Investigation on Crystal-Structure, Thermal and Electrical Properties of PVDF Nanocomposites with Cobalt Oxide and Functionalized Multi-Wall-Carbon-Nanotubes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of 1D Co3O4 Nanostructures
2.3. Functionalization of the MWCNTs
2.4. Preparation of Co3O4/PVDF and Co3O4/f-CNTs/PVDF Nanocomposites
2.5. Characterization of the Co3O4 and Its Nanocomposites
3. Results and Discussion
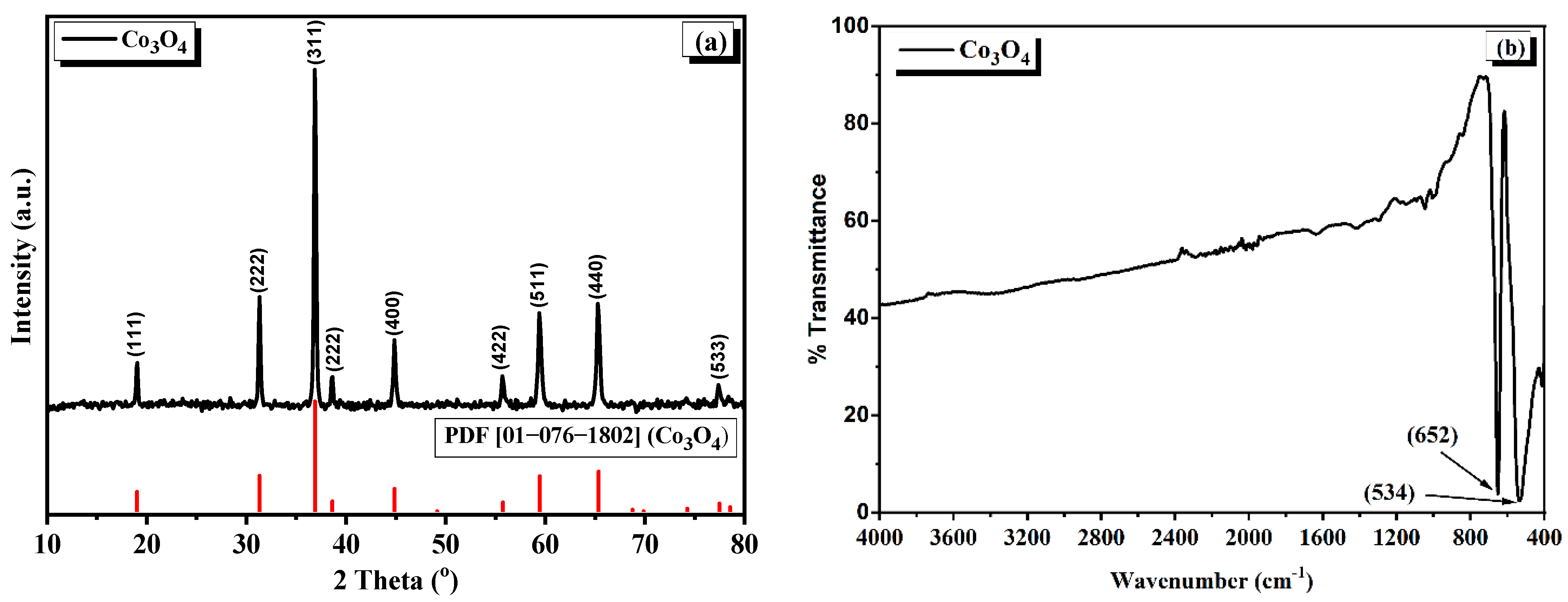
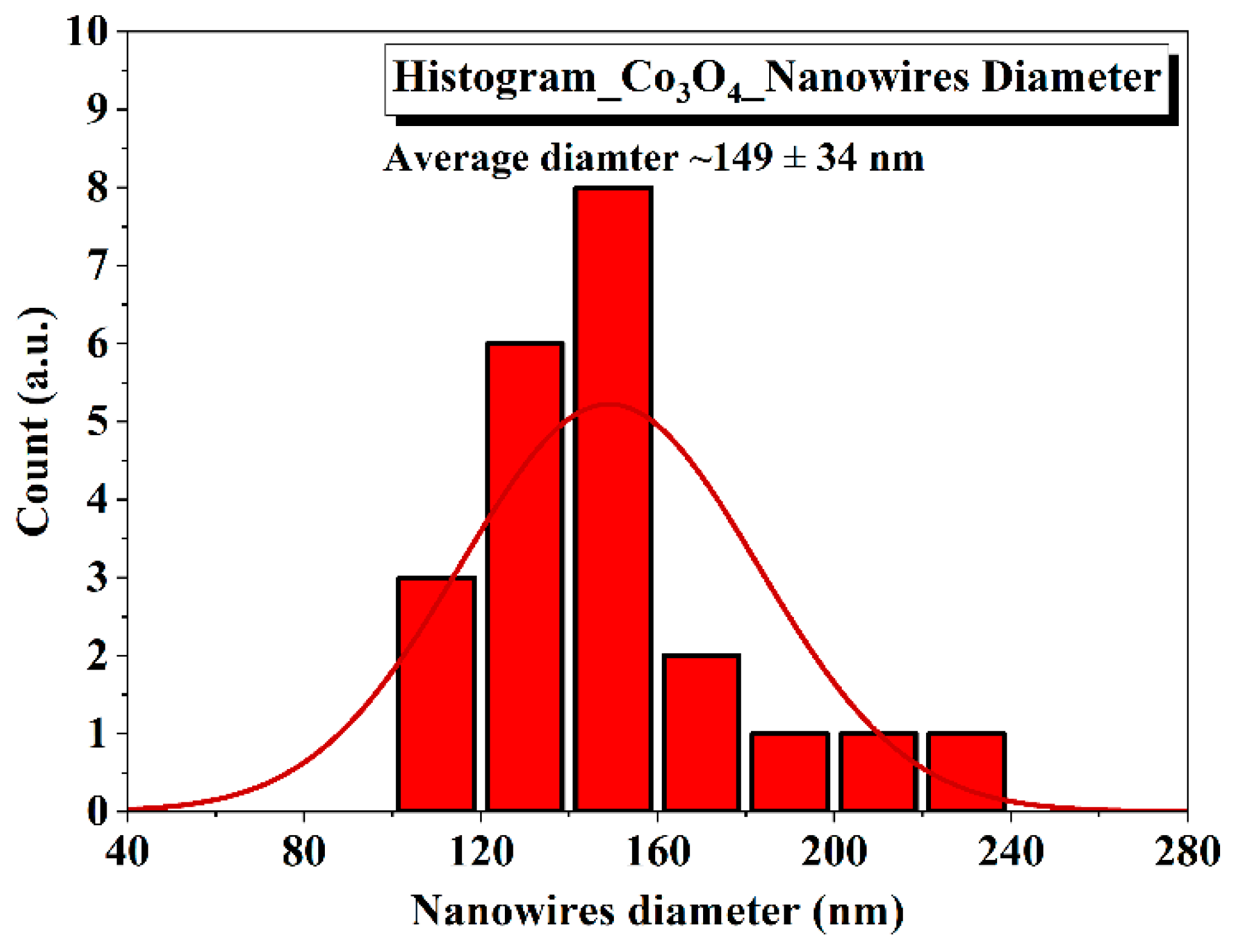
3.1. Analysis of Co3O4 Nanostructures
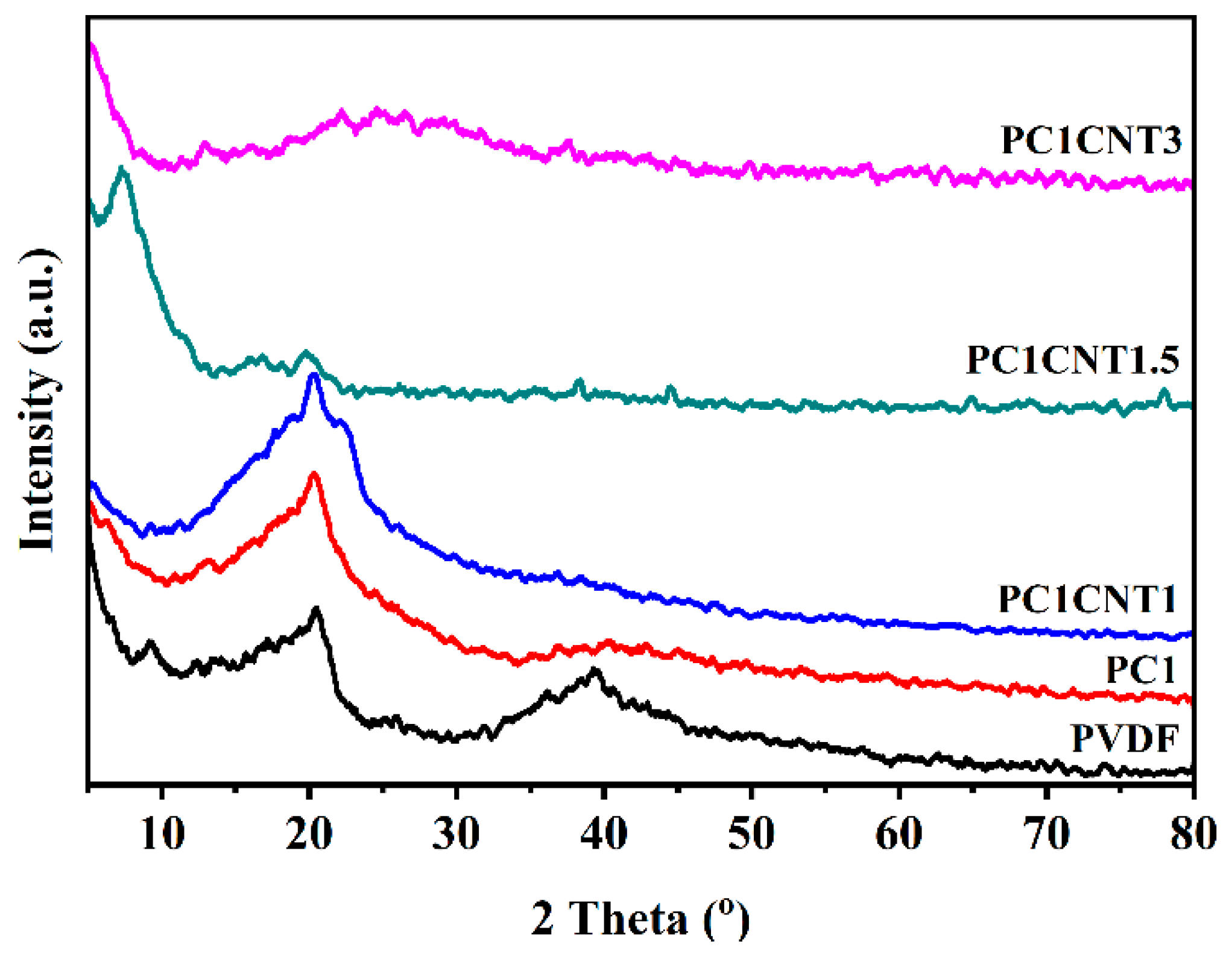
3.2. Structural Analysis of PVDF Nanocomposite Films
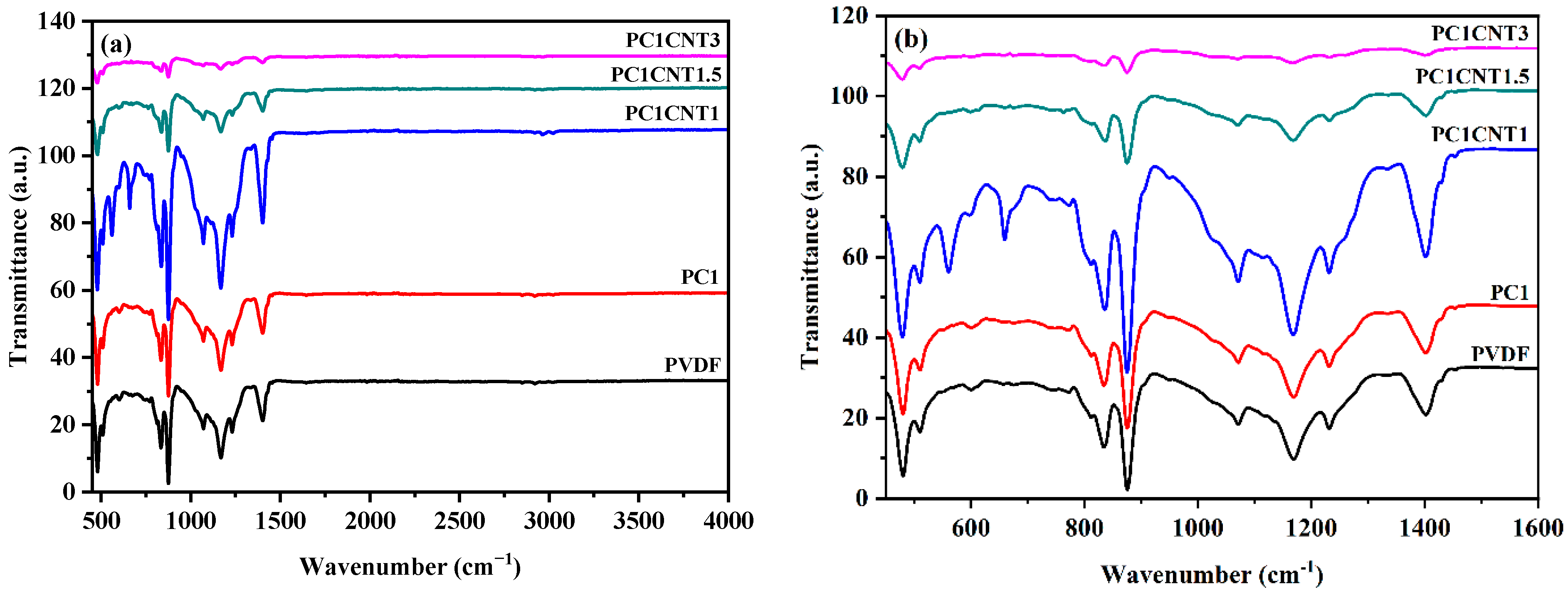
3.3. FTIR Analysis
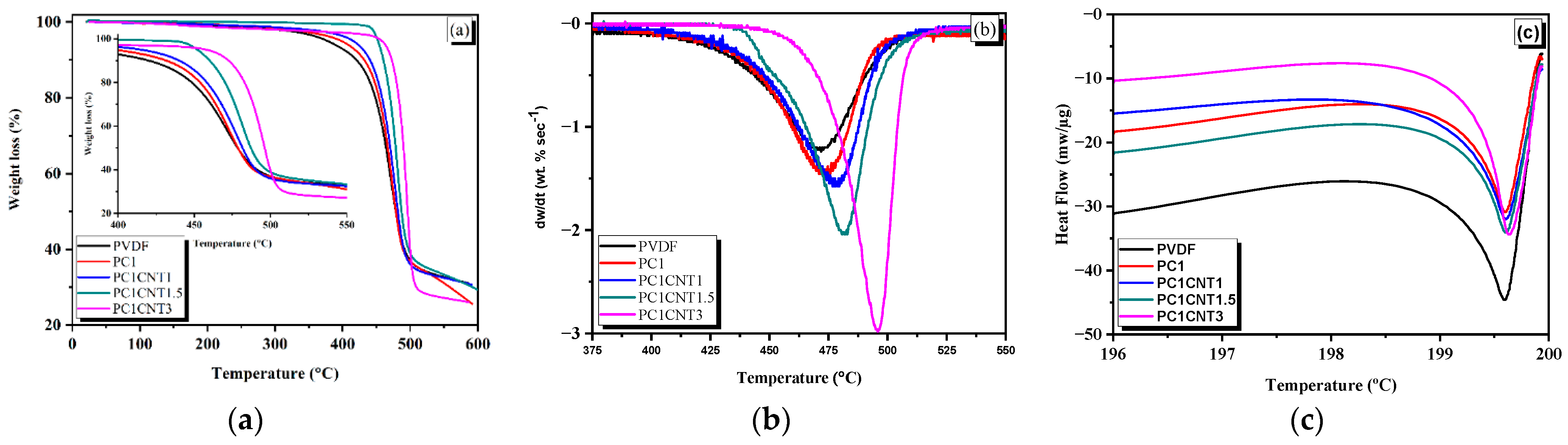
3.4. Thermal Analysis of PVDF Nanocomposite Films
3.5. Electrical Behavior of PVDF Nanocomposites Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safarpour, M.; Khataee, A.; Vatanpour, V. Effect of reduced graphene oxide/TiO2 nanocomposite with different molar ratios on the performance of PVDF ultrafiltration membranes. Sep. Purif. Technol. 2015, 140, 32–42. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Z.; Quan, S.; Xu, Y.; Jiang, Z.; Shao, L. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly(vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl. Surf. Sci. 2014, 316, 537–548. [Google Scholar] [CrossRef]
- Gregorio, R., Jr. Determination of the α, β, and γ crystalline phases of poly (vinylidene fluoride) films prepared at different conditions. J. Appl. Polym. Sci. 2006, 100, 3272–3279. [Google Scholar] [CrossRef]
- Kim, I.-H.; Baik, D.H.; Jeong, Y.G. Structures, electrical, and dielectric properties of PVDF-based nanocomposite films reinforced with neat multi-walled carbon nanotube. Macromol. Res. 2012, 20, 920–927. [Google Scholar] [CrossRef]
