A Novel Method to Synthesize Co/Fe3O4 Nanocomposites with Optimal Magnetic and Microwave Performance
Abstract
:1. Introduction
2. Materials and Methods
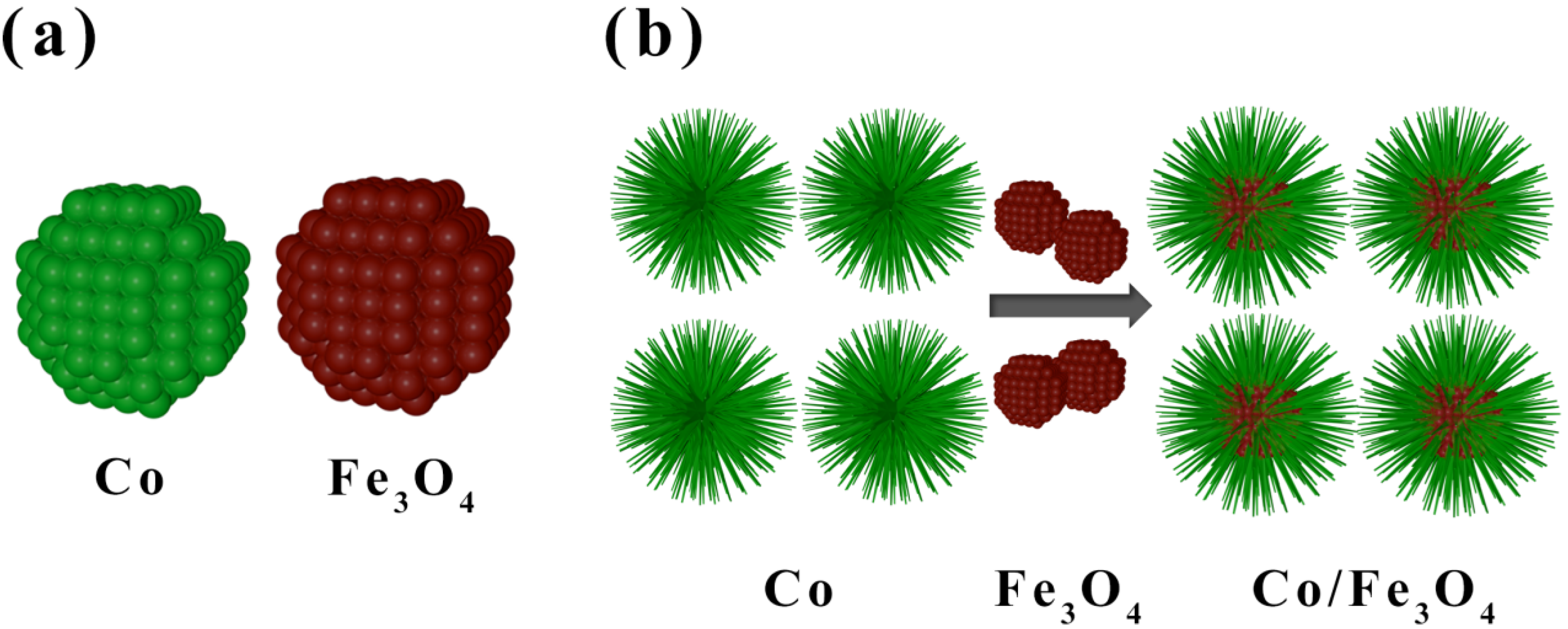
2.1. Synthesis of Co/Fe3O4 Composite Particles
2.2. Characterization
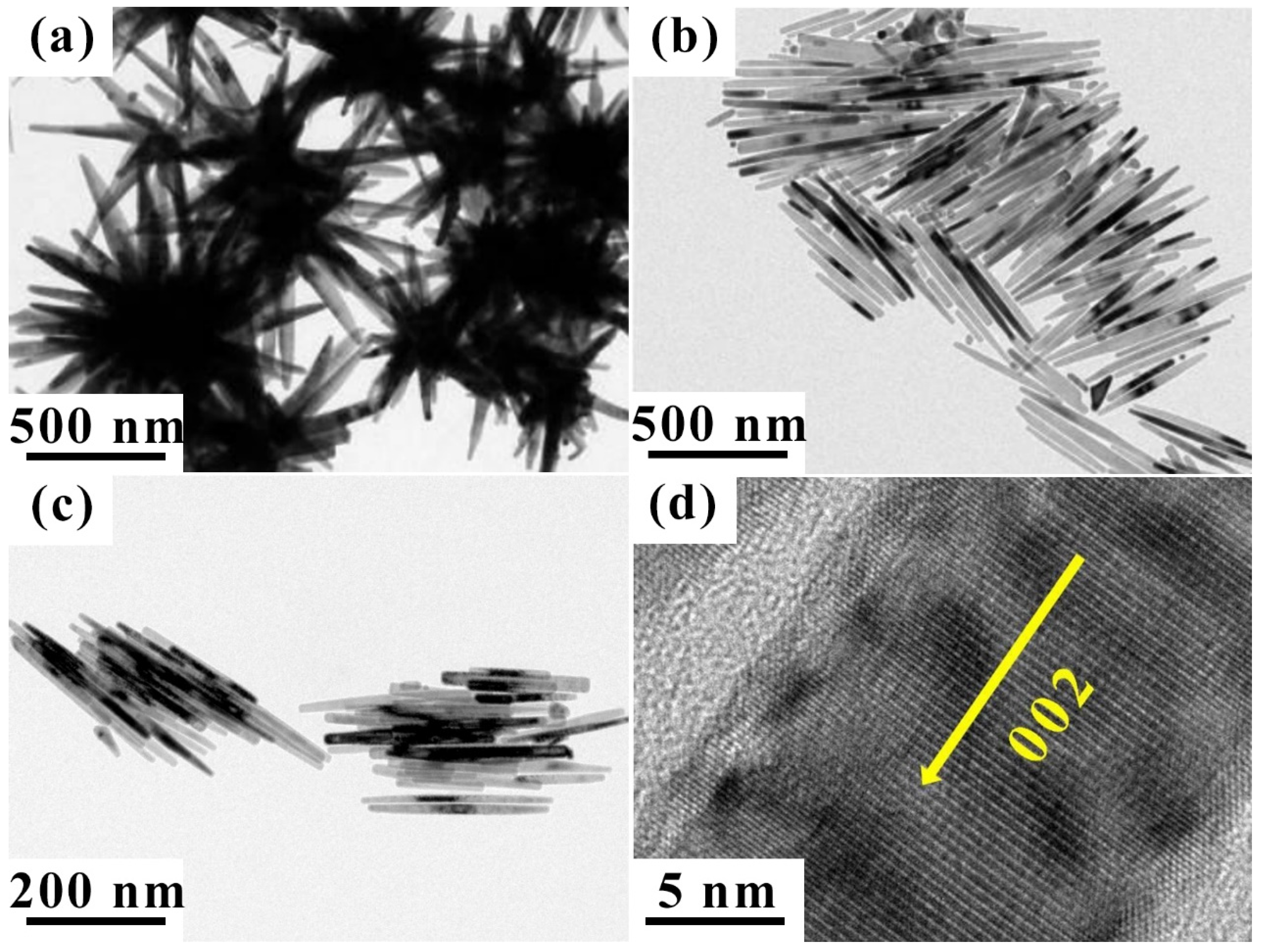
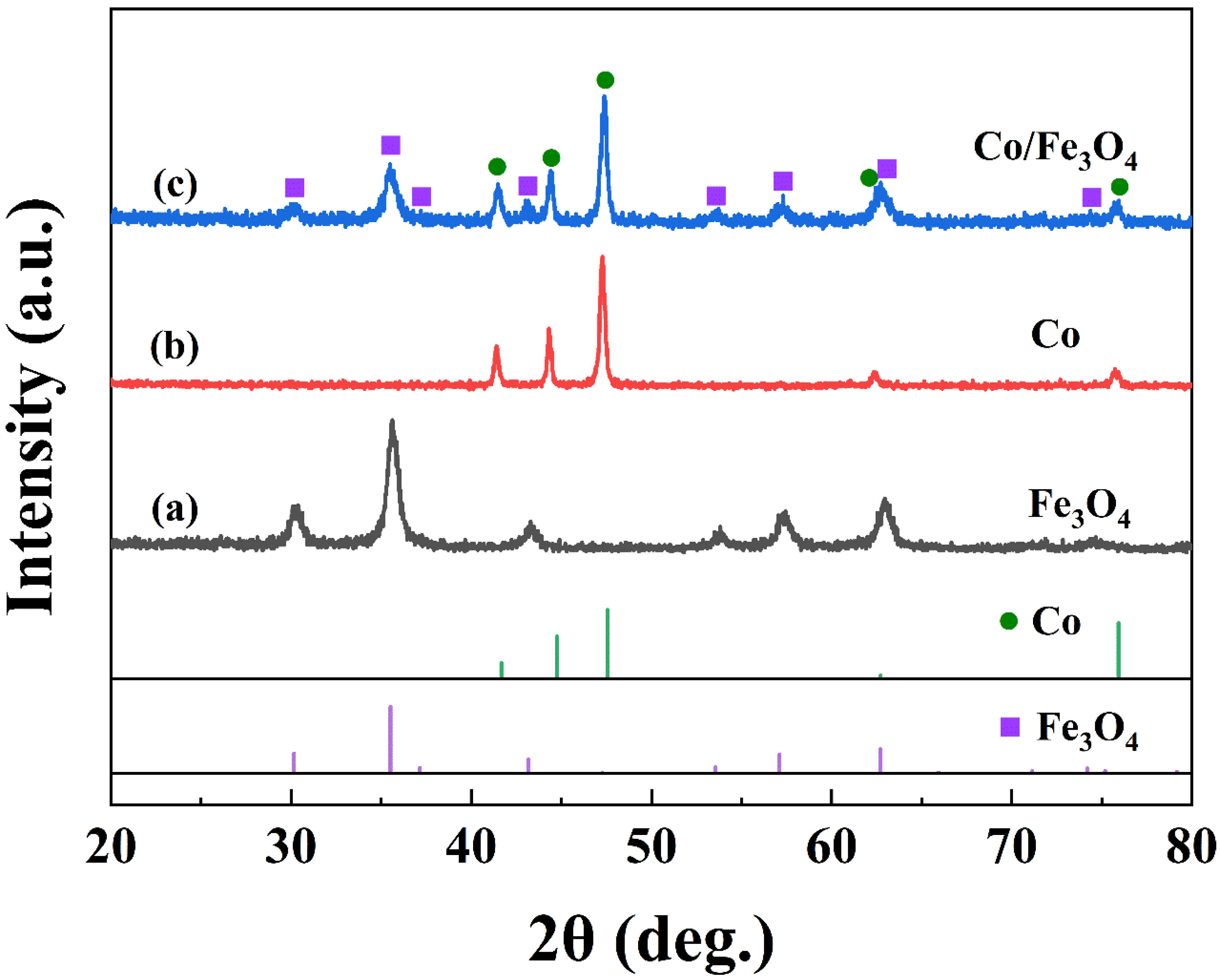
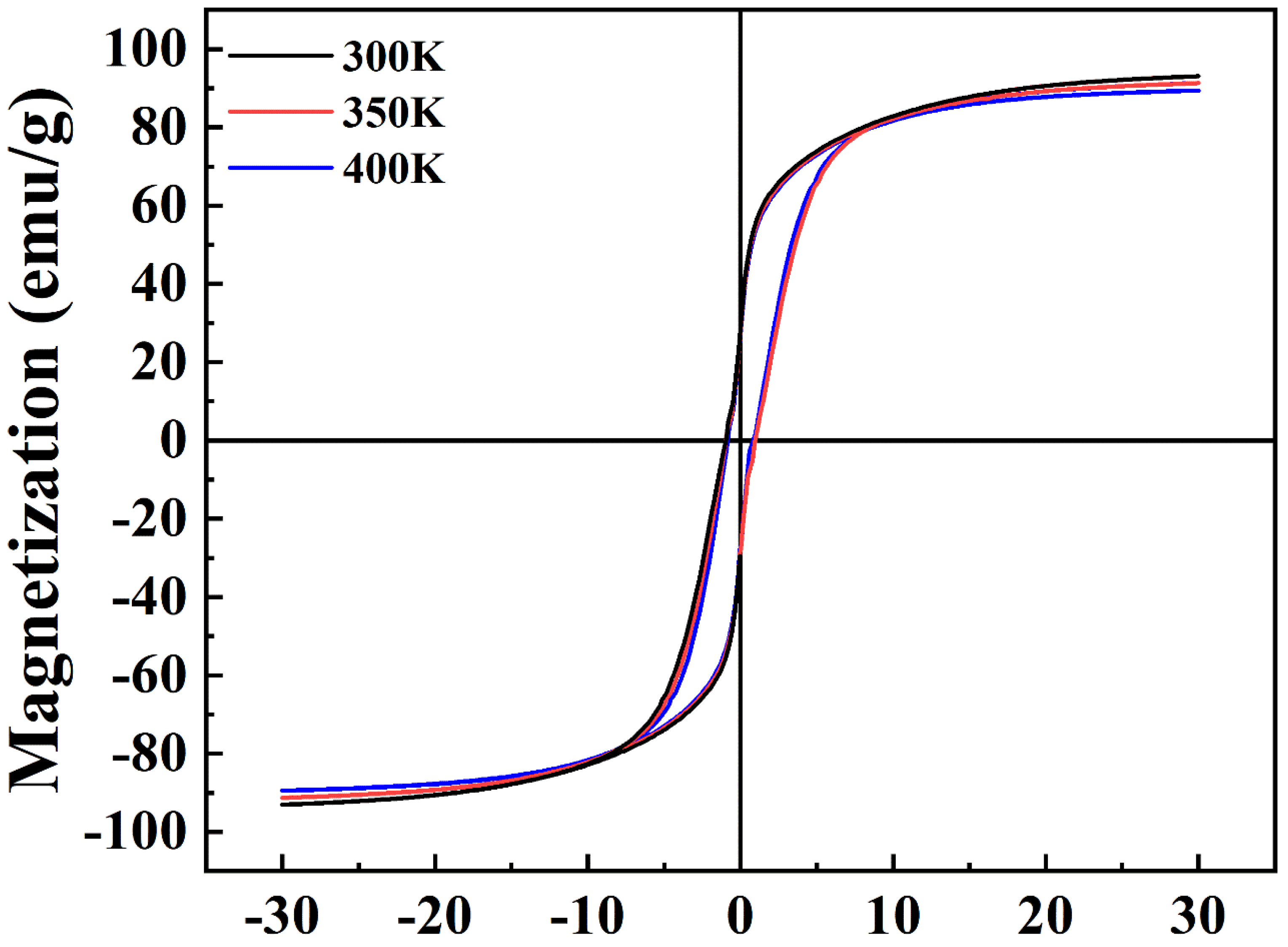
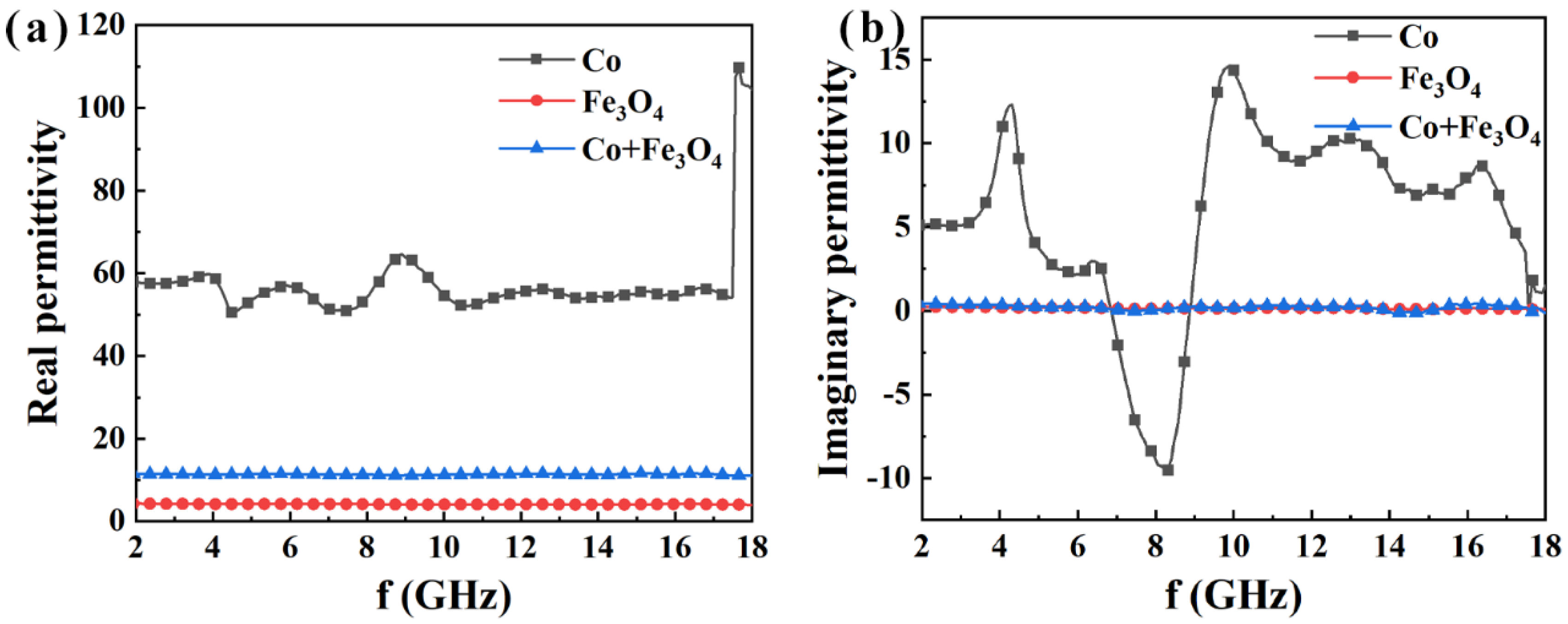
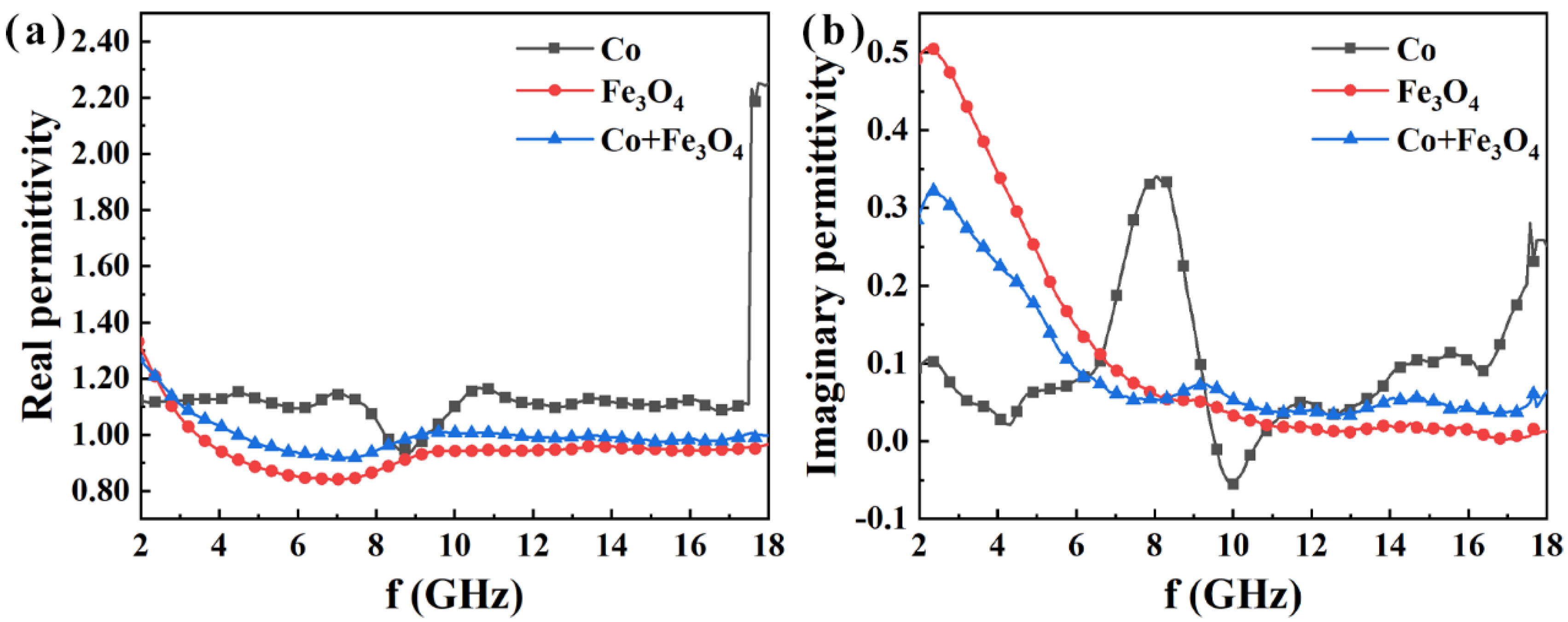
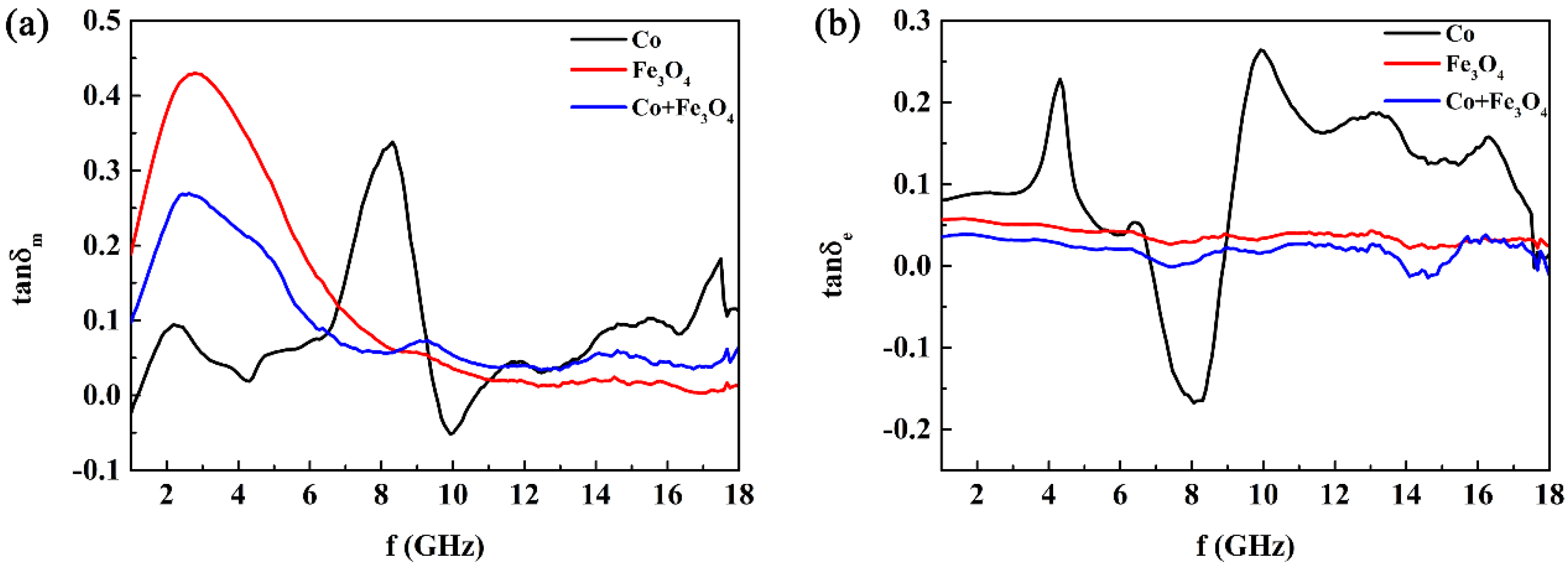
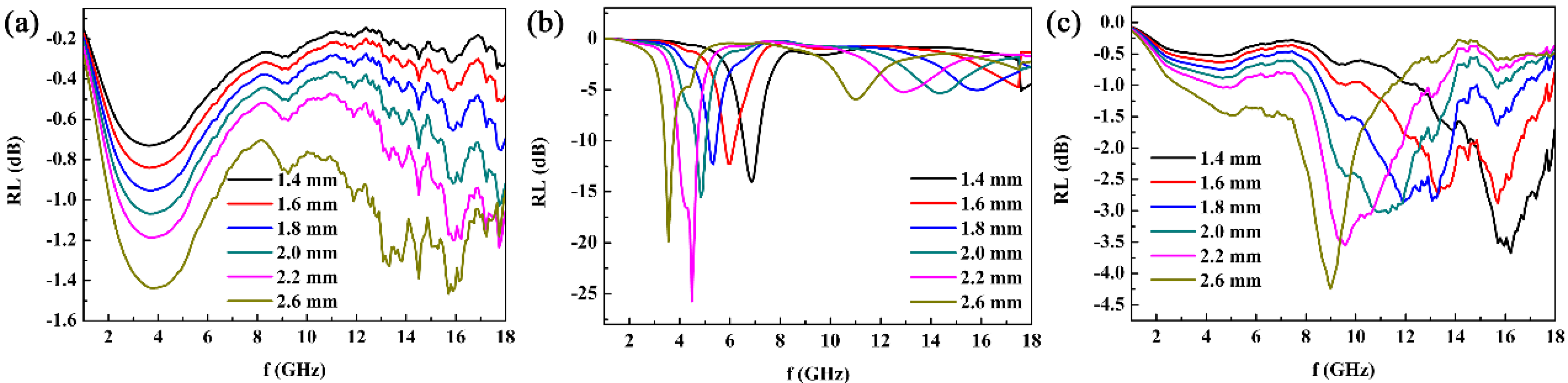
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Chang, Y.; Wang, L.; Liu, C. Synthesis, characterization and microwave absorption properties of Fe3O4/Co core/shell-type nanoparticles. Adv. Powder Technol. 2012, 23, 861–865. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Zhang, Q.; Zheng, Z.; Wang, L.S.; Peng, D.L. Facile synthesis of Fe3O4/C composites for broadband microwave absorption properties. Appl. Surf. Sci. 2018, 445, 82–88. [Google Scholar] [CrossRef]
- Deng, Y.; Zheng, Y.; Zhang, D.; Han, C.; Cheng, A.; Shen, J.; Zeng, G.; Zhang, H. A novel and facile-to-synthesize three-dimensional honeycomb-like nano-Fe3O4@C composite: Electromagnetic wave absorption with wide bandwidth. Carbon 2020, 169, 118–128. [Google Scholar] [CrossRef]
- Sui, M.; Fu, T.; Sun, X.; Cui, G.; Lv, X.; Gu, G. Unary and binary doping effect of M2+ (M=Mn, Co, Ni, Zn) substituted hollow Fe3O4 approach for enhancing microwave attenuation. Ceram. Int. 2018, 44, 17138–17146. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Cao, W.; Han, C.; Yang, H.; Yuan, J.; Cao, M. A facile fabrication and highly tunable microwave absorption of 3D flower-like Co3O4-rGO hybrid-architectures. Chem. Eng. J. 2018, 339, 487–498. [Google Scholar] [CrossRef]
