Comparative Physicochemical and Catalytic Study of Nanocrystalline Mg-Al Hydrotalcites Precipitated with Inorganic and Organic Bases
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Physicochemical Characterization
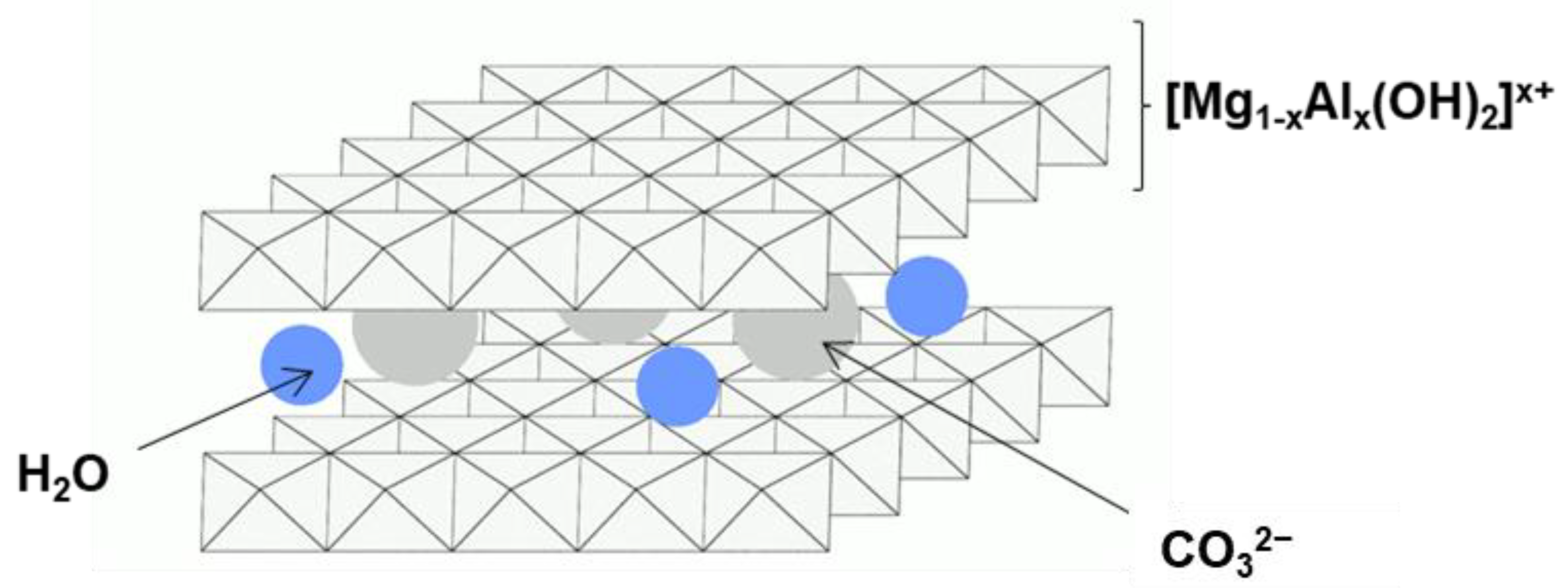
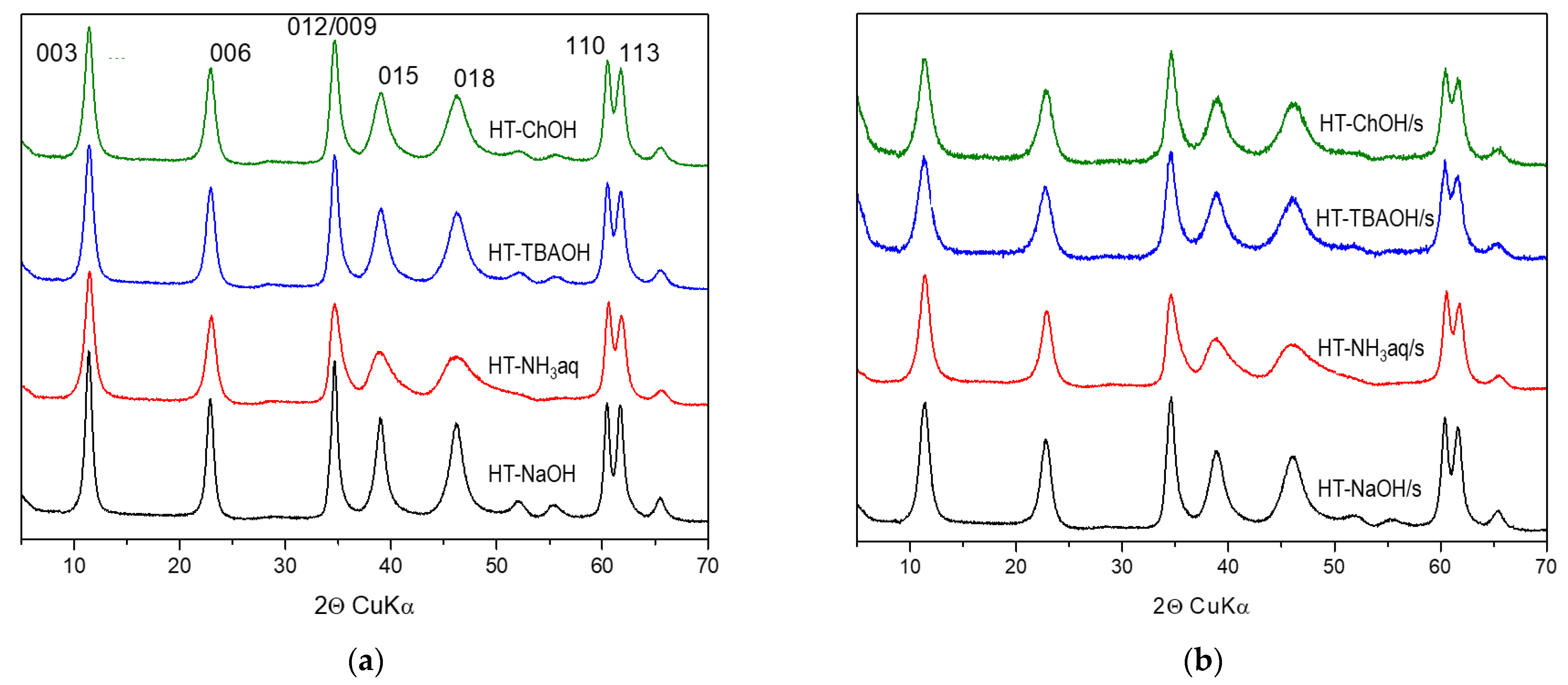
3.1.1. XRD Analysis
3.1.2. QM Analysis of NH3 Interaction with Mg2+ and Al3+ Aquo complexes
3.1.3. SEM Analysis
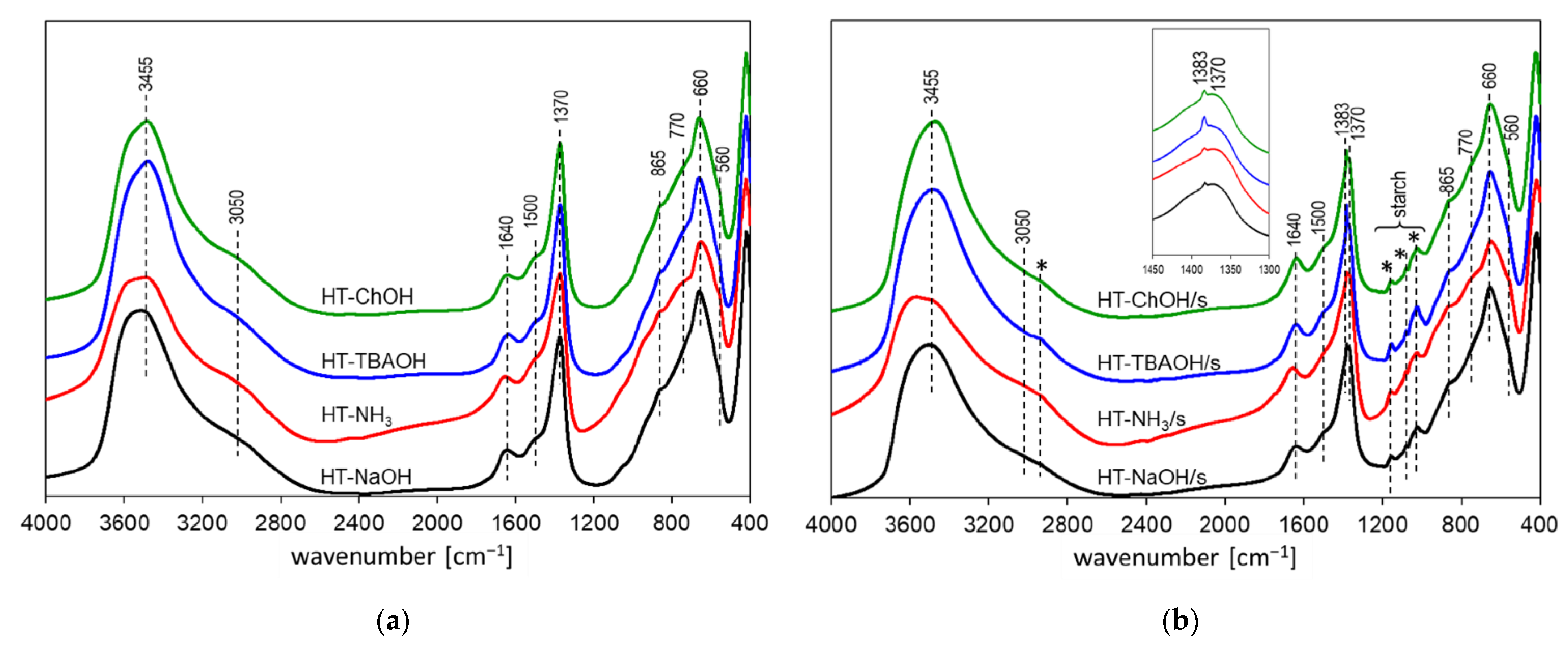
3.1.4. FTIR Analysis
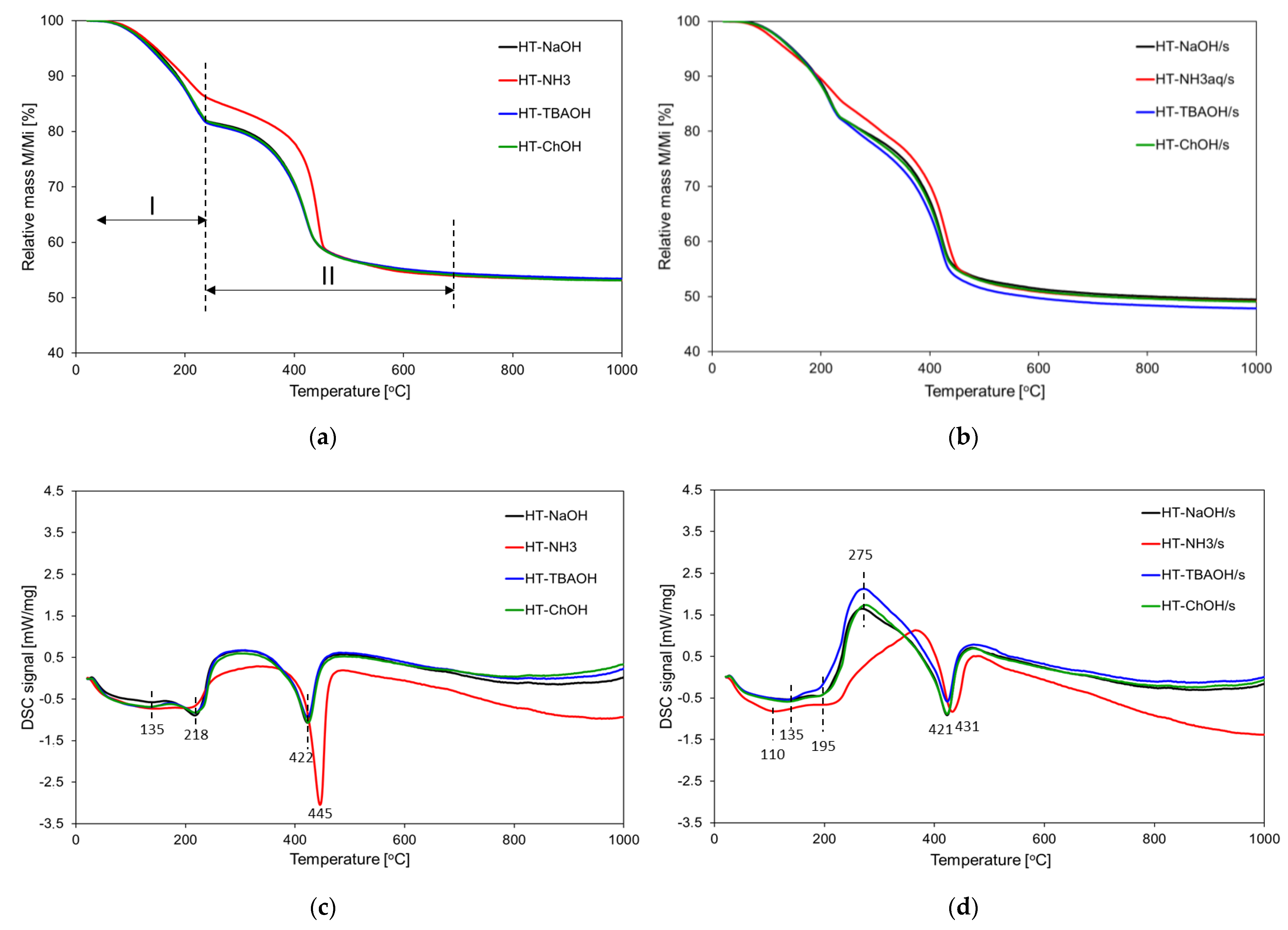
3.1.5. Thermal Analysis
3.2. Catalytic Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhitova, E.S.; Krivovichev, S.V.; Pekov, I.V.; Greenwell, H.C. Crystal chemistry of natural layered double hydroxides. 5. Single-crystal structure refinement of hydrotalcite, [Mg6Al2(OH)16](CO3)(H2O)4. Miner. Mag. 2019, 83, 269–280. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Rives, V. Layered Double Hydroxides: Present and Future; Nova Science Publishers: New York, NY, USA, 2001; pp. 2–3. [Google Scholar]
- Adachi-Pagano, M.; Forano, C.; Besse, J.P. Synthesis of Al-rich hydrotalcite-like compounds by using the urea hydrolysis reaction—Control of size and morphology. J. Mater. Chem. 2003, 13, 1988–1993. [Google Scholar] [CrossRef]
- Rao, M.M.; Reddy, B.R.; Jayalakshmi, M.; Jaya, V.S.; Sridhar, B. Hydrothermal synthesis of Mg–Al hydrotalcites by urea hydrolysis. Mater. Res. Bull. 2005, 40, 347–359. [Google Scholar] [CrossRef]
- Olszówka, J.E.; Karcz, R.; Bielańska, E.; Kryściak-Czerwenka, J.; Napruszewska, B.D.; Sulikowski, B.; Socha, R.P.; Gaweł, A.; Bahranowski, K.; Olejniczak, Z.; et al. New insight into the preferred valency of interlayer anions in hydrotalcite-like compounds: The effect of Mg/Al ratio. Appl. Clay Sci. 2018, 155, 84–94. [Google Scholar] [CrossRef]
- Baskaran, T.; Christopher, J.; Sakthivel, A. Progress on layered hydrotalcite (HT) materials as potential support and catalytic materials. RSC Adv. 2015, 5, 98853–98875. [Google Scholar] [CrossRef]
- Xu, M.; Wei, M. Layered double hydroxide-based catalysts: Recent advances in preparation, structure, and applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Costantino, U.; Ambrogi, V.; Nocchetti, M.; Perioli, L. Hydrotalcite-like compounds: Versatile layered hosts of molecular anions with biological activity. Microporous Mesoporous Mater. 2008, 107, 149–160. [Google Scholar] [CrossRef]
