Synthesis and Characterization of Coordination Compound [Eu(µ2-OC2H5)(btfa)(NO3)(phen)]2phen with High Luminescence Efficiency
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Methods for Characterization of the Complex
3. Results and Discussion
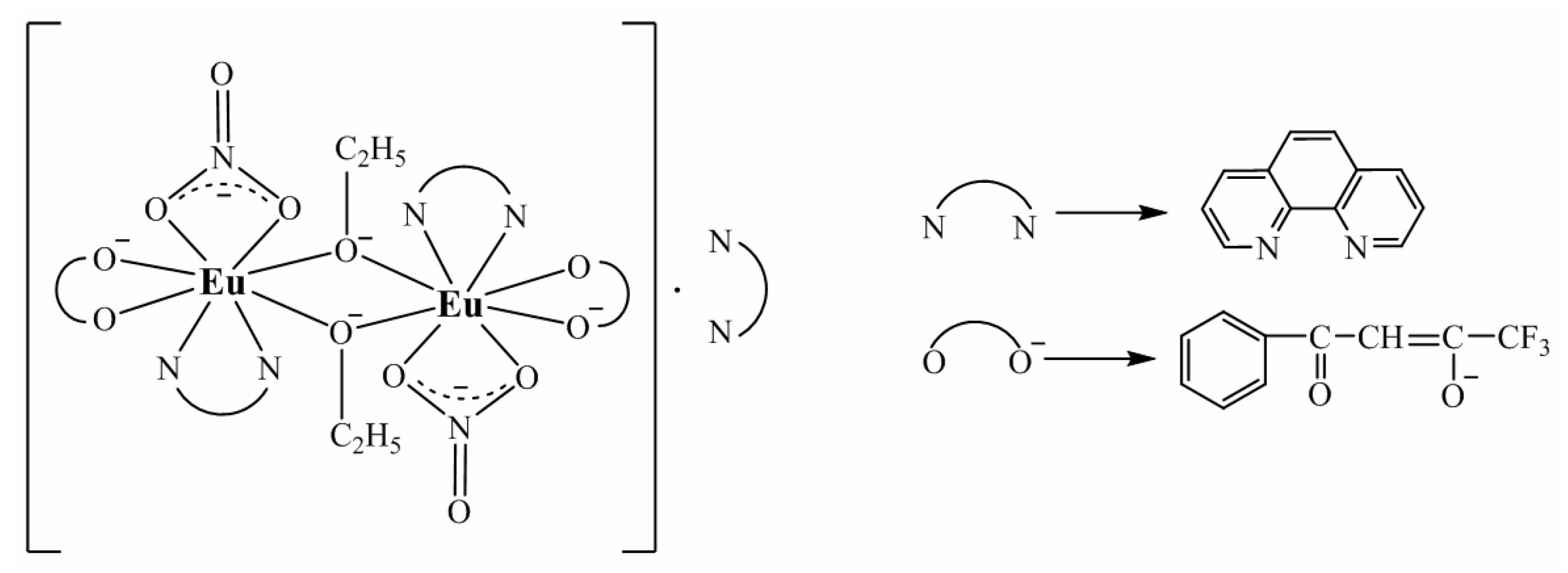
3.1. Infrared Spectra
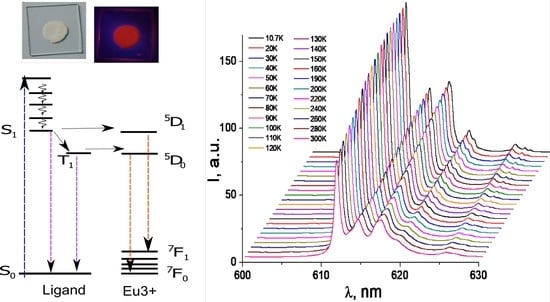
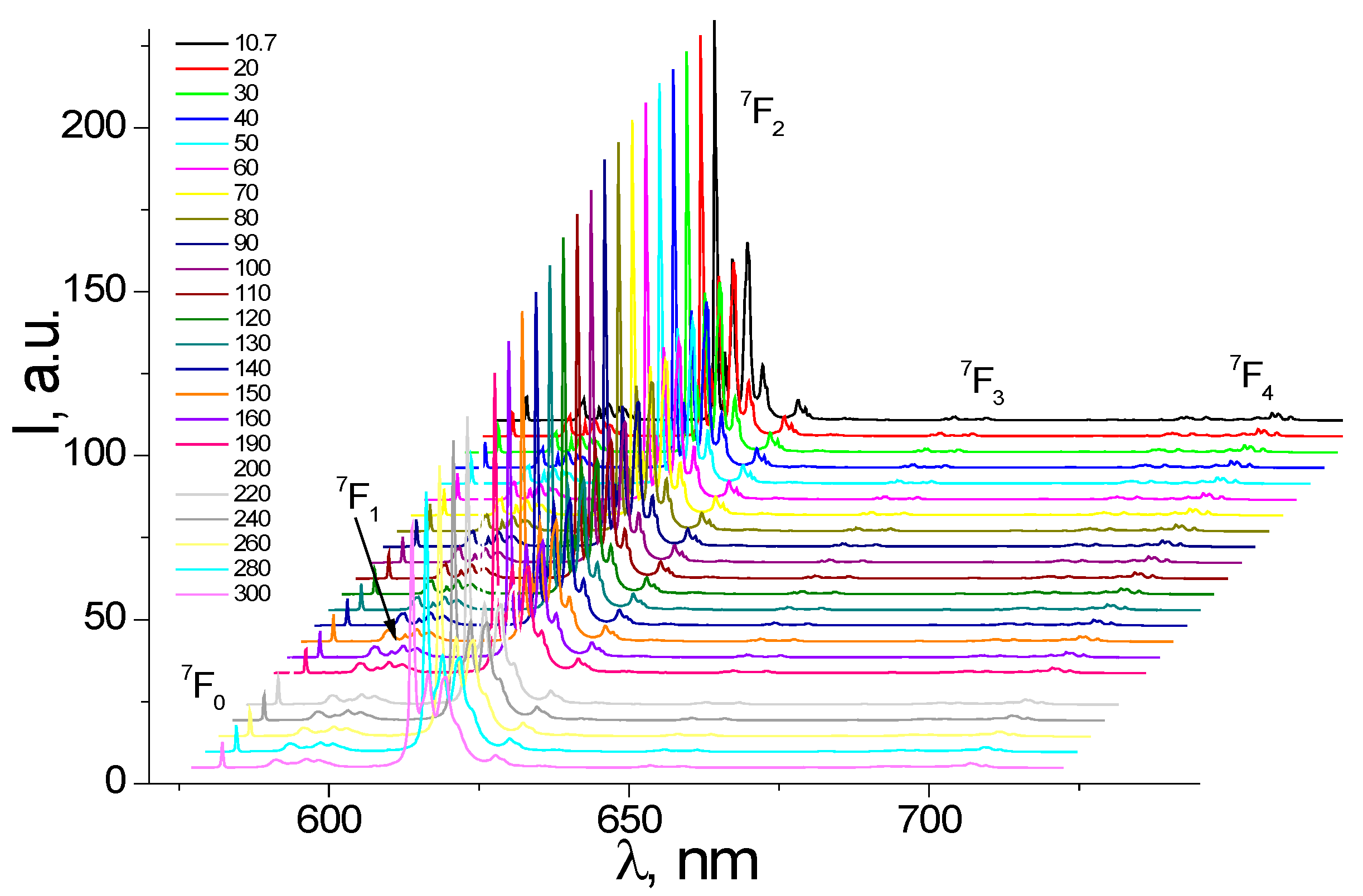
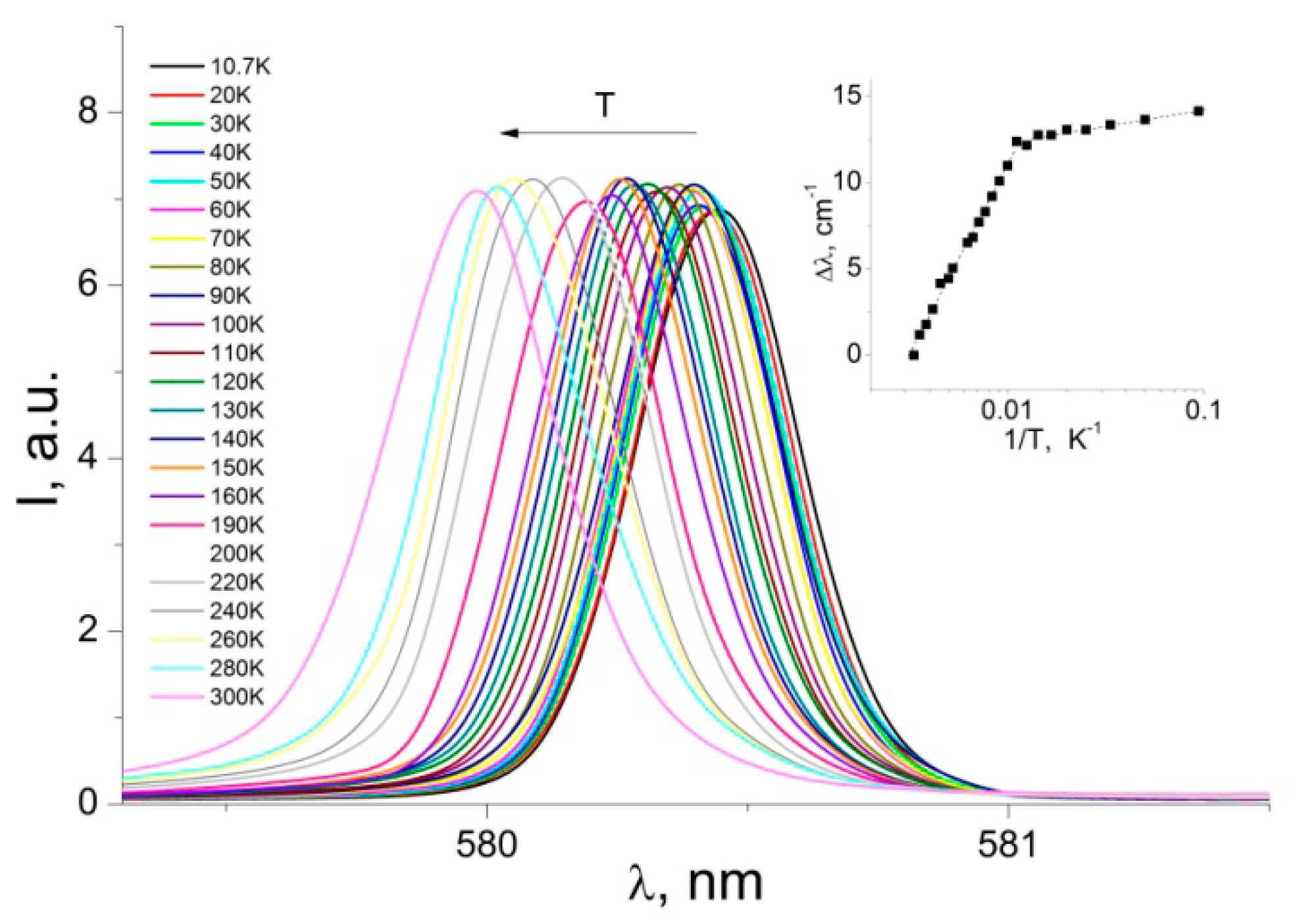
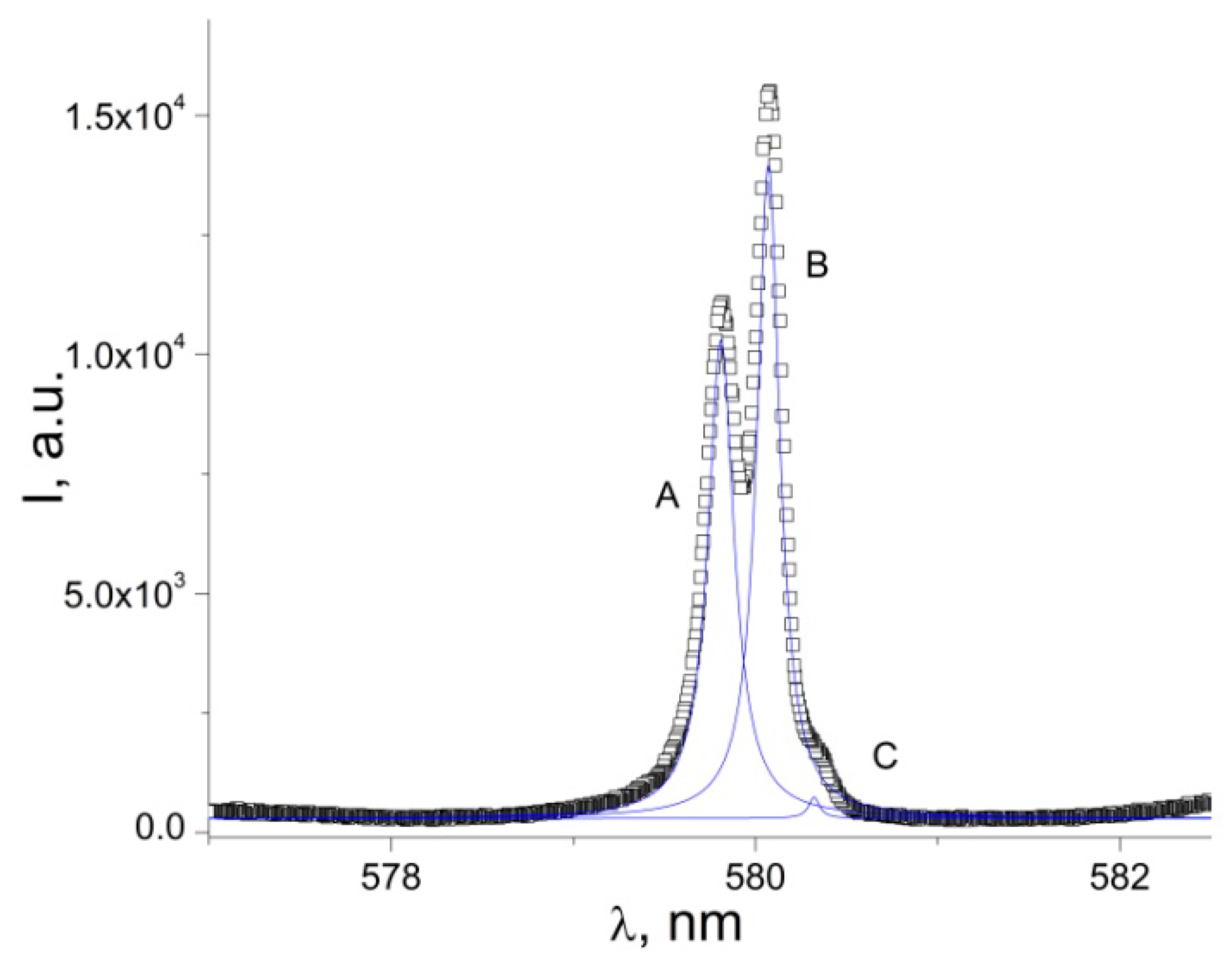
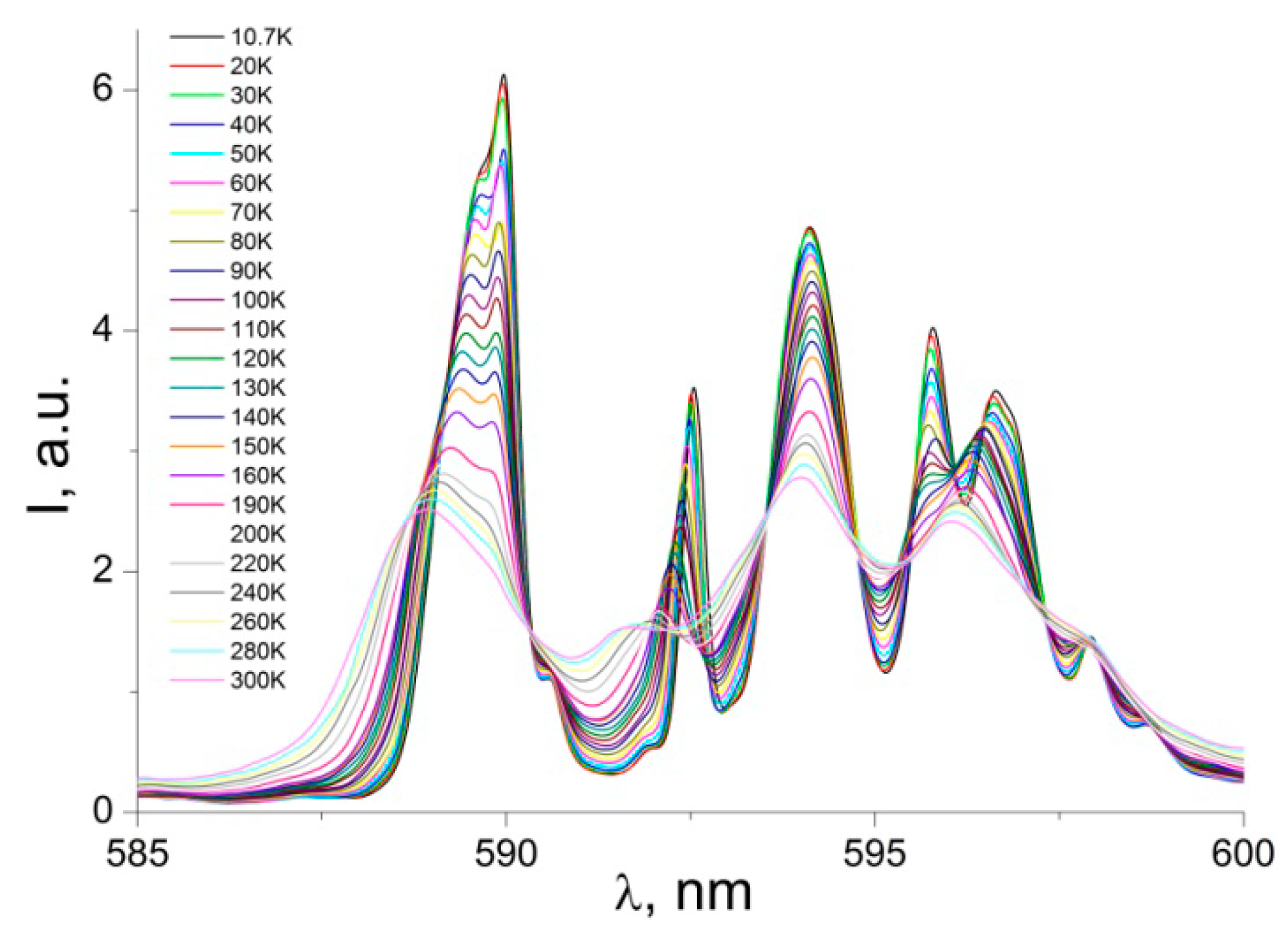
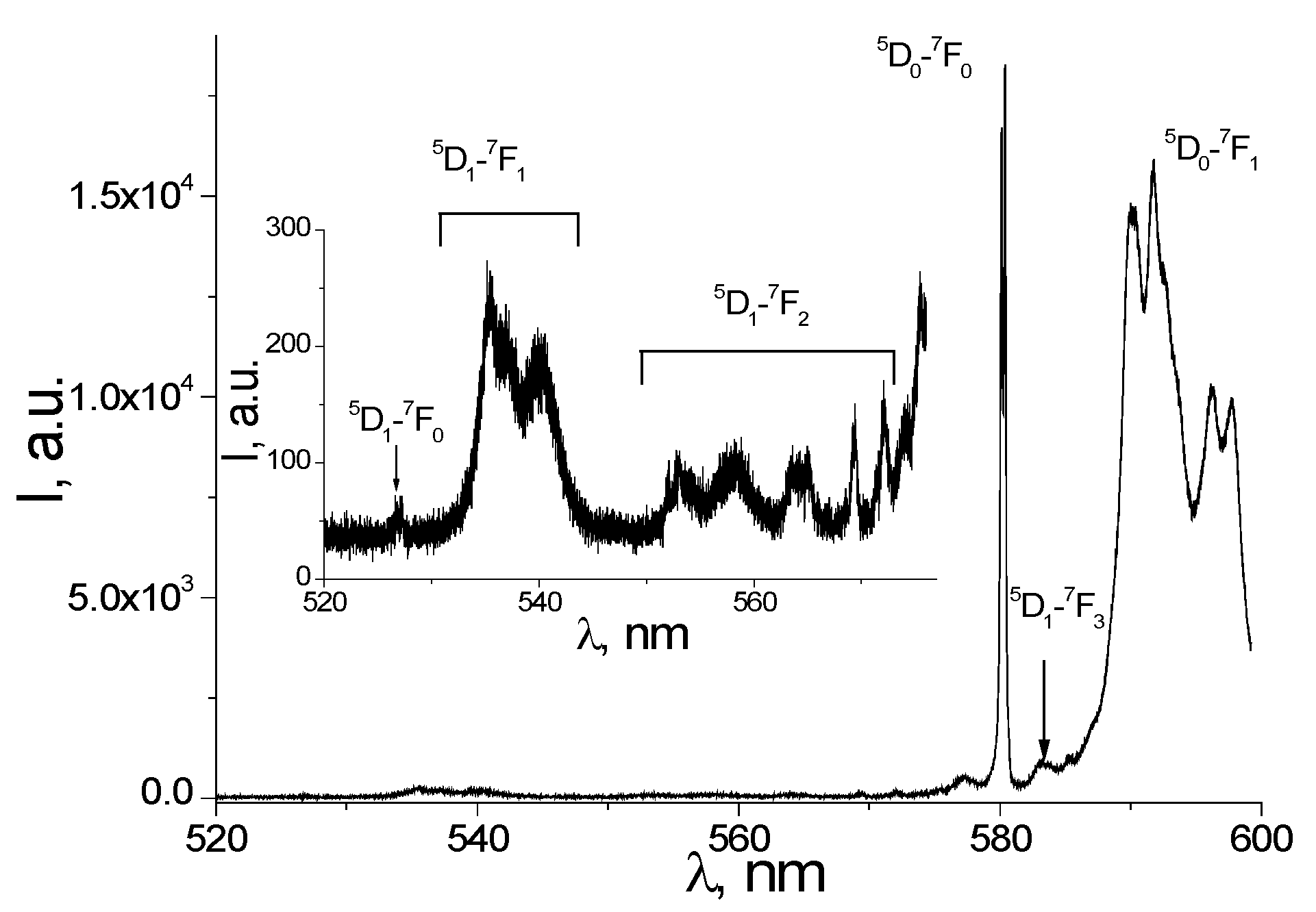
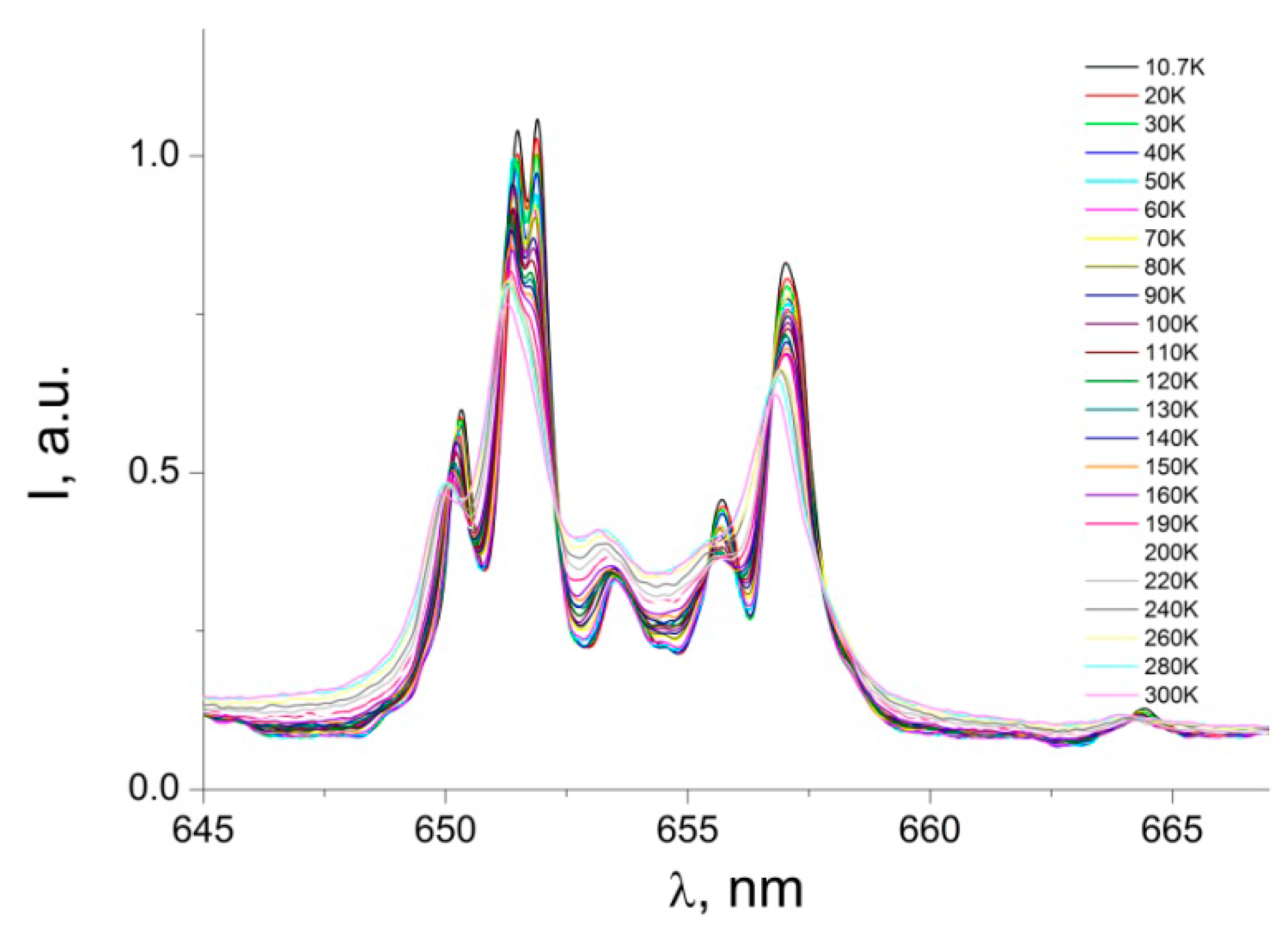
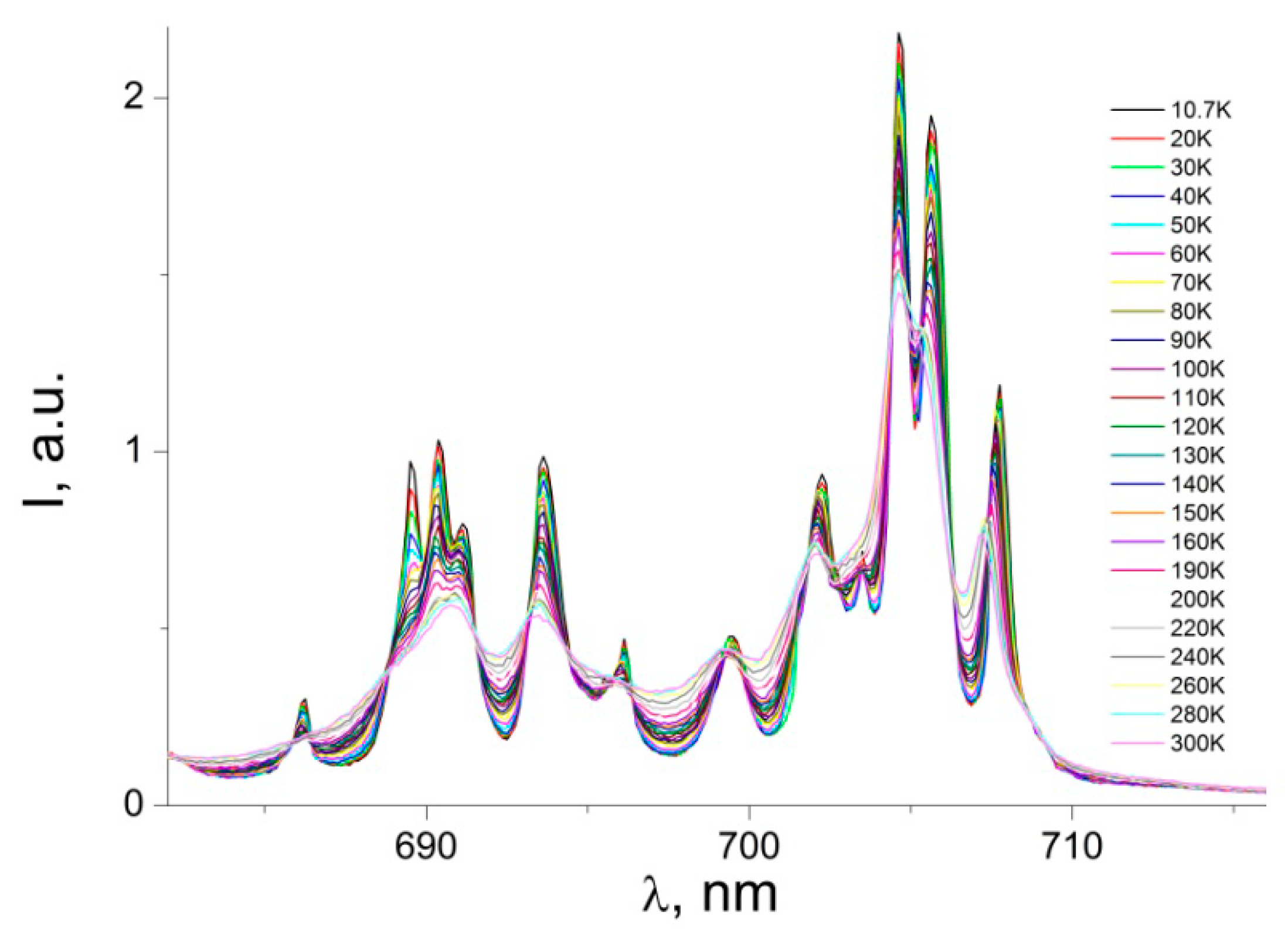
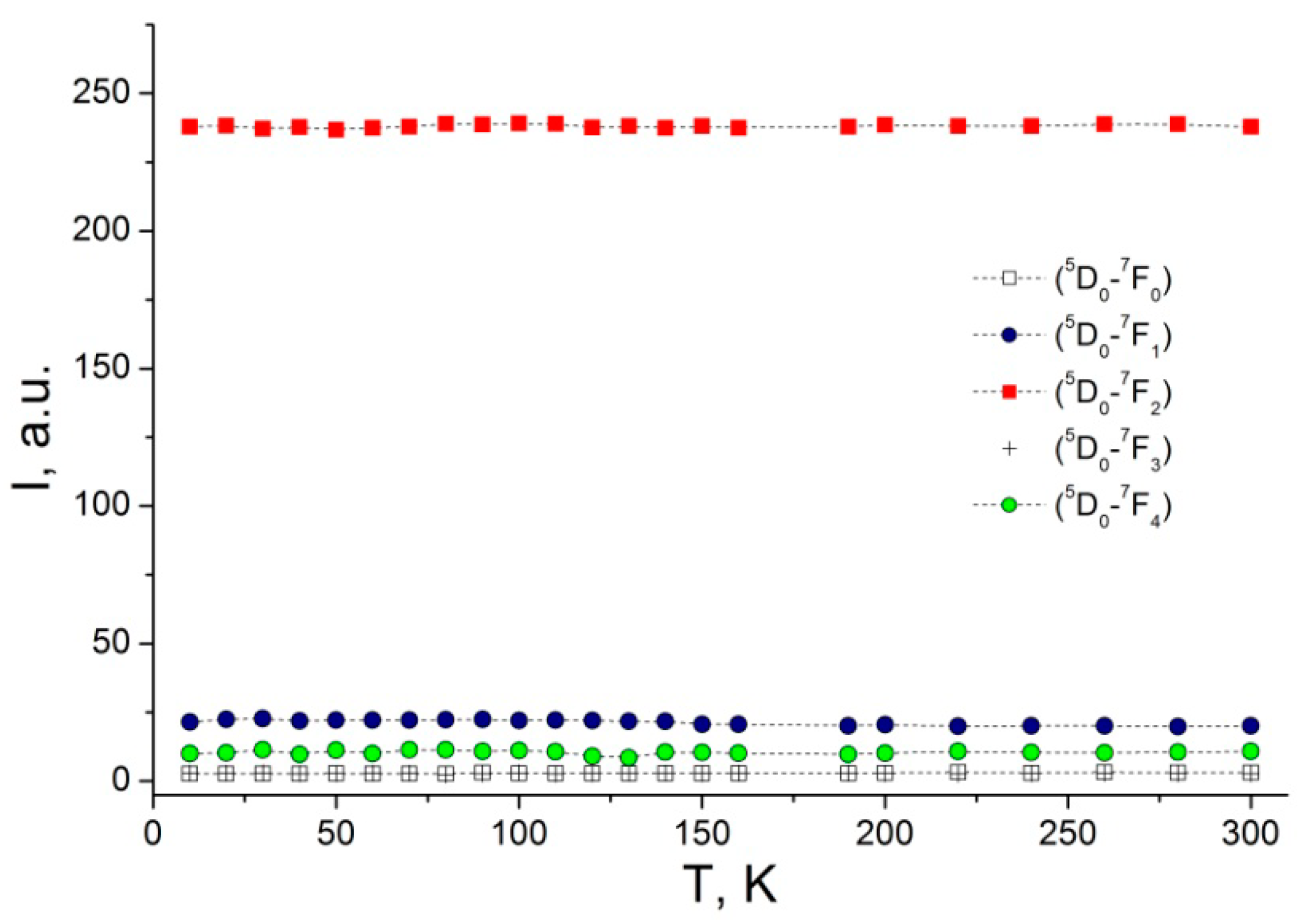
3.2. Photoluminescence and Discussion
4. Conclusions
5. Patent MD 4677 B1 2020.02.29 (Republic of Moldova)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ungur, L.; Szabo, B.; ALOthman, Z.A.; Al-Kahtani, A.A.S.; Chibotaru, L.F. Mechanisms of Luminescence in Lanthanide Complexes: A Crucial Role of Metal−Ligand Covalency. Inorg. Chem. 2022, 61, 5972–5976. [Google Scholar] [CrossRef] [PubMed]
- Denisenko, Y.G.; Atuchin, V.V.; Molokeev, M.S.; Aleksandrovsky, A.S.; Krylov, A.S.; Oreshonkov, A.S.; Volkova, S.S.; Andreev, O.V. Structure, Thermal Stability, and Spectroscopic Properties of Triclinic Double Sulfate AgEu(SO4)2 with Isolated SO4 Groups. Inorg. Chem. 2018, 57, 13279–13288. [Google Scholar] [CrossRef] [PubMed]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Eliseeva, S.V.; Bunzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Bordian, O.; Verlan, V.; Culeac, I.; Bulhac, I.; Zubarev, V. Managing the luminescence efficiency of the organic compounds of Europium(III) through preparation technology. In Proceedings of the SPIE 11718, Advanced Topics in Optoelectronics, Microelectronics and Nanotechnologies X, Online Only, Romania, 31 December 2020. [Google Scholar] [CrossRef]
