Efficient Removal of Siloxane from Biogas by Using β-Cyclodextrin-Modified Reduced Graphene Oxide Aerogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
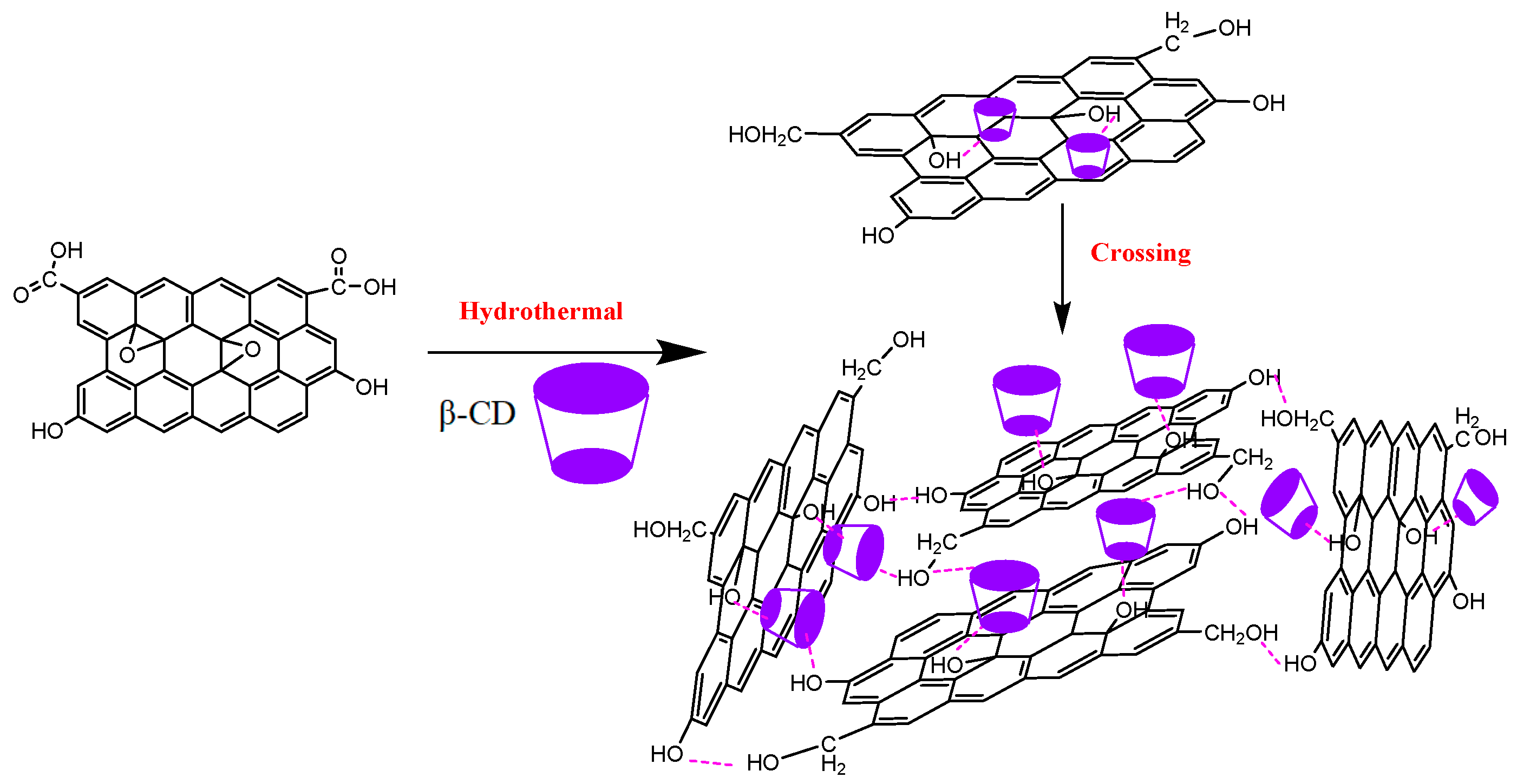
2.2. Preparation of β-CD-rGOAs
2.3. Characterization
2.4. Adsorption and Regeneration
2.5. Mathematical Models
3. Results
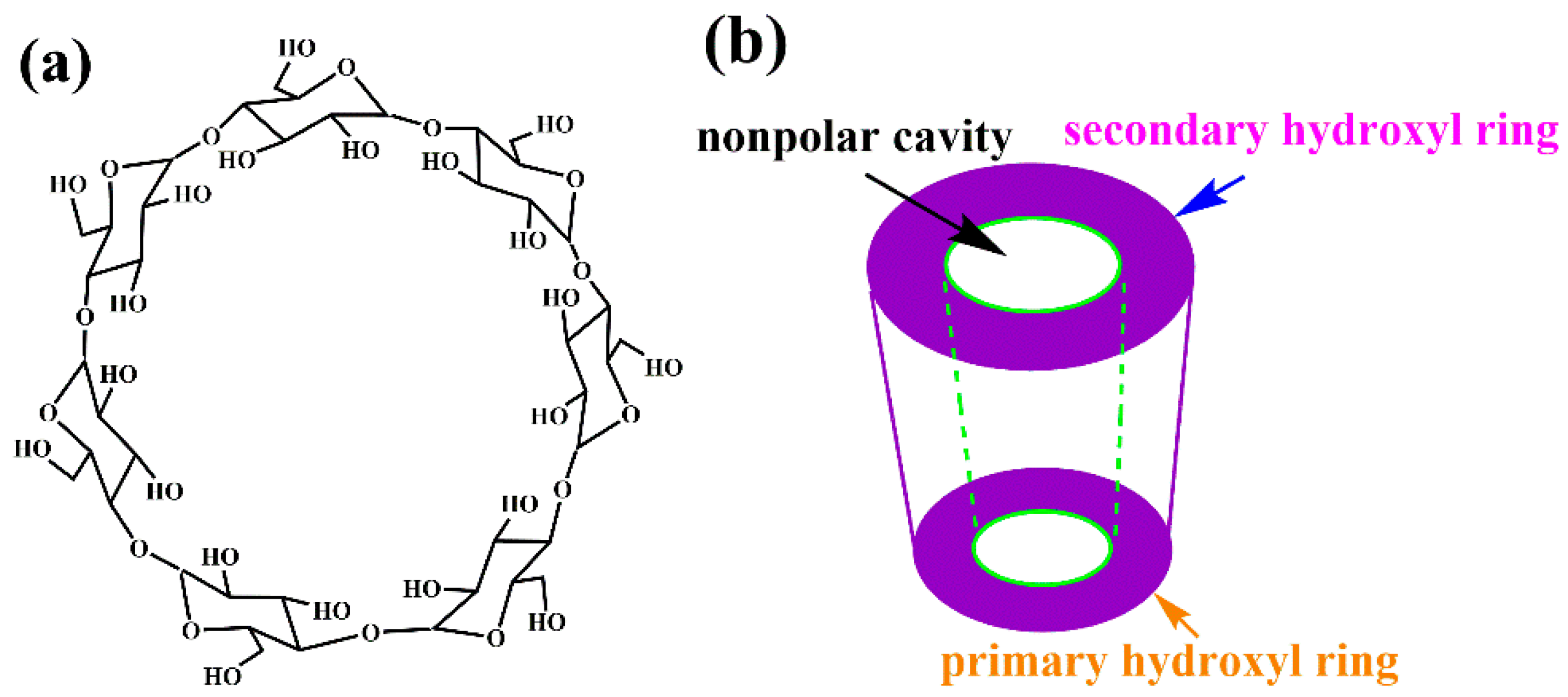
3.1. Effect of Modifier β-CD
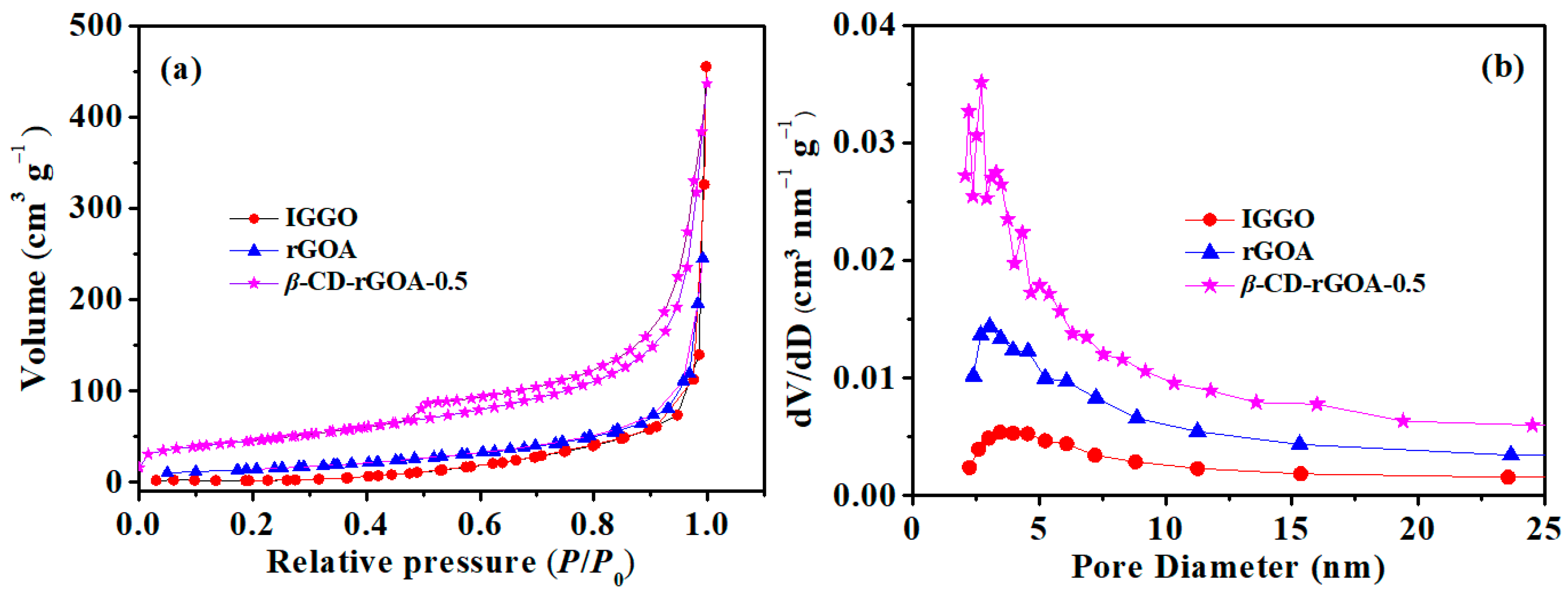
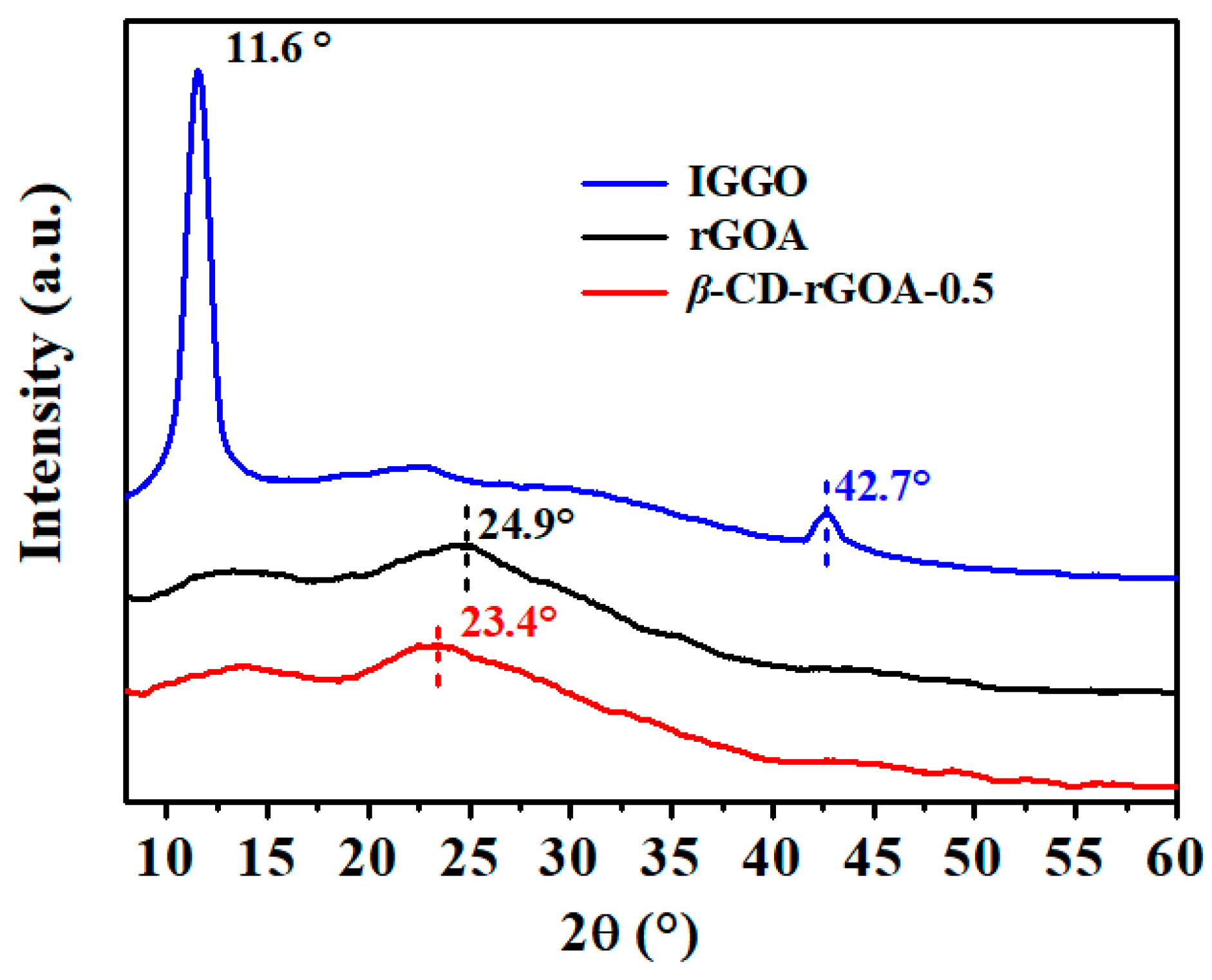
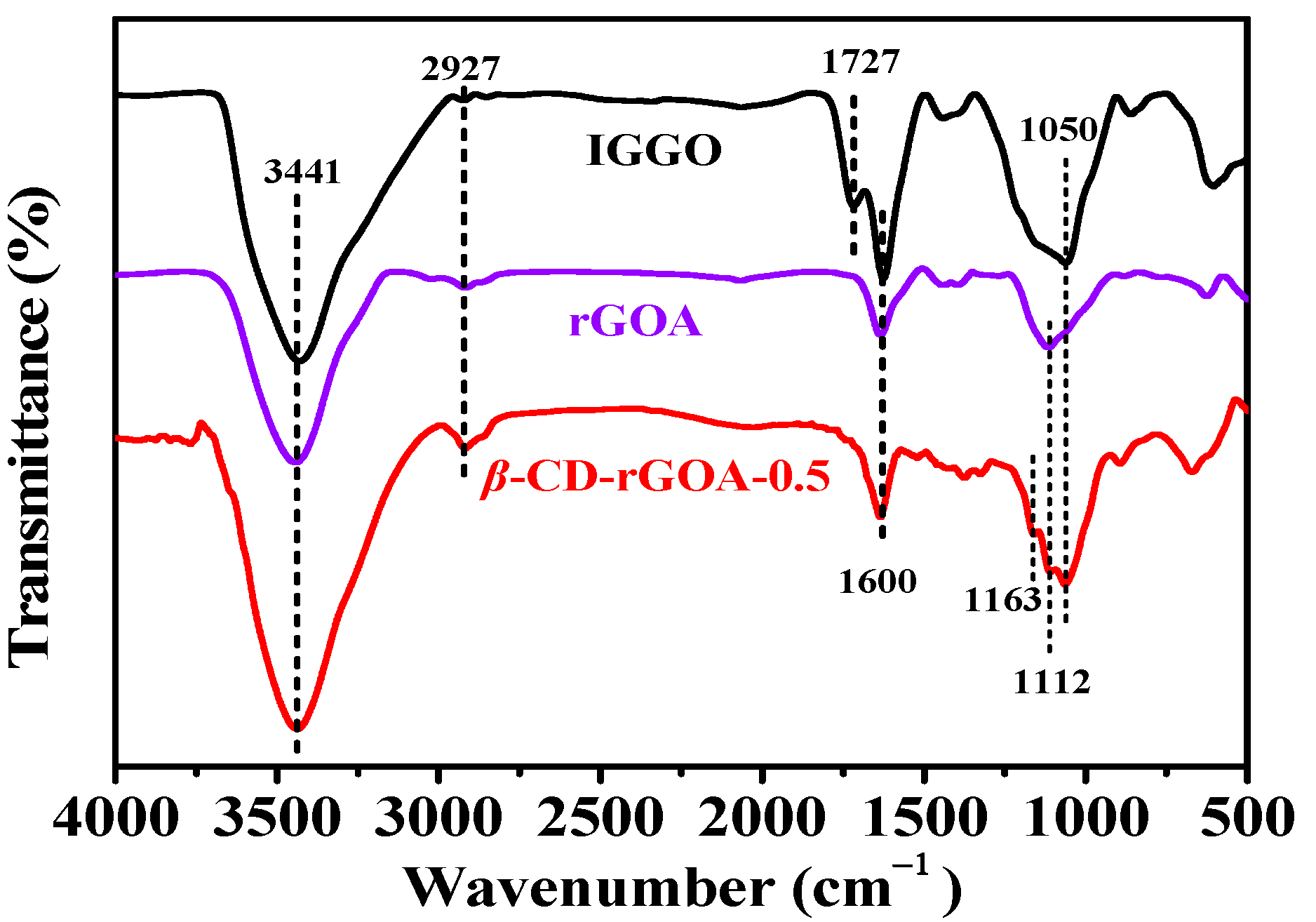
3.2. Characterization of Adsorbents
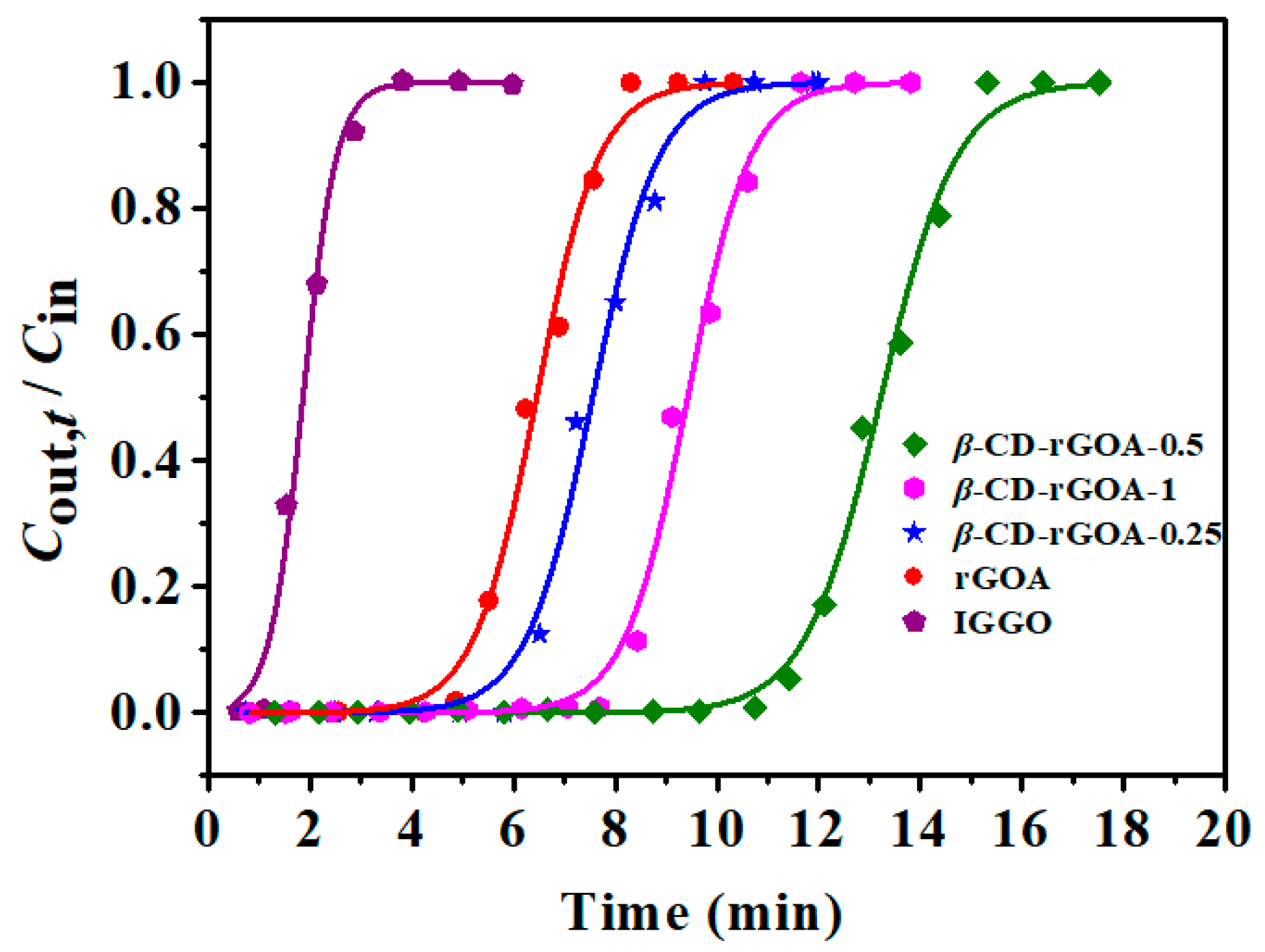
3.3. Comparison of Dynamic Adsorption Performances
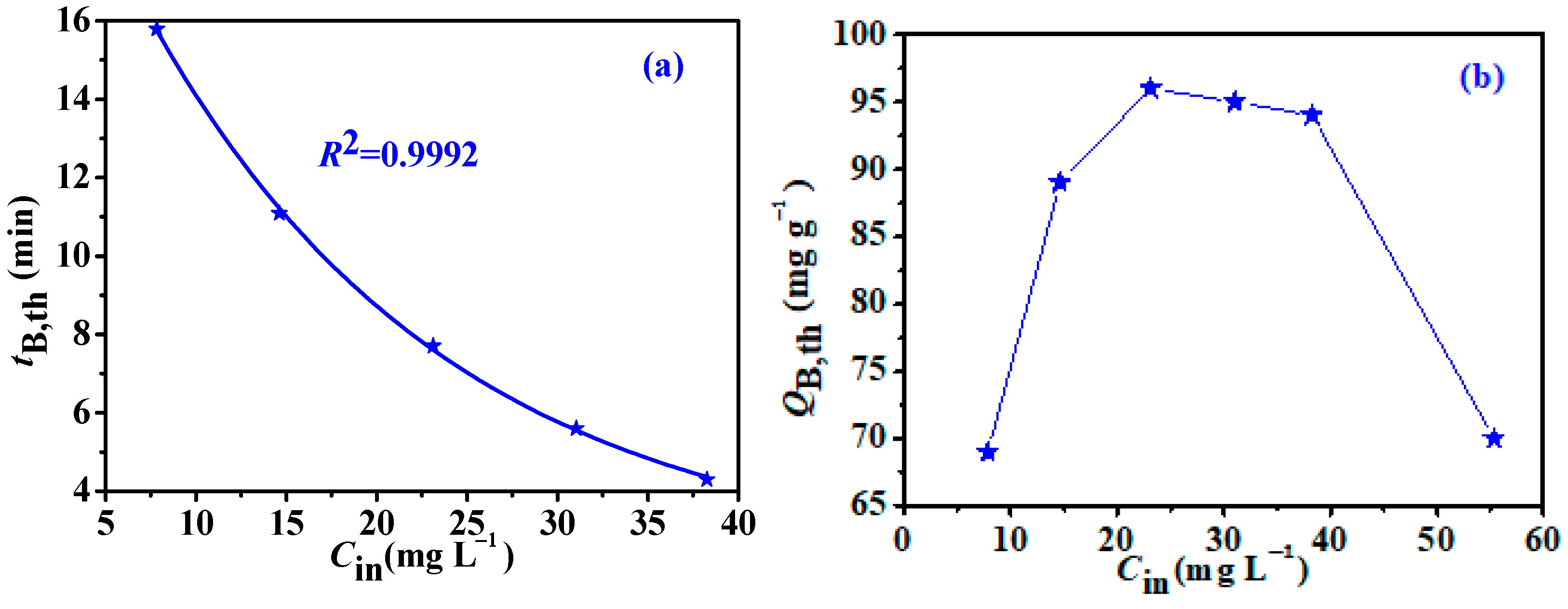
3.4. Influence of Inlet Concentration and Bed Temperature
3.5. Recycling Performance of β-CD-rGOA-0.5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, T.; Zhang, Z.; Lei, Z.; Shimizu, K.; Lee, D.J. A review on biogas upgrading in anaerobic digestion systems treating organic solids and wastewaters via biogas recirculation. Bioresour. Technol. 2022, 344, 126412. [Google Scholar] [CrossRef] [PubMed]
- Kunatsa, T.; Xia, X. A review on anaerobic digestion with focus on the role of biomass co-digestion, modelling and optimisation on biogas production and enhancement. Bioresour. Technol. 2022, 344, 126311. [Google Scholar] [CrossRef]
- Das, J.; Ravishankar, H.; Lens, P.N.L. Biological biogas purification: Recent developments, challenges and future prospects. J. Environ. Manag. 2022, 304, 114198. [Google Scholar] [CrossRef] [PubMed]
