Effective Removal of Methylene Blue by Surface Alteration of TiO2 with Ficus Carica Leaf Extract under Visible Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of Leaf Extract
2.3. The Surface Alteration of Bulk TiO2 Material by Ficus Carica (FC) Leaf Extract
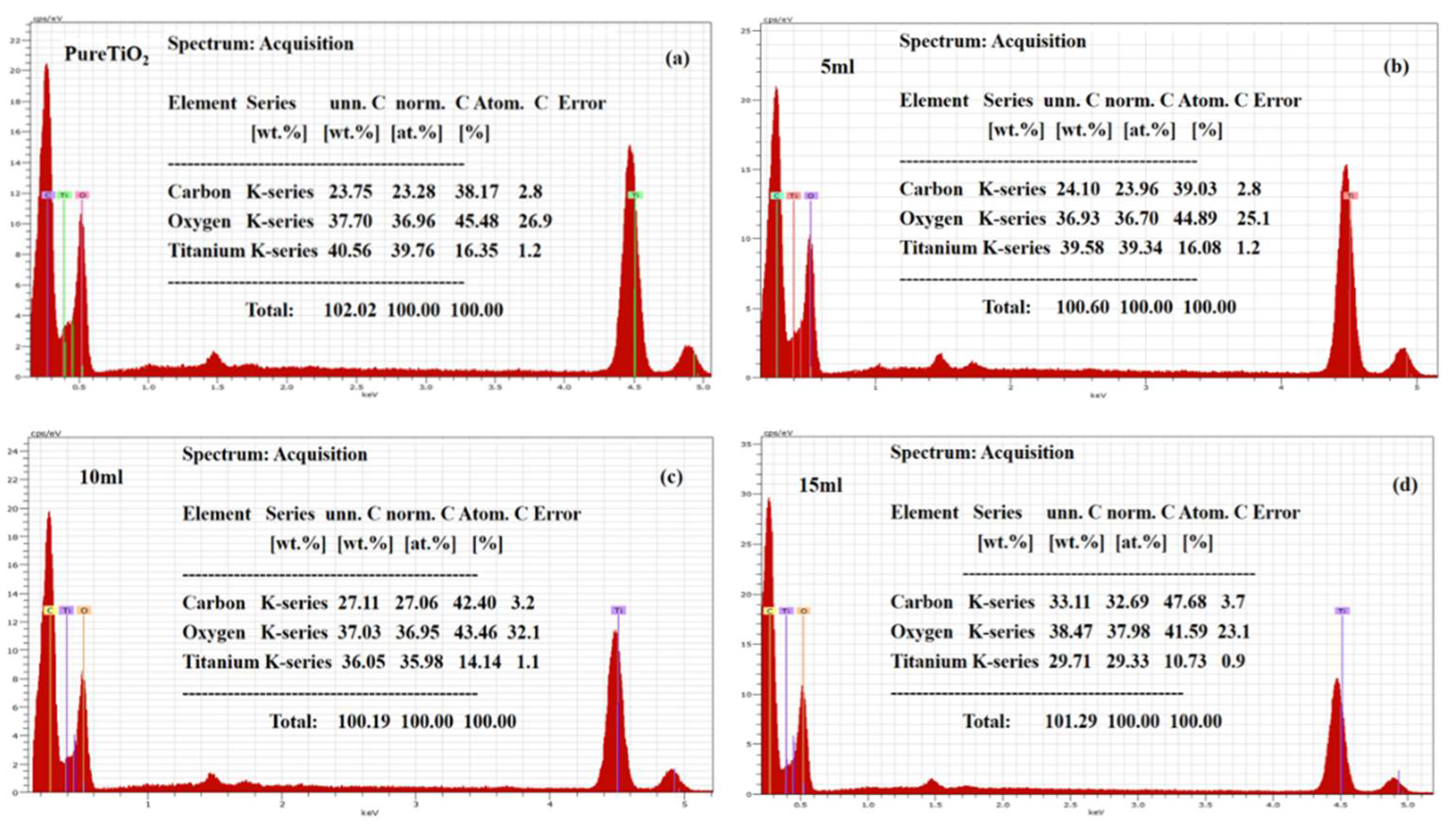
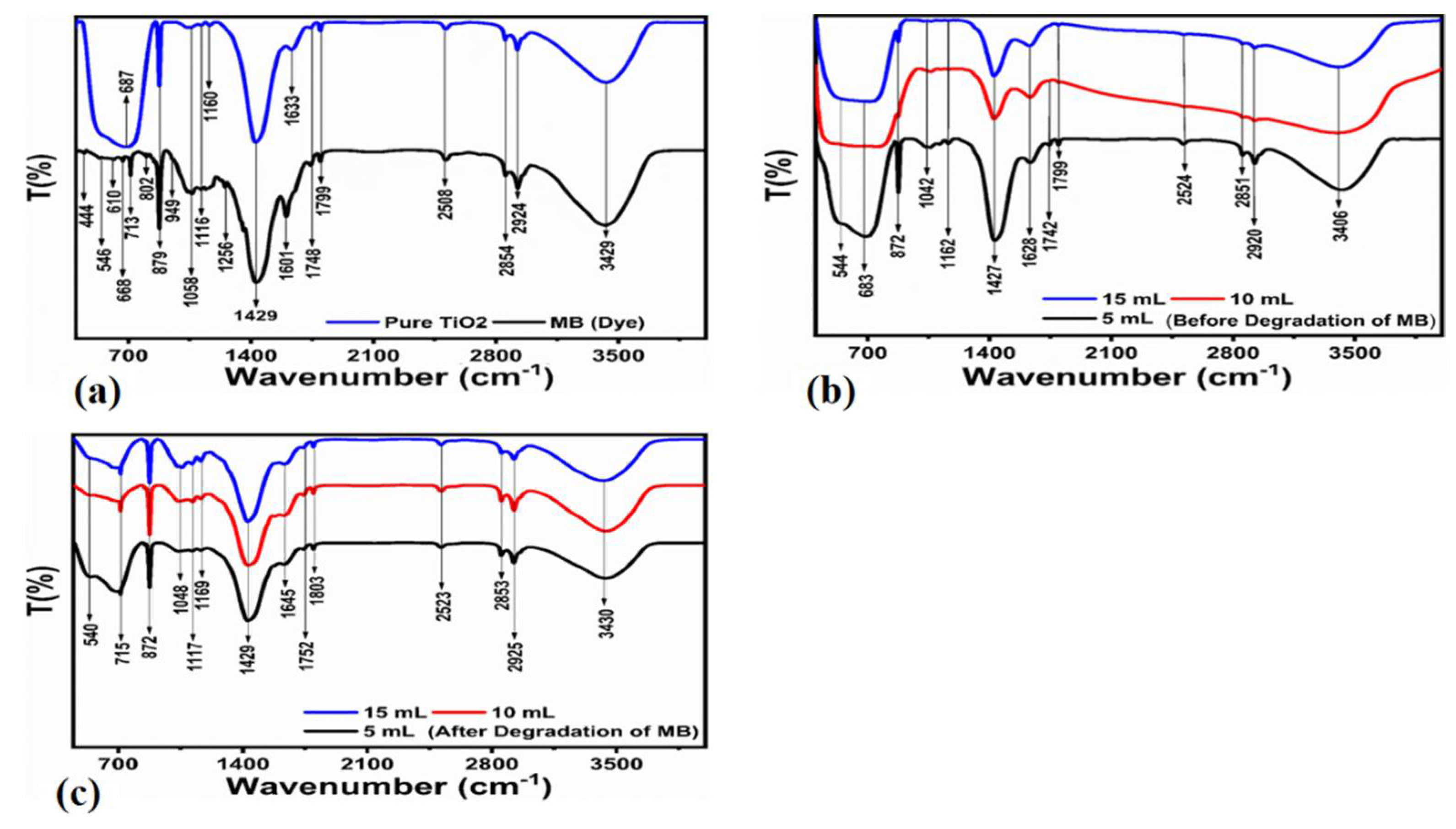
2.4. Characterization
2.5. Photo Catalytic Measurements
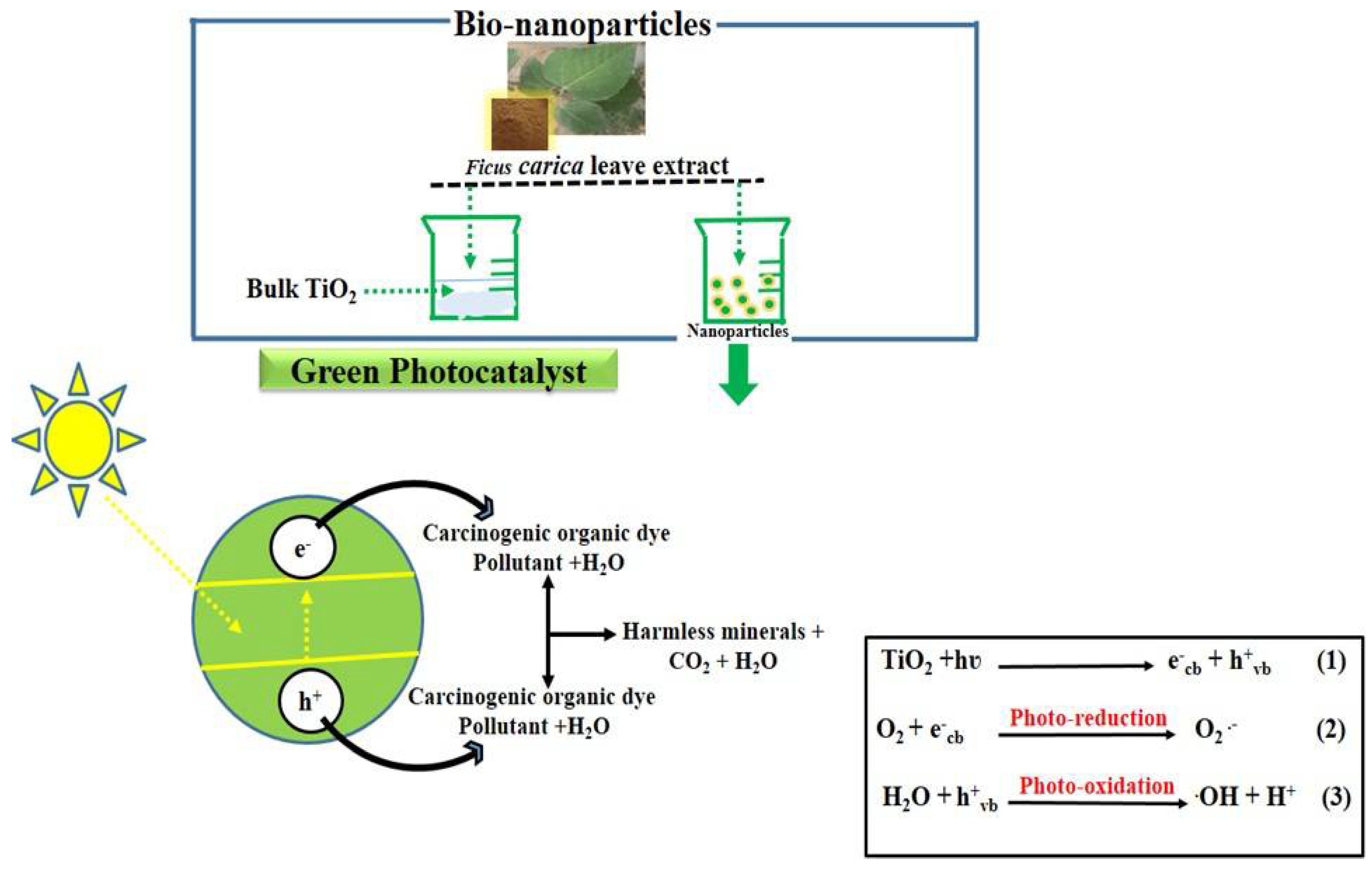
3. Results and Discussion
3.1. Analysis of Structure, Phase and Composition of the Surface Modified Bulk TiO2 Material after Treated with FC Leaf Extract
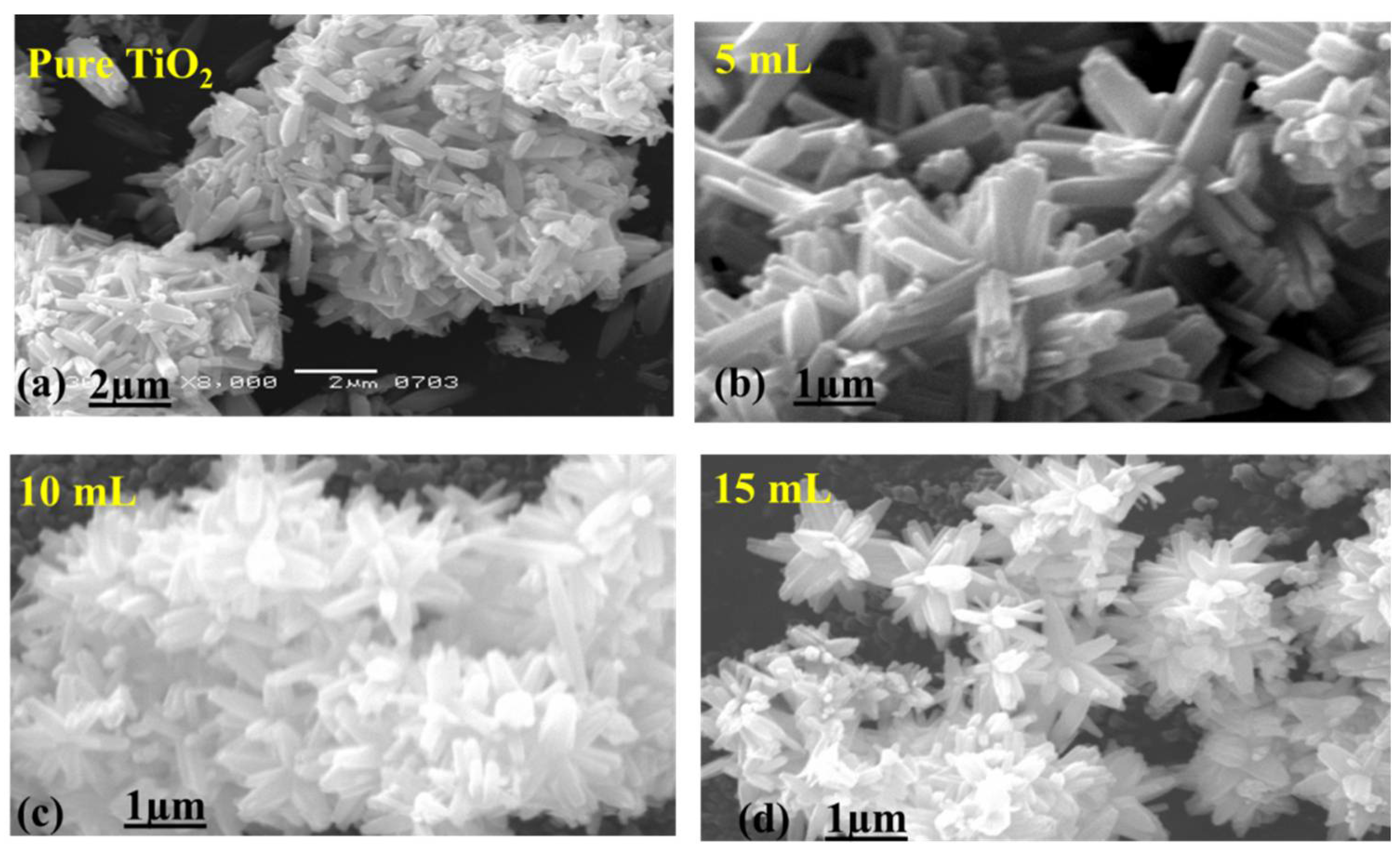
3.1.1. Morphological Investigation
3.1.2. XRD Investigation
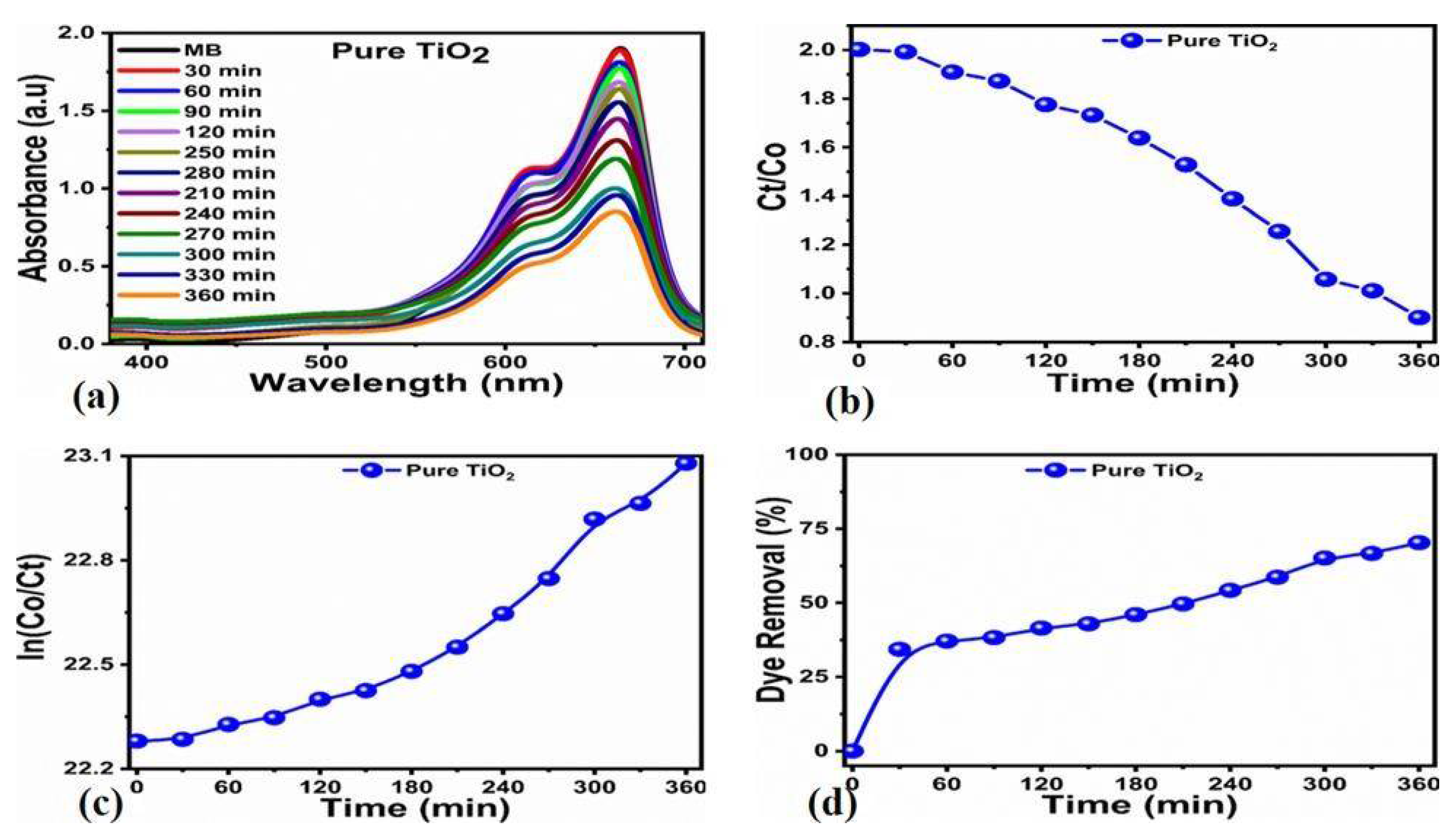
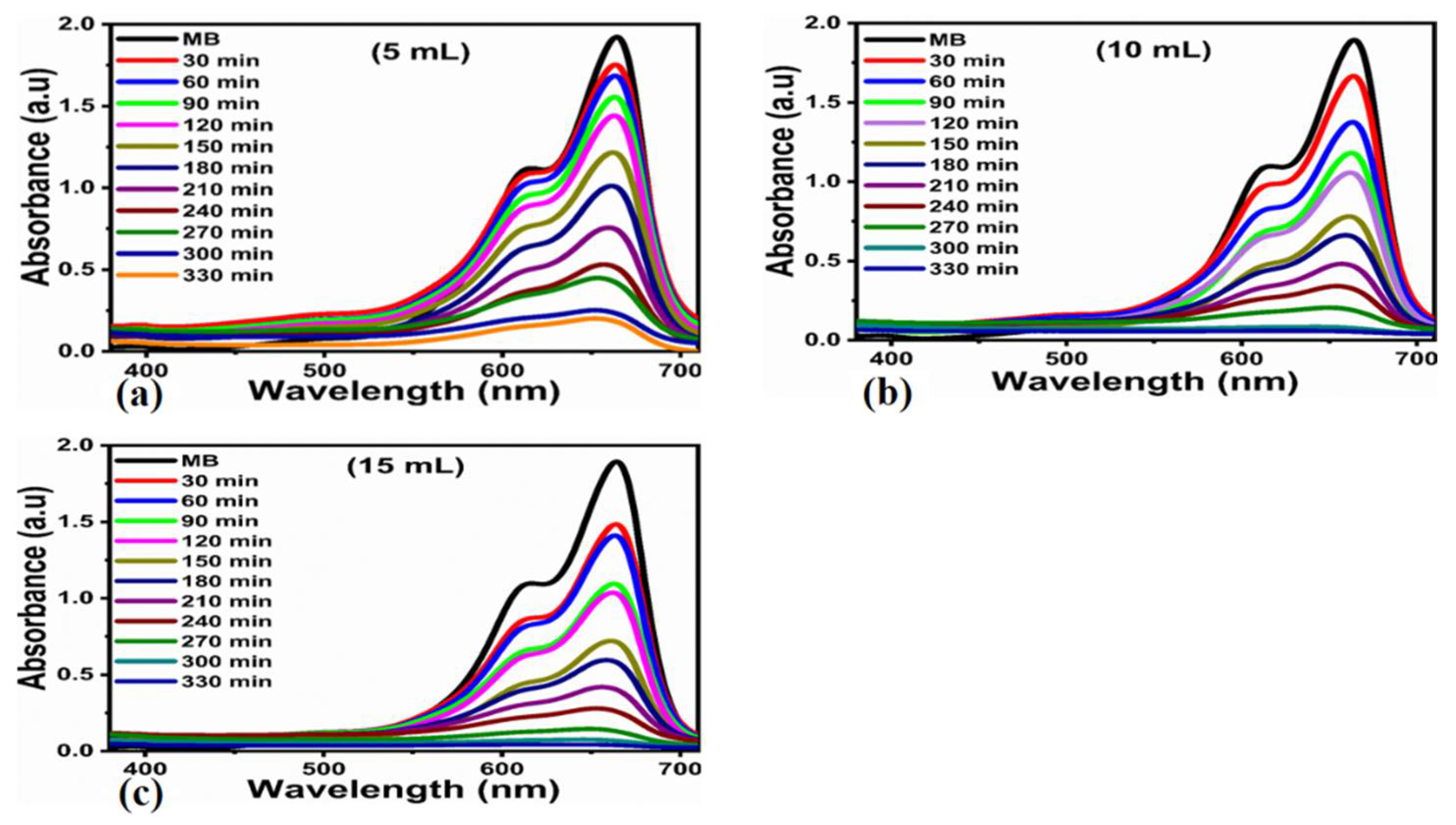
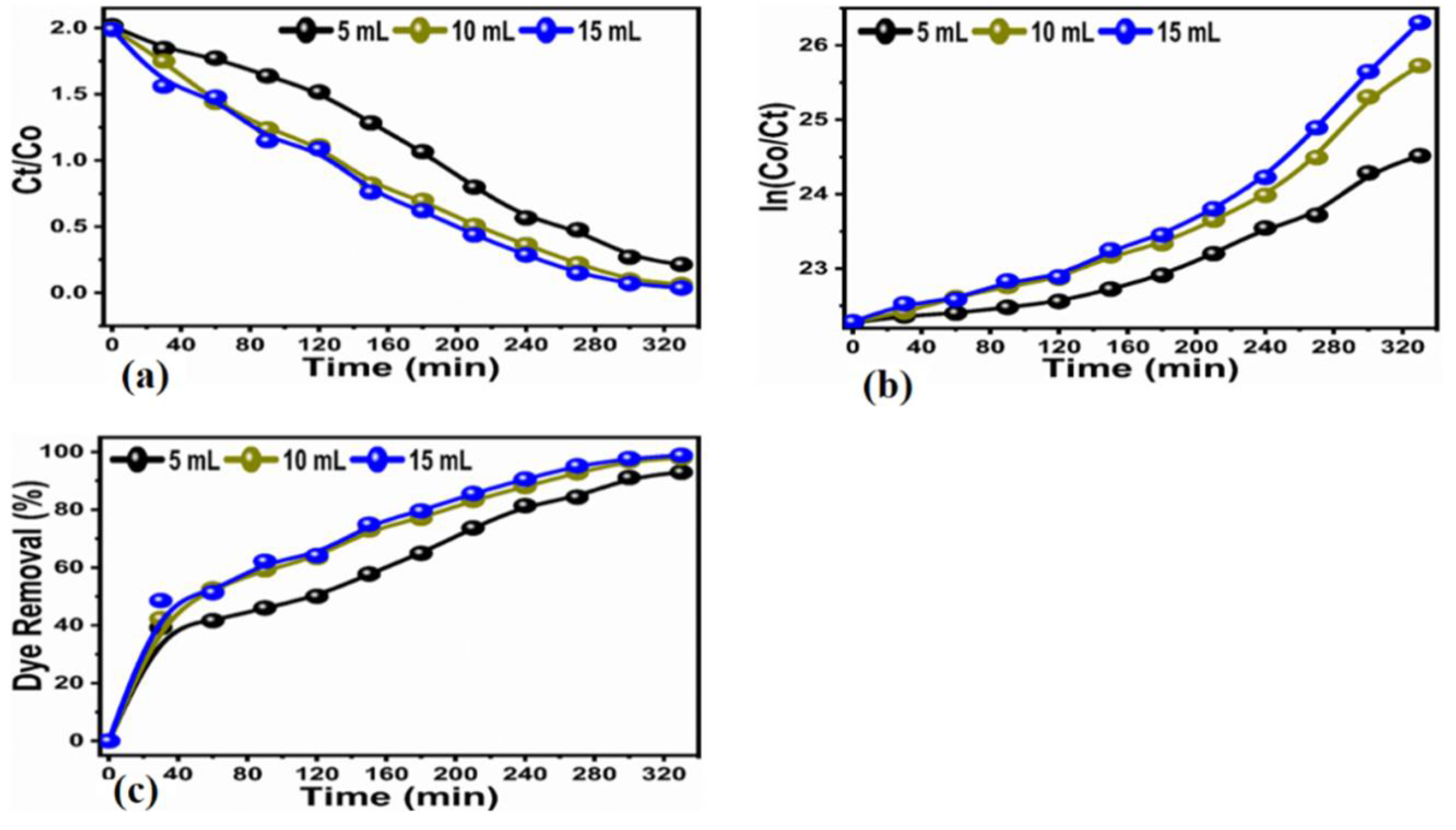
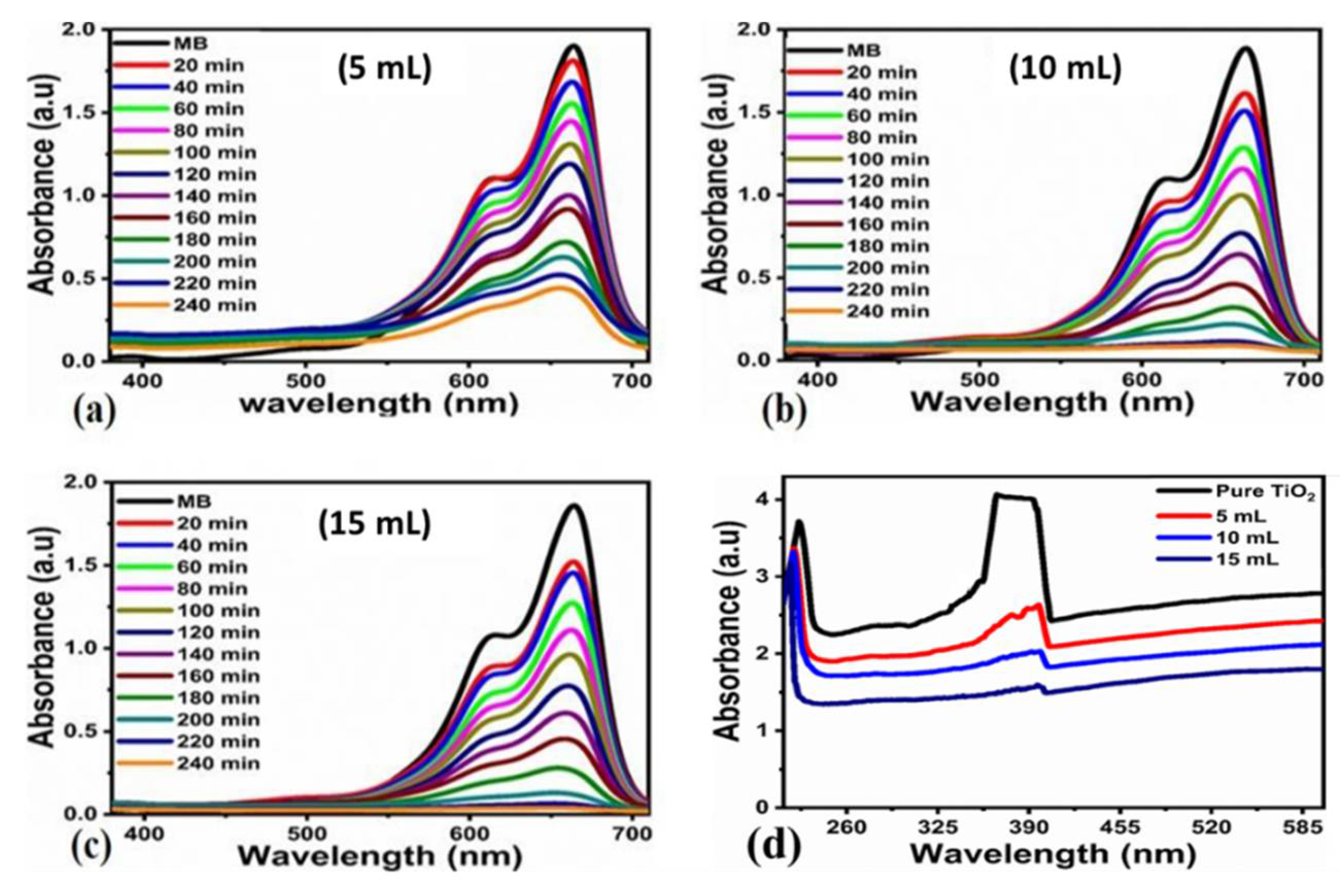
3.2. Photocatalytic Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, S.; Vaya, D.; Punjabi, P.B.; Ameta, S.C. Enhancing photocatalytic activity of zinc oxide by coating with some natural Pigments. Arab. J. Chem. 2011, 4, 205–209. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Khaleel, A.; Ahmed, A. Photocatalytic degradation of methylene blue using a mixed catalyst and product analysis by LC/MS. Chem. Eng. J. 2010, 157, 373–378. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Nazir, M.; Kanwal, S.; Zaman, S.; Sarwar, M.N.; Haroon, S.M. Efficient template based synthesis of Ni nanorods by etching porous alumina for their enhanced photocatalytic activities against Methyl Red and Methyl Orange dyes. J. Mol. Struct. 2019, 1184, 316–323. [Google Scholar] [CrossRef]
- Coughlin, M.F.; Kinkle, B.K.; Bishop, P.L. Degradation of acid orange 7 in an aerobic biofilm. Chemosphere 2002, 46, 11–19. [Google Scholar] [CrossRef]
- Khan, S.A.; Noreen, F.; Kanwal, S.; Hussain, G. Comparative synthesis, characterization of Cu-doped ZnO nanoparticles and their antioxidant, antibacterial, antifungal and photocatalytic dye degradation activities. Dig. J. Nanomater. Biostruct. 2017, 12, 877–889. [Google Scholar]
- Ijaz, F.; Shahid, S.; Khan, S.A.; Ahmad, W.; Zaman, S. Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: Antimicrobial, antioxidant and photocatalytic dye degradation activitie. Trop. J. Pharm. Res. 2017, 16, 743–753. [Google Scholar] [CrossRef]
