Charge Regulation and pH Effects on Thermo-Osmotic Conversion
Abstract
:1. Introduction
2. Methodology
2.1. Theoretical Model and Mechanism
2.2. Numerical Modelling
3. Results and Discussion
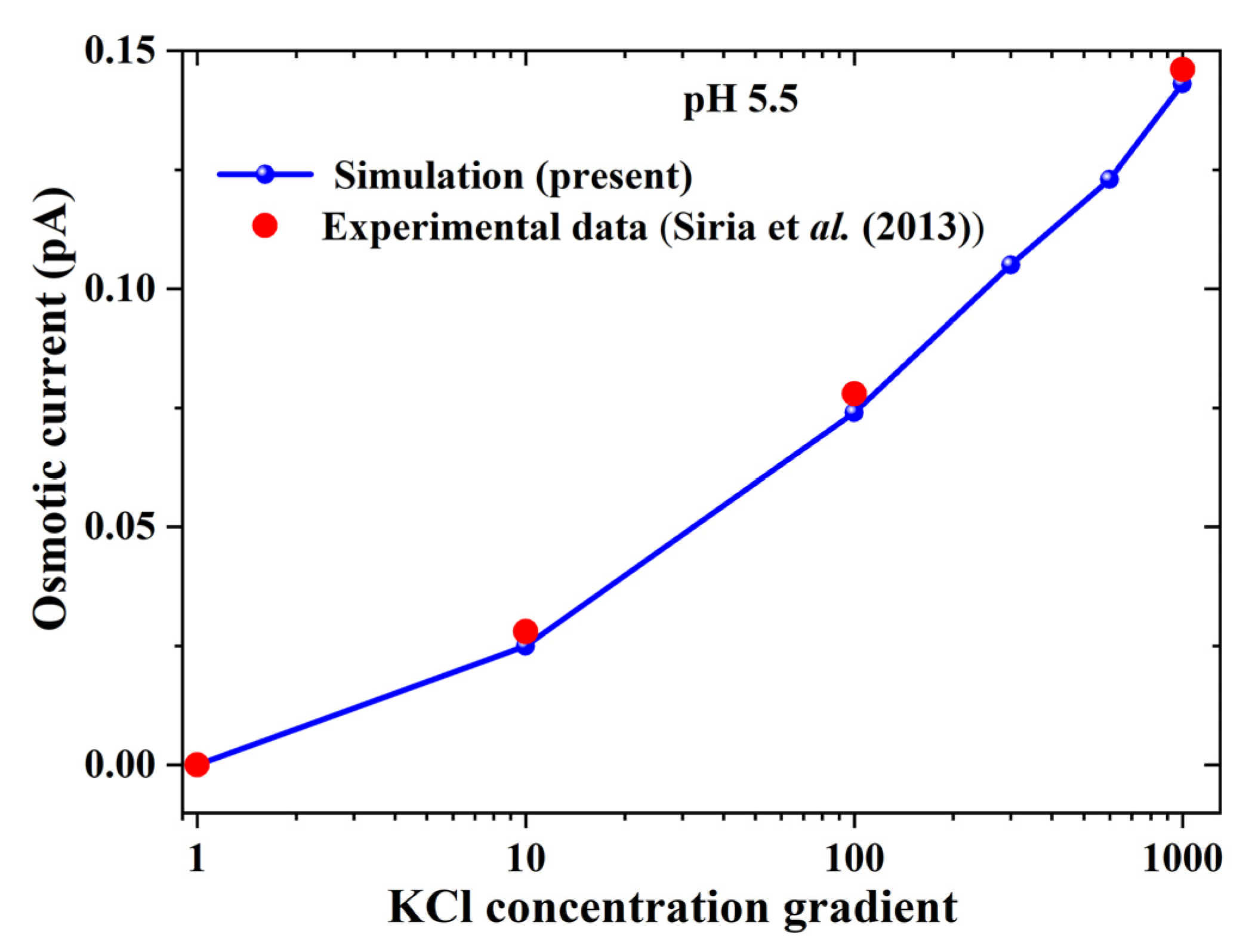
3.1. Verifying Numerical Simulation
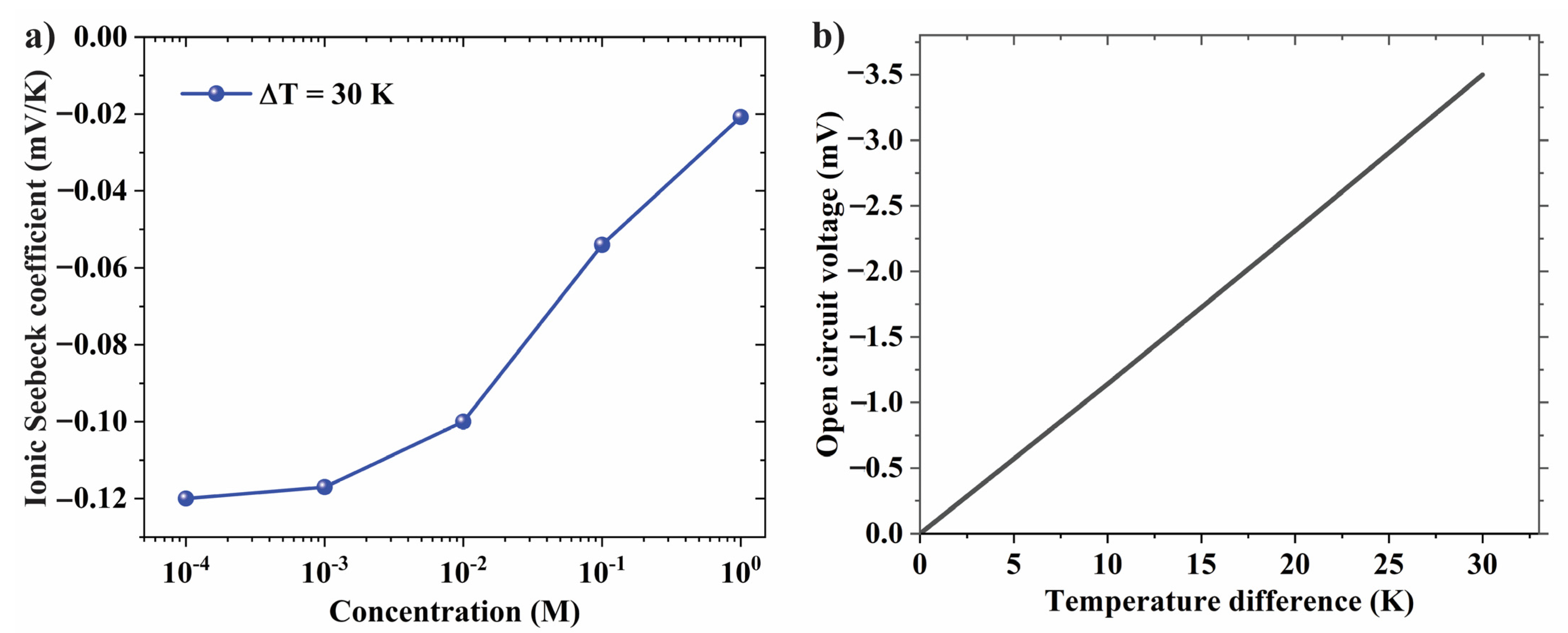
3.2. Effect of Electrolyte Concentration on Ionic Seebeck Coefficient
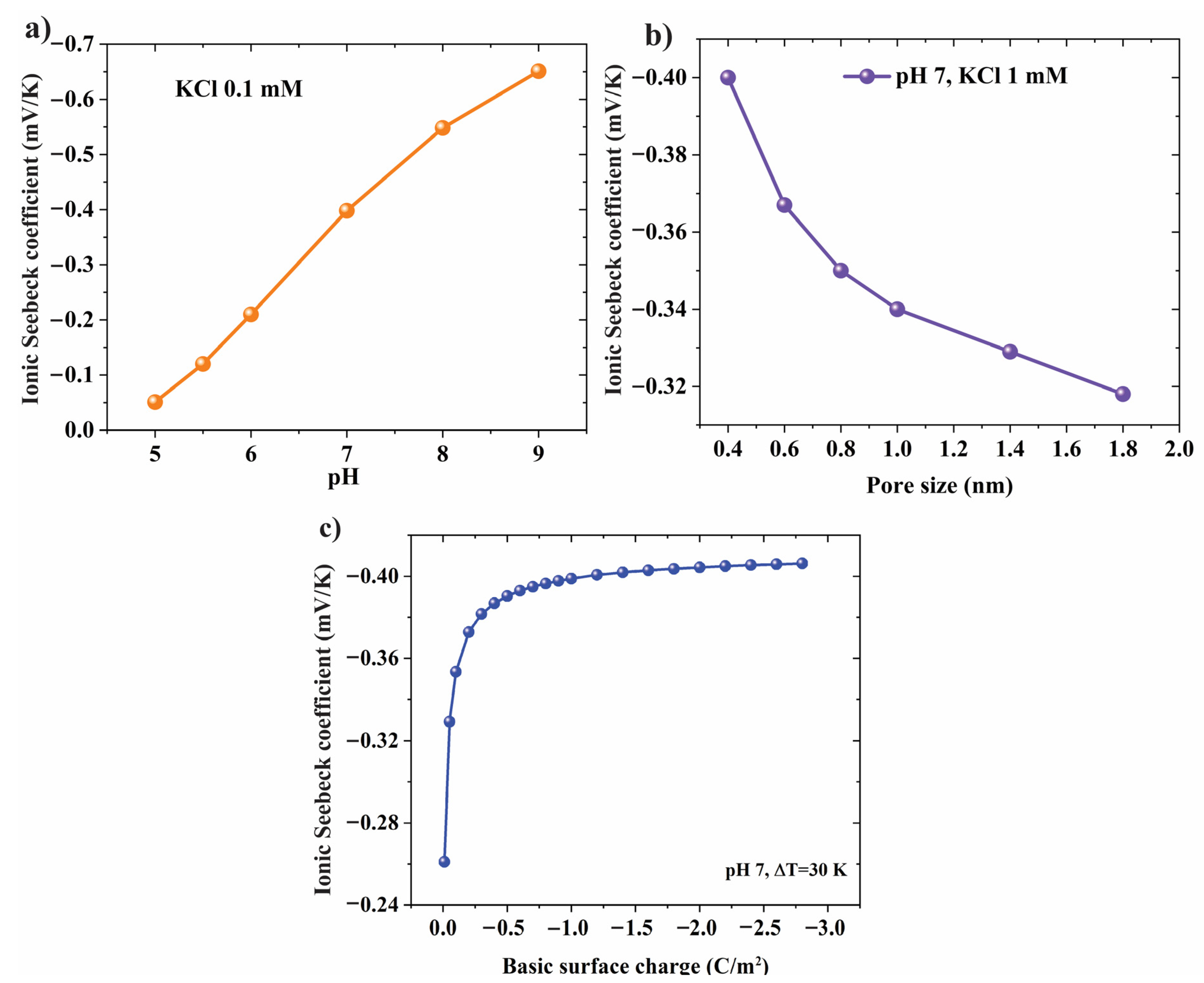
3.3. Effect of pH Level and Pore Structure on Ionic Seebeck Coefficient
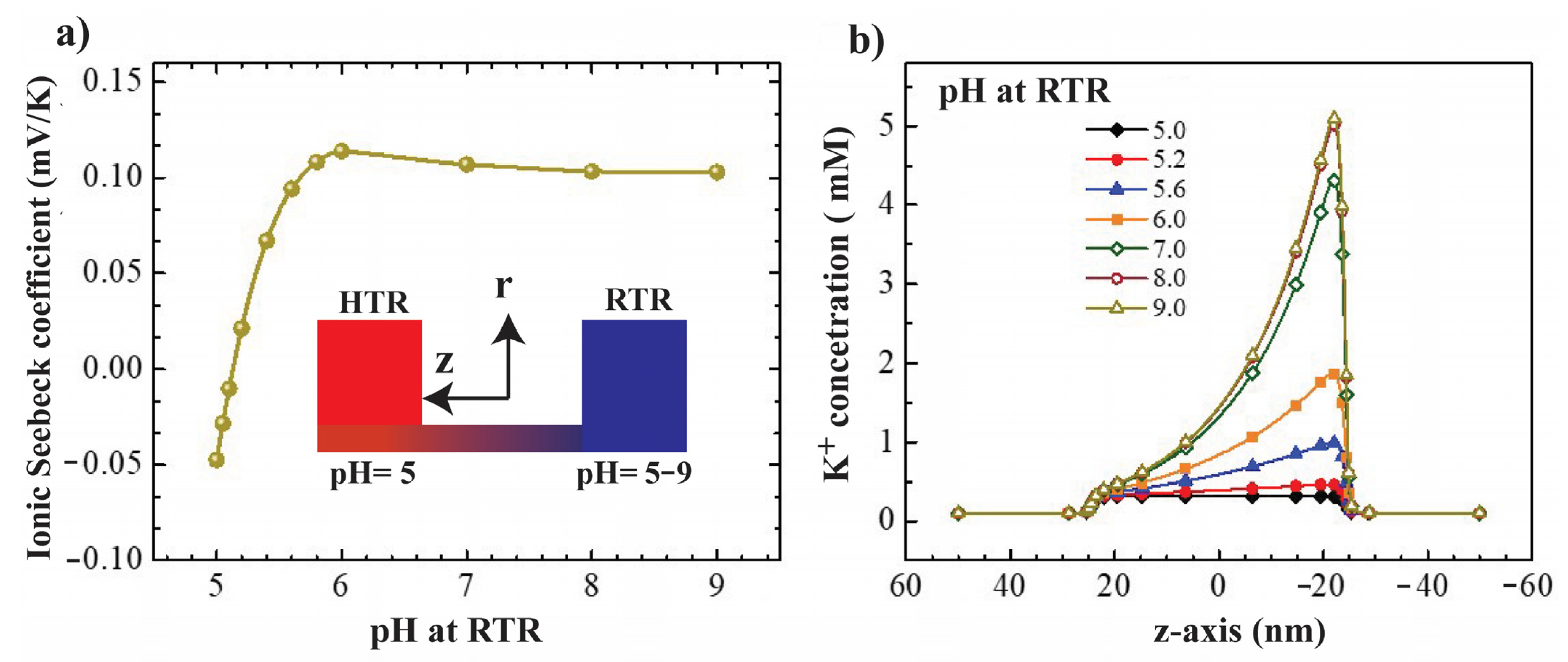
3.4. Effect of pH Gradient on Thermo-Osmosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laing, T. Solar power challenges. Nat. Sustain. 2022, 5, 285–286. [Google Scholar] [CrossRef]
- Bu, Z.; Zhang, X.; Hu, Y.; Chen, Z.; Lin, S.; Li, W.; Xiao, C.; Pei, Y. A record thermoelectric efficiency in tellurium-free modules for low-grade waste heat recovery. Nat. Commun. 2022, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Jouhara, H.; Khordehgah, N.; Almahmoud, S.; Delpech, B.; Chauhan, A.; Tassou, S.A. Waste heat recovery technologies and applications. Therm. Sci. Eng. Prog. 2018, 6, 268–289. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, I.; Bedin, K.C.; Mouriño, B.; Souza Junior, J.B.; Souza, F.L. Advances in Engineered Metal Oxide Thin Films by Low-Cost, Solution-Based Techniques for Green Hydrogen Production. Nanomaterials 2022, 12, 1957. [Google Scholar] [CrossRef]