- Golmakani, A.; Ali Nabavi, S.; Wadi, B.; Manovic, V. Advances, challenges, and perspectives of biogas cleaning, upgrading, and utilisation. Fuel 2022, 317, 123085. [Google Scholar] [CrossRef]
- Bragança, I.; Sánchez-Soberón, F.; Pantuzza, G.F.; Alves, A.; Ratola, N. Impurities in biogas: Analytical strategies, occurrence, effects and removal technologies. Biomass Bioenergy 2020, 143, 105878. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Mukumba, P.; Chikukwa, E.; Makaka, G. Biogas upgrading approaches with special focus on siloxane removal-A review. Energies 2020, 13, 6088. [Google Scholar] [CrossRef]
- Mendiara, T.; Cabello, A.; Izquierdo, M.T.; Abad, A.; Mattisson, T.; Adánez, J. Effect of the Presence of Siloxanes in Biogas Chemical Looping Combustion. Energy Fuels 2021, 35, 14984–14994. [Google Scholar] [CrossRef]
- Nair, N.; Zhang, X.; Gutierrez, J.; Chen, J.; Egolfopoulos, F.; Tsotsis, T. Impact of siloxane impurities on the performance of an engine operating on renewable natural gas. Ind. Eng. Chem. Res. 2012, 51, 15786–15795. [Google Scholar] [CrossRef]
- Wang, N.; Tan, L.; Xie, L.; Wang, Y.; Ellis, T. Investigation of volatile methyl siloxanes in biogas and the ambient environment in a landfill. J. Environ. Sci. (China) 2020, 91, 54–61. [Google Scholar] [CrossRef]