- Agrawal, P.R.; Kumar, R.; Teotia, S.; Kumari, S.; Mondal, D.P.; Dhakate, S.R. Lightweight, high electrical and thermal conducting carbon-rGO composites foam for superior electromagnetic interference shielding. Compos. Part B 2019, 160, 131–139. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Yan, X.; Qin, G.; Liu, Y.; Zhong, B.; Xia, L.; Liu, D.; Zhou, Y.; Huang, X. Mesoscopically ordered Fe3O4/C nano-composite for superior broadband electromagnetic wave absorption. Compos. Part A 2022, 158, 106983. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.; Ding, L.; Zhao, X.; Liu, P.; Li, T. Synthesis of covalently bonded reduced graphene oxide-Fe3O4 nanocomposites for efficient electromagnetic wave absorption. J. Mater. Sci. Technol. 2021, 72, 93–103. [Google Scholar] [CrossRef]
- Adebayo, L.L.; Soleimani, H.; Yahya, N.; Abbas, Z.; Wahaab, F.A.; Ayinla, R.T.; Ali, H. Recent advances in the development OF Fe3O4-BASED microwave absorbing materials. Ceram. Int. 2020, 46, 1249–1268. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, C.; Xue, Z.; Jiang, T.; Fang, G.; Peng, K.; Zhang, Y. Excellent microwave absorption of Fe3O4/Ag composites attained by synergy of considerable magnetic loss and dielectric loss. Ceram. Int. 2022, 48, 5824–5830. [Google Scholar] [CrossRef]
- Cui, G.; Lu, Y.; Zhou, W.; Lv, X.; Hu, J.; Zhang, G.; Gu, G. Excellent Microwave Absorption Properties Derived from the Synthesis of Hollow Fe3O4@Reduced Graphite Oxide (RGO) Nanocomposites. Nanomaterials 2019, 9, 141. [Google Scholar] [CrossRef]