- Chang, C.-C. Asymmetric Electrokinetic Energy Conversion in Slip Conical Nanopores. Nanomaterials 2022, 12, 1100. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Mai, V.P.; Yang, R.J. Effects of electrode placement position and tilt angles of a platform on voltage induced by NaCl electrolyte flowing over graphene wafer. Appl. Energy 2020, 261, 114435. [Google Scholar] [CrossRef]
- Yeh, L.H.; Huang, Z.Y.; Liu, Y.C.; Deng, M.J.; Chou, T.H.; Yang, H.C.; Ahamad, T.; Alshehri, S.M.; Wu, K.C. A nanofluidic osmotic power generator demonstrated in polymer gel electrolytes with substantially enhanced performance. J. Mater. Chem. A 2019, 7, 26791–26796. [Google Scholar] [CrossRef]
- Jiang, T.; Pang, H.; An, J.; Lu, P.; Feng, Y.; Liang, X.; Zhong, W.; Wang, Z.L. Robust Swing-Structured Triboelectric Nanogenerator for Efficient Blue Energy Harvesting. Adv. Energy Mater. 2020, 10, 2000064. [Google Scholar] [CrossRef]
- Zhai, L.; Gao, L.; Wang, Z.; Dai, K.; Wu, S.; Mu, X. An Energy Harvester Coupled with a Triboelectric Mechanism and Electrostatic Mechanism for Biomechanical Energy Harvesting. Nanomaterials 2022, 12, 933. [Google Scholar] [CrossRef]