- Jin, W.; Lee, D.; Jeon, Y.; Park, D.-H. Biocompatible Hydrotalcite Nanohybrids for Medical Functions. Minerals 2020, 10, 172. [Google Scholar] [CrossRef]
- Machej, T.; Serwicka, E.M.; Zimowska, M.; Dula, R.; Michalik-Zym, A.; Napruszewska, B.; Rojek, W.; Socha, R. Cu/Mn-based mixed oxides derived from hydrotalcite-like precursors as catalysts for methane combustion. Appl. Catal. A-Gen. 2014, 474, 87–94. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods—A review. Appl. Surf. Sci. 2016, 383, 200–213. [Google Scholar] [CrossRef]
- Conterosito, E.; Gianotti, V.; Palin, L.; Boccaleri, E.; Viterbo, D.; Milanesio, M. Facile preparation methods of hydrotalcite layered materials and their structural characterization by combined techniques. Inorg. Chim. Acta 2018, 470, 36–50. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2019, 269, 494–596. [Google Scholar] [CrossRef]
- Cantrell, D.G.; Gillie, L.J.; Lee, A.F.; Wilson, K. Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis. Appl. Catal. A-Gen. 2005, 287, 183–190. [Google Scholar] [CrossRef]
- Abelló, S.; Medina, F.; Tichit, D.; Pérez-Ramírez, J.; Rodríguez, X.; Sueiras, J.E.; Salagre, P.; Cesteros, Y. Study of alkaline-doping agents on the performance of reconstructed Mg–Al hydrotalcites in aldol condensations. Appl. Catal. A-Gen. 2005, 281, 191–198. [Google Scholar] [CrossRef]
- Mališová, M.; Hájek, M.; Kocián, D.; Malina, J.; Peller, A.; Horňáček, M. The influence of various anions in Mg-Al mixed oxides on presence of sodium ions in transesterification of oil. Fuel 2022, 319, 123781. [Google Scholar] [CrossRef]
- Kruissink, E.C.; Pelt, H.L.; Ross, J.R.H.; van Reüen, L.L. The effect of sodium on the methanation activity of nickel/alumina coprecipitated catalysts. Appl. Catal. 1981, 1, 23–29. [Google Scholar] [CrossRef]
- Kung, H.H. Deactivation of methanol synthesis catalysts-a review. Catal. Today 1992, 11, 443–453. [Google Scholar] [CrossRef]
- Napruszewska, B.D.; Michalik-Zym, A.; Rogowska, M.; Bielańska, E.; Rojek, W.; Gaweł, A.; Wójcik-Bania, M.; Bahranowski, K.; Serwicka, E.M. Novel Montmorillonite/TiO2/MnAl-Mixed Oxide Composites Prepared from Inverse Microemulsions as Combustion Catalysts. Materials 2017, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.; Zhang, W.; Guo, Y.; Valverde, J.L.; Giroir-Fendler, A. The Influence of Residual Sodium on the Catalytic Oxidation of Propane and Toluene over Co3O4 Catalysts. Catalysts 2020, 10, 867. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Durand, R.; Tichit, D. Investigation of Acid−Base Properties of Catalysts Obtained from Layered Double Hydroxides. J. Phys. Chem. B 2000, 104, 11117–11126. [Google Scholar] [CrossRef]