- Shang, T.; Lu, Q.; Zhao, J.; Chao, L.; Qin, Y.; Ren, N.; Yun, Y.; Yun, G. Novel Three-Dimensional Graphene-Like Networks Loaded with Fe3O4 Nanoparticles for Efficient Microwave Absorption. Nanomaterials 2021, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Feng, N.; Xu, X.; Li, W.; Zhang, Q.; Lan, S.; Liu, Y.; Sha, H.; Bi, K.; Xu, B.; et al. Enhanced electric resistivity and dielectric energy storage by vacancy defect complex. Energy Storage Mater. 2021, 42, 836–844. [Google Scholar] [CrossRef]
- Thiabgoh, O.; Eggers, T.; Albrecht, C.; Jimenez, V.O.; Shen, H.; Jiang, S.D.; Sun, J.F.; Lam, D.S.; Lam, V.D.; Phan, M.H. Optimization of the high-frequency magnetoimpedance response in melt-extracted Co-rich microwires through novel multiple-step Joule heating. J. Sci. Adv. Mater. Devices 2021, 6, 364–371. [Google Scholar] [CrossRef]
- Green, M.; Chen, X. Recent progress of nanomaterials for microwave absorption. J. Mater. 2019, 5, 503–541. [Google Scholar] [CrossRef]
- Kaur, P.; Bahel, S.; Narang, S.B. Microwave absorption behavior and electromagnetic properties of Ni-Zr doped La-Sr hexagonal ferrite synthesized by auto-combustion method. Mater. Res. Bull. 2018, 100, 275–281. [Google Scholar] [CrossRef]
- Zeng, X.; Sang, Y.; Xia, G.; Jiang, G.; Xie, N.; Zheng, N.; Cheng, Y.; Yu, R. 3-D hierarchical urchin-like Fe3O4/CNTs architectures enable efficient electromagnetic microwave absorption. Mater. Sci. Eng. B 2022, 281, 115721. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Kim, H.; Lee, H.; Ryu, C.; Young Lee, J.; Yoon, J. Engineering Core-Shell Structures of Magnetic Ferrite Nanoparticles for High Hyperthermia Performance. Nanomaterials 2020, 10, 991. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, C.; Xia, Y.; Wang, T.; Li, F. Synthesis and microwave absorption properties of FeCo nanoplates. J. Alloys Compd. 2010, 493, 549–552. [Google Scholar] [CrossRef]