- Mai, V.-P.; Yang, R.-J. Boosting power generation from salinity gradient on high-density nanoporous membrane using thermal effect. Appl. Energy 2020, 274, 115294. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Zhao, R.; Zhang, B.; Liang, Y.; Long, R.; Liu, W.; Liu, Z. Stack Thermo-Osmotic System for Low-Grade Thermal Energy Conversion. ACS Appl. Mater. Interfaces 2021, 13, 21371–21378. [Google Scholar] [CrossRef]
- Chen, K.; Yao, L.; Yan, F.; Liu, S.; Yang, R.; Su, B. Thermo-osmotic energy conversion and storage by nanochannels. J. Mater. Chem. A 2019, 7, 25258–25261. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Q.; Zeng, M.; Zhao, C. Thermoelectric effect and temperature-gradient-driven electrokinetic flow of electrolyte solutions in charged nanocapillaries. Int. J. Heat Mass Transfer. 2019, 143, 118569. [Google Scholar] [CrossRef]
- Mai, V.-P.; Yang, R.-J. Active control of salinity-based power generation in nanopores using thermal and pH effects. RSC Adv. 2020, 10, 18624–18631. [Google Scholar] [CrossRef]
- Yeh, L.-H.; Chen, F.; Chiou, Y.-T.; Su, Y.-S. Anomalous pH-Dependent Nanofluidic Salinity Gradient Power. Small 2017, 13, 1702691. [Google Scholar] [CrossRef]