- Javed, M.S.; Shah, S.S.A.; Najam, T.; Siyal, S.H.; Hussain, S.; Saleem, M.; Zhao, Z.; Mai, W. Achieving high-energy density and superior cyclic stability in flexible and lightweight pseudocapacitor through synergic effects of binder-free CoGa2O4 2D-hexagonal nanoplates. Nano Energy 2020, 77, 105276. [Google Scholar] [CrossRef]
- Ameduri, B. From Vinylidene Fluoride (VDF) to the Applications of VDF-Containing Polymers and Copolymers: Recent Developments and Future Trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Ullah, H.; Kausar, A.; Siddiq, M.; Aleem, M.A. Epoxy functionalized multi-walled carbon nanotubes/polyvinylidene fluoride nanocomposites: Microstructure, morphology, thermal, piezoelectricity and conductivity investigations. Polym. Compos. 2019, 40, e776–e794. [Google Scholar] [CrossRef]
- Begum, S.; Ullah, H.; Kausar, A.; Siddiq, M.; Aleem, M.A. Fabrication of epoxy functionalized MWCNTs reinforced PVDF nanocomposites with high dielectric permittivity, low dielectric loss and high electrical conductivity. Compos. Sci. Technol. 2018, 167, 497–506. [Google Scholar] [CrossRef]
- Sorayani Bafqi, M.S.; Bagherzadeh, R.; Latifi, M. Fabrication of composite PVDF-ZnO nanofiber mats by electrospinning for energy scavenging application with enhanced efficiency. J. Polym. Res. 2015, 22, 130. [Google Scholar] [CrossRef]
- Levi, N.; Czerw, R.; Xing, S.; Iyer, P.; Carroll, D.L. Properties of Polyvinylidene Difluoride−Carbon Nanotube Blends. Nano Lett. 2004, 4, 1267–1271. [Google Scholar] [CrossRef]