- Gao, J.; Ma, Z.; Liu, F.; Weng, X.; Meng, K. Preparation and microwave absorption properties of Gd–Co ferrite@silica@carbon multilayer core–shell structure composites. Chem. Eng. J. 2022, 446, 137157. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Wang, D.; Wang, P.; Duan, B.; Qiao, L.; Wang, T. Electromagnetic interference shielding and microwave absorption performance of magnetic Co@C/Na2SiO3 composite at 673 K. Ceram. Int. 2019, 45, 23172–23179. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Li, N.; Sun, R.; Tang, J.; Gong, Z.Z.; Li, Y.; Yang, X.; Xie, Z.K.; Gul, Q.; et al. Optical determination of spin diffusion length and interfacial spin mixing conductance in epitaxial Pd/Fe bilayers. J. Phys. Condens. Matter 2019, 31, 305802. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Su, H.; Wang, G.; Tang, X.; Liang, X.; Huang, X.; Li, Y.; Zhang, H.; Ye, C.; Wang, X.R. Improved microwave dielectric properties of CaMgSi2O6 ceramics through CuO doping. J. Alloys Compd. 2019, 772, 40–48. [Google Scholar] [CrossRef]
- Cui, C.; Guo, R.; Ren, E.; Xiao, H.; Zhou, M.; Lai, X.; Qin, Q.; Jiang, S.; Qin, W. MXene-based rGO/Nb2CTx/Fe3O4 composite for high absorption of electromagnetic wave. Chem. Eng. J. 2021, 405, 126626. [Google Scholar] [CrossRef]
- Qiao, L.; Wen, F.; Wei, J.; Wang, J.; Li, F. Microwave permeability spectra of flake-shaped FeCuNbSiB particle composites. J. Appl. Phys. 2008, 103, 063903. [Google Scholar] [CrossRef]