- De Arespacochaga, N.; Valderrama, C.; Raich-Montiu, J.; Crest, M.; Mehta, S.; Cortina, J.L. Understanding the effects of the origin, occurrence, monitoring, control, fate and removal of siloxanes on the energetic valorization of sewage biogas—A review. Renew. Sustain. Energy Rev. 2015, 52, 366–381. [Google Scholar] [CrossRef] [Green Version]
- Piechota, G. Removal of siloxanes from biogas upgraded to biomethane by Cryogenic Temperature Condensation System. J. Clean. Prod. 2021, 308, 127404. [Google Scholar] [CrossRef]
- Charry Prada, I.D.; Rivera-Tinoco, R.; Bouallou, C. Biogas industry: Novel acid gas removal technology using a superacid solvent. Process design, unit specification and feasibility study compared with other existing technologies. Chem. Eng. Res. Des. 2020, 154, 212–231. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Xu, X.; Chen, C.; Xu, J.; Kong, L.; Xie, P.; Zheng, C.; Ren, N.; Lee, D. Effective removal of methyl siloxane from water by sewage activated sludge microbes: Biodegradation behavior and characteristics of microbial community. Bioresour. Technol. Rep. 2019, 7, 100209. [Google Scholar] [CrossRef]
- Santos-Clotas, E.; Cabrera-Codony, A.; Comas, J.; Martín, M.J. Biogas purification through membrane bioreactors: Experimental study on siloxane separation and biodegradation. Sep. Purif. Technol. 2020, 238, 116440. [Google Scholar] [CrossRef]
- Silva, E.N.; Cantillo-Castrillon, M.; Dantas, T.M.; Mesquita, Y.M.; Maia, D.A.S.; Bastos-Neto, M.; Barcellos, W.M.; Azevedo, D.C.S. Siloxane adsorption by porous silica synthesized from residual sand of wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 104805. [Google Scholar] [CrossRef]