- Ojha, S.; Paria, S.; Karan, S.K.; Si, S.K.; Maitra, A.; Das, A.K.; Halder, L.; Bera, A.; De, A.; Khatua, B.B. Morphological interference of two different cobalt oxides derived from a hydrothermal protocol and a single two-dimensional metal organic framework precursor to stabilize the β-phase of PVDF for flexible piezoelectric nanogenerators. Nanoscale 2019, 11, 22989–22999. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.M.; Seo, D.M.; Yun, H.D.; Thangavel, G.; Park, L.S.; Nam, S.Y. Transparent Conducting Film Fabricated by Metal Mesh Method with Ag and Cu@Ag Mixture Nanoparticle Pastes. Metals 2017, 7, 176. [Google Scholar] [CrossRef]
- Wei, Y.; Chu, H.-Q.; Dong, B.-Z.; Li, X.; Xia, S.-J.; Qiang, Z.-M. Effect of TiO2 nanowire addition on PVDF ultrafiltration membrane performance. Desalination 2011, 272, 90–97. [Google Scholar] [CrossRef]
- Huang, W.; Edenzon, K.; Fernandez, L.; Razmpour, S.; Woodburn, J.; Cebe, P. Nanocomposites of poly (vinylidene fluoride) with multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2010, 115, 3238–3248. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yan, H. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Joshi, R.K.; Schneider, J.J. Assembly of one dimensional inorganic nanostructures into functional 2D and 3D architectures. Synthesis, arrangement and functionality. Chem. Soc. Rev. 2012, 41, 5285–5312. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Petukhova, A.; Kumacheva, E. Properties and emerging applications of self-assembled structures made from inorganic nanoparticles. Nat. Nanotechnol. 2010, 5, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Martins, P.M.; Caparrós, C.; Martins, P.; Benelmekki, M.; Botelho, G.; Barandiarán, J.M. Nucleation of the electroactive β-phase, dielectric and magnetic response of poly (vinylidene fluoride) composites with Fe2O3 nanoparticles. J. Non-Cryst. Solids 2013, 361, 93–99. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Shen, B.; Zhai, J. Enhanced discharged energy density and efficiency of poly (vinylidene fluoride) nanocomposites through a small loading of core-shell structured BaTiO3@Al2O3 nanofibers. Ceram. Int. 2017, 43, 585–589. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Y.; Liu, W.; Zhang, S.; Liu, G.; Chen, H.; Qiu, J. Hydrothermal synthesis and electrochemical performance of Co3O4/reduced graphene oxide nanosheet composites for supercapacitors. Electrochim. Acta 2013, 112, 120–126. [Google Scholar] [CrossRef]
- Son, M.Y.; Kim, J.H.; Kang, Y.C. Study of Co3O4 mesoporous nanosheets prepared by a simple spray-drying process and their electrochemical properties as anode material for lithium secondary batteries. Electrochim. Acta 2014, 116, 44–50. [Google Scholar] [CrossRef]
- Park, K.; Seo, D.; Lee, J. Conductivity of silver paste prepared from nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 351–354. [Google Scholar] [CrossRef]
- Siyal, S.H.; Javed, M.S.; Ahmad, A.; Sajjad, M.; Batool, S.; Khan, A.J.; Akram, S.; Alothman, A.A.; Alshgari, R.A.; Najam, T. Free-standing 3D Co3O4@NF micro-flowers composed of porous ultra-long nanowires as an advanced cathode material for supercapacitor. Curr. Appl. Phys. 2021, 31, 221–227. [Google Scholar] [CrossRef]
- Datta, J.; Nandi, A.K. Cocrystallization of poly (vinylidene fluoride) and vinylidene fluoride-tetrafluoro-ethylene copolymer blends: 3. Structural study. Polymer 1997, 38, 2719–2724. [Google Scholar] [CrossRef]
