4, 031104. [Google Scholar] [CrossRef]
- Yao, H.; Yun, G.; Bai, N.; Li, J. Surface elasticity effect on the size-dependent elastic property of nanowires. J. Appl. Phys. 2012, 111, 083506. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, H.; Li, X.; Chen, C. Size effects on tensile and compressive strengths in metallic glass nanowires. J. Mech. Phys. Solids 2015, 84, 130–144. [Google Scholar] [CrossRef]
- Tang, C.; Dávila, L.P. Anomalous surface states modify the size-dependent mechanical properties and fracture of silica nanowires. Nanotechnology 2014, 25, 435702. [Google Scholar] [CrossRef]
- Miller, R.E.; Shenoy, V.B. Size-dependent elastic properties of nanosized structural elements. Nanotechnology 2000, 11, 139–147. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Chen, X. Effect of surface stress on the asymmetric yield strength of nanowires. J. Appl. Phys. 2008, 103, 123527. [Google Scholar] [CrossRef]
- Weinberger, C.R.; Cai, W. Plasticity of metal nanowires. J. Mater. Chem. 2012, 22, 3277. [Google Scholar] [CrossRef]
- Ferrari, A.C. Diamond-like carbon for magnetic storage disks. Surf. Coat. Technol. 2004, 180–181, 190–206. [Google Scholar] [CrossRef]
- Price, M.; Ovcharenko, A.; Raeymaekers, B. Qualitative Evaluation of Ultra-thin Multi-layer Diamond-Like Carbon Coatings Using Molecular Dynamics Nanoindentation Simulations. Tribol. Lett. 2016, 62, 3. [Google Scholar] [CrossRef]
- Farkas, D.; Caro, A.; Bringa, E.; Crowson, D. Mechanical response of nanoporous gold. Acta Mater. 2013, 61, 3249–3256. [Google Scholar] [CrossRef]
- Saffarini, M.H.; Voyiadjis, G.Z.; Ruestes, C.J. Scaling laws for nanoporous metals under uniaxial loading. J. Mater. Res. 2021, 36, 2729–2741. [Google Scholar] [CrossRef]
- Lukes, J.R.; Zhong, H. Thermal Conductivity of Individual Single-Wall Carbon Nanotubes. J. Heat Transf. 2007, 129, 705–716. [Google Scholar] [CrossRef]
- Khan, A.; Navid, I.; Noshin, M.; Uddin, H.; Hossain, F.; Subrina, S. Equilibrium Molecular Dynamics (MD) Simulation Study of Thermal Conductivity of Graphene Nanoribbon: A Comparative Study on MD Potentials. Electronics 2015, 4, 1109–1124. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Chen, R.; Delgado, R.D.; Liang, W.; Garnett, E.C.; Najarian, M.; Majumdar, A.; Yang, P. Enhanced thermoelectric performance of rough silicon nanowires. Nature 2008, 451, 163–167. [Google Scholar] [CrossRef]
- Li, R.; Lee, E.; Luo, T. A unified deep neural network potential capable of predicting thermal conductivity of silicon in different phases. Mater. Today Phys. 2020, 12, 100181. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Pei, Q.X.; Zhang, Y.W. Surface morphology and strain coupling effects on phonon transport in silicon nanowires. Mater. Today Proc. 2016, 3, 2759–2765. [Google Scholar] [CrossRef]
- He, H.; Thorpe, M.F. Elastic properties of glasses. Phys. Rev. Lett. 1985, 54, 2107. [Google Scholar] [CrossRef]
- Jana, R.; Savio, D.; Deringer, V.; Pastewka, L. Structural and elastic properties of amorphous carbon from simulated quenching at low rates. Model. Simul. Mater. Sci. Eng. 2019, 27, 085009. [Google Scholar] [CrossRef]
- Fujii, A. Effect of Nanoporosity on the Thermal Conductivity of Amorphous Carbon. Ph.D. Thesis, UCLA, Los Angeles, CA, USA, 2014. [Google Scholar]
- Falabella, S.; Boercker, D.; Sanders, D. Fabrication of amorphous diamond films. Thin Solid Films 1993, 236, 82–86. [Google Scholar] [CrossRef]