- Joseph, P. Leonard and Thorfinnur Gunnlaugsson, Luminescent Eu(III) and Tb(III) Complexes: Developing Lanthanide Luminescent-Based Devices. J. Fluoresc. 2005, 15, 585–595. [Google Scholar] [CrossRef]
- Bunzli, J.-C.G.; Chauvin, A.-S.; Vandevyver, C.D.B.; Song, B.O.; Comby, S. Lanthanide Bimetallic Helicates for in Vitro Imaging and Sensing. Ann. N. Y. Acad. Sci. 2008, 1130, 97–105. [Google Scholar] [CrossRef]
- Bodman, S.E.; Butler, S.J. Advances in anion binding and sensing using luminescent lanthanide complexes. Chem. Sci. 2021, 12, 2716–2734. [Google Scholar] [CrossRef]
- Demchenko, A.P. Introduction to Fluorescence Sensing, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-20779-7. ISBN 978-3-319-20780-3 (eBook). [Google Scholar] [CrossRef]
- Räsänen, M.; Takalo, H.; Soukka, T.; Haapakka, K.; Kankare, J. Photophysicalstudy of blue-lightexcitableternaryEu(III)complexes and their encapsulation into polystyrene nanoparticles. J. Lumin. 2015, 160, 128–133. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, Z.; Snow, C.D.; Xu, Y.; Wang, Y.; Xiu, D.; Belfiore, L.A.; Tang, J. Stable Fluorescence of Eu3+ Complex Nanostructures Beneath a Protein Skin for Potential Biometric Recognition. Nanomaterials 2021, 11, 2462. [Google Scholar] [CrossRef]
- González-Mancebo, D.; Becerro, A.I.; Corral, A.; Balcerzyk, M.; Ocaña, M. Luminescence and X-ray Absorption Properties of Uniform Eu3+: (H3O)Lu3F10 Nanoprobes. Nanomaterials 2019, 9, 1153. [Google Scholar] [CrossRef]