- Khan, S.A.; Noreen, F.; Kanwal, S.; Iqbal, A.; Hussain, G. Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon indicum, Clerodendrum infortunatum, Clerodendrum inerme and investigation of their biological and photocatalytic activities. Mater. Sci. Eng. C 2018, 82, 46–59. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Madzivire, G.; Petrik, L.F. A review of combined advanced oxidation technologies for the removal of organic pollutants from water. Water Air Soil Pollut. 2014, 225, 2102. [Google Scholar] [CrossRef]
- Hisaindee, S.; Meetani, M.A.; Rauf, M.A. Application of LC-MS to the analysis of advanced oxidation process (AOP) degradation of dye products and reaction mechanisms. TrAC Trends Anal. Chem. 2013, 49, 31–44. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Z.; Huang, S.; Huang, X.; Xu, B.; Hu, L.; Cui, H.; Ruan, S.; Zeng, Y.J. Bio-inspired carbon doped graphitic carbon nitride with booming photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 246, 61–71. [Google Scholar] [CrossRef]
- He, Y.; Peng, G.; Jiang, Y.; Zhao, M.; Wang, X.; Chen, M.; Lin, S. Environmental Hazard Potential of Nano-Photocatalysts Determined by Nano-Bio Interactions and Exposure Conditions. Small 2020, 16, 1907690. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Bora, L.V.; Mewada, R.K. Visible/solar light active photocatalysts for organic effluent treatment: Fundamentals, mechanisms and parametric review. Renew. Sustain. Energy Rev. 2017, 76, 1393–1421. [Google Scholar] [CrossRef]
- Saien, J.; Mesgari, Z. Highly efficient visible-light photocatalyst of nitrogen-doped TiO2 nanoparticles sensitized by Hematoporphyrin. J. Mol. Catal. A Chem. 2016, 14, 108–115. [Google Scholar] [CrossRef]
- Khairy, M.; Zakaria, W. Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egypt. J. Pet. 2014, 23, 419–426. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Niu, H.; Han, X.; Zhang, T.; Liu, J.; Lin, H.; Qu, F. The synthesis of CdS/TiO2 hetero-nanofibers with enhanced visible photocatalytic activity. J. Colloid Interface Sci. 2015, 452, 89–97. [Google Scholar] [CrossRef]
- Kanagaraj, T.; Thiripuranthagan, S. Photocatalytic activities of novel SrTiO3–BiOBr heterojunction catalysts towards the degradation of reactive dyes. Appl. Catal. B Environ. 2017, 207, 218–232. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S.; Rao, N.N. Visible light assisted photodegradation of halocarbons on the dye modified TiO2 surface using visible light. Sol. Energy Mater. Sol. Cells 2006, 90, 1013–1020. [Google Scholar] [CrossRef]