- Mai, V.-P.; Huang, W.-H.; Yang, R.-J. Enhancing Ion Transport through Nanopores in Membranes for Salinity Gradient Power Generation. ACS EST Eng. 2021, 1, 1725–1752. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, W.; Lin, L.; Wang, M.; Li, N.; Zheng, Z.; Liu, F.; Cao, L. Vertically Transported Graphene Oxide for High-Performance Osmotic Energy Conversion. Adv. Sci. 2020, 7, 2000286. [Google Scholar]
- Su, S.; Sun, Q.; Gu, X.; Xu, Y.; Shen, J.; Zhu, D.; Chao, J.; Fan, C.; Wang, L. Two-dimensional nanomaterials for biosensing applications. TrAC Trends Anal. Chem. 2019, 119, 115610. [Google Scholar] [CrossRef]
- Dietzel, M.; Hardt, S. Thermoelectricity in confined liquid electrolytes. Phys. Rev. Lett. 2016, 116, 225901. [Google Scholar] [CrossRef] [PubMed]
- Ghonge, T.; Chakraborty, J.; Dey, R.; Chakraborty, S. Electrohydrodynamics within the electrical double layer in the presence of finite temperature gradients. Phys. Rev. E 2013, 88, 053020. [Google Scholar] [CrossRef]
- Di Lecce, S.; Bresme, F. Thermal polarization of water influences the thermoelectric response of aqueous solutions. J. Phys. Chem. B 2018, 122, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Saghir, M. Thermodiffusion or Soret effect: Historical review. Int. J. Heat Mass Transfer. 2014, 73, 693–705. [Google Scholar] [CrossRef]
- Hernández, A.; Arcos, J.; Martínez-Trinidad, J.; Bautista, O.; Sánchez, S.; Méndez, F. Thermodiffusive effect on the local Debye-length in an electroosmotic flow of a viscoelastic fluid in a slit microchannel. Int. J. Heat Mass Transf. 2022, 187, 122522. [Google Scholar] [CrossRef]