- Piccinelli, F.; De Rosa, C.; Melchior, A.; Faura, G.; Tolazzi, M.; Bettinelli, M. Eu(III) and Tb(III) complexes of 6-fold coordinating ligands showing high affinity for the hydrogen carbonate ion: A spectroscopic and thermodynamic study. Dalton Trans. 2018, 48, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Spangler, C.; Schaferling, M. Luminescent Chemical and Physical Sensors Based on Lanthanide Complexes. In Lanthanide Luminescence. Photophysical, Analytical and Biological Aspects; Hanninen, P., Harma, H., Eds.; Springer Series on Fluorescence, Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2011; Volume 7, pp. 235–240. [Google Scholar] [CrossRef]
- Lanthanide Probes in Life, Chemical and Earth Sciences: Theory and Practice; Bunzil, J.C.; Choppin, G.R. (Eds.) Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Selvin, P.R. Principles and biophysical applications of lanthanide-based probes. Annu. Rev. Biophys. Biomol. Struct. 2002, 31, 275–302. [Google Scholar] [CrossRef]
- Binnemans, K.; van Deun, R.; Simon, C.; Collinson, R.; Gorller, W.; FrancÓ, O.M.; Duncan, W.; Bruce, D.W.; Wickleder, C. Spectroscopic behavior of lanthanide(III) coordination compounds with Schiff base ligands. Phys. Chem. Chem. Phys. 2000, 2, 3753–3757. [Google Scholar] [CrossRef]
- Binnemans, K.; Gorller-Walrand, C. Application of the Eu3+ ion for site symmetry determination. J. Rare Earths 1996, 14, 173–180. [Google Scholar]