- Papurello, D.; Gandiglio, M.; Kafashan, J.; Lanzini, A. Biogas Purification: A Comparison of Adsorption Performance in D4 Siloxane Removal Between Commercial Activated Carbons and Waste Wood-Derived Char Using Isotherm Equations. Processes 2019, 7, 774. [Google Scholar] [CrossRef] [Green Version]
- Gai, K. Adsorptive Biogas Purification from Siloxanes—A Critical Review. Energies 2020, 13, 2605. [Google Scholar] [CrossRef]
- Cabrera-Codony, A.; Santos-Clotas, E.; Ania, C.O.; Martín, M.J. Competitive siloxane adsorption in multicomponent gas streams for biogas upgrading. Chem. Eng. J. 2018, 344, 565–573. [Google Scholar] [CrossRef]
- Sigot, L.; Ducom, G.; Germain, P. Adsorption of octamethylcyclotetrasiloxane (D4) on silica gel (SG): Retention mechanism. Microporous Mesoporous Mater. 2015, 213, 118–124. [Google Scholar] [CrossRef]
- Wei, D.; Liu, X.; Lv, S.; Liu, L.; Wu, L.; Li, Z.; Hou, Y. Fabrication, Structure, Performance, and Application of Graphene-Based Composite Aerogel. Materials 2022, 15, 299. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A. Graphene/Reduced Graphene Oxide-Carbon Nanotubes Composite Electrodes: From Capacitive to Battery-Type Behaviour. Nanomaterials 2021, 11, 1240. [Google Scholar] [CrossRef]