- Verdier, M.; Han, Y.; Lacroix, D.; Chapuis, P.O.; Termentzidis, K. Radial dependence of thermal transport in silicon nanowires. J. Phys. Mater. 2018, 2, 015002. [Google Scholar] [CrossRef]
- Baggioli, M.; Zaccone, A. Unified theory of vibrational spectra in hard amorphous materials. Phys. Rev. Res. 2020, 2, 013267–013271. [Google Scholar] [CrossRef]
- Kumagai, T.; Choi, J.; Izumi, S.; Kato, T. Structures and phonon properties of nanoscale fractional graphitic structures in amorphous carbon determined by molecular simulations. J. Appl. Phys. 2010, 107, 104307. [Google Scholar] [CrossRef]
- Matsubara, H.; Kikugawa, G.; Bessho, T.; Ohara, T. Evaluation of thermal conductivity and its structural dependence of a single nanodiamond using molecular dynamics simulation. Diam. Relat. Mater. 2020, 102, 107669. [Google Scholar] [CrossRef]
- Alben, R.; Weaire, D.; Smith, J.; Brodsky, M. Vibrational properties of amorphous Si and Ge. Phys. Rev. B 1975, 11, 2271–2296. [Google Scholar] [CrossRef]
- Pardanaud, C.; Cartry, G.; Lajaunie, L.; Arenal, R.; Buijnsters, J.G. Investigating the Possible Origin of Raman Bands in Defective sp2/sp3 Carbons below 900 cm−1: Phonon Density of States or Double Resonance Mechanism at Play? C 2019, 5, 79. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14108. [Google Scholar] [CrossRef]
- Lopinski, G.P.; Merkulov, V.I.; Lannin, J.S. Vibrational states of tetrahedral amorphous carbon. Appl. Phys. Lett. 1996, 69, 3348–3350. [Google Scholar] [CrossRef]
- Bhattarai, B.; Pandey, A.; Drabold, D. Evolution of amorphous carbon across densities: An inferential study. Carbon 2018, 131, 168–174. [Google Scholar] [CrossRef]
- Donadio, D.; Galli, G. Temperature Dependence of the Thermal Conductivity of Thin Silicon Nanowires. Nano Lett. 2010, 10, 847–851. [Google Scholar] [CrossRef]
- Malhotra, A.; Maldovan, M. Phononic pathways towards rational design of nanowire heat conduction. Nanotechnology 2019, 30, 372002. [Google Scholar] [CrossRef]
- Santiago, J.; Fernández-Martínez, I.; Sánchez-López, J.C.; Rojas, T.C.; Wennberg, A.; Bellido-González, V.; Molina-Aldareguia, J.M.; Monclús, A.; González-Arrabal, R. Tribomechanical properties of hard Cr-doped DLC coatings deposited by low-frequency HiPIMS. Surf. Coat. Technol. 2020, 382, 124899–124903. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Barzaga, G.; Valencia, F.J.; Carrasco, M.I.; González, R.I.; Parlanti, M.G.; Miranda, E.N.; Bringa, E.M. Enhancing the Thermal Conductivity of Amorphous Carbon with Nanowires and Nanotubes. Nanomaterials 2022, 12, 2835. https://doi.org/10.3390/nano12162835
Mora-Barzaga G, Valencia FJ, Carrasco MI, González RI, Parlanti MG, Miranda EN, Bringa EM. Enhancing the Thermal Conductivity of Amorphous Carbon with Nanowires and Nanotubes. Nanomaterials. 2022; 12(16):2835. https://doi.org/10.3390/nano12162835
Chicago/Turabian StyleMora-Barzaga, Geraudys, Felipe J. Valencia, Matías I. Carrasco, Rafael I. González, Martín G. Parlanti, Enrique N. Miranda, and Eduardo M. Bringa. 2022. "Enhancing the Thermal Conductivity of Amorphous Carbon with Nanowires and Nanotubes" Nanomaterials 12, no. 16: 2835. https://doi.org/10.3390/nano12162835
APA StyleMora-Barzaga, G., Valencia, F. J., Carrasco, M. I., González, R. I., Parlanti, M. G., Miranda, E. N., & Bringa, E. M. (2022). Enhancing the Thermal Conductivity of Amorphous Carbon with Nanowires and Nanotubes. Nanomaterials, 12(16), 2835. https://doi.org/10.3390/nano12162835