- León, M.; Díaz, E.; Bennici, S.; Vega, A.; Ordóñez, S.; Auroux, A. Adsorption of CO2 on Hydrotalcite-Derived Mixed Oxides: Sorption Mechanisms and Consequences for Adsorption Irreversibility. Ind. Eng. Chem. Res. 2010, 49, 3663–3671. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Structure–performance correlations of Mg–Al hydrotalcite catalysts for the isomerization of glucose into fructose. J. Catal. 2015, 327, 1–9. [Google Scholar] [CrossRef]
- Obalová, L.; Karásková, K.; Wach, A.; Kustrowski, P.; Mamulová-Kutláková, K.; Michalik, S.; Jirátová, K. Alkali metals as promoters in Co–Mn–Al mixed oxide for N2O decomposition. Appl. Catal. A Gen. 2013, 462–463, 227–235. [Google Scholar] [CrossRef]
- Olanrewaju, J.; Newalkar, B.L.; Mancino, C.; Komarneni, S. Simplified synthesis of nitrate form of layered double hydroxide. Mater. Lett. 2000, 45, 307–310. [Google Scholar] [CrossRef]
- Olfs, H.W.; Torres-Dorante, L.O.; Eckelt, R.; Kosslick, H. Comparison of different synthesis routes for Mg–Al layered double hydroxides (LDH): Characterization of the structural phases and anion exchange properties. Appl. Clay Sci. 2009, 43, 459–464. [Google Scholar] [CrossRef]
- Costantino, U.; Marmottini, F.; Nocchetti, M.; Vivani, R. New Synthetic Routes to Hydrotalcite-Like Compounds−Characterisation and Properties of the Obtained Materials. Eur. J. Inorg. Chem. 1998, 1998, 1439–1446. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Graffin, P.; Tichit, D. Synthesis and characterization of sol–gel Mg/Al and Ni/Al layered double hydroxides and comparison with co-precipitated samples. Microporous Mesoporous Mater. 2000, 39, 229–247. [Google Scholar] [CrossRef]
- Tittabut, T.; Trakarnpruk, W. Metal-Loaded MgAl Oxides for Transesterification of Glyceryl Tributyrate and Palm Oil. Ind. Eng. Chem. Res. 2008, 47, 2176–2181. [Google Scholar] [CrossRef]
- Abderrazek, K.; Srasra, N.F.; Srasra, E. Synthesis and characterization of [Zn-Al] layered double hydroxides: Effect of the operating parameters. J. Chin. Chem. Soc. 2017, 64, 346–353. [Google Scholar] [CrossRef]
- Michalik, A.; Napruszewska, B.D.; Walczyk, A.; Kryściak-Czerwenka, J.; Duraczyńska, D.; Serwicka, E.M. Synthesis of Nanocrystalline Mg-Al Hydrotalcites in the Presence of Starch—The Effect on Structure and Composition. Materials 2020, 13, 602. [Google Scholar] [CrossRef]