- Würger, A. Transport in charged colloids driven by thermoelectricity. Phys. Rev. Lett. 2008, 101, 108302. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Merabia, S.; Joly, L. What controls thermo-osmosis? Molecular simulations show the critical role of interfacial hydrodynamics. Phys. Rev. Lett. 2017, 119, 214501. [Google Scholar] [CrossRef]
- Fu, L.; Joly, L.; Merabia, S. Giant thermoelectric response of nanofluidic systems driven by water excess enthalpy. Phys. Rev. Lett. 2019, 123, 138001. [Google Scholar] [CrossRef]
- Herrero, C.; de San Feliciano, M.; Merabia, S.; Joly, L. Fast and versatile thermo-osmotic flows with a pinch of salt. Nanoscale 2022, 14, 626–631. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Jing, D.; Mohamad, A.; Prezhdo, O. Net unidirectional fluid transport in locally heated nanochannel by thermo-osmosis. Nano Lett. 2020, 20, 8965–8971. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, C.; Xian, W.; Meng, Q.W.; Guo, Q.; Zhu, X.; Wang, S.; Wang, Y.; Ma, S.; Sun, Q. Thermo-Osmotic Energy Conversion Enabled by Covalent-Organic-Framework Membranes with Record Output Power Density. Angew. Chem. Int. Ed. 2022, 61, e202116910. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Lacey, S.D.; Mi, R.; Zhao, X.; Jiang, F.; Song, J.; Liu, Z.; Chen, G.; Dai, J.; et al. Cellulose ionic conductors with high differential thermal voltage for low-grade heat harvesting. Nat. Mater. 2019, 18, 608–613. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, H.; Le, Q.; Chen, R.; Li, J.; Ouyang, J. Giant Thermoelectric Properties of Ionogels with Cationic Doping. Adv. Energy Mater. 2022, 12, 2200858. [Google Scholar] [CrossRef]
- Chen, W.Q.; Sedighi, M.; Jivkov, A.P. Thermo-osmosis in hydrophilic nanochannels: Mechanism and size effect. Nanoscale 2021, 13, 1696–1716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Farhan, M.; Jiao, K.; Qian, F.; Guo, P.; Wang, Q.; Yang, C.C.; Zhao, C. Simultaneous thermoosmotic and thermoelectric responses in nanoconfined electrolyte solutions: Effects of nanopore structures and membrane properties. J. Colloid. Interface Sci. 2022, 618, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guan, K.; Ji, Y.; Liu, G.; Jin, W.; Xu, N. Controllable ion transport by surface-charged graphene oxide membrane. Nat. Commun. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Su, Y.-S.; Hsu, S.-C.; Peng, P.-H.; Yang, J.-Y.; Gao, M.; Yeh, L.-H. Unraveling the anomalous channel-length-dependent blue energy conversion using engineered alumina nanochannels. Nano Energy 2021, 84, 105930. [Google Scholar] [CrossRef]