- Okada, D.; Kaneko, H.; Kato, K.; Furumi, S.; Takeguchi, M.; Yamamoto, Y. Colloidal Crystallization and Ionic Liquid Induced Partial β-Phase Transformation of Poly (vinylidene fluoride) Nanoparticles. Macromolecules 2015, 48, 2570–2575. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A critical analysis of the α, β and γ phases in poly (vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]
- Mohammadi, B.; Yousefi, A.A.; Bellah, S.M. Effect of tensile strain rate and elongation on crystalline structure and piezoelectric properties of PVDF thin films. Polym. Test. 2007, 26, 42–50. [Google Scholar] [CrossRef]
- Kim, G.H.; Hong, S.M.; Seo, Y. Piezoelectric properties of poly (vinylidene fluoride) and carbon nanotube blends: β-phase development. Phys. Chem. Chem. Phys. 2009, 11, 10506–10512. [Google Scholar] [CrossRef]
- Chatterjee, J.; Nash, N.; Cottinet, P.J.; Wang, B. Synthesis and characterization of poly (vinylidene fluoride)/carbon nanotube composite piezoelectric powders. J. Mater. Res. 2012, 27, 2352–2359. [Google Scholar] [CrossRef]
- Xu, L.; Ma, J.; Wei, G.; Gu, C.; Tao, J. Improved surface-enhanced Raman scattering performance enabled by hydrophilic-hydrophobic piezoelectric PVDF-silver substrate. Sens. Actuators B Chem. 2022, 132431. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tashiro, K.; Tadokoro, H. Molecular Vibrations of Three Crystal Forms of Poly (vinylidene fluoride). Macromolecules 1975, 8, 158–171. [Google Scholar] [CrossRef]
- Ke, K.; Petra, P.; Dieter, J.; Dieter, F.; Brigitte, V. Achieving β-phase poly (vinylidene fluoride) from melt cooling: Effect of surface functionalized carbon nanotubes. Polymer 2014, 55, 611–619. [Google Scholar] [CrossRef]
- Olmos, D.; Rodríguez-Gutiérrez, E.; González-Benito, J. Polymer structure and morphology of low density polyethylene filled with silica nanoparticles. Polym. Compos. 2012, 33, 2009–2021. [Google Scholar] [CrossRef]