- Karcz, R.; Napruszewska, B.D.; Michalik, A.; Kryściak-Czerwenka, J.; Duraczyńska, D.; Serwicka, E.M. Fine Crystalline Mg-Al Hydrotalcites as Catalysts for Baeyer-Villiger Oxidation of Cyclohexanone with H2O2. Catalysts 2021, 11, 1493. [Google Scholar] [CrossRef]
- Roelofs, J.C.A.A.; Lensveld, D.J.; van Dillen, A.J.; de Jong, K.P. On the Structure of Activated Hydrotalcites as Solid Base Catalysts for Liquid-Phase Aldol Condensation. J. Catal. 2001, 203, 184–191. [Google Scholar] [CrossRef]
- Climent, M.; Corma, A.; Iborra, S.; Epping, K.; Velty, A. Increasing the basicity and catalytic activity of hydrotalcites by different synthesis procedures. J. Catal. 2004, 225, 316–326. [Google Scholar] [CrossRef]
- Abelló, S.; Medina, F.; Tichit, D.; Pérez-Ramírez, J.; Groen, J.C.; Sueiras, J.E.; Salagre, P.; Cesteros, Y. Aldol Condensations over Reconstructed Mg-Al Hydrotalcites: Structure-Activity Relationships Related to the Rehydration Method. Chem. Eur. J. 2004, 11, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Winter, F.; van Dillen, A.J.; de Jong, K.P. Supported hydrotalcites as highly active solid base catalysts. Chem. Commun. 2005, 3977–3979. [Google Scholar] [CrossRef]
- Lee, G.; Jeong, Y.; Takagaki, A.; Jung, J.C. Sonication assisted rehydration of hydrotalcite catalyst for isomerization of glucose to fructose. J. Mol. Catal. A-Chem. 2014, 393, 289–295. [Google Scholar] [CrossRef]
- Olszówka, J.E.; Karcz, R.; Napruszewska, B.D.; Michalik-Zym, A.; Duraczyńska, D.; Kryściak-Czerwenka, J.; Niecikowska, A.; Bahranowski, K.; Serwicka, E.M. Effect of Mg-Al hydrotalcite crystallinity on catalytic Baeyer-Villiger oxidation of cyclohexanone with H2O2/acetonitrile. Catal. Commun. 2018, 107, 48–52. [Google Scholar] [CrossRef]
- Fokema, M.D.; Chiu, E.; Ying, J.Y. Synthesis and Characterization of Nanocrystalline Yttrium Oxide Prepared with Tetraalkylammonium Hydroxides. Langmuir 2000, 16, 3154–3159. [Google Scholar] [CrossRef]
- Yang, J.; Mei, S.; Ferreira, J.M.F. Hydrothermal Synthesis of Nanosized Titania Powders: Influence of Tetraalkyl Ammonium Hydroxides on Particle Characteristics. J. Am. Ceram. Soc. 2001, 84, 1696–1702. [Google Scholar] [CrossRef]
- Xi, X.; Abe, H.; Kuruma, K.; Harada, R.; Shui, A.; Naito, M. Novel Co-precipitation method to synthesize NiO–YSZ nanocomposite powder for solid oxide fuel cell. Adv. Powder Technol. 2014, 25, 490–494. [Google Scholar] [CrossRef]