- Liu, W.-D.; Yang, L.; Chen, Z.-G. Cu2Se thermoelectrics: Property, methodology, and device. Nano Today 2020, 35, 100938. [Google Scholar] [CrossRef]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through pKa Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef]
- Orth, E.S.; Ferreira, J.G.; Fonsaca, J.E.; Blaskievicz, S.F.; Domingues, S.H.; Dasgupta, A.; Terrones, M.; Zarbin, A.J. pKa determination of graphene-like materials: Validating chemical functionalization. J. Colloid. Interface Sci. 2016, 467, 239–244. [Google Scholar] [CrossRef]
- Hsu, J.-P.; Su, T.-C.; Peng, P.-H.; Hsu, S.-C.; Zheng, M.-J.; Yeh, L.-H. Unraveling the Anomalous Surface-Charge-Dependent Osmotic Power Using a Single Funnel-Shaped Nanochannel. ACS Nano 2019, 13, 13374–13381. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Su, Y.-S.; Gao, M.; Yeh, L.-H. Realization of robust mesoscale ionic diodes for ultrahigh osmotic energy generation at mild neutral pH. J. Mater. Chem. A 2021, 9, 20502–20509. [Google Scholar] [CrossRef]
- Siria, A.; Poncharal, P.; Biance, A.L.; Fulcrand, R.; Blase, X.; Purcell, S.T.; Bocquet, L. Giant osmotic energy conversion measured in a single transmembrane boron nitride nanotube. Nature 2013, 494, 455–458. [Google Scholar] [CrossRef] [PubMed]