- Swarnkar, A.K.; Sahare, S.; Chander, N.; Gangwar, R.K.; Bhoraskar, S.V.; Bhave, T.M. Nanocrystalline titanium dioxide sensitised with natural dyes for eco-friendly solar cell application. J. Exp. Nanosci. 2015, 10, 1001–1011. [Google Scholar] [CrossRef]
- Barolo, M.I.; Mostacero, N.R.; Lopez, S.N. Ficus carica, L. (Moraceae): An ancient source of food and health. Food Chem. 2014, 164, 119–127. [Google Scholar] [CrossRef]
- Jeong, W.S.; Lachance, P.A. Phytosterols and fatty acids in fig (Ficus carica, var. Mission) fruit and tree components. J. Food Sci. 2001, 66, 278–281. [Google Scholar] [CrossRef]
- Seong-Kuk, K.; Dong-Ok, C.; Hee-Jong, C. Purification and identification of antimicrobial substances in phenolic fraction of fig leaves. Appl. Biol. Chem. 1995, 38, 293–296. [Google Scholar]
- Khodarahmi, G.A.; Ghasemi, N.; Hassanzadeh, F.; Safaie, M. Cytotoxic effects of different extracts and latex of Ficus carica L. on HeLa cell line. Iran. J. Pharm. Res. IJPR 2011, 10, 273. [Google Scholar] [PubMed]
- Saeed, M.A.; Sabir, A.W. Irritant potential of triterpenoids from Ficus carica leaves. Fitoterapia 2002, 73, 417–420. [Google Scholar] [CrossRef]
- Shai, R.; Yoel, K.; Ruth, R.; Michael, S.; Raphael, M. Suppressors of cancer cell proliferation from fig (Ficus carica) resin: Isolation and structure elucidation. J. Nat. Prod. 2001, 64, 993–996. [Google Scholar]
- Weiping, Y.; Hongming, C.; Tianxin, W.; Mengshen, C. A new coumarin compound with anticancer activity. Chin. Trad. Herb. Drug 1997, 28, 3–4. [Google Scholar]
- Weiping, Y.; Hongming, C.; Tianxin, W.; Mengshen, C. Research on the chemical structure and anticancer activity of 9,19-cyclopropane-24, 25 ethyleneoxide-5-en-3β-spirostol. Chin. J. Med. Chem. 1997, 7, 46–47. [Google Scholar]
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.E.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef]
- Bhatti, M.A.; Tahira, A.; dad Chandio, A.; Almani, K.F.; Bhatti, A.L.; Waryani, B.; Nafady, A.; Ibupoto, Z.H. Enzymes and phytochemicals from neem extract robustly tuned the photocatalytic activity of ZnO for the degradation of malachite green (MG) in aqueous media. Res. Chem. Intermed. 2021, 47, 1581–1599. [Google Scholar] [CrossRef]