- Li, H.; Sun, L.; Wang, Z.; Zhang, Y.; Tan, T.; Wang, G.; Bakenov, Z. Three-Dimensionally Hierarchical Graphene Based Aerogel Encapsulated Sulfur as Cathode for Lithium/Sulfur Batteries. Nanomaterials 2018, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, M.; Xie, J. Self-Assembled Nano-Fe3C Embedded in Reduced Graphene Oxide Aerogel with Efficient Fenton-Like Catalysis. Nanomaterials 2020, 10, 2348. [Google Scholar] [CrossRef]
- Zhou, J.; Du, E.; Fan, Y.; Ye, Y.; Tang, B. Preparation of Carbonized Kapok Fiber/Reduced Graphene Oxide Aerogel forOil-Water Separation. Chem. Eng. Tech. 2020, 43, 2418–2427. [Google Scholar] [CrossRef]
- Lamberti, A.; Gigot, A.; Bianco, S.; Fontana, M.; Castellino, M.; Tresso, E.; Pirri, C.F. Self-assembly of graphene aerogel on copper wire for wearable fiber-shaped supercapacitors. Carbon 2016, 105, 649–654. [Google Scholar] [CrossRef]
- Bashir, S.; Hasan, K.; Hina, M.; Ali Soomro, R.; Mujtaba, M.A.; Ramesh, S.; Ramesh, K.; Duraisamy, N.; Manikam, R. Conducting polymer/graphene hydrogel electrodes based aqueous smart Supercapacitors: A review and future prospects. J. Electroanal. Chem. 2021, 898, 115626. [Google Scholar] [CrossRef]
- Liang, W.; Rong, Y.; Fan, L.; Dong, W.; Dong, Q.; Yang, C.; Zhong, Z.; Dong, C.; Shuang, S.; Wong, W. 3D graphene/hydroxypropyl-β-cyclodextrin nanocomposite as an electrochemical chiral sensor for the recognition of tryptophan enantiomers. J. Mater. Chem. C 2018, 6, 12822–12829. [Google Scholar] [CrossRef]