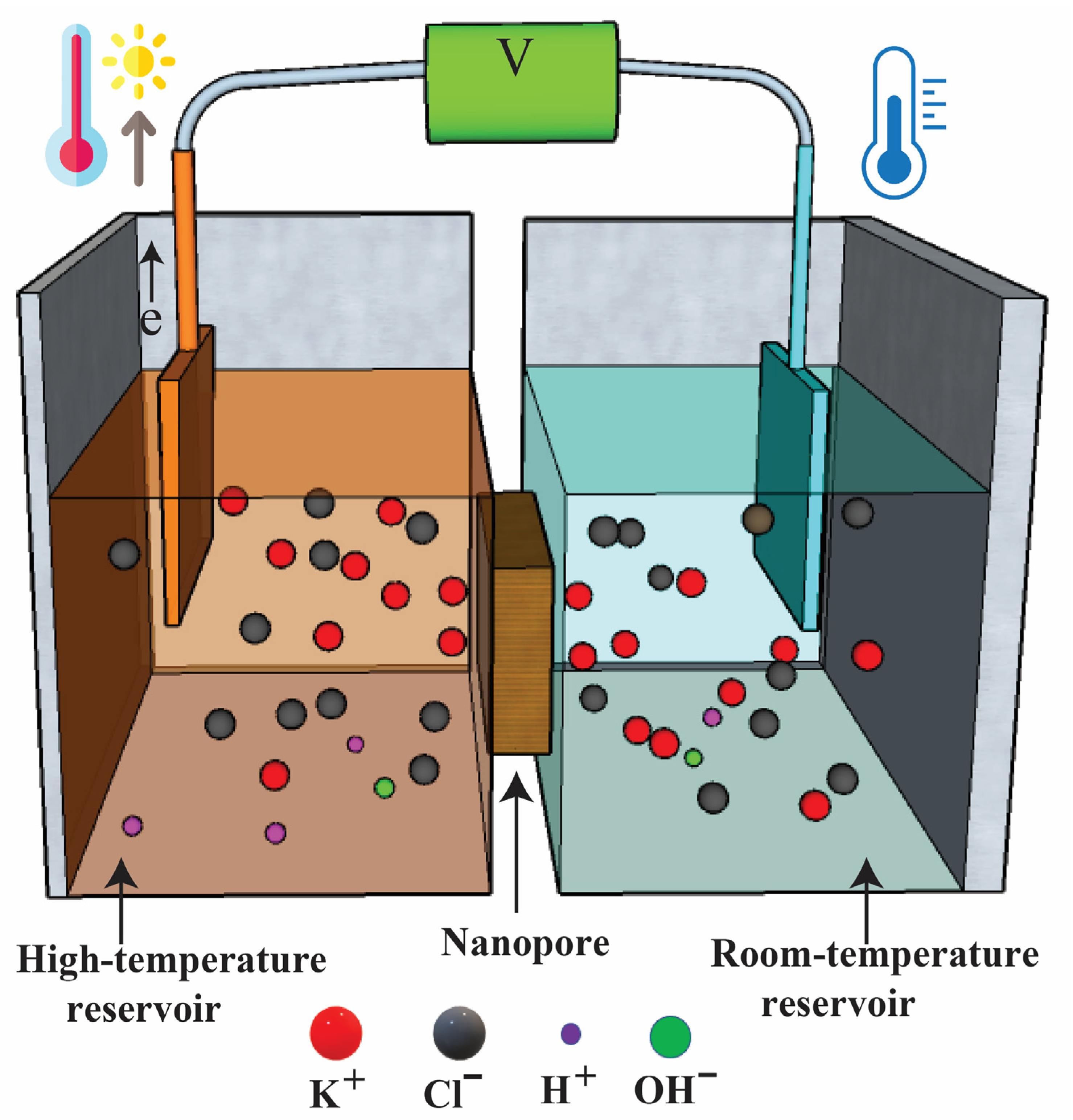
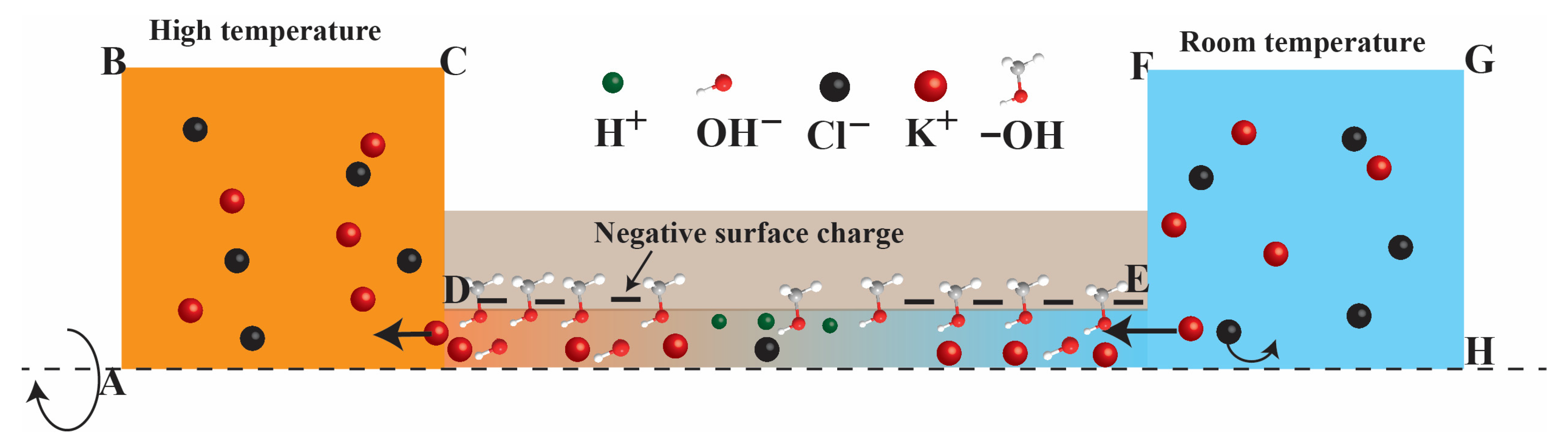

| Surface | Electric Potential | Ion Transport | Flow Field | Heat Transfer |
|---|---|---|---|---|
| AB | Open circuit voltage | Concentration of K+, Cl−, H+, and OH− | Pressure = 0 | 298 + ΔT K |
| BC, FG | Zero charge | No flux | Slip | Thermal insulation |
| CD, EF | Zero charge | No flux | No slip | Thermal insulation |
| DE | Surface charge density | No flux | No slip | Thermal insulation |
| GH | Ground | Concentration of K+, Cl−, H+, and OH− | Pressure = 0 | 298 K |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, V.-P.; Huang, W.-H.; Yang, R.-J. Charge Regulation and pH Effects on Thermo-Osmotic Conversion. Nanomaterials 2022, 12, 2774. https://doi.org/10.3390/nano12162774
Mai V-P, Huang W-H, Yang R-J. Charge Regulation and pH Effects on Thermo-Osmotic Conversion. Nanomaterials. 2022; 12(16):2774. https://doi.org/10.3390/nano12162774
Chicago/Turabian StyleMai, Van-Phung, Wei-Hao Huang, and Ruey-Jen Yang. 2022. "Charge Regulation and pH Effects on Thermo-Osmotic Conversion" Nanomaterials 12, no. 16: 2774. https://doi.org/10.3390/nano12162774
APA StyleMai, V.-P., Huang, W.-H., & Yang, R.-J. (2022). Charge Regulation and pH Effects on Thermo-Osmotic Conversion. Nanomaterials, 12(16), 2774. https://doi.org/10.3390/nano12162774