- Sagadevan, S.; Podder, J. Investigations on structural, optical, morphological and electrical properties of nickel oxide nanoparticles. Int. J. Nanopart. 2015, 8, 289–301. [Google Scholar] [CrossRef]
- Banerjee, I.; Karmakar, S.; Kulkarni, N.V.; Nawale, A.B.; Mathe, V.L.; Das, A.K.; Bhoraskar, S.V. Effect of ambient pressure on the crystalline phase of nano TiO2 particles synthesized by a dc thermal plasma reactor. J. Nanopart. Res. 2010, 12, 581–590. [Google Scholar] [CrossRef]
- Tauc, J.; Menth, A. States in the gap. J. Non-Cryst. Solids 1972, 8, 569–585. [Google Scholar] [CrossRef]
- Avciata, O.; Benli, Y.; Gorduk, S.; Koyun, O. Ag doped TiO2 nanoparticles prepared by hydrothermal method and coating of the nanoparticles on the ceramic pellets for photocatalytic study: Surface properties and photoactivity. J. Eng. Technol. Appl. Sci. 2016, 1, 34–50. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Mathpal, M.C.; Kumar, P.; Singh, M.K.; Mishra, S.K.; Srivastava, R.K.; Chung, J.S.; Verma, G.; Ahmad, M.M.; Agarwal, A. Synthesis based structural and optical behavior of anatase TiO2 nanoparticles. Mater. Sci. Semicond. Process. 2014, 23, 136–143. [Google Scholar] [CrossRef]
- Vaya, J.; Mahmood, S. Flavonoid content in leaf extracts of the fig (Ficus carica L.), carob (Ceratonia siliqua L.) and pistachio (Pistacia lentiscus L.). Biofactors 2006, 28, 169–175. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ollis, D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Al-Sayyed, G.; D’Oliveira, J.C.; Pichat, P. Semiconductor-sensitized photodegradation of 4-chlorophenol in water. J. Photochem. Photobiol. A Chem. 1991, 58, 99–114. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of nanomaterials in the treatment of wastewater: A review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Efficient degradation of organic pollutants by novel titanium dioxide coupled bismuth oxide nanocomposite: Green synthesis, kinetics and photoactivity. J. Environ. Manag. 2021, 300, 113777. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Mary, M.S.M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Khade, G.V.; Suwarnkar, M.B.; Gavade, N.L.; Garadkar, K.M. Green synthesis of TiO2 and its photocatalytic activity. J. Mater. Sci. Mater. Electron. 2015, 26, 3309–3315. [Google Scholar] [CrossRef]