- Binnemans, K. Review: Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Baldoví, J.J.; Kondinski, A. Exploring High-Symmetry Lanthanide—Functionalized Polyoxopalladates as Building Blocks for Quantum Computing. Inorganics 2018, 6, 101. [Google Scholar] [CrossRef]
- Aguilà, D.; Barrios, L.A.; Velasco, V.; Roubeau, O.; Repollés, A.; Alonso, P.J.; Sesé, J.; Teat, S.J.; Luis, F.; Aromí, G. Heterodimetallic [LnLn’] Lanthanide Complexes: Toward a Chemical Design of Two-Qubit Molecular Spin Quantum Gates. J. Am. Chem. Soc. 2014, 136, 14215–14222. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Xu, C.-J.; Wan, J. Mono- and dinuclear europium(III) complexes with thenoyltrifluoroacetone and 1, 10-phenanthroline-5, 6-dione. Int. J. Chem. 2014, 145, 1913–1917. [Google Scholar]
- Wang, Z.; Yang, H.; He, P.; He, Y.; Zhaoa, J.; Tang, H. A highly-efficient blue-light excitable red phosphor: Intramolecular π-stacking interactions in one dinuclear europium(III) complex. Dalton Trans. 2015, 45, 2839–2844. [Google Scholar] [CrossRef]
- Reddy, M.L.P.; Divyaa, V.; Pavithran, R. Visible-light sensitized luminescent europium(III)-β-diketonate complexes: Bioprobes for cellular imaging. Dalton Trans. 2013, 42, 15249–15262. [Google Scholar] [CrossRef]
- Zubareva, V.; Bulhac, I.; Bordian, O.; Verlan, V.; Culeac, I.; Enachescu, M.; Moise, C. Europium(III) Dinuclear Coordinating Compound with Mixed Ligands, Which Show Luminescent Properties. (Compus coordinativ dinuclear al europiului(III) cu liganzi micşti, care manifestă proprietăţi luminescente). Patent MD 4677 B1, 29 February 2020. [Google Scholar]