- Zhao, H.; Han, X.; Li, Z.; Liu, D.; Wang, Y.; Wang, Y.; Zhou, W.; Du, Y. Reduced graphene oxide decorated with carbon nanopolyhedrons as an efficient and lightweight microwave absorber. J. Colloid Interface Sci. 2018, 528, 174–183. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, C.; Qin, Z.; Chen, G.; Xia, L.; Zhong, B. Preparation of cauliflower shaped hp-Co/GNs composite microwave absorbing materials. Mater. Charact. 2022, 189, 495002. [Google Scholar] [CrossRef]
- Park, J.H.; Ro, J.C.; Suh, S.J. Fe/Co ratio dependent excellent microwave absorption of FeCo alloys with a wide bandwidth in the high-frequency region. Mater. Res. Bull. 2022, 145, 111513. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Li, Y.; Lv, J.; Qin, R.; Wang, X.; Liu, X.; Liu, Y. Enhanced microwave absorption property of ferroferric Oxide: The role of magnetoelectric resonance. Chem. Eng. J. 2022, 433, 134455. [Google Scholar] [CrossRef]
- Morales, I.; Costo, R.; Mille, N.; Silva, G.B.D.; Carrey, J.; Hernando, A.; Presa, P. High Frequency Hysteresis Losses on γ-Fe2O3 and Fe3O4: Susceptibility as a Magnetic Stamp for Chain Formation. Nanomaterials 2018, 8, 970. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Sun, R.; Liu, J.N.; Li, N.; Yang, X.; Gong, Z.Z.; Xie, Z.K.; He, W.; Zhang, X.Q.; et al. Drag effect induced large anisotropic damping behavior in magnetic thin films with strong magnetic anisotropy. J. Phys. Condens. Matter 2021, 33, 175801. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, C.; Wang, M.; Yuan, Y.; Liu, H.; Dillemans, L.; Homm, P.; Menghini, M.; Locquet, J.P.; Van Haesendonck, C.; et al. Coupling of ferromagnetism and structural phase transition in V2O3/Co bilayers. J. Phys. D Appl. Phys. 2017, 50, 495002. [Google Scholar] [CrossRef]
- Qing, X.; Yue, X.; Wang, B.; Lu, Y. Facile synthesis of size-tunable, multilevel nanoporous Fe3O4 microspheres for application in electromagnetic wave absorption. J. Alloys Compd. 2014, 595, 131–137. [Google Scholar] [CrossRef]
- Wang, X.; Geng, Q.; Shi, G.; Xu, G.; Yu, J.; Guan, Y.; Zhang, Y.; Li, D. One-pot solvothermal synthesis of Fe/Fe3O4 composites with broadband microwave absorption. J. Alloys Compd. 2019, 803, 818–825. [Google Scholar] [CrossRef]
- Fu, L.; Jiang, J.; Xu, C.; Zhen, L. Synthesis of hexagonal Fe microflakes with excellent microwave absorption performance. CrystEngComm 2012, 14, 6827–6832. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, L.; Zhang, L. Lightweight, flexible SiCN ceramic nanowires applied as effective microwave absorbers in high frequency. Chem. Eng. J. 2018, 338, 248–260. [Google Scholar] [CrossRef]
- Li, J.; Zhou, D.; Wang, P.; Du, C.; Liu, W.; Su, J.; Pang, L.; Cao, M.; Kong, L. Recent progress in two-dimensional materials for microwave absorption applications. Chem. Eng. J. 2021, 425, 131558. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kusada, K.; Kitagawa, H. Creation of Novel Solid-Solution Alloy Nanoparticles on the Basis of Density-of-States Engineering by Interelement Fusion. Acc. Chem. Res. 2015, 48, 1551–1559. [Google Scholar] [CrossRef]
- Baldelli, A.; Ou, J.; Barona, D.; Li, W.; Amirfazli, A. Sprayable, Superhydrophobic, Electrically, and Thermally Conductive Coating. Adv. Mater. Interfaces 2020, 8, 1902110. [Google Scholar] [CrossRef]
- Shen, B.; Sun, S. Chemical Synthesis of Magnetic Nanoparticles for Permanent Magnet Applications. Chem. Eur. J. 2019, 26, 6757–6766. [Google Scholar] [CrossRef]
- Jiang, S.; Qian, K.; Yu, K.; Zhou, H.; Weng, Y.; Zhang, Z. Controllable synthesis and microwave absorption properties of Fe3O4@f-GNPs nanocomposites. Compos. Commun. 2020, 20, 100363. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, P.; Wang, J.; Feng, X.; Dai, J. Low-frequency microwave absorption of MOF-derived Co/CoO/SrCO3@C composites. Mater. Chem. Phys. 2021, 264, 124457. [Google Scholar] [CrossRef]
- Abbas, S.M.; Dixit, A.K.; Chatterjee, R.; Goel, T.C. Complex permittivity and microwave absorption properties of BaTiO3–polyaniline composite. Mater. Sci. Eng. B 2005, 123, 167–171. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Wang, G.; Duan, B.; He, D.; Wang, T.; Li, F. Synthesis and characterization of MoS2/Fe@Fe3O4 nanocomposites exhibiting enhanced microwave absorption performance at normal and oblique incidences. J. Mater. Sci. Technol. 2019, 35, 1931–1939. [Google Scholar] [CrossRef]
- Song, N.N.; Yang, H.T.; Liu, H.L.; Ren, X.; Ding, H.F.; Zhang, X.Q.; Cheng, Z.H. Exceeding natural resonance frequency limit of monodisperse Fe3O4 nanoparticles via superparamagnetic relaxation. Sci. Rep. 2013, 3, 3161. [Google Scholar] [CrossRef]
- Zhang, Y.; Piao, M.; Zhang, H.; Zhang, F.; Chu, J.; Wang, X.; Shi, H.; Li, C. Synthesis of mesoporous hexagonal cobalt nanosheets with low permittivity for enhancing microwave absorption performances. J. Magn. Magn. Mater. 2019, 486, 165272. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, Y.; Ma, Y.; Zheng, H.; Wang, L.-S.; Zhang, Q.; Chen, Y.; Peng, D.-L. Facile preparation and microwave absorption properties of porous Co/CoO microrods. J. Alloys Compd. 2017, 721, 411–418. [Google Scholar] [CrossRef]
- Sun, W.S.; Li, H.; Liu, Y.; Zhao, X.C.; Cheng, J.W.; Wen, S.L. Preparation and Microwave Absorption Properties of Spherical Cobalt Particles. Rare Met. Mater. Eng. 2016, 45, 3099–3103. [Google Scholar]
- Han, R.; Li, W.; Pan, W.; Zhu, M.; Zhou, D.; Li, F.-S. 1D Magnetic Materials of Fe3O4 and Fe with High Performance of Microwave Absorption Fabricated by Electrospinning Method. Sci. Rep. 2014, 4, 07493. [Google Scholar] [CrossRef]
- Ni, S.; Lin, S.; Pan, Q.; Yang, F.; Huang, K.; He, D. Hydrothermal synthesis and microwave absorption properties of Fe3O4nanocrystals. J. Phys. D Appl. Phys. 2009, 42, 055004. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, Y.; Chen, Y.; Wang, Y.; Wang, P.; Wu, Q. A Novel Method to Synthesize Co/Fe3O4 Nanocomposites with Optimal Magnetic and Microwave Performance. Nanomaterials 2022, 12, 2764. https://doi.org/10.3390/nano12162764
Zhang C, Wang Y, Chen Y, Wang Y, Wang P, Wu Q. A Novel Method to Synthesize Co/Fe3O4 Nanocomposites with Optimal Magnetic and Microwave Performance. Nanomaterials. 2022; 12(16):2764. https://doi.org/10.3390/nano12162764
Chicago/Turabian StyleZhang, Chi, Yao Wang, Yun Chen, Yatao Wang, Peng Wang, and Qiong Wu. 2022. "A Novel Method to Synthesize Co/Fe3O4 Nanocomposites with Optimal Magnetic and Microwave Performance" Nanomaterials 12, no. 16: 2764. https://doi.org/10.3390/nano12162764
APA StyleZhang, C., Wang, Y., Chen, Y., Wang, Y., Wang, P., & Wu, Q. (2022). A Novel Method to Synthesize Co/Fe3O4 Nanocomposites with Optimal Magnetic and Microwave Performance. Nanomaterials, 12(16), 2764. https://doi.org/10.3390/nano12162764