- Sanchez, F.A.; González-Benito, J. PVDF BaTiO3/carbon nanotubes ternary nanocomposites: Effect of nanofillers and processing. Polym. Compos. 2017, 38, 227–235. [Google Scholar] [CrossRef]
- Gurunathan, T.; Nayak, S.K. The influence of reactive organoclay on a biorenewable castor oil-based polyurethane prepolymers toughened polylactide nanocomposites. Polym. Adv. Technol. 2016, 27, 1484–1493. [Google Scholar] [CrossRef]
- Gurunathan, T.; Chung, J.S. Physicochemical Properties of Amino–Silane-Terminated Vegetable Oil-Based Waterborne Polyurethane Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 4645–4653. [Google Scholar] [CrossRef]
- Ma, J.; Nan, X.; Liu, J. Investigation of the dielectric, mechanical, and thermal properties of noncovalent functionalized MWCNTs/polyvinylidene fluoride (PVDF) composites. Polym. Adv. Technol. 2017, 28, 166–173. [Google Scholar] [CrossRef]
- Bahader, A.; Gui, H.; Li, Y.; Xu, P.; Ding, Y. Crystallization kinetics of PVDF filled with multi wall carbon nanotubes modified by amphiphilic ionic liquid. Macromol. Res. 2015, 23, 273–283. [Google Scholar] [CrossRef]
- Al-Solamy, F.R.; Al-Ghamdi, A.A.; Mahmoud, W.E. Piezoresistive behavior of graphite nanoplatelets based rubber nanocomposites. Polym. Adv. Technol. 2012, 23, 478–482. [Google Scholar] [CrossRef]
- Mahmoud, W.E.; Al-Ghamdi, A.A. Charge transport mechanism of graphite-nanosheet-loaded rubber nanocomposites. Polym. Int. 2012, 61, 51–54. [Google Scholar] [CrossRef]


| Sample Code | PVDF (wt.%) | Co3O4 (wt.%) | f-MWCNTs |
|---|---|---|---|
| PVDF | 100 | 0.0 | 0.0 |
| PC1 | 99.9 | 0.1 | 0.0 |
| PC1CNT1 | 99.8 | 0.1 | 0.1 |
| PC1CNT1.5 | 99.75 | 0.1 | 0.15 |
| PC1CNT3 | 99.6 | 0.1 | 0.3 |
| Temp | PVDF | PC1 | PC1CNT1 | PC1CNT1.5 | PC1CNT3 |