- Gaigalas, A.K.; Wang, L. Measurement of the fluorescence quantum yield using a spectrometer with an integrating sphere detector. J. Res. Natl. Inst. Stand. Technol. 2008, 113, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Rurack, K. Fluorescence Quantum Yields: Methods of Determination and Standards. In Springer Series on Fluorescence; Springer: Berlin/Heidelberg, Germany, 2008; Volume 5, pp. 101–145. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules; Methuen & Co. LTD: London, UK; Willey: New York, NY, USA, 1971. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley: New York, NY, USA, 1978; p. 239. [Google Scholar]
- Gordon, A.J.; Ford, R.A. A Handbook of Practical Data, Techniques and References; John Wiley and Sons: New York, NY, USA, 1972. [Google Scholar]
- Ullmann, S.; Hahn, P.; Blömer, L.; Mehnert, A.; Laube, C.; Abel, B.; Kersting, B. Dinuclear lanthanide complexes supported by a hybrid salicylaldiminato/calix[4]arene-ligand: Synthesis, structure, and magnetic and luminescence properties of (HNEt3)[Ln2(HL)(L)] (Ln = SmIII, EuIII, GdIII, TbIII). Dalton Trans. 2019, 48, 3893–3905. [Google Scholar] [CrossRef] [PubMed]
- Biju, S.; Ambili Raj, D.B.; Reddy, M.L.P.; Kariuki, B.M. Synthesis, Crystal Structure, and Luminescent Properties of Novel Eu3+ Heterocyclic â-Diketonate Complexes with Bidentate Nitrogen Donors. Inorg. Chem. 2006, 45, 10659. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, P.P.F.; Miyazaki, S.; Sakamoto, H.; Kitagawa, Y.; Miyata, K.; Akama, T.; Kobayashi, M.; Fushimi, K.; Onda, K.; Taketsugu, T.; et al. Coordination Geometrical Effect on Ligand-to-Metal Charge Transfer-Dependent Energy Transfer Processes of Luminescent Eu(III) Complexes. J. Phys. Chem. A 2021, 125, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Puntus, L.N.; Lyssenko, K.A.; Antipin, M.Y.; Bünzli, J.-C.G. Role of Inner- and Outer-Sphere Bonding in the Sensitization of EuIII-Luminescence Deciphered by Combined Analysis of Experimental Electron Density Distribution Function and Photophysical Data. Inorg. Chem. 2008, 47, 11095. [Google Scholar] [CrossRef] [PubMed]
- Mautner, F.A.; Bierbaumer, F.; Fischer, R.C.; Torvisco, A.; Vicente, R.; Font-Bardía, M.; Tubau, À.; Speed, S.; Massoud, S.S. Diverse Coordination Numbers and Geometries in Pyridyl Adducts of Lanthanide(III) Complexes Based on β-Diketonate. Inorganics 2021, 9, 74. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Chimitova, O.D.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Bazarov, B.G.; Bazarova, J.G. Synthesis and Spectroscopic Properties of Monoclinic α-Eu2(MoO4)3. J. Phys. Chem. C 2014, 118, 15404–15411. [Google Scholar] [CrossRef]
- Coelho, A.C.; Gomes, A.C.; Fernandes, A.J.; Paz, F.A.A.; Braga, S.S.; Rino, L.; Pereira, L.; Pillinger, M.; Gonçalves, I.S. Crystal Structure and Spectroscopic Studies of a Dimeric Europium(III) β-Diketonate Complex Containing [3-(2-Pyridyl)-1-pyrazolyl]acetate. Eur. J. Inorg. Chem. 2014, 2014, 1284–1288. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Eliseeva, S.V.; Scopelliti, R.; Bunzli, J.-C.G. Influence of Symmetry on the Luminescence and Radiative Lifetime of Nine-Coordinate Europium Complexes. Inorg. Chem. 2015, 54, 9166–9173. [Google Scholar] [CrossRef]
- Holz, R.C.; Thompson, L.C. Spectroscopically Distinct Geometrical Isomers in a Single Crystal. Characterization of the Eight-Coordinate Adducts of Tris(dipivaloylmethanato) lanthanide(III) with 2,9-Dimethyl-l,lO-phenanthrohe. Inorg. Chem. 1993, 32, 5251–5256. [Google Scholar] [CrossRef]
- Tanner, P.A. Lanthanide Luminescence in Solids. In Lanthanide Luminescence. Photophysical, Analytical and Biological Aspects; Hanninen, P., Harma, H., Eds.; Springer Series on Fluorescence, Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2011; Volume 7, p. 203. [Google Scholar] [CrossRef]
- Arnaud, N.; Georges, J. Comprehensive study of the luminescent properties and lifetimes of Eu3+ and Tb3+ chelated with various ligands in aqueous solutions: Influence of the synergic agent, the surfactant and the energy level of the ligand triplet. Spectrochim. Acta 2003, 59, 1829–1840. [Google Scholar] [CrossRef]
- Judd, B.R. Optical absorbtion intensities of rare-earth ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of crystal spectra of rare earth ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Werts, M.H.V. Luminescent Lanthanide Complexes: Visible Light Sensitised Red and Near-infrared Luminescence; University of Amsterdam, UvA-DARE (Digital Academic Repository): Amsterdam, The Netherlands, 2000. [Google Scholar]
- Gawryszewska, P.P.; Jerzykiewicz, L.; Sobota, P.; Legendziewicz, J. Spectroscopy and structure of Eu(III) complex with N-methylglycine. J. Alloys Compd. 2000, 300–301, 275–282. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, J.; Lu, S.; Zhao, H.; Zhang, X.; Wang, X. Fabrication of Eu3+ and Sm3+ Co-doped Micro/Nanosized MMoO4 (M) Ca, Ba, and Sr) via Facile Hydrothermal Method and their Photoluminescence Properties through Energy Transfer. J. Phys. Chem. C 2008, 112, 5860–5864. [Google Scholar] [CrossRef]