- Alkaykh, S.; Mbarek, A.; Ali-Shattle, E.E. Photocatalytic degradation of methylene blue dye in aqueous solution by MnTiO3 nanoparticles under sunlight irradiation. Heliyon 2020, 6, e03663. [Google Scholar] [CrossRef]
- Vasiljevic, Z.Z.; Dojcinovic, M.P.; Vujancevic, J.D.; Jankovic-Castvan, I.; Ognjanovic, M.; Tadic, N.B.; Stojadinovic, S.; Brankovic, G.O.; Nikolic, M.V. Photocatalytic degradation of methylene blue under natural sunlight using iron titanate nanoparticles prepared by a modified sol–gel method. R. Soc. Open Sci. 2020, 7, 200708. [Google Scholar] [CrossRef]
- Xing, Z.; Li, J.; Wang, Q.; Zhou, W.; Tian, G.; Pan, K.; Tian, C.; Zou, J.; Fu, H. A floating porous crystalline TiO2 ceramic with enhanced photocatalytic performance for wastewater decontamination. Eur. J. Inorg. Chem. 2013, 13, 2411–2417. [Google Scholar] [CrossRef]
- Xing, Z.; Zhou, W.; Du, F.; Qu, Y.; Tian, G.; Pan, K.; Tian, C.; Fu, H. A floating macro/mesoporous crystalline anatase TiO2 ceramic with enhanced photocatalytic performance for recalcitrant wastewater degradation. Dalton Trans. 2014, 43, 790–798. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Bu, Y.; Zhang, J.; Wang, X.; Huang, J.; Chen, J.; Zhao, J. Preparation, characterization, and photocatalytic activity evaluation of Fe–N-codoped TiO2/fly ash cenospheres floating photocatalyst. Environ. Sci. Pollut. Res. 2016, 23, 22793–22802. [Google Scholar] [CrossRef]
- Wang, B.; Li, C.; Pang, J.; Qing, X.; Zhai, J.; Li, Q. Novel polypyrrole-sensitized hollow TiO2/fly ash cenospheres: Synthesis, characterization, and photocatalytic ability under visible light. Appl. Surf. Sci. 2012, 258, 9989–9996. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.K.; Mahalingam, H. Novel floating Ag+-doped TiO2/polystyrene photocatalysts for the treatment of dye wastewater. Ind. Eng. Chem. Res. 2014, 53, 16332–16340. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, Z.; Zhang, H.; Li, Z.; Zhang, X.; Zhang, Y.; Li, L.; Zhou, W. Multifunctional floating titania-coated macro/mesoporous photocatalyst for efficient contaminant removal. ChemPlusChem 2015, 80, 623. [Google Scholar] [CrossRef]
- Magalhães, F.; Moura, F.C.; Lago, R.M. TiO2/LDPE composites: A new floating photocatalyst for solar degradation of organ iccontaminants. Desalination 2011, 276, 266–271. [Google Scholar] [CrossRef]