- Ding, S.; Zhu, C.; Hojo, H.; Einaga, H. Enhanced catalytic performance of spinel-type Cu-Mn oxides for benzene oxidation under microwave irradiation. J. Hazard. Mater. 2022, 424, 127523. [Google Scholar] [CrossRef]
- Pavel, O.D.; Stamate, A.E.; Bacalum, E.; Cojocaru, B.; Zăvoianu, R.; Pârvulescu, V.I. Catalytic behavior of Li-Al-LDH prepared via mechanochemical and co-precipitation routes for cyanoethylation reaction. Catal. Today 2021, 366, 227–234. [Google Scholar] [CrossRef]
- Shuck, C.E.; Ventura-Martinez, K.; Goad, A.; Uzun, S.; Shekhirev, M.; Gogotsi, Y. Safe Synthesis of MAX and MXene: Guidelines to Reduce Risk During Synthesis. ACS Chem. Health Saf. 2021, 28, 326–338. [Google Scholar] [CrossRef]
- Kalla, R.M.N.; Zhang, Y.; Kim, I. Highly efficient green synthesis of α-hydroxyphosphonates using a recyclable choline hydroxide catalyst. N. J. Chem. 2017, 41, 5373–5379. [Google Scholar] [CrossRef]
- Olszówka, J.E.; Karcz, R.; Michalik-Zym, A.; Napruszewska, B.D.; Bielańska, E.; Kryściak-Czerwenka, J.; Socha, R.P.; Nattich-Rak, M.; Krzan, M.; Klimek, A.; et al. Effect of grinding on the physico-chemical properties of Mg-Al hydrotalcite and its performance as a catalyst for Baeyer-Villiger oxidation of cyclohexanone. Catal. Today 2019, 333, 147–153. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Derr, P.F.; Vosburgh, W. Complex Ions. VII. A Solubility Method for the Determination of Instability Constants in Solution and the Ammines of Nickel, Cadmium and Magnesium. J. Am. Chem. Soc. 1943, 65, 2408–2411. [Google Scholar] [CrossRef]
- Bjerrum, J. Metal Ammine Formation in Aqueous Solution: Theory of the Reversible Step Reactions; Haase and Son: Copenhagen, Denmark, 1957. [Google Scholar]
- Tongraar, A.; Sagarik, K.; Rode, B.M. Effects of Many-Body Interactions on the Preferential Solvation of Mg2+ in Aqueous Ammonia Solution: A Born−Oppenheimer ab Initio QM/MM Dynamics Study. J. Phys. Chem. B 2001, 105, 10559–10564. [Google Scholar] [CrossRef]
- Wang, Q.; Tay, H.H.; Guo, Z.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. Morphology and composition controllable synthesis of Mg–Al–CO3 hydrotalcites by tuning the synthesis pH and the CO2 capture capacity. Appl. Clay Sci. 2012, 55, 18–26. [Google Scholar] [CrossRef]
- Hernandez-Moreno, M.J.; Ulibarri, M.A.; Rendon, J.L.; Serna, C.J. IR characteristics of hydrotalcite-like compounds. Phys. Chem. Miner. 1985, 12, 34–38. [Google Scholar]
- Kloprogge, J.T.; Frost, R.L. Fourier transform infrared and Raman spectroscopic study of the local structure of Mg-, Ni-, and Co-hydrotalcites. J. Solid State Chem. 1999, 146, 506–515. [Google Scholar] [CrossRef]
- Kagunya, W.; Baddour-Hadjean, R.; Kooli, F.; Jones, W. Vibrational modes in layered double hydroxides and their calcined derivatives. Chem. Phys. 1998, 236, 225–234. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zeng, H.C. Abrupt structural transformation in hydrotalcite-like compounds Mg1−xAlx(OH)2(NO3)x·nH2O as a continuous function of nitrate anions. J. Phys. Chem. B 2001, 105, 1743–1749. [Google Scholar] [CrossRef]
- Pesic, L.; Salipurovic, S.; Markovic, V.; Vucelic, D.; Kagunya, W.; Jones, W. Thermal characteristics of a synthetic hydrotalcite-like material. J. Mater. Chem. 1992, 2, 1069–1073. [Google Scholar] [CrossRef]
- Lopez, T.; Ramos, E.; Bosch, P.; Asomoza, M.; Gomez, R. DTA and TGA characterization of sol-gel hydrotalcites. Mater. Lett. 1997, 30, 279–282. [Google Scholar] [CrossRef]
- Stanimirova, T.; Piperov, N.; Petrova, N.; Kirov, G. Thermal evolution of Mg-Al-CO3 hydrotalcites. Clay Miner. 2004, 39, 177–191. [Google Scholar] [CrossRef]
- Zeng, H.; Feng, Z.; Deng, X.; Li, Y. Activation of Mg–Al hydrotalcite catalysts for transesterification of rape oil. Fuel 2008, 87, 3071–3076. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.F.; Qian, G.; Xu, Z.P.; Chen, C.; Liu, Q. Reinvestigation of Dehydration and Dehydroxylation of Hydrotalcite-like Compounds through Combined TG-DTA-MS Analyses. J. Phys. Chem. C 2010, 114, 10768–10774. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, C.; Aoki, Y.; Habazaki, H. Starch-Derived Hierarchical Porous Carbon with Controlled Porosity for High Performance Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 7292–7303. [Google Scholar] [CrossRef]
- Nishimura, S.; Takagaki, A.; Ebitani, K. Characterization, synthesis and catalysis of hydrotalcite-related materials for highly efficient materials transformations. Green Chem. 2013, 15, 2026. [Google Scholar] [CrossRef]
- Kaneda, K.; Mizugaki, T. Design of High-Performance Heterogeneous Catalysts Using Hydrotalcite for Selective Organic Transformations. Green Chem. 2019, 21, 1361–1389. [Google Scholar] [CrossRef]
- Song, Y.; Beaumont, S.; Zhang, X.; Wilson, K.; Lee, A.F. Catalytic applications of layered double hydroxides in biomass valorisation. Curr. Opin. Green Sustain. Chem. 2020, 22, 29–38. [Google Scholar] [CrossRef]
- Kaneda, K.; Ueno, S. Development of Hydrotalcite Catalysts in Heterogeneous Baeyer-Villiger Oxidation. ACS Symp. Ser. 1996, 638, 300–318. [Google Scholar]
- Pillai, U. Sn-exchanged hydrotalcites as catalysts for clean and selective Baeyer–Villiger oxidation of ketones using hydrogen peroxide. J. Mol. Catal. A-Chem. 2003, 191, 93–100. [Google Scholar] [CrossRef]
- Kawabata, T.; Fujisaki, N.; Shishido, T.; Nomura, K.; Sano, T.; Takehira, K. Improved Fe/Mg-Al hydrotalcite catalyst for Baeyer–Villiger oxidation of ketones with molecular oxygen and benzaldehyde. J. Mol. Catal. A-Chem. 2006, 253, 279–289. [Google Scholar] [CrossRef]
- Llamas, R.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Heterogeneous Baeyer–Villiger oxidation of ketones with H2O2/nitrile, using Mg/Al hydrotalcite as catalyst. Tetrahedron 2007, 63, 1435–1439. [Google Scholar] [CrossRef]
- Jiménez-Sanchidrián, C.; Ruiz, J.R. The Baeyer–Villiger reaction on heterogeneous catalysts. Tetrahedron 2008, 64, 2011–2026. [Google Scholar] [CrossRef]
- Chen, C.; Peng, J.; Li, B.; Wang, L. The Catalytic Baeyer–Villiger Oxidation of Cyclohexanone to ε-Caprolactone over Stibium-Containing Hydrotalcite. Catal. Lett. 2009, 131, 618–623. [Google Scholar] [CrossRef]
- Olszówka, J.; Karcz, R.; Napruszewska, B.D.; Duraczyńska, D.; Gaweł, A.; Bahranowski, K.; Serwicka, E.M. Baeyer-Villiger oxidation of cyclohexanone with H2O2/acetonitrile over hydrotalcite-like catalysts: Effect of Mg/Al ratio on the ε-caprolactone yield. Catal. Commun. 2017, 100, 196–201. [Google Scholar] [CrossRef]
- Karcz, R.; Olszówka, J.E.; Napruszewska, B.D.; Kryściak-Czerwenka, J.; Serwicka, E.M.; Klimek, A.; Bahranowski, K. Combined H2O2/nitrile/bicarbonate system for catalytic Baeyer-Villiger oxidation of cyclohexanone to ε-caprolactone over Mg Al hydrotalcite catalysts. Catal. Commun. 2019, 132, 105821. [Google Scholar] [CrossRef]
- Payne, G.B.; Deming, P.H.; Williams, P.H. Reactions of hydrogen peroxide. VII. Alkali-catalyzed Epoxidation and oxidation using a nitrile as co-reactant. J. Org. Chem. 1961, 26, 659–663. [Google Scholar] [CrossRef]
- Prihod’ko, R.; Sychev, M.; Kolomitsyn, I.; Stobbelaar, P.J.; Hensen, E.J.M.; van Santen, R.A. Layered double hydroxides as catalysts for aromatic nitrile hydrolysis. Micropor. Mesopor. Mat. 2002, 56, 241–255. [Google Scholar] [CrossRef]
- Jiménez-Sanchidrián, C.; Hidalgo, J.M.; Llamas, R.; Ruiz, J.R. Baeyer–Villiger oxidation of cyclohexanone with hydrogen peroxide/benzonitrile over hydrotalcites as catalysts. Appl. Catal. A-Gen. 2006, 312, 86–94. [Google Scholar] [CrossRef]

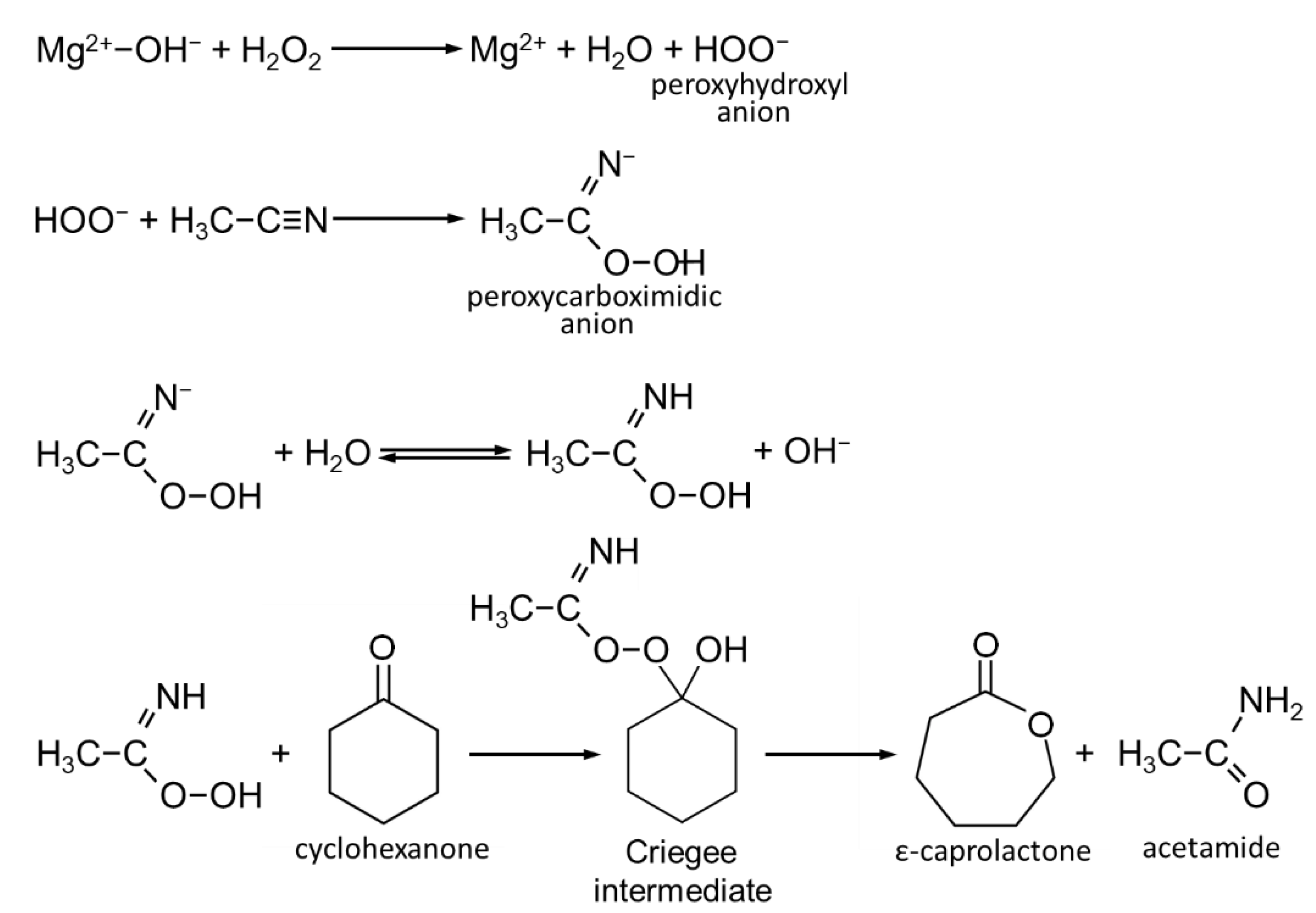
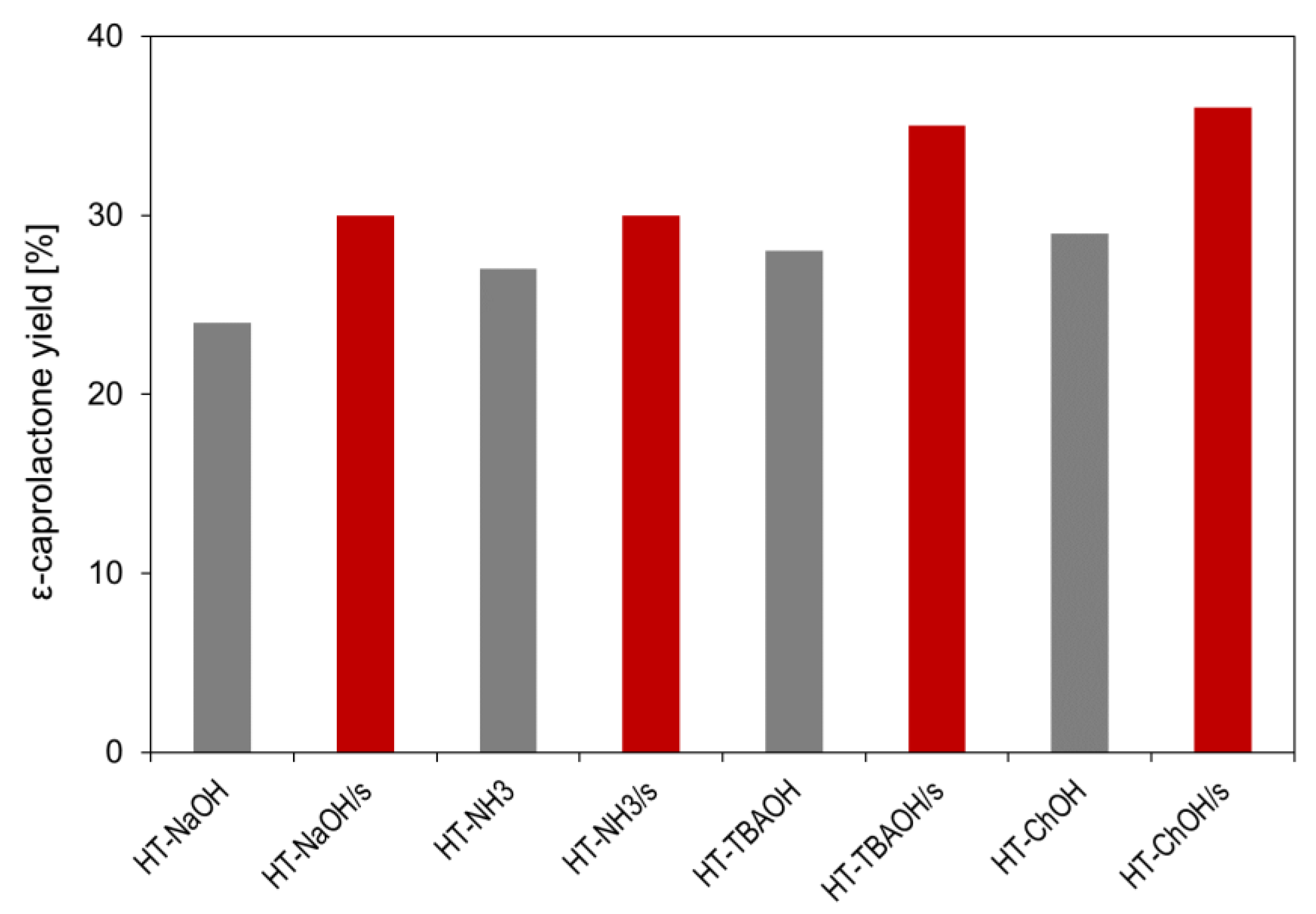
| Sample | d003 [nm] | d110 [nm] | D003 [nm] | D110 [nm] | D110/D003 | Mg/Al | Na [wt.%] | C [wt.%] | N [wt.%] |
|---|---|---|---|---|---|---|---|---|---|
| HT-NaOH | 0.778 | 0.1532 | 14.0 | 20.6 | 1.47 | 3.03 | 0.15 | 2.28 | 0 |
| HT-NaOH/s | 0.778 | 0.1532 | 8.8 (−37%) | 15.2 (−26%) | 1.72 | 3.05 | 0.21 | 5.18 | 0.04 |
| HT-NH3 | 0.774 | 0.1528 | 9.8 | 15.1 | 1.54 | 2.54 | - | 2.34 | 0 |
| HT-NH3/s | 0.775 | 0.1528 | 7.3 (−25%) | 13.8 (−9%) | 1.89 | 2.51 | - | 5.12 | 0.04 |
| HT-TBAOH | 0.776 | 0.1531 | 10.9 | 16.7 | 1.53 | 2.97 | - | 2.37 | 0 |
| HT-TBAOH/s | 0.779 | 0.1531 | 6.9 (−37%) | 11.8 (−29%) | 1.71 | 3.00 | - | 6.71 | 0.11 |
| HT-ChOH | 0.777 | 0.1531 | 10.4 | 16.9 | 1.63 | 2.93 | - | 2.39 | 0 |
| HT-ChOH/s | 0.778 | 0.1531 | 6.9 (−34%) | 13.3 (−21%) | 1.93 | 2.97 | - | 5.44 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karcz, R.; Napruszewska, B.D.; Walczyk, A.; Kryściak-Czerwenka, J.; Duraczyńska, D.; Płaziński, W.; Serwicka, E.M. Comparative Physicochemical and Catalytic Study of Nanocrystalline Mg-Al Hydrotalcites Precipitated with Inorganic and Organic Bases. Nanomaterials 2022, 12, 2775. https://doi.org/10.3390/nano12162775
Karcz R, Napruszewska BD, Walczyk A, Kryściak-Czerwenka J, Duraczyńska D, Płaziński W, Serwicka EM. Comparative Physicochemical and Catalytic Study of Nanocrystalline Mg-Al Hydrotalcites Precipitated with Inorganic and Organic Bases. Nanomaterials. 2022; 12(16):2775. https://doi.org/10.3390/nano12162775
Chicago/Turabian StyleKarcz, Robert, Bogna D. Napruszewska, Anna Walczyk, Joanna Kryściak-Czerwenka, Dorota Duraczyńska, Wojciech Płaziński, and Ewa M. Serwicka. 2022. "Comparative Physicochemical and Catalytic Study of Nanocrystalline Mg-Al Hydrotalcites Precipitated with Inorganic and Organic Bases" Nanomaterials 12, no. 16: 2775. https://doi.org/10.3390/nano12162775
APA StyleKarcz, R., Napruszewska, B. D., Walczyk, A., Kryściak-Czerwenka, J., Duraczyńska, D., Płaziński, W., & Serwicka, E. M. (2022). Comparative Physicochemical and Catalytic Study of Nanocrystalline Mg-Al Hydrotalcites Precipitated with Inorganic and Organic Bases. Nanomaterials, 12(16), 2775. https://doi.org/10.3390/nano12162775