- Weirts, M.H.V.; Junkes, R.T.F.; Verhoven, J.W. The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes. Phys. Chem. 2002, 4, 1542–1548. [Google Scholar] [CrossRef]


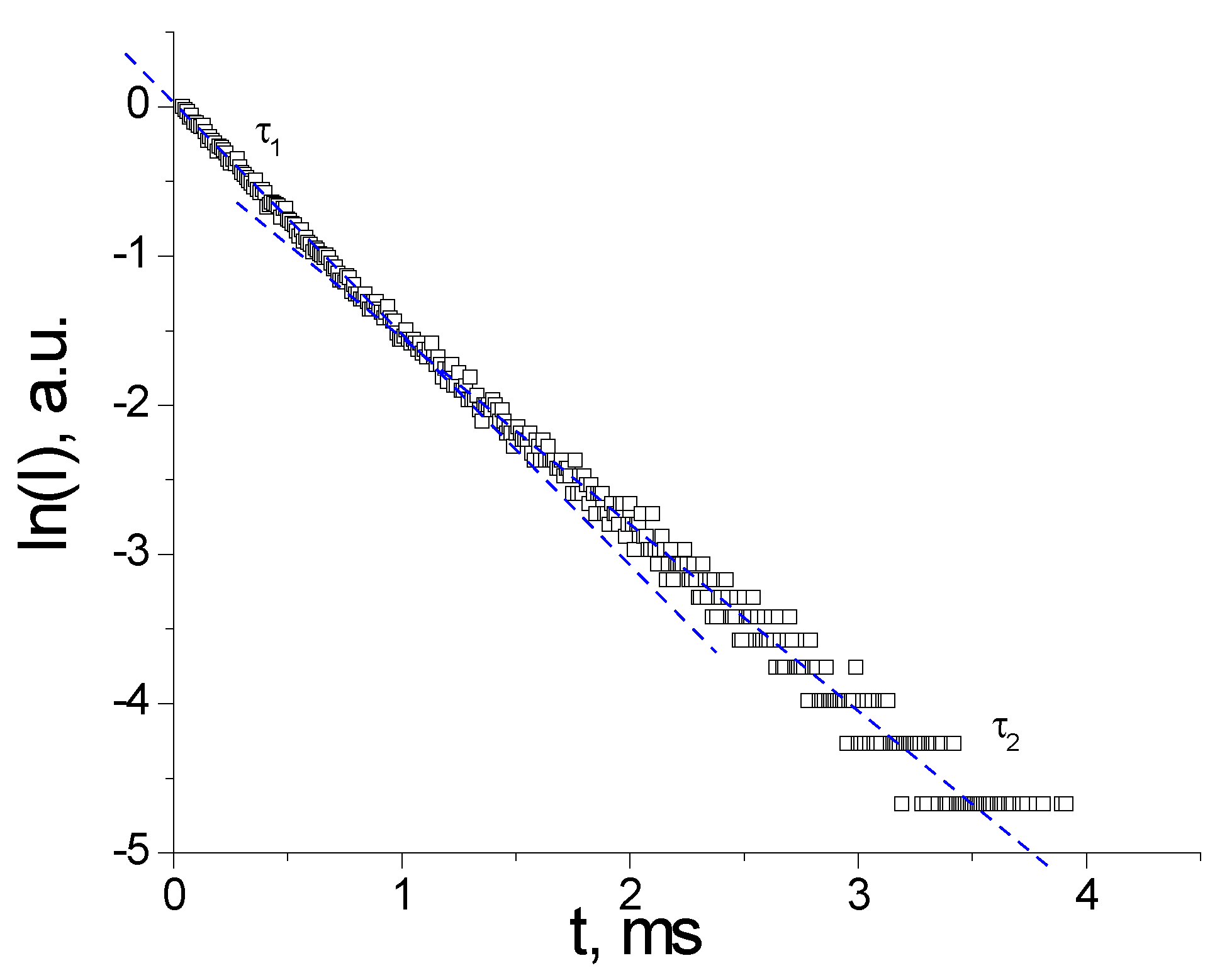
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Culeac, I.P.; Verlan, V.I.; Bordian, O.T.; Zubareva, V.E.; Iovu, M.S.; Bulhac, I.I.; Siminel, N.A.; Siminel, A.V.; Mihai, G.; Enachescu, M. Synthesis and Characterization of Coordination Compound [Eu(µ2-OC2H5)(btfa)(NO3)(phen)]2phen with High Luminescence Efficiency. Nanomaterials 2022, 12, 2788. https://doi.org/10.3390/nano12162788
Culeac IP, Verlan VI, Bordian OT, Zubareva VE, Iovu MS, Bulhac II, Siminel NA, Siminel AV, Mihai G, Enachescu M. Synthesis and Characterization of Coordination Compound [Eu(µ2-OC2H5)(btfa)(NO3)(phen)]2phen with High Luminescence Efficiency. Nanomaterials. 2022; 12(16):2788. https://doi.org/10.3390/nano12162788
Chicago/Turabian StyleCuleac, Ion P., Victor I. Verlan, Olga T. Bordian, Vera E. Zubareva, Mihail S. Iovu, Ion I. Bulhac, Nichita A. Siminel, Anatolii V. Siminel, Geanina Mihai, and Marius Enachescu. 2022. "Synthesis and Characterization of Coordination Compound [Eu(µ2-OC2H5)(btfa)(NO3)(phen)]2phen with High Luminescence Efficiency" Nanomaterials 12, no. 16: 2788. https://doi.org/10.3390/nano12162788
APA StyleCuleac, I. P., Verlan, V. I., Bordian, O. T., Zubareva, V. E., Iovu, M. S., Bulhac, I. I., Siminel, N. A., Siminel, A. V., Mihai, G., & Enachescu, M. (2022). Synthesis and Characterization of Coordination Compound [Eu(µ2-OC2H5)(btfa)(NO3)(phen)]2phen with High Luminescence Efficiency. Nanomaterials, 12(16), 2788. https://doi.org/10.3390/nano12162788