- Samuel, M.S.; Selvarajan, E.; Subramaniam, K.; Mathimani, T.; Seethappan, S.; Pugazhendhi, A. Synthesized β-cyclodextrin modified graphene oxide (β-CD-GO) composite for adsorption of cadmium and their toxicity profile in cervical cancer (HeLa) cell lines. Process Biochem. 2020, 93, 28–35. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Wang, X.; Shi, G. On the Gelation of Graphene Oxide. J. Physic. Chem. C 2011, 115, 5545–5551. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, L.; Liu, C.; Zhen, Y.; Zhang, W.; Ma, J. A novel 3D adsorbent of reduced graphene oxide-β-cyclodextrin aerogel coupled hardness with softness for efficient removal of bisphenol A. Chem. Eng. J. 2019, 372, 896–904. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Feng, X.; Qiu, B.; Dang, Y.; Sun, D. Enhanced adsorption of naproxen from aquatic environments by β-cyclodextrin-immobilized reduced graphene oxide. Chem. Eng. J. 2021, 412, 128710. [Google Scholar] [CrossRef]
- Song, W.; Hu, J.; Zhao, Y.; Shao, D.; Li, J. Efficient removal of cobalt from aqueous solution using β-cyclodextrin modified graphene oxide. RSC Adv. 2013, 3, 9514–9521. [Google Scholar] [CrossRef]
- Meng, Z.; Hou, X.; Liu, Y.; Ma, Z.; Shen, H. Facile fabrication of iron-modified biochar as a renewable adsorbent for efficient siloxane (L2) removal. J. Environ. Chem. Eng. 2021, 9, 105799. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, Y.; Li, X.; Ma, Z. Removal of siloxane (L2) from biogas using methyl-functionalised silica gel as adsorbent. Chem. Eng. J. 2020, 389, 124440. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, X.; Ma, X.; Hao, Z.; Ma, Z. Vitamin C-Assisted Fabrication of Aerogels from Industrial Graphene Oxide for Gaseous Hexamethyldisiloxane Adsorption. Appl. Sci. 2021, 11, 8486. [Google Scholar] [CrossRef]
- Hou, X.; Zheng, Y.; Ma, X.; Liu, Y.; Ma, Z. The Effects of Hydrophobicity and Textural Properties on Hexamethyldisiloxane Adsorption in Reduced Graphene Oxide Aerogels. Molecules 2021, 26, 1130. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, X.; Liu, Y.; Ma, Z. Hexamethyldisiloxane removal from biogas using reduced graphene-oxide aerogels as adsorbents. Renew. Energy 2021, 178, 153–161. [Google Scholar] [CrossRef]
- Wang, J.; Duan, X.; Dong, Q.; Meng, F.; Tan, X.; Liu, S.; Wang, S. Facile synthesis of N-doped 3D graphene aerogel and its excellent performance in catalytic degradation of antibiotic contaminants in water. Carbon 2019, 144, 781–790. [Google Scholar] [CrossRef]
- Mauro, M.; Maggio, M.; Antonelli, A.; Acocella, M.; Guerra, G. Intercalation and Exfoliation Compounds of Graphite Oxide with Quaternary Phosphonium Ions. Chem. Mater. 2015, 27, 1590–1596. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, H.; Hou, P.; Qian, B.; Wang, X.; Guo, C.; Wang, L. Efficient Graphene/Cyclodextrin-Based Nanocontainer: Synthesis and Host-Guest Inclusion for Self-Healing Anticorrosion Application. ACS Appl. Mater. Interfaces 2018, 10, 36229–36239. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, W.; Ren, J.; Qu, X. Electrochemically and DNA-triggered cell release from ferrocene/β-cyclodextrin and aptamer modified dualfunctionalized graphene substrate. Nano Res. 2014, 8, 887–899. [Google Scholar] [CrossRef]
