Simply Prepared Magnesium Vanadium Oxides as Cathode Materials for Rechargeable Aqueous Magnesium Ion Batteries
Abstract
:1. Introduction
2. Experimental Part
3. Results and Discussion
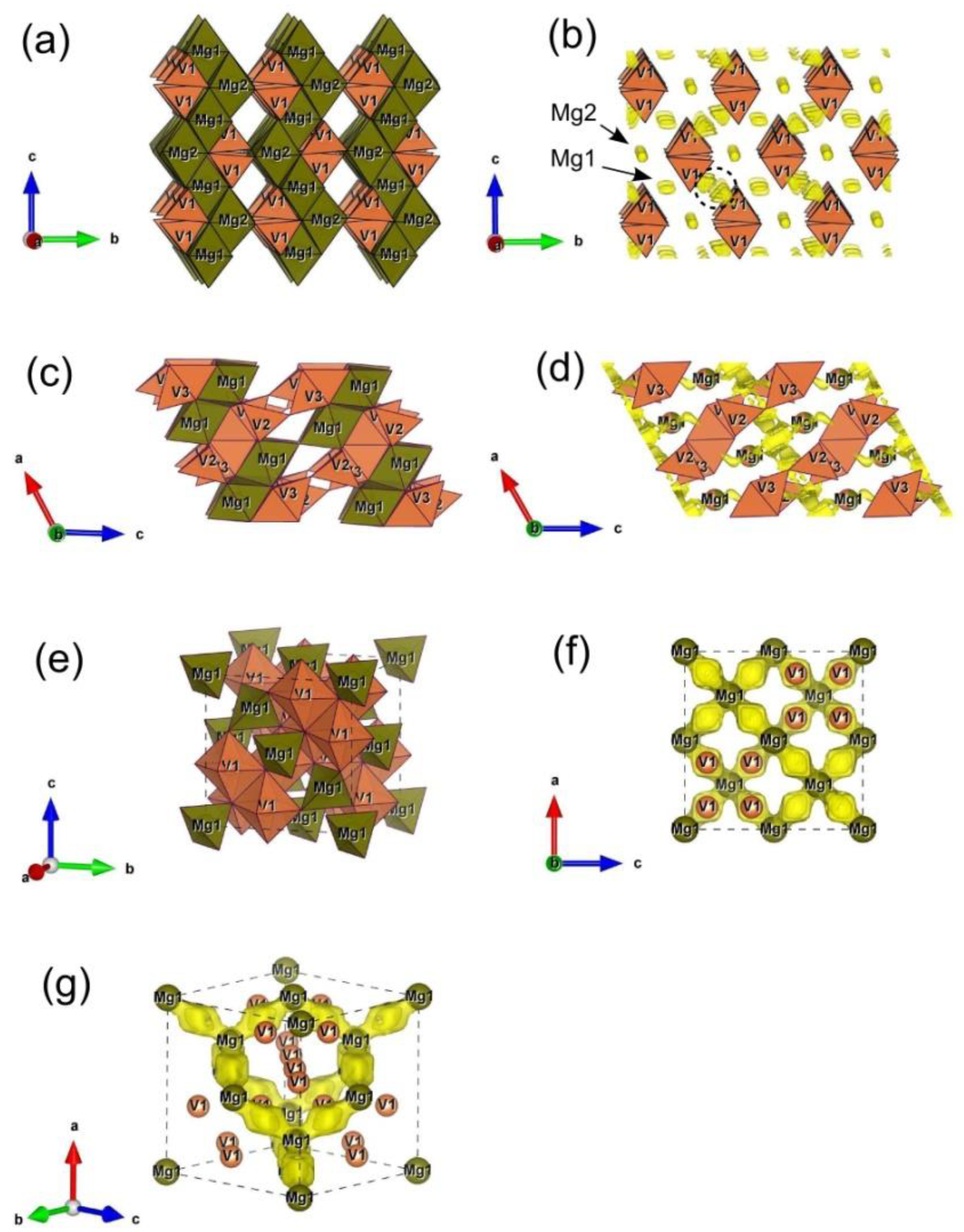
3.1. Structural Characterization
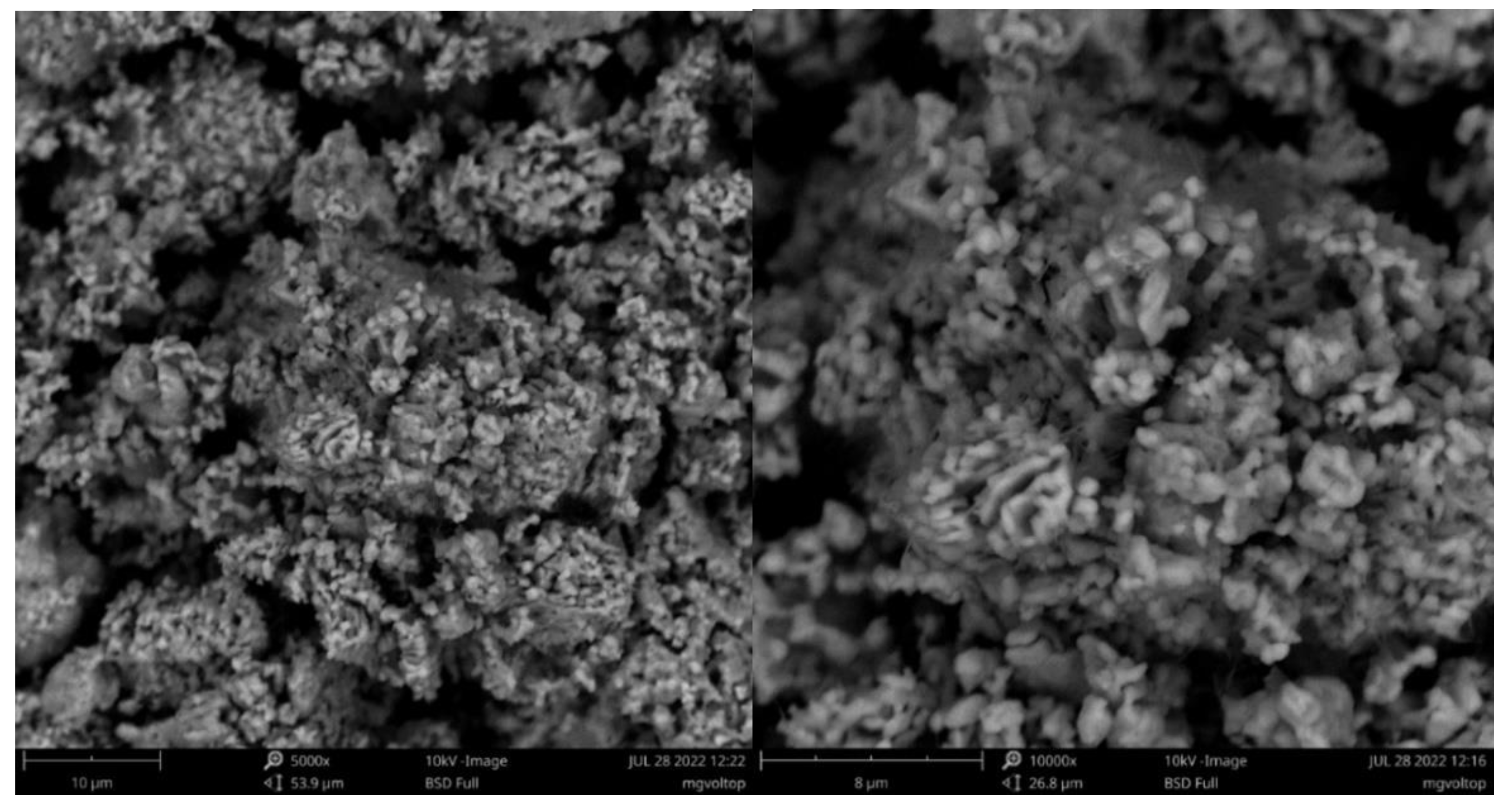
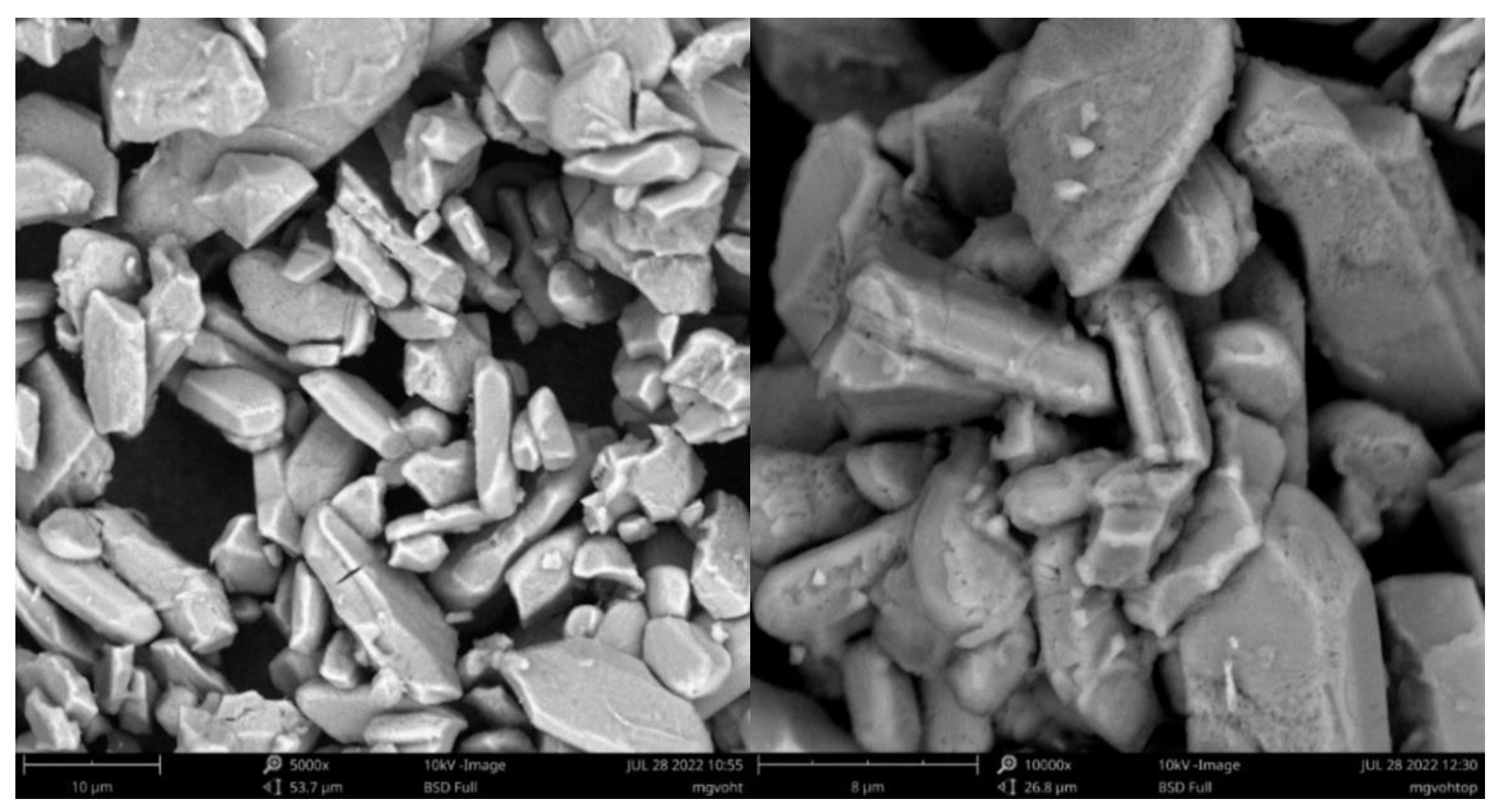
3.2. Morphological Characterization
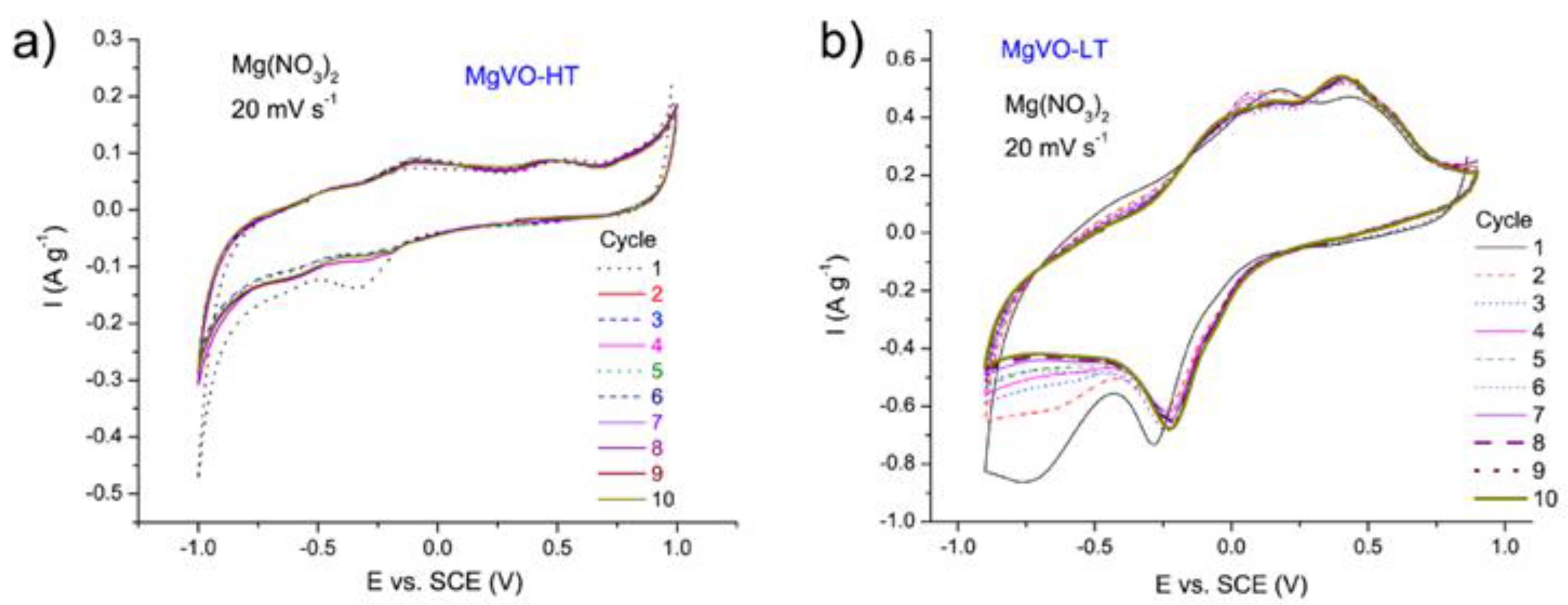
3.3. Electrochemical Characterization
3.3.1. MgVO-HT Material
3.3.2. MgVO-LT Material
3.4. Characterization of the MgVO-LT Material after Electrochemical Treatment
Bond Valence Sum (BVS) Calculations for the Phases Present in the MgVO-HT and MgVO-LT
3.5. Structural, Morphological, and Electrochemical Characterization of the MgVO/C Material with Improved Performances
3.5.1. BVS Calculations for the Phases Present in the MgVO/C
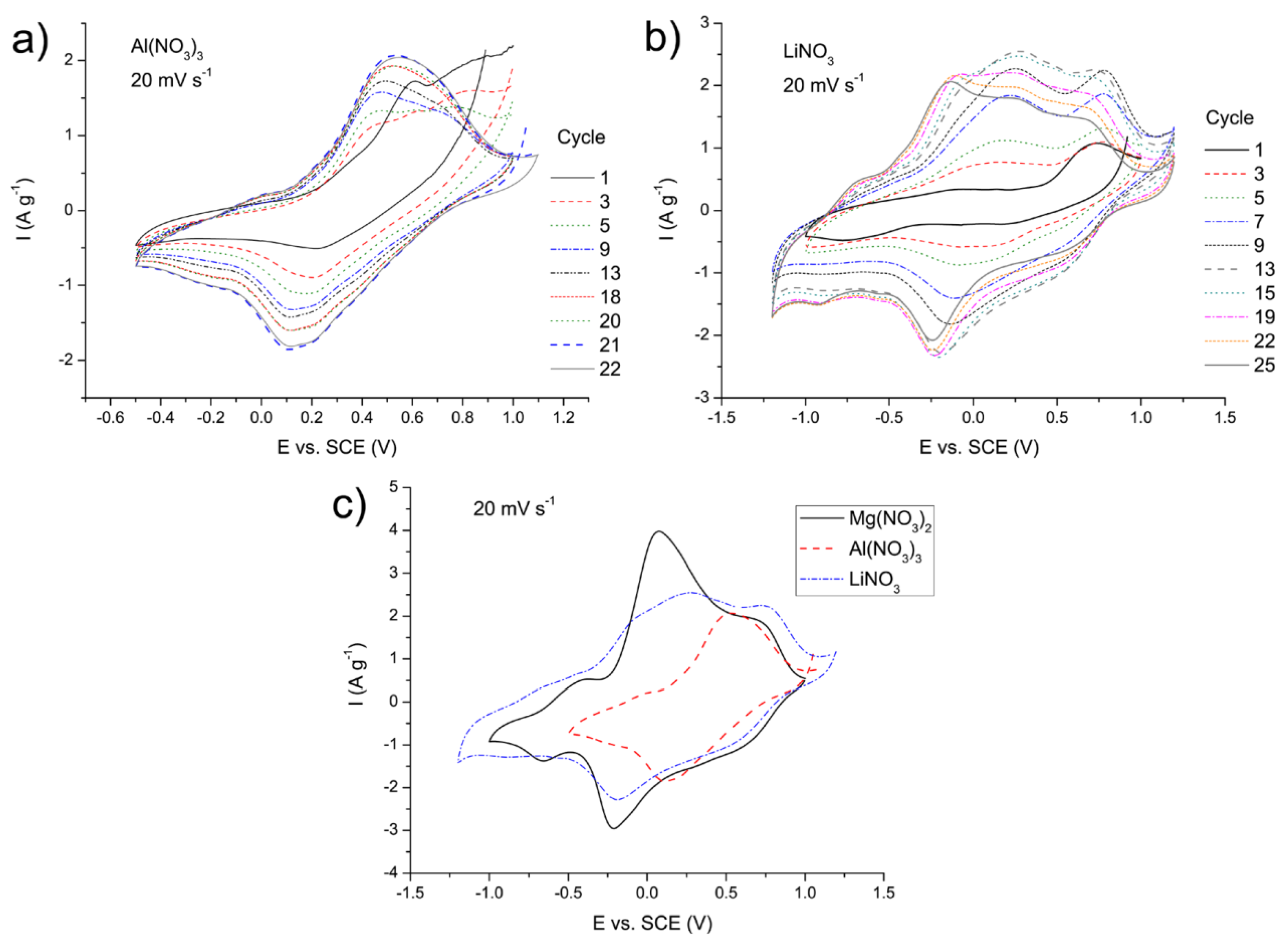
3.5.2. Comparison of the MgVO/C Performance in the Mg2+, Al3+, and Li+ Electrolytes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Bao, C.; Xie, L.; Yang, X.; Cao, X. Review of synthesis and structural optimization of LiNi1/3Co1/3Mn1/3O2 cathode materials for lithium-ion batteries applications. J. Alloy. Compd. 2020, 831, 154864. [Google Scholar] [CrossRef]
- Wen, J.; Yu, Y.; Chen, C. A review on lithium-ion batteries safety issues: Existing problems and possible solutions. Mater. Express 2012, 2, 197–212. [Google Scholar] [CrossRef]
- Heubner, C.; Nikolowski, K.; Reuber, S.; Schneider, M.; Wolter, M.; Michaelis, A. Recent insights into rate performance limitations of Li-ion batteries. Batter. Supercaps 2021, 4, 268–285. [Google Scholar] [CrossRef]
- Li, L.; Lu, Y.; Zhang, Q.; Zhao, S.; Hu, Z.; Chou, S.L. Recent progress on layered cathode materials for nonaqueous rechargeable magnesium batteries. Small 2019, 17, 1902767. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Chou, S.L.; Dou, S.X. Recent progress of layered transition metal oxide cathodes for sodium-ion batteries. Small 2019, 15, 1805381. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Ji, Y.; Ma, J.; Yu, H. Emerging nonaqueous aluminum-ion batteries: Challenges, status, and perspectives. Adv. Mater. 2018, 30, 1706310. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, Y.; Jia, M.; Bian, X.; Wang, Y.; Qiu, M.; Xu, J.; Liu, Y.; Jiao, L.; Cheng, F. Rechargeable aqueous Zn−V2O5 battery with high energy density and long cycle life. ACS Energy Lett. 2018, 3, 1366–1372. [Google Scholar] [CrossRef]
- Walter, M.; Kovalenko, M.V.; Kravchyk, K.V. Challenges and benefits of post-lithium-ion batteries. New J. Chem. 2020, 44, 1677–1683. [Google Scholar] [CrossRef]
- Pan, W.; Liu, C.; Wang, M.-Y.; Zhang, Z.-J.; Yan, X.-Y.; Yang, S.-C.; Liu, X.-H.; Wang, Y.-F.; Leung, D. Non-aqueous Al-ion batteries: Cathode materials and corresponding underlying ion storage mechanisms. Rare Met. 2022, 41, 762–774. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Wang, Z.; Zhao, W.; Qin, C.; Yu, H.; Wang, X.; Bakenov, Z.; Zhang, Y. Defective ZnOx@porous carbon nanofiber network inducing dendrite-free zinc plating as zinc metal anode for high-performance aqueous rechargeable Zn/Na4Mn9O18 battery based on hybrid electrolyte. J. Power Sources 2022, 518, 230761. [Google Scholar] [CrossRef]
- Karapidakis, E.; Vernardou, D. Progress on V2O5 cathodes for multivalent aqueous batteries. Materials 2021, 14, 2310. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Tsai, C.-L.; Tietz, F.; Wei, X.-K.; Heggen, M.; Dunin-Borkowskic, R.E.; Wang, R.; Xiao, Y.; Ma, Q.; Guillon, O. Room-temperature all-solid-state sodium batteries with robust ceramic interface between rigid electrolyte and electrode materials. Nano Energy 2019, 65, 104040. [Google Scholar] [CrossRef]
- Legeein, C.; Morgan, B.; Fayon, F.; Koketsu, T.; Ma, J.; Body, M.; Sarou-Kanian, V.; Wei, X.; Heggen, M.; Borkiewicz, O.; et al. Atomic Insights into Aluminium-Ion Insertion in Defective Anatase for Batteries. Angew. Chem. 2020, 59, 19247. [Google Scholar] [CrossRef]
- Yan, C.; Lv, C.; Wang, L.; Cui, W.; Zhang, L.; Dinh, K.N.; Tan, H.; Wu, C.; Wu, T.; Ren, Y.; et al. Architecting a stable high-energy aqueous Al-ion battery. J. Am. Chem. Soc. 2020, 142, 15295–15304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, M.; Qin, L.; Chen, M.; Chen, Z.; Guo, S.; Wang, L.; Fang, G.; Liang, S. Simultaneous regulation of cations and anions in an electrolyte for high-capacity, high-stability aqueous zinc–vanadium batteries. Escience 2022, 2, 209–218. [Google Scholar] [CrossRef]
- Novak, P.; Desilvestro, J. Electrochemical insertion of magnesium in metal oxides and sulfides from aprotic electrolytes. J. Electrochem. Soc. 1993, 140, 140–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, H.; Wei, W.; Ma, J.; Chen, V.; Li, C.C. Challenges and recent progress in the design of advanced electrode materials for rechargeable Mg batteries. Energy Storage Mater. 2019, 20, 118–138. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Zhang, Z.; Zhao, R.; Zhao, D.; Ma, R.; Yin, L. Layered materials for supercapacitors and batteries: Applications and challenges. Prog. Mater. Sci. 2021, 118, 100763. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Zhao, X.; Yao, S.; Chen, A.; Zhou, Z. Computational screening of layered materials for multivalent ion batteries. ACS Omega 2019, 4, 7822–7828. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, G.; Yin, J.; Lei, Y.; Emwas, A.H.; Yu, X.; Mohammed, O.F.; Alshareef, H.N. Hydrated MgxV5O12 cathode with improved Mg2+ storage performance. Adv. Energy Mater. 2020, 10, 2002128. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, L.; Cao, X.; Zhou, J.; Pan, A.; Fang, G.; Wang, S.; Liang, S. Ion migration and defect effect of electrode materials in multivalent-ion batteries. Prog. Mater. Sci. 2022, 125, 100911. [Google Scholar] [CrossRef]
- Mao, M.; Gao, T.; Hou, S.; Chunsheng, W. A critical review of cathodes for rechargeable Mg batteries. Chem. Soc. Rev. 2018, 47, 8804. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Xie, J.; Luo, T.; Huang, L.; Zhou, Y.; Yu, D.; Chen, C.; Liu, Y. Phase transformation and diffusion kinetics of V2O5 electrode in rechargeable Li and Mg batteries: A first-principle study. J. Phys. Chem. C 2018, 122, 1513–1521. [Google Scholar] [CrossRef]
- Kaveevivitchai, W.; Jacobson, A.J. High capacity rechargeable magnesium-ion batteries based on a microporous molybdenum-vanadium oxide cathode. Chem. Mater. 2016, 28, 4593–4601. [Google Scholar] [CrossRef]
- Kim, R.H.; Kim, J.S.; Kim, H.J.; Chang, W.S.; Han, D.W.; Lee, S.S.; Doo, S.G. Highly reduced VOx nanotube cathode materials with ultra-high capacity for magnesium ion batteries. J. Mater. Chem. A 2014, 2, 20636–20641. [Google Scholar] [CrossRef]
- Johnson, I.D.; Stapleton, N.; Nolis, G.; Bauer, D.; Parajuli, P.; Yoo, H.D.; Yin, L.; Ingram, B.J.; Klie, R.F.; Lapidus, S.; et al. Control of crystal size tailors the electrochemical performance of α-V2O5 as a Mg2+ intercalation host. Nanoscale 2021, 13, 10081–10091. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Jokisaari, J.R.; Kwon, B.J.; Yin, L.; Kim, S.; Park, H.; Lapidus, S.H.; Klie, R.F.; Key, B.; Zapol, P.; et al. High capacity for Mg2+ deintercalation in spinel vanadium oxide nanocrystals. ACS Energy Lett. 2020, 5, 2721–2727. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, X.; Li, Q.; Zhang, G.; Xiong, F.; Tan, S.; Wei, Q.; Lu, J.; Li, J.; An, Q.; et al. Vanadium oxide pillared by interlayer Mg2+ ions and water as ultralong-life cathodes for magnesium-ion batteries. Chem 2019, 5, 1194–1209. [Google Scholar] [CrossRef]
- Du, X.; Huang, G.; Qin, Y.; Wang, L. Solvothermal synthesis of GO/V2O5 composites as a cathode material for rechargeable magnesium batteries. RSC Adv. 2015, 5, 76352–76355. [Google Scholar] [CrossRef]
- Inamoto, M.; Kurihara, H.; Yajima, T. Electrode performance of vanadium pentoxide xerogel prepared by microwave irradiation as an active cathode material for rechargeable magnesium batteries. Electrochemistry 2012, 80, 421–422. [Google Scholar] [CrossRef]
- Yin, J.; Pelliccione, C.J.; Lee, S.H.; Takeuchi, E.S.; Takeuchi, K.J.; Marschilok, A.C. Communication—Sol-gel synthesized magnesium vanadium oxide, MgxV2O5·nH2O: The role of structural Mg2+ on battery performance. J. Electrochem. Soc. 2016, 163, A1941–A1943. [Google Scholar] [CrossRef]
- Sun, J.Z. Study of MgV2O6 as cathode material for secondary magnesium batteries. Asian J. Chem. 2011, 23, 1399–1400. [Google Scholar]
- Inamoto, M.; Kurihara, H.; Yajima, T. Vanadium pentoxide-based composite synthesized using microwave water plasma for cathode material in rechargeable magnesium batteries. Materials 2013, 6, 4514–4522. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.F.; Yuan, H.T.; Si, Y.C.; Wang, Y.J.; Wang, Y.M. Synthesis of Cu0.1-doped vanadium oxide nanotubes and their application as cathode materials for rechargeable magnesium batteries. Electrochem. Commun. 2006, 8, 1041–1044. [Google Scholar] [CrossRef]
- Demir-Cakan, R.; Palacín, M.R.; Croguennec, L. Rechargeable aqueous electrolyte batteries: From univalent to multivalent cation chemistry. J. Mater. Chem. A 2019, 7, 20519–20539. [Google Scholar] [CrossRef]
- Chao, D.; Zhou, W.; Xie, F.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S.Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098. [Google Scholar] [CrossRef]
- Wang, F.; Fan, X.; Gao, T.; Sun, W.; Ma, Z.; Yang, C.; Han, F.; Xu, K.; Wang, C. High-voltage aqueous magnesium ion batteries. ACS Central Sci. 2017, 3, 1121–1128. [Google Scholar] [CrossRef]
- Song, J.; Sahadeo, E.; Noked, M.; Lee, S.B. Mapping the challenges of magnesium battery. J. Phys. Chem. Lett. 2016, 7, 1736–1749. [Google Scholar] [CrossRef]
- Vujković, M.; Pašti, I.; Simatović, I.S.; Šljukić, B.; Milenković, M.; Mentus, S. The influence of intercalated ions on cyclic stability of V2O5/graphite composite in aqueous electrolytic solutions: Experimental and theoretical approach. Electrochim. Acta 2015, 176, 130–140. [Google Scholar] [CrossRef]
- Vujković, M.J.; Mladenović, D.; Milović, M.; Petrović, T.; Bogdanović, D.B.; Paunković, B.Š.; Mentus, S. Sodium-pillared vanadium oxides as next-gen materials: Does co-inserted water control the cyclic stability of vanadates in an aqueous electrolyte? Electrochim. Acta 2022, 425, 140603. [Google Scholar] [CrossRef]
- Charles, D.S.; Feygenson, M.; Page, K.; Neuefeind, J.; Xu, W.; Teng, X. Structural water engaged disordered vanadium oxide nanosheets for high capacity aqueous potassium-ion storage. Nat. Commun. 2017, 8, 15520. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; et al. Water-lubricated intercalation in V2O5·nH2O for high-capacity and high-rate aqueous rechargeable zinc batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Wang, L.; Huang, K.W.; Chen, J.; Zheng, J. Ultralong cycle stability of aqueous zinc-ion batteries with zinc vanadium oxide cathodes. Sci. Adv. 2019, 5, eaax4279. [Google Scholar] [CrossRef]
- Dong, S.; Shin, W.; Jiang, H.; Wu, X.; Li, Z.; Holoubek, J.; Stickle, W.F.; Key, B.; Liu, C.; Lu, J.; et al. Ultra-fast NH4+ storage: Strong H bonding between NH4+ and bi-layered V2O5. Chem 2019, 5, 1537–1551. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X. Review of vanadium-based electrode materials for rechargeable aqueous zinc ion batteries. J. Energy Chem. 2021, 56, 223–237. [Google Scholar] [CrossRef]
- Jo, J.H.; Sun, Y.K.; Myung, S.T. Hollandite-type Al-doped VO1.52(OH)0.77 as a zinc ion insertion host material. J. Mater. Chem. A 2017, 5, 8367–8375. [Google Scholar] [CrossRef]
- González, J.R.; Nacimiento, F.; Cabello, M.; Alcántara, R.; Lavela, P.; Tirado, J.L. Reversible intercalation of aluminium into vanadium pentoxide xerogel for aqueous rechargeable batteries. RSC Adv. 2016, 6, 62157–62164. [Google Scholar] [CrossRef]
- Soenen, V.; Herrmann, J.M.; Volta, J.C. In situ electrical characterization of magnesium vanadate reference phases (meta-MgV2O6, pyro-Mg2V2O7, and ortho-Mg3V2O8) used in oxidative dehydrogenation of propane to propene. J. Catal. 1996, 159, 410–417. [Google Scholar] [CrossRef]
- Rybarczyk, P.; Berndt, H.; Radnik, J.; Pohl, M.M.; Buyevskaya, O.; Baerns, M.; Brückner, A. The structure of active sites in Me–V–O catalysts (Me = Mg, Zn, Pb) and its influence on the catalytic performance in the oxidative dehydrogenation (ODH) of propane. J. Catal. 2001, 202, 45–58. [Google Scholar] [CrossRef]
- ICSD. Inorganic Crystals Structure Database; FIZ Karlsruhe: Eggenstein-Leopoldshafen, Germany, 2014. [Google Scholar]
- Scherrer, P. Bestimmung der größe und der inneren struktur von kolloidteilchen mittels röntgenstrahlen, Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen. Abh. Akad. Wiss. Gött. Math.-Phys. Kl. 1918, 2, 98–100. [Google Scholar]
- Sale, M.; Avdeev, M. 3DBVSMAPPER: A program for automatically generating bond-valence sum landscapes. J. Appl. Crystallogr. 2012, 45, 1054–1056. [Google Scholar] [CrossRef]
- Adams, S. Practical Considerations in Determining Bond Valence Parameters. In Bond Valence; Brown, I.D., Poeppelmeier, K.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 91–128. [Google Scholar]
- Mojet, B.L.; Ebbesen, S.D.; Lefferts, L. Light at the interface: The potential of attenuated total reflection infrared spectroscopy for understanding heterogeneous catalysis in water. Chem. Soc. Rev. 2010, 39, 4643–4655. [Google Scholar] [CrossRef]
- Hanuza, J.; Jezowska-Trzebiatowska, B.; Oganowski, W. Structure of the active layer and catalytic mechanism of the V2O5/MgO catalysts in the oxidative dehydrogenation of ethylbenzene to styrene. J. Mol. Catal. 1985, 29, 109–143. [Google Scholar] [CrossRef]
- Gao, X.; Ruiz, P.; Xin, Q.; Guo, X.; Delmon, B. Effect of coexistence of magnesium vanadate phases in the selective oxidation of propane to propene. J. Catal. 1994, 148, 56–67. [Google Scholar] [CrossRef]
- Corma, A.; Nieto, J.M.L.; Paredes, N. Influence of the preparation methods of V-Mg-O catalysts on their catalytic properties for the oxidative dehydrogenation of propane. J. Catal. 1993, 144, 425–438. [Google Scholar] [CrossRef]
- Jin, M.; Cheng, Z.M. Oxidative dehydrogenation of cyclohexane to cyclohexene over Mg-V-O catalysts. Catal. Lett. 2009, 131, 266–278. [Google Scholar] [CrossRef]
- Heyns, A.M.; Venter, M.W.; Range, K.J. The vibrational spectra of NH4VO3 at elevated temperatures and pressures. Z. Naturforsch. 1987, 42b, 843–852. [Google Scholar] [CrossRef]
- Rahman, M.A.; Sarker, M.A.R. Synthesis, characterization and physical properties of high quality MgV2O6 crystals by solid-state reaction and ab-initio methods. J. Alloy. Compd. 2019, 797, 630–639. [Google Scholar] [CrossRef]
- Imamura, D.; Miyayama, M. Characterization of magnesium-intercalated V2O5/carbon composites. Solid State Ion. 2003, 161, 173–180. [Google Scholar] [CrossRef]
- Keen, O.S.; Love, N.G.; Linden, K.G. Nitrate photochemistry in the context of water reclamation. In Water Reclamation and Sustainability; Ahuja, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 229–246. [Google Scholar]
- Li, P.; Zhou, W.; Wang, X.; Zhang, Y.; Umezawa, N.; Abe, H.; Ye, J.; Wang, D. Effects of cation concentration on photocatalytic performance over magnesium vanadates. APL Mater. 2015, 3, 104405. [Google Scholar] [CrossRef]
- Choi, N.H.; Kwon, S.; Kim, H. Analysis of the oxidation of the V(II) by dissolved oxygen using UV-visible spectrophotometry in a vanadium redox flow battery. J. Electrochem. Soc. 2013, 160, A973–A979. [Google Scholar] [CrossRef]
- Venkatesan, A.; Chandar, N.R.K.; Pradeeswari, K.; Pandi, P.; Kandasamy, A.; Kumar, R.M.; Jayavel, R. Influence of Al doping on structural, luminescence and electrochemical properties of V2O5 nanostructures synthesized via non-hydrolytic sol-gel technique. Mater. Res. Express. 2019, 6, 015017. [Google Scholar] [CrossRef]
- Zhang, W.; Zuo, C.; Tang, C.; Tang, W.; Lan, B.; Fu, X.; Dong, S.; Luo, P. The current developments and perspectives of V2O5 as cathode for rechargeable aqueous zinc-ion batteries. Energy Technol. 2021, 9, 2000789. [Google Scholar] [CrossRef]
- Rastgoo-Deylami, M.; Chae, M.S.; Hong, S.T. H2V3O8 as a high energy cathode material for nonaqueous magnesium-ion batteries. Chem. Mater. 2018, 30, 7464–7472. [Google Scholar] [CrossRef]
- Ng, H.N.; Calvo, C. Crystal Structure of and Electron Spin Resonance of Mn2+ in MgV2O6. Can. J. Chem. 1972, 50, 3619–3624. [Google Scholar] [CrossRef]
- Shklover, V.; Haibach, T.; Ried, F.; Nesper, R.; Novák, P. Crystal Structure of the Product of Mg2+ Insertion into V2O5 Single Crystals. J. Solid State Chem. 1996, 123, 317–323. [Google Scholar] [CrossRef]
- Nielsen, U.G.; Jakobsen, H.J.; Skibsted, J.; Norby, P. Crystal structure of α-Mg2V2O7 from synchrotron X-ray powder diffraction and characterization by 51V MAS NMR spectroscopy. J. Chem. Soc. Dalton Trans. 2001, 21, 3214–3218. [Google Scholar] [CrossRef]
- Sault, A.G.; Mudd, J.E.; Miller, J.E.; Ruffner, J.A.; Rodriquez, M.A.; Tissot, R.G. Thin Film Models of Magnesium Orthovanadate Catalysts for Oxidative Dehydrogenation; Sandia Report SAND2000-3157, Unlimited Release; Sandia National Laboratories: Albuquerque, NM, USA, 2001. [Google Scholar]
- Fang, G.; Wang, Q.; Zhou, J.; Lei, Y.; Chen, Z.; Wang, Z.; Pan, A.; Liang, S. Metal organic framework-templated synthesis of bimetallic selenides with rich phase boundaries for sodium-ion storage and oxygen evolution reaction. ACS Nano 2019, 13, 5635–5645. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xu, N.; Pei, C.; Xiong, F.; Tan, S.; Luo, W.; An, Q.; Mai, L. H2V3O8 nanowires as high-capacity cathode materials for magnesium-based battery. ACS Appl. Mater. Interfaces 2017, 9, 28667–28673. [Google Scholar] [CrossRef]
- Drosos, C.; Jia, C.; Mathew, S.; Palgrave, R.G.; Moss, B.; Kafizas, A.; Vernardou, D. Aerosol-assisted chemical vapor deposition of V2O5 cathodes with high ratecapabilities for magnesium-ion batteries. J. Power Sources 2018, 384, 355–359. [Google Scholar] [CrossRef]
- Drosos, C.; Moss, B.; Kafizas, A.; Vernardou, D. V2O5 as magnesium cathode material with extended cyclic stability. J. Electrochem. Sci. Eng. 2020, 10, 257–262. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, K.; Zhu, K.; Cang, R.; Yan, J.; Cheng, K.; Wang, G.; Cao, D. High-energy-density aqueous magnesium-ion battery based on a carbon-coated FeVO4 anode and a Mg-OMS-1 cathode. Chem. Eur. J. 2017, 23, 17118–17126. [Google Scholar] [CrossRef]
- Krishnamachari, N.; Calvo, C. Refinement of the Structure of Mg3(VO4)2. Can. J. Chem. 1971, 49, 1629–1637. [Google Scholar] [CrossRef]
- Saux, M.; Galy, J. Structure cristalline de MgV3O8 beta. Comptes Rendus Hebd. Seances Acad. Sci. 1973, 276, 81–84. [Google Scholar]
- Reuter, B.; Aust, R.; Colsmann, G.; Neuwald, C. Über Oxidsysteme mit Übergangsmetallionen in verschiedenen Oxydationsstufen. XIX. Darstellung und Eigenschaften Vanadium(II)-haltiger und damit n-leitender Vanadium(III)-Spinelle. Z. Für Anorg. Allg. Chem. 1983, 500, 188–198. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Li, F.; Guan, Z.; Wang, R.; He, B.; Gong, Y.; Hu, X. Conformal conducting polymer shells on V2O5 nanosheet arrays as a high-rate and stable zinc-ion battery cathode. Adv. Mater. Interfaces 2019, 6, 1801506. [Google Scholar] [CrossRef]

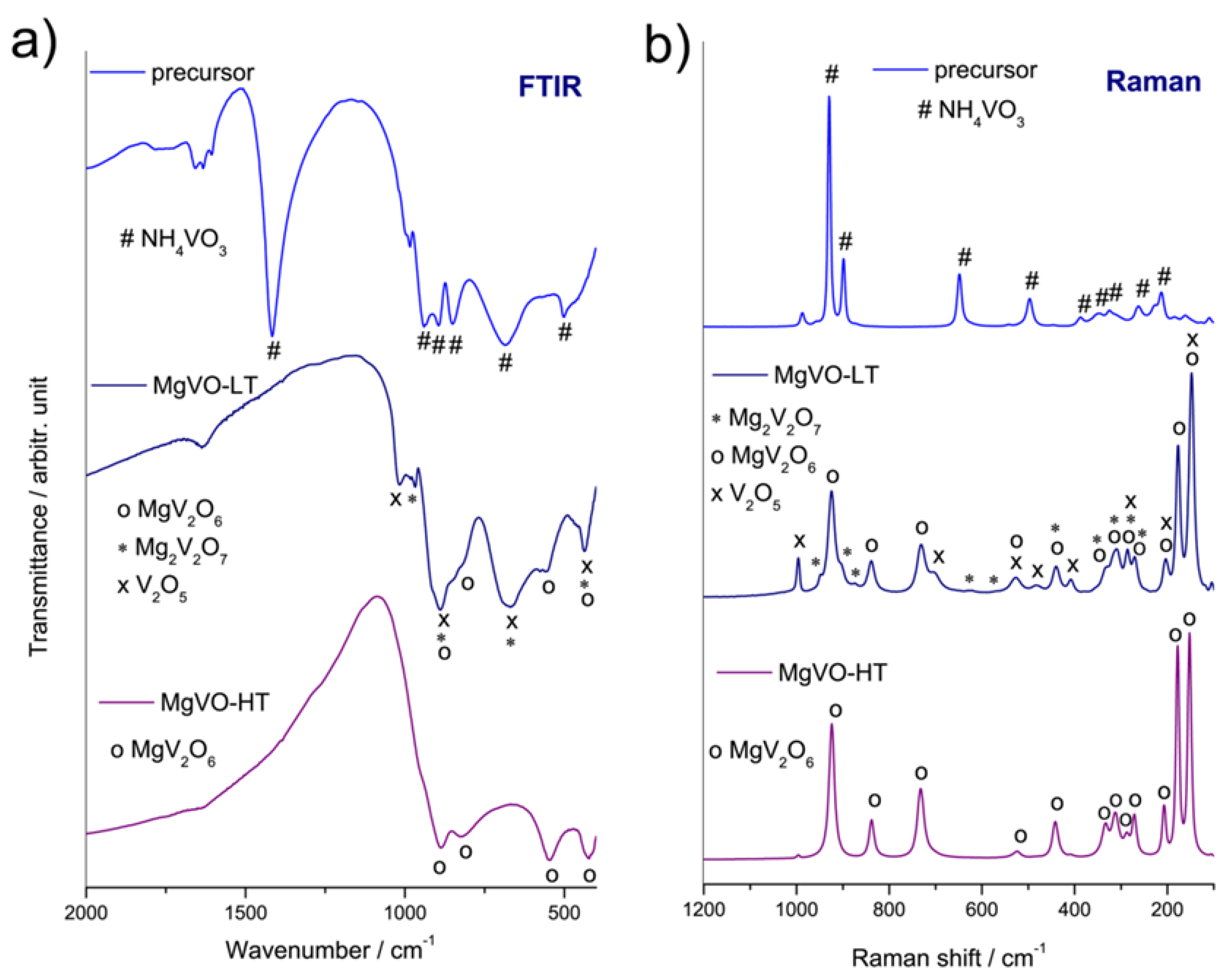

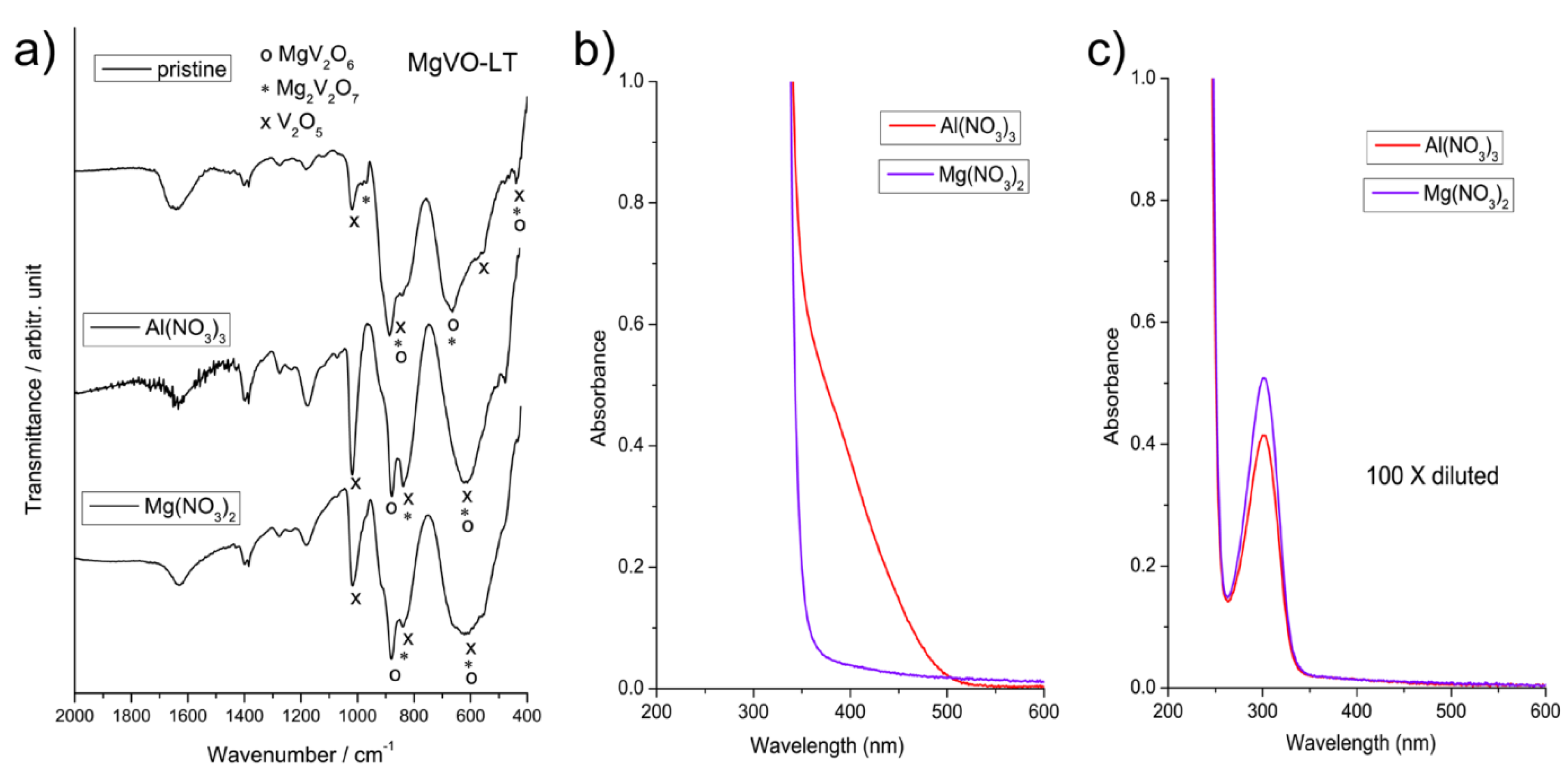

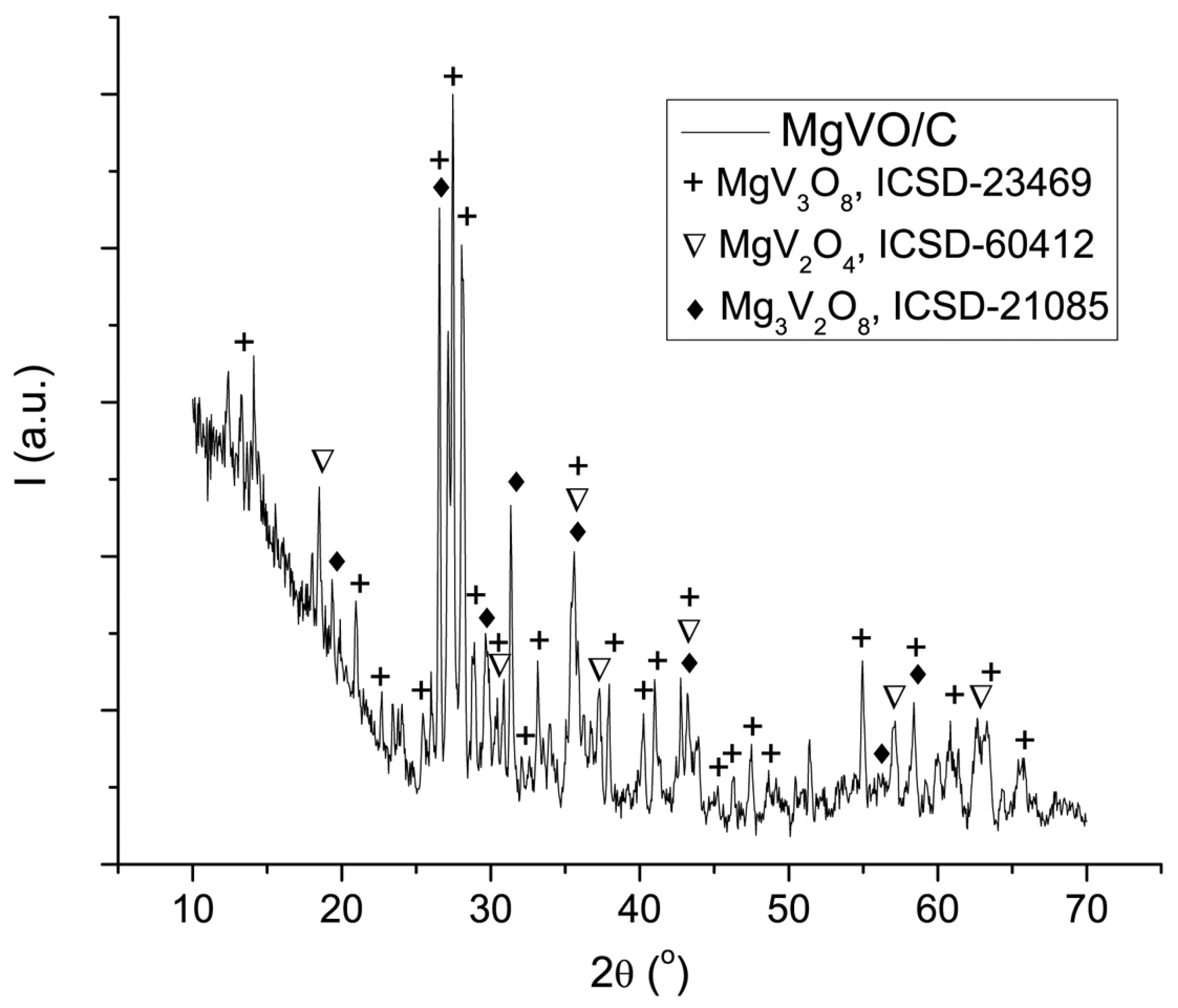

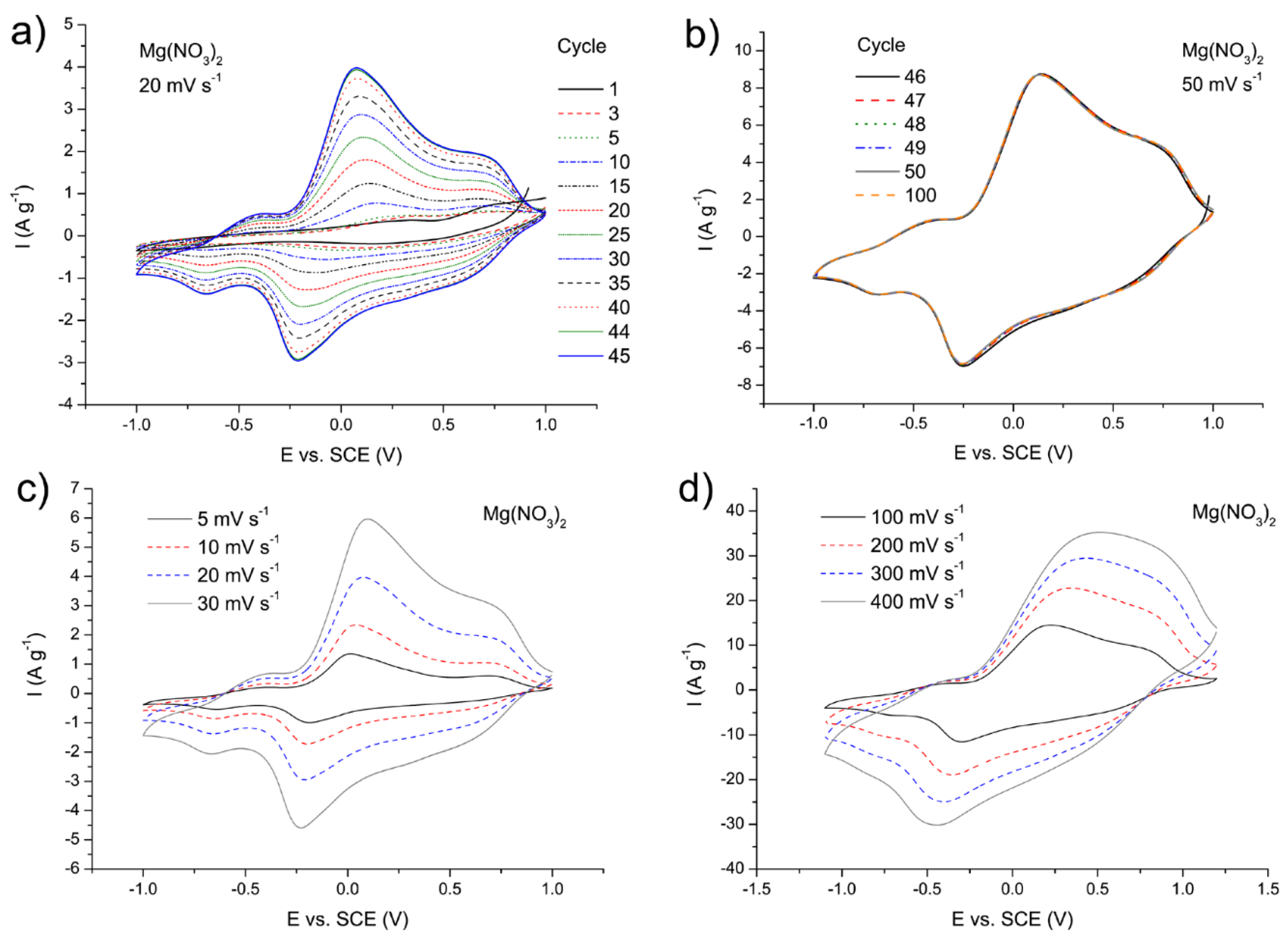
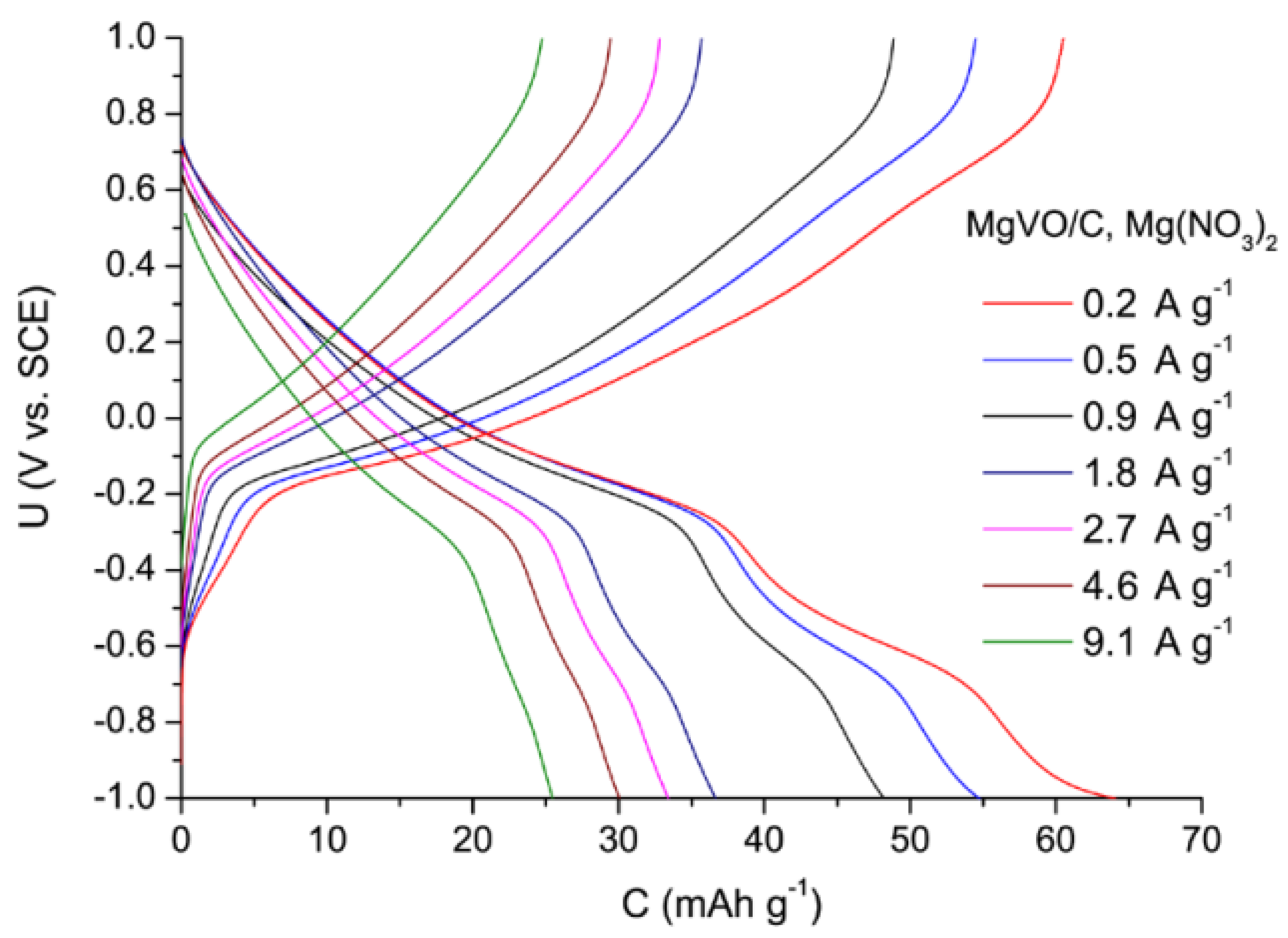
| Band Position | Assignation | Reference |
|---|---|---|
| FTIR spectra | ||
| 885 and 815 cm−1 | MgV2O6-stretching asymmetric νas(VO4) and νas(VO6) | [58,63] |
| 540 cm−1 | MgV2O6-stretching symmetric νs(VOV) | [58] |
| 425 cm−1 | MgV2O6-bending symmetric δs(VO4) and δs(VO6); and Mg-O vibrations | [58,63] |
| Raman spectra | ||
| 925 cm−1 | MgV2O6-stretching ν(VV=O) and νs(VO4) (symmetric) | [58] |
| 840 cm−1 | MgV2O6-stretching asymmetric νas(VO4) and νas(VO6) | [58] |
| 733 cm−1 | MgV2O6-stretching asymmetric νas(VOV) | [58] |
| 524 cm−1 | MgV2O6-stretching symmetric νs(VOV) | [58] |
| 444 cm−1 | MgV2O6-bending symmetric δs(VO4) and δs(VO6) | [58] |
| 270–330 cm−1 | MgV2O6-bending asymmetric δas(VO4) and δas(VO6) | [58,61] |
| 205 cm−1; 175 cm−1; 150 cm−1 | MgV2O6 | [59,61] |
| Band Position | Assignation | Reference |
|---|---|---|
| FTIR spectra | ||
| 1020 cm−1 | V2O5-stretching ν(V-O) | [58] |
| 975 cm−1 | Mg2V2O7-stretching ν(VV=O) and νs(VO4) (symmetric) | [58] |
| 815–920 cm−1 | V2O5, MgV2O6, and Mg2V2O7-stretching asymmetric νas(VO4) and νas(VO6) | [58] |
| 620–720 cm−1 | V2O5 and Mg2V2O7-stretching asymmetric νas(VOV) | [58,60,61] |
| 540 cm−1 | MgV2O6-stretching symmetric νs(VOV) | [58] |
| 420–450 cm−1 | V2O5, MgV2O6, and Mg2V2O7-bending symmetric δs(VO4) and δs(VO6) | [58] |
| Raman spectra | ||
| 996 cm−1 | V2O5-stretching ν(VV=O) and νs(VO4) (symmetric) | [58] |
| 922 cm−1 | MgV2O6-stretching ν(VV=O) and νs(VO4) (symmetric) | [58,59] |
| 840 cm−1 | V2O5 and MgV2O6-stretching asymmetric νas(VO4) and νas(VO6); Mg2V2O7- stretching asymmetric νas(VOV) | [58] |
| 730 cm−1 | V2O5 and MgV2O6-stretching asymmetric νas(VOV) | [58,59] |
| 523 cm−1 | MgV2O6 and Mg2V2O7-stretching symmetric νs(VOV) | [58,59] |
| 440 cm−1 | MgV2O6 and Mg2V2O7-bending symmetric δs(VO4) and δs(VO6) | [58] |
| 412 cm−1 | V2O5-bending symmetric δs(VO4) and δs(VO6) | [58,59] |
| 335 cm−1 | V2O5 and Mg2V2O7-bending asymmetric δas(VO4) and δas(VO6) | [58] |
| 310 cm−1 | MgV2O6 and Mg2V2O7-bending asymmetric δas(VO4) and δas(VO6) | [58] |
| 285 cm−1 | Mg2V2O7-bending asymmetric δas(VO4) and δas(VO6) | [58] |
| 272 cm−1 | MgV2O6-bending asymmetric δas(VO4) and δas(VO6) | [58] |
| 205 cm−1; 175 cm−1; 150 cm−1 | MgV2O6 | [59,61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasić, M.M.; Milović, M.; Bajuk-Bogdanović, D.; Petrović, T.; Vujković, M.J. Simply Prepared Magnesium Vanadium Oxides as Cathode Materials for Rechargeable Aqueous Magnesium Ion Batteries. Nanomaterials 2022, 12, 2767. https://doi.org/10.3390/nano12162767
Vasić MM, Milović M, Bajuk-Bogdanović D, Petrović T, Vujković MJ. Simply Prepared Magnesium Vanadium Oxides as Cathode Materials for Rechargeable Aqueous Magnesium Ion Batteries. Nanomaterials. 2022; 12(16):2767. https://doi.org/10.3390/nano12162767
Chicago/Turabian StyleVasić, Milica M., Miloš Milović, Danica Bajuk-Bogdanović, Tamara Petrović, and Milica J. Vujković. 2022. "Simply Prepared Magnesium Vanadium Oxides as Cathode Materials for Rechargeable Aqueous Magnesium Ion Batteries" Nanomaterials 12, no. 16: 2767. https://doi.org/10.3390/nano12162767
APA StyleVasić, M. M., Milović, M., Bajuk-Bogdanović, D., Petrović, T., & Vujković, M. J. (2022). Simply Prepared Magnesium Vanadium Oxides as Cathode Materials for Rechargeable Aqueous Magnesium Ion Batteries. Nanomaterials, 12(16), 2767. https://doi.org/10.3390/nano12162767