- Zhong, W.; Yu, Y.; Du, C.; Li, W.; Wang, Y.; He, G.; Xie, Y.; He, Q. Characterization and high pollutant removal ability of buoyant (C, N)–TiO2/PTFE flakes prepared by high-energy ball-milling. RSC Adv. 2014, 4, 40019–40028. [Google Scholar] [CrossRef]
- Xue, H.; Jiang, Y.; Yuan, K.; Yang, T.; Hou, J.; Cao, C.; Feng, K.; Wang, X. Floating photocatalyst of B–N–TiO2/expanded perlite: A sol–gel synthesis with optimized mesoporous and high photocatalytic activity. Sci. Rep. 2016, 6, 29902. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.A.; Almaani, K.F.; Shah, A.A.; Tahira, A.; Chandio, A.D.; Mugheri, A.Q.; Bhatti, L.L.; Waryani, B.; Medany, S.S.; Nafady, A.; et al. Low Temperature Aqueous Chemical Growth Method for the Doping of W into ZnO Nanostructures and Their Photocatalytic Role in the Degradration of Methylene Blue. J. Clust. Sci. 2022, 33, 1445–1456. [Google Scholar] [CrossRef]
- Modestov, A.; Glezer, V.; Marjasin, I.; Lev, O. Photocatalytic degradation of chlorinated phenoxyacetic acids by a new buoyant titania-exfoliated graphite composite photocatalyst. J. Phys. Chem. B 1997, 101, 623–4629. [Google Scholar] [CrossRef]
- Han, H.; Bai, R. Buoyant photocatalyst with greatly enhanced visible-light activity prepared through a low temperature hydrothermal method. Ind. Eng. Chem. Res. 2009, 48, 2891–2898. [Google Scholar] [CrossRef]
- Han, H.; Bai, R. Highly effective buoyant photocatalyst prepared with a novel layered-TiO2 configuration on polypropylene fabric and the degradation performance for methyl orange dye under UV–Vis and Vis lights. Sep. Purif. Technol. 2010, 73, 142–150. [Google Scholar] [CrossRef]
- Pinna, M.; Binda, G.; Altomare, M.; Marelli, M.; Dossi, C.; Monticelli, D.; Spanu, D.; Recchia, S. Biochar nanoparticles over TiO2 nanotube arrays: A green co-catalyst to boost the photocatalytic degradation of organic pollutants. Catalysts 2021, 11, 1048. [Google Scholar] [CrossRef]
- Dalto, F.; Kuźniarska-Biernacka, I.; Pereira, C.; Mesquita, E.; Soares, O.S.G.; Pereira, M.F.R.; Rosa, M.J.; Mestre, A.S.; Carvalho, A.P.; Freire, C. Solar light-induced methylene blue removal over TiO2/AC composites and photocatalytic regeneration. Nanomaterials 2021, 11, 3016. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.; Chen, J.; Wang, S.; Ju, J.; Kang, W. Preparing Biomass Carbon Fiber Derived from Waste Rabbit Hair as a Carrier of TiO2 for Photocatalytic Degradation of Methylene Blue. Polymers 2022, 14, 1593. [Google Scholar] [CrossRef]
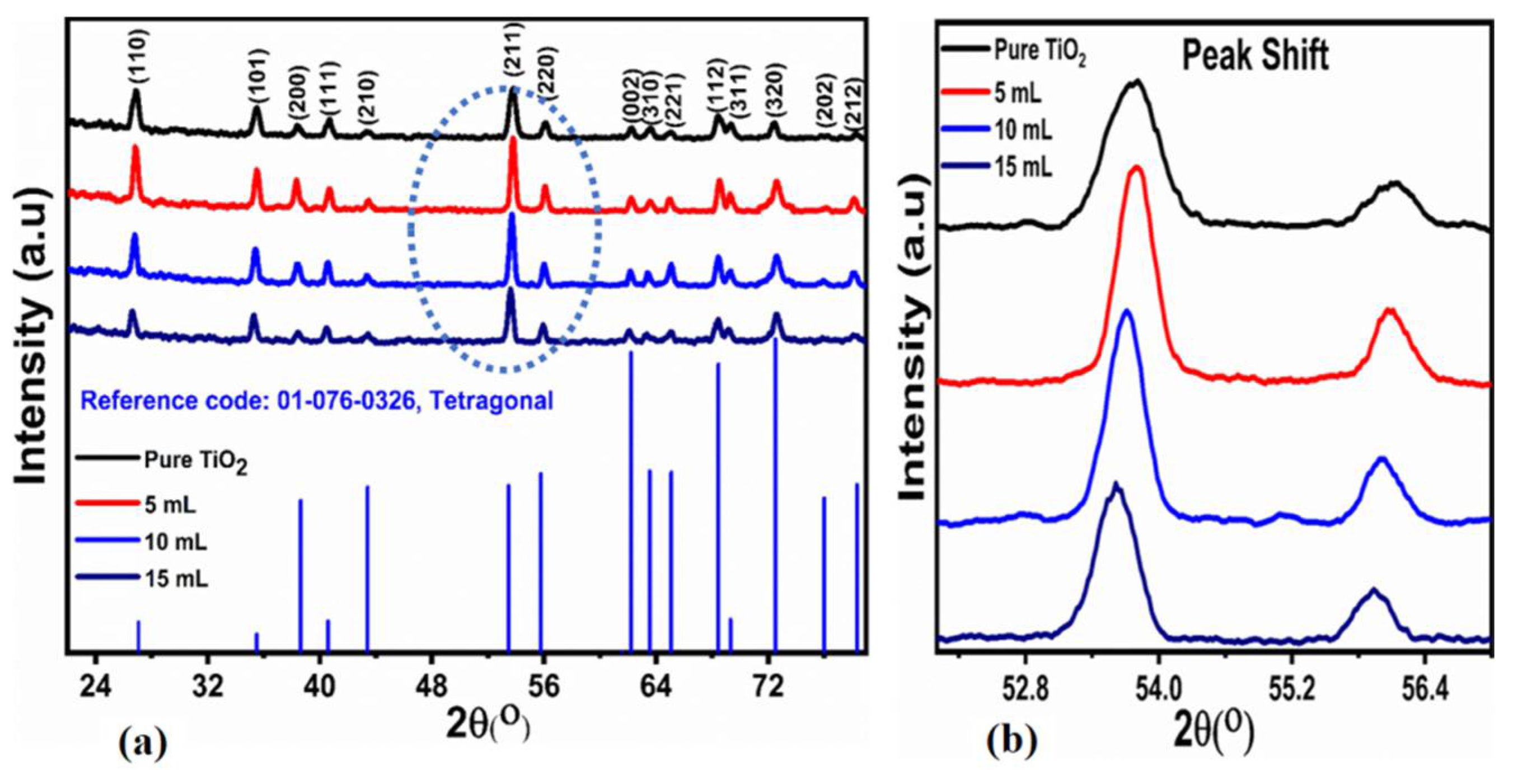
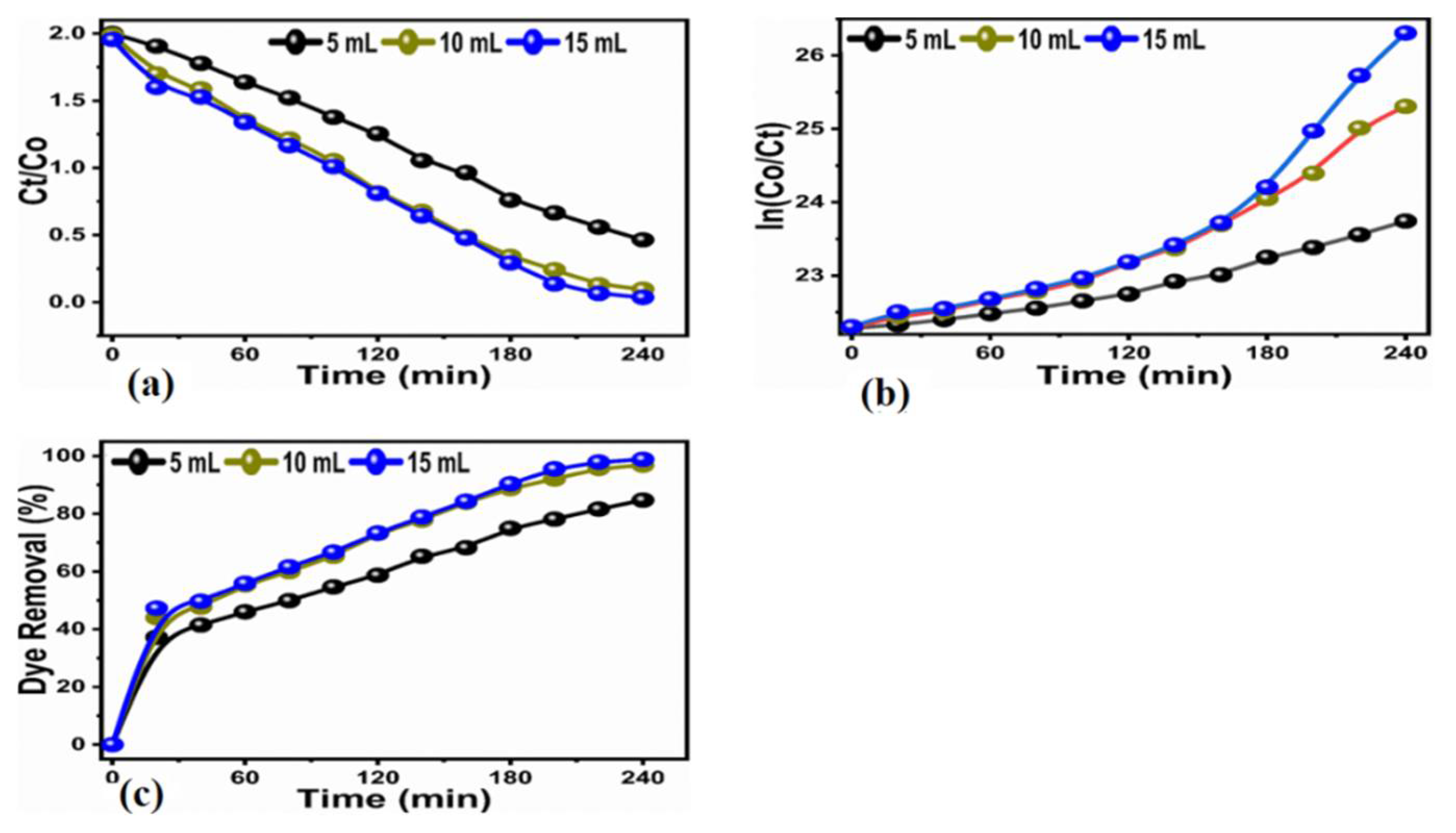
| Sample | 2θ (°) (211) | FWHM | Height | Crystalline Size (nm) | Constant (K) 1 mg | Constant (K) 2 mg |
|---|---|---|---|---|---|---|
| Pure TiO2 | 53.81 | 0.55333 | 36.55 | 18.1 | - | 2.27 × 10−3 min−1 |
| 5 mL | 53.78 | 0.37074 | 51.55 | 16.1 | 6.84 × 10−3 min−1 | 6.13 × 10−3 min−1 |
| 10 mL | 53.71 | 0.38377 | 51.33 | 13.1 | 9.99 × 10−3 min−1 | 1.23 × 10−2 min−1 |
| 15 mL | 53.62 | 0.41464 | 38.77 | 10.2 | 1.14 × 10−2 min−1 | 1.55 × 10−2 min−1 |
| Catalysts | Weight | Light Source | Dye Concentrati on | Time (min) | Dye Removal (%) | Method | Ref. | |
|---|---|---|---|---|---|---|---|---|
| TiO2@Bi2O3 | 35 mg | Sun light | 2 mg of catalyst (50 mL of dye solution) | 250 | 94% | Green synthesis | [39] | |
| TiO2 NPs | 100 mg | UV irradiation | 100 mL MB | 120 | 92% | Chemical and green synthesis methods | [40] | |
| TiOSO4 | 0.1 g | UV irradiation | 20 ppm MO | 150 | 94% | Sol-gel method | [41] | |
| MnTiO3 | 5 mg | Sun light | MB 1 × 10−5 M | 250 | 75% | Sol-gel | [42] | |
| Fe2TiO5 | 50 mg | Sunlight | MB 10 mg L−1 | 250 | 97% | Sol-gel | [43] | |
| porous TiO2 ceramic s | eighty pieces (20 mg each piece) | UV light | RhB 10 mg L−1 | 300 min | 99.3% | camphene-based freeze-casting process | [44] | |
| macro/mesop orous anatase TiO2 ceramic | eighty pieces (20 mg each piece) | UV light | RhB 10 mg mL−1 | 180 | 99.4% | a camphene-based freeze-casting process | [45] | |
| Fe–N- codoped TiO2/fly ashecnospheres | 0.2 g | Sun light | RhB mg L−1 | 4 h | 89% | Sol-gel method | [46] | |
| hollow TiO2/fly ashecnospheres | 1.5 g | Sun light | MB 30 mg L−1 | 9 h | 0.0922 (min−1) | Sol-gel | [47] | |
| Ag+-doped TiO2/ploystyrene | 0.05 g | Sun light | MB 5 mg L−1 | 5 h | 83% | Impregnation and strewing based on a simple solvent-cast method | [48] | |
| TiO2/polyuret hane foam | forty pieces | Sun light | MB + CrVI 1 0 mg L−1 | 150 | 90% | a low-temperature ultrasonic and deposition approach | [49] | |
| TiO2/low density polyethylene | 1 g | Sun light | MB 0.16 mmol L−1 | 225 | 30% | [50] | ||
| C,N- TiO2/polytetrafluoroethylene | 1 gL−2 | Visible light | MO 20 mg L−1 | 4 h | 96.9% | y a simple high-energy ball-milling process | [51] | |
| B–N– TiO2/expanded perlite | (6 mg/g, 24 wt% TiO2 | Solar light | RhB | 5 h | 99.1% | Sol-gel | [52] | |
| W doped ZnO | 20 mg | UV light | MB | 3 h | 96.9% | Low temperature aqueous chemical growth method | [53] | |
| TiO2- graphene | 0.2 g | UV light | RhB 0.02 mM | 2–4 h | 95% | Sol-gel | [54] | |
| TiO2/polypro pylene | 15– 20 mg | UV light | MO 15 mg L−1 | 4 h | 65% | Low temperature Hydrothermal method | [55] | |
| TiO2/polypro pylene fabric | a piece (d = 47 mm) | UV light | MO 15 mg L−1 | 240 | 100% | Hydrothermal Process | [56] | |
| BC-TiO2 | --------- | UV light | MB 10 mg/L | 180 | 90% | Low temperature Pyrolysis | [57] | |
| TiO2/AC | 10 g | Sun light | 25 mg dm−3 | 90 | 98.1% | in situ immobilization process | [58] | |
| TiO2/CRFs | 10 mg | Solar light | 10 mg L−1 | 80 | 98.1% | Impregnation and Calcination method | [59] | |
| TiO2 | Ficus | 2 mg | Solar Light | 5 × 10−5 M (MB) | 330 | 98.82% | Simple Hydrothermal Process | Present Work |
| carica leave extract | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatti, M.A.; Gilani, S.J.; Shah, A.A.; Channa, I.A.; Almani, K.F.; Chandio, A.D.; Halepoto, I.A.; Tahira, A.; Bin Jumah, M.N.; Ibupoto, Z.H. Effective Removal of Methylene Blue by Surface Alteration of TiO2 with Ficus Carica Leaf Extract under Visible Light. Nanomaterials 2022, 12, 2766. https://doi.org/10.3390/nano12162766
Bhatti MA, Gilani SJ, Shah AA, Channa IA, Almani KF, Chandio AD, Halepoto IA, Tahira A, Bin Jumah MN, Ibupoto ZH. Effective Removal of Methylene Blue by Surface Alteration of TiO2 with Ficus Carica Leaf Extract under Visible Light. Nanomaterials. 2022; 12(16):2766. https://doi.org/10.3390/nano12162766
Chicago/Turabian StyleBhatti, Muhammad Ali, Sadaf Jamal Gilani, Aqeel Ahmed Shah, Iftikhar Ahmed Channa, Khalida Faryal Almani, Ali Dad Chandio, Imran Ali Halepoto, Aneela Tahira, May Nasser Bin Jumah, and Zafar Hussain Ibupoto. 2022. "Effective Removal of Methylene Blue by Surface Alteration of TiO2 with Ficus Carica Leaf Extract under Visible Light" Nanomaterials 12, no. 16: 2766. https://doi.org/10.3390/nano12162766
APA StyleBhatti, M. A., Gilani, S. J., Shah, A. A., Channa, I. A., Almani, K. F., Chandio, A. D., Halepoto, I. A., Tahira, A., Bin Jumah, M. N., & Ibupoto, Z. H. (2022). Effective Removal of Methylene Blue by Surface Alteration of TiO2 with Ficus Carica Leaf Extract under Visible Light. Nanomaterials, 12(16), 2766. https://doi.org/10.3390/nano12162766