|---|---|---|---|---|---|
| Tonset (°C) | 340 | 374 | 401 | 440 | 449 |
| Tend (°C) | 496 | 498 | 503 | 508 | 510 |
| TP (°C) | 471 | 473 | 478 | 481 | 495 |
| Tm (°C) | 199.59 | 199.6 | 199.60 | 199.61 | 199.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhan; Ahmad, S.; Ullah, H.; Rehman, Z.U.; Nawaz, M.; Uddin, I.; Parkash, A.; Alamri, H.R.; Alsaiari, N.S.; Javed, M.S. Investigation on Crystal-Structure, Thermal and Electrical Properties of PVDF Nanocomposites with Cobalt Oxide and Functionalized Multi-Wall-Carbon-Nanotubes. Nanomaterials 2022, 12, 2796. https://doi.org/10.3390/nano12162796
Farhan, Ahmad S, Ullah H, Rehman ZU, Nawaz M, Uddin I, Parkash A, Alamri HR, Alsaiari NS, Javed MS. Investigation on Crystal-Structure, Thermal and Electrical Properties of PVDF Nanocomposites with Cobalt Oxide and Functionalized Multi-Wall-Carbon-Nanotubes. Nanomaterials. 2022; 12(16):2796. https://doi.org/10.3390/nano12162796
Chicago/Turabian StyleFarhan, Shabir Ahmad, Hameed Ullah, Zia Ur Rehman, Mohsan Nawaz, Imad Uddin, Anand Parkash, Hatem R Alamri, Norah Salem Alsaiari, and Muhammad Sufyan Javed. 2022. "Investigation on Crystal-Structure, Thermal and Electrical Properties of PVDF Nanocomposites with Cobalt Oxide and Functionalized Multi-Wall-Carbon-Nanotubes" Nanomaterials 12, no. 16: 2796. https://doi.org/10.3390/nano12162796
APA StyleFarhan, Ahmad, S., Ullah, H., Rehman, Z. U., Nawaz, M., Uddin, I., Parkash, A., Alamri, H. R., Alsaiari, N. S., & Javed, M. S. (2022). Investigation on Crystal-Structure, Thermal and Electrical Properties of PVDF Nanocomposites with Cobalt Oxide and Functionalized Multi-Wall-Carbon-Nanotubes. Nanomaterials, 12(16), 2796. https://doi.org/10.3390/nano12162796