- Xiao, Q.; Lu, S.; Huang, C.; Su, W.; Huang, S. Novel N-Doped Carbon Dots/beta-Cyclodextrin Nanocomposites for Enantioselective Recognition of Tryptophan Enantiomers. Sensors 2016, 16, 1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Z.; Liu, Y.; Ma, Z.; Hou, X. The regulation of micro/mesoporous silica gel by polyethylene imine for enhancing the siloxane removal. Inorg. Chem. Commun. 2020, 112, 107754. [Google Scholar] [CrossRef]
- Wang, Z.; Muhammad, Y.; Tang, R.; Lu, C.; Yu, S.; Song, R.; Tong, Z.; Han, B.; Zhang, H. Dually organic modified bentonite with enhanced adsorption and desorption of tetracycline and ciprofloxacine. Sep. Purif. Technol. 2021, 274, 119059. [Google Scholar] [CrossRef]
- Darwish, A.; Rashad, M.; AL-Aoh, H. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dyes Pigm. 2019, 160, 563–571. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, Z.; Wang, J.; Dong, Y.; Ma, Z. Removal of siloxanes from biogas using acetylated silica gel as adsorbent. Pet. Sci. 2019, 16, 920–928. [Google Scholar] [CrossRef] [Green Version]





| Sample | Specific Surface Area (m2 g−1) | Total Pore Volume (cm3 g−1) | Average Pore Diameter (nm) | Contact Angle (°) |
|---|---|---|---|---|
| IGGO | 7.4 | 0.23 | 13.18 | 76.6 |
| rGOