Nanoporous Carbon Electrodes Derived from Coffee Side Streams for Supercapacitors in Aqueous Electrolytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dry and Ash Content Determination
2.2. Simultaneous Thermal Analysis (STA)
2.3. Scanning Electron Microscopy (SEM)
2.4. Pre-Carbonization and Activation
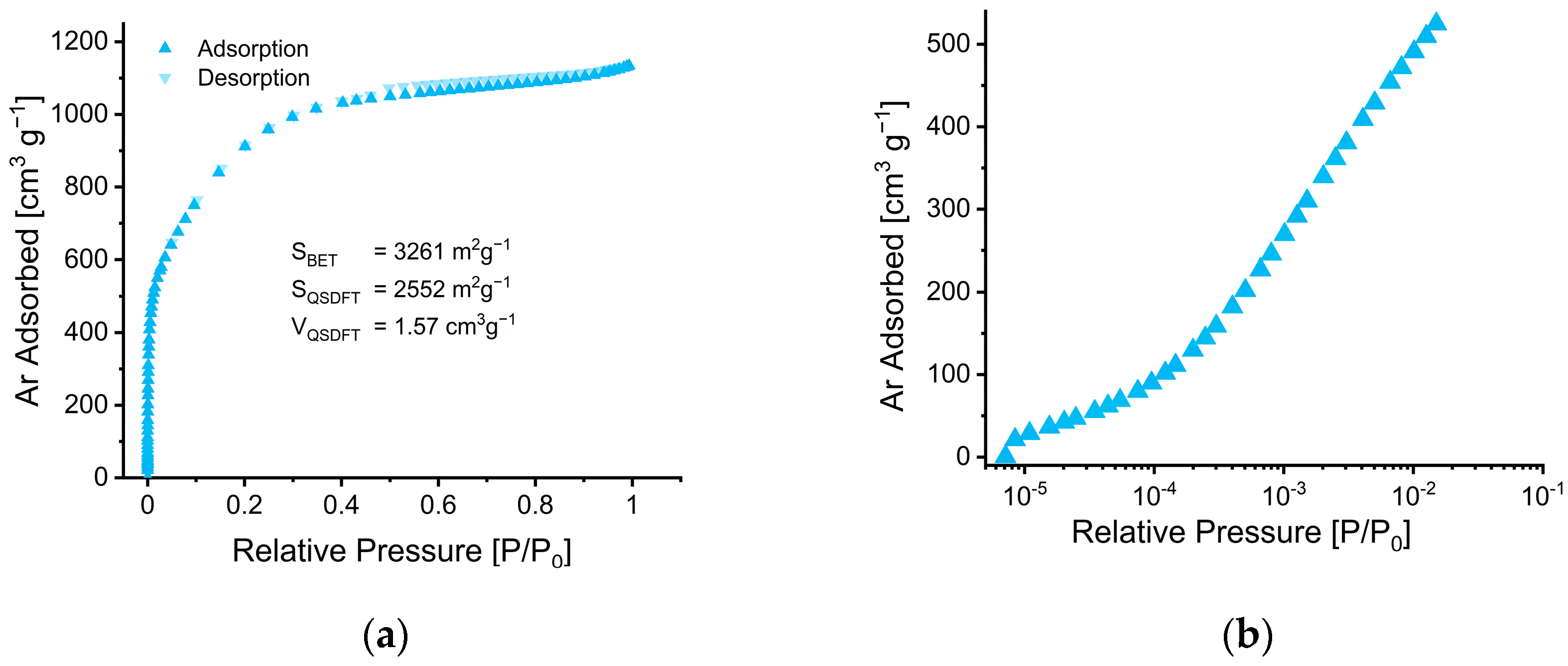
2.5. Gas Adsorption/Desorption Analysis
2.6. X-ray Diffraction (XRD)
2.7. Raman Spectroscopy
2.8. Elemental Analysis
2.9. Electrode Preparation
2.10. Supercapacitor Assembly
2.11. Electrochemical Characterization
3. Results and Discussion
3.1. Thermal Conversion of Coffee Silver Skins
3.2. Characterization of CSS and AC—CSS
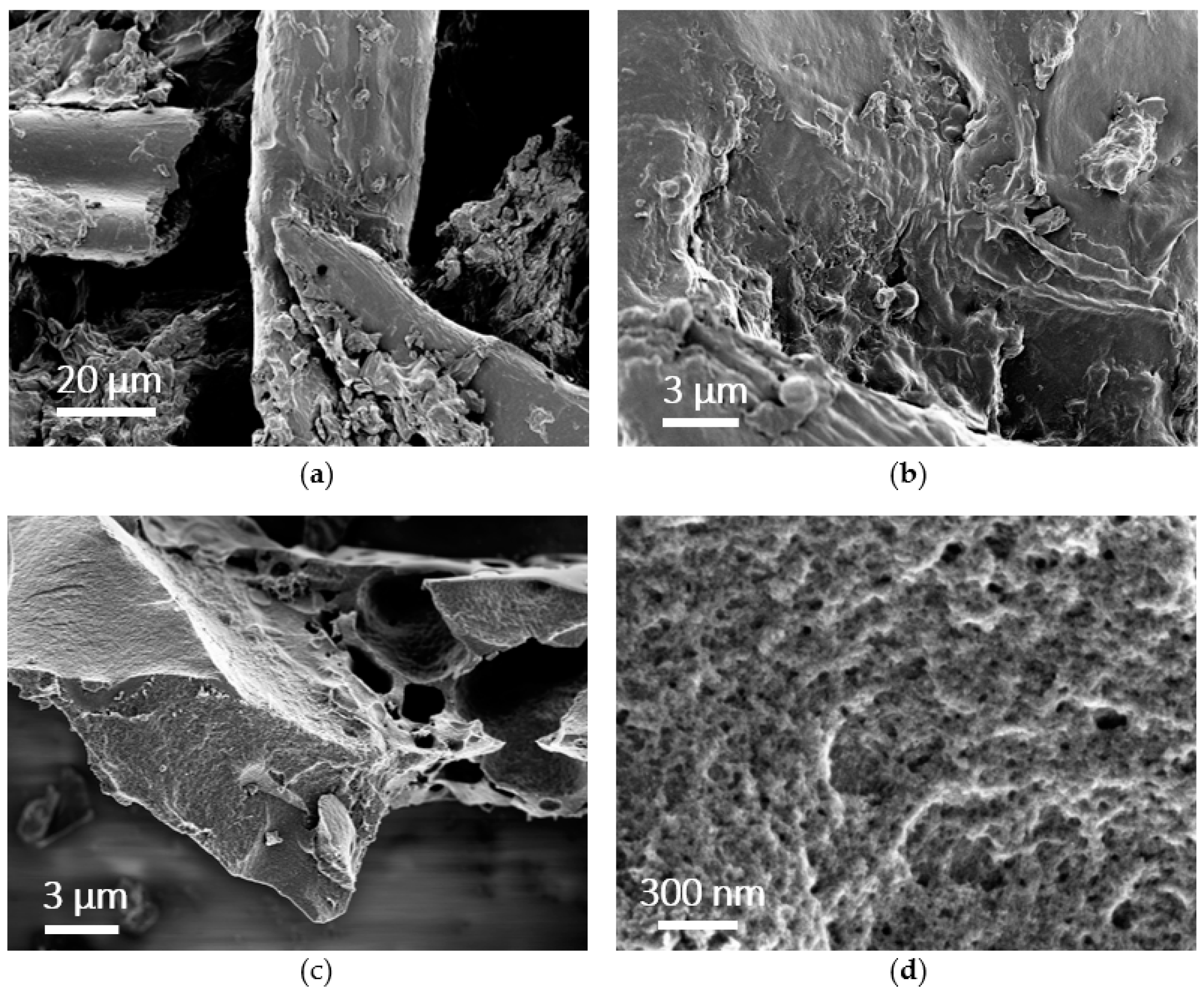
3.2.1. Morphology and Porosity
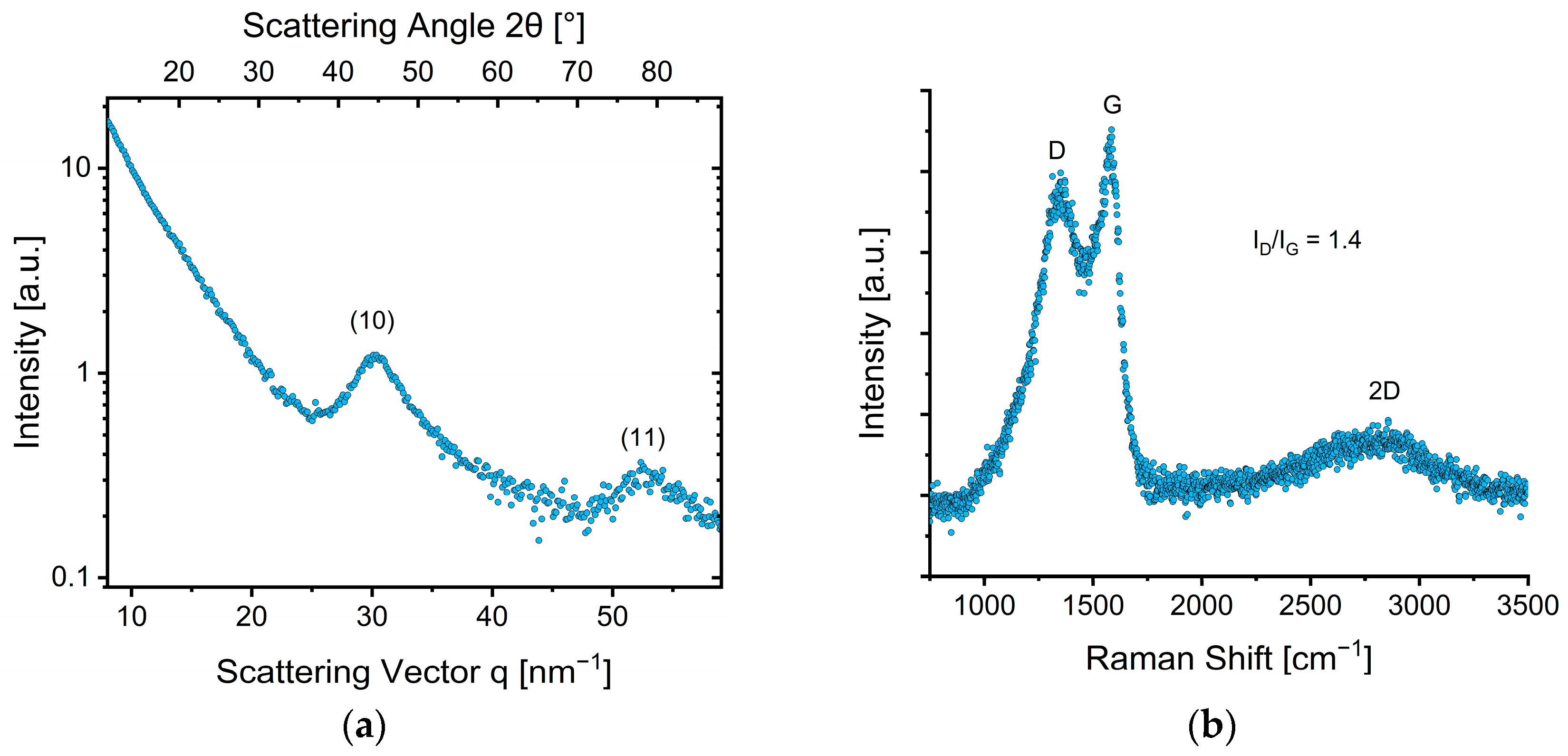
3.2.2. Structural Properties
3.2.3. Chemical Composition
3.3. Supercapacitor Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roussos, S.; de los Angeles Aquiáhuatl, M.; del Refugio Trejo-Hernández, M.; Gaime Perraud, I.; Favela, E.; Ramakrishna, M.; Raimbault, M.; Viniegra-González, G. Biotechnological management of coffee pulp—Isolation, screening, characterization, selection of caffeine-degrading fungi and natural microflora present in coffee pulp and husk. Appl. Microbiol. Biotechnol. 1995, 42, 756–762. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.P.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crops Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Low, J.H.; Rahman, W.A.W.A.; Jamaluddin, J. The influence of extraction parameters on spent coffee grounds as a renewable tannin resource. J. Clean. Prod. 2015, 101, 222–228. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Gottstein, V.; Bernhardt, M.; Dilger, E.; Keller, J.; Breitling-Utzmann, C.M.; Schwarz, S.; Kuballa, T.; Lachenmeier, D.W.; Bunzel, M. Coffee Silver Skin: Chemical Characterization with Special Consideration of Dietary Fiber and Heat-Induced Contaminants. Foods 2021, 10, 1705. [Google Scholar] [CrossRef] [PubMed]
- Espindola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.H.; Murthy, H.N.; Hahn, E.J.; Lee, H.L.; Paek, K.Y. Efficient extraction of caffeic acid derivatives from adventitious roots of Echinacea purpurea. Czech J. Food Sci. 2008, 26, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A Knowledge Base for The Recovery of Natural Phenols with Different Solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef] [Green Version]
- Schlemmer, W.; Selinger, J.; Hobisch, M.A.; Spirk, S. Polysaccharides for sustainable energy storage—A review. Carbohydr. Polym. 2021, 265, 118063. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Guo, L. Renewable-Biomolecule-Based Electrochemical Energy-Storage Materials. Adv. Energy Mater. 2017, 7, 1700663. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yan, B.; Feng, L.; Zheng, J.; You, B.; Chen, J.; Zhao, X.; Zhang, C.; Jiang, S.; He, S. Progress in the use of organic potassium salts for the synthesis of porous carbon nanomaterials: Microstructure engineering for advanced supercapacitors. Nanoscale 2022, 14, 8216–8244. [Google Scholar] [CrossRef] [PubMed]
- Hobisch, M.A.; Phiri, J.; Dou, J.; Gane, P.; Vuorinen, T.; Bauer, W.; Prehal, C.; Maloney, T.; Spirk, S. Willow Bark for Sustainable Energy Storage Systems. Materials 2020, 13, 1016. [Google Scholar] [CrossRef] [Green Version]
- Breitenbach, S.; Lumetzberger, A.; Hobisch, M.A.; Unterweger, C.; Spirk, S.; Stifter, D.; Fürst, C.; Hassel, A.W. Supercapacitor Electrodes from Viscose-Based Activated Carbon Fibers: Significant Yield and Performance Improvement Using Diammonium Hydrogen Phosphate as Impregnating Agent. C 2020, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Sezali, N.A.A.; Ong, H.L.; Jullok, N.; Villagracia, A.R.; Doong, R.A. A Review on Nanocellulose and Its Application in Supercapacitors. Macromol. Mater. Eng. 2021, 306, 2100556. [Google Scholar] [CrossRef]
- Tafete, G.A.; Abera, M.K.; Thothadri, G. Review on nanocellulose-based materials for supercapacitors applications. J. Energy Storage 2022, 48, 103938. [Google Scholar] [CrossRef]
- Aristizábal-Marulanda, V.; Chacón-Perez, Y.; Cardona Alzate, C.A. Chapter 3—The biorefinery concept for the industrial valorization of coffee processing by-products. In Handbook of Coffee Processing By-Products, 1st ed.; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 63–92. [Google Scholar] [CrossRef]
- Trogen, M.; Le, N.-D.; Sawada, D.; Guizani, C.; Lourençon, T.V.; Pitkänen, L.; Sixta, H.; Shah, R.; O’Neill, H.; Balakshin, M.; et al. Cellulose-lignin composite fibres as precursors for carbon fibres. Part 1—Manufacturing and properties of precursor fibres. Carbohydr. Polym. 2021, 252, 117133. [Google Scholar] [CrossRef]
- Ryu, S.; Kim, E.; Yoo, J. Optimization of redox-active anthraquinone as electrode and electrolyte materials in supercapacitors. Electrochim. Acta 2021, 370, 137809. [Google Scholar] [CrossRef]
- Qin, W.; Zhou, N.; Wu, C.; Xie, M.; Sun, H.; Guo, Y.; Pan, L. Mini-Review on the Redox Additives in Aqueous Electrolyte for High Performance Supercapacitors. ACS Omega 2020, 5, 3801–3808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yang, S.; Chang, J.; Zhao, D.; Wang, J.; Yang, C.; Cao, B. A Review of Redox Electrolytes for Supercapacitors. Front. Chem. 2020, 8, 413. [Google Scholar] [CrossRef]
- Schlemmer, W.; Nothdurft, P.; Petzold, A.; Riess, G.; Fruhwirt, P.; Schmallegger, M.; Gescheidt-Demner, G.; Fischer, R.; Freunberger, S.A.; Kern, W.; et al. 2-Methoxyhydroquinone from Vanillin for Aqueous Redox-Flow Batteries. Angew. Chem. Int. Ed. Engl. 2020, 59, 22943–22946. [Google Scholar] [CrossRef] [PubMed]
- Neimark, A.V.; Lin, Y.; Ravikovitch, P.I.; Thommes, M. Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon 2009, 47, 1617–1628. [Google Scholar] [CrossRef]
- Koczwara, C.; Rumswinkel, S.; Hammerschmidt, L.; Salihovic, M.; Elsaesser, M.S.; Amenitsch, H.; Paris, O.; Huesing, N. Nanofibers versus Nanopores: A Comparison of the Electrochemical Performance of Hierarchically Ordered Porous Carbons. ACS Appl. Energy Mater. 2019, 2, 5279–5291. [Google Scholar] [CrossRef]
- Paris, O.; Peterlik, H. Single Carbon Fibres: Structure from X -ray Diffraction and Nanomechanical Properties. In Structure and Multiscale Mechanics of Carbon Nanomaterials, 1st ed.; Paris, O., Ed.; Springer: Vienna, Austria, 2016; Volume 563, pp. 1–28. [Google Scholar]
- Zickler, G.A.; Smarsly, B.; Gierlinger, N.; Peterlik, H.; Paris, O. A reconsideration of the relationship between the crystallite size La of carbons determined by X-ray diffraction and Raman spectroscopy. Carbon 2006, 44, 3239–3246. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Habibi, A.; Khoie, S.M.M.; Mahboubi, F.; Urgen, M. Fast synthesis of turbostratic carbon thin coating by cathodic plasma electrolysis. Thin Solid Films 2017, 621, 253–258. [Google Scholar] [CrossRef]
- Matthews, M.J.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Endo, M. Origin of dispersive effects of the Raman D band in carbon materials. Phys. Rev. B 1999, 59, R6585–R6588. [Google Scholar] [CrossRef]
- Ge, Y.; Xie, X.; Roscher, J.; Holze, R.; Qu, Q. How to measure and report the capacity of electrochemical double layers, supercapacitors, and their electrode materials. J. Solid State Electrochem. 2020, 24, 3215–3230. [Google Scholar] [CrossRef]
- Baptista, J.M.; Sagu, J.S.; Kg, U.W.; Lobato, K. State-of-the-art materials for high power and high energy supercapacitors: Performance metrics and obstacles for the transition from lab to industrial scale—A critical approach. Chem. Eng. J. 2019, 374, 1153–1179. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Flexible supercapacitors based on carbon nanomaterials. J. Mater. Chem. A 2014, 2, 10756–10775. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294. [Google Scholar] [CrossRef]
- Polidoro, A.d.S.; Scapin, E.; Lazzari, E.; Silva, A.N.; dos Santos, A.L.; Caramão, E.B.; Jacques, R.A. Valorization of coffee silverskin industrial waste by pyrolysis: From optimization of bio-oil production to chemical characterization by GC × GC/qMS. J. Anal. Appl. Pyrolysis 2018, 129, 43–52. [Google Scholar] [CrossRef]
- Del Pozo, C.; Bartroli, J.; Alier, S.; Puy, N.; Fabregas, E. Production of antioxidants and other value-added compounds from coffee silverskin via pyrolysis under a biorefinery approach. Waste Manag. 2020, 109, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Venderbosch, R.H.; Prins, W. Fast pyrolysis technology development. Biofuels Bioprod. Biorefin. 2010, 4, 178–208. [Google Scholar] [CrossRef]
- Wang, X.; Ren, Q.; Li, L.; Li, S.; Lu, Q. TG–MS analysis of nitrogen transformation during combustion of biomass with municipal sewage sludge. J. Therm. Anal. Calorim. 2015, 123, 2061–2068. [Google Scholar] [CrossRef]
- Nan, H.; Xiao, Z.; Zhao, L.; Yang, F.; Xu, H.; Xu, X.; Qiu, H. Nitrogen Transformation during Pyrolysis of Various N-Containing Biowastes with Participation of Mineral Calcium. ACS Sustain. Chem. Eng. 2020, 8, 12197–12207. [Google Scholar] [CrossRef]
- Chen, W.; Yang, H.; Chen, Y.; Xia, M.; Chen, X.; Chen, H. Transformation of Nitrogen and Evolution of N-Containing Species during Algae Pyrolysis. Environ. Sci. Technol. 2017, 51, 6570–6579. [Google Scholar] [CrossRef]
- Hansson, K.-M.; Samuelsson, J.; Tullin, C.; Åmand, L.-E. Formation of HNCO, HCN, and NH3 from the pyrolysis of bark and nitrogen-containing model compounds. Combust. Flame 2004, 137, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Sumpter, B.G.; Meunier, V. A universal model for nanoporous carbon supercapacitors applicable to diverse pore regimes, carbon materials, and electrolytes. Eur. J. Chem. 2008, 14, 6614–6626. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520. [Google Scholar] [CrossRef] [PubMed]
- Raymundo-Piñero, E.; Kierzek, K.; Machnikowski, J.; Béguin, F. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon 2006, 44, 2498–2507. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Kostoglou, N.; Koczwara, C.; Stock, S.; Tampaxis, C.; Charalambopoulou, G.; Steriotis, T.; Paris, O.; Rebholz, C.; Mitterer, C. Nanoporous polymer-derived activated carbon for hydrogen adsorption and electrochemical energy storage. Chem. Eng. J. 2022, 427, 131730. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, S.B.; Liu, Y.G.; Gu, Y.L.; Zeng, G.M.; Hu, X.J.; Wang, X.; Liu, S.H.; Jiang, L.H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Boonpakdee, D.; Guajardo Yévenes, C.F.; Surareungchai, W.; La-O-Vorakiat, C. Exploring non-linearities of carbon-based microsupercapacitors from an equivalent circuit perspective. J. Mater. Chem. A 2018, 6, 7162–7167. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, H.-J.; Yoo, J.J.; Yoo, C.-Y.; Park, J.H.; Lee, Y.A.; Cho, W.K.; Han, Y.-K.; Kim, D.H. Pseudocapacitive slurry electrodes using redox-active quinone for high-performance flow capacitors: An atomic-level understanding of pore texture and capacitance enhancement. J. Mater. Chem. A. 2015, 3, 23323–23332. [Google Scholar] [CrossRef]
- Pognon, G.; Brousse, T.; Bélanger, D. Effect of molecular grafting on the pore size distribution and the double layer capacitance of activated carbon for electrochemical double layer capacitors. Carbon 2011, 49, 1340–1348. [Google Scholar] [CrossRef]





| Element | CSS [%] | AC—CSS [%] |
|---|---|---|
| Carbon | 48.6 ± 0.3 | 89.2 ± 0.2 |
| Oxygen | 38.6 ± 0.3 | 6.7 ± 0.01 |
| Hydrogen | 6.1 ± 0.1 | 0.2 ± 0.01 |
| Nitrogen | 2.9 ± 0.2 | 0.1 ± 0.01 |
| Sulfur | n.d. | n.d. |
| N/C | 0.060 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selinger, J.; Stock, S.; Schlemmer, W.; Hobisch, M.; Kostoglou, N.; Abbas, Q.; Paris, O.; Mitterer, C.; Hummel, M.; Spirk, S. Nanoporous Carbon Electrodes Derived from Coffee Side Streams for Supercapacitors in Aqueous Electrolytes. Nanomaterials 2022, 12, 2647. https://doi.org/10.3390/nano12152647
Selinger J, Stock S, Schlemmer W, Hobisch M, Kostoglou N, Abbas Q, Paris O, Mitterer C, Hummel M, Spirk S. Nanoporous Carbon Electrodes Derived from Coffee Side Streams for Supercapacitors in Aqueous Electrolytes. Nanomaterials. 2022; 12(15):2647. https://doi.org/10.3390/nano12152647
Chicago/Turabian StyleSelinger, Julian, Sebastian Stock, Werner Schlemmer, Mathias Hobisch, Nikolaos Kostoglou, Qamar Abbas, Oskar Paris, Christian Mitterer, Michael Hummel, and Stefan Spirk. 2022. "Nanoporous Carbon Electrodes Derived from Coffee Side Streams for Supercapacitors in Aqueous Electrolytes" Nanomaterials 12, no. 15: 2647. https://doi.org/10.3390/nano12152647
APA StyleSelinger, J., Stock, S., Schlemmer, W., Hobisch, M., Kostoglou, N., Abbas, Q., Paris, O., Mitterer, C., Hummel, M., & Spirk, S. (2022). Nanoporous Carbon Electrodes Derived from Coffee Side Streams for Supercapacitors in Aqueous Electrolytes. Nanomaterials, 12(15), 2647. https://doi.org/10.3390/nano12152647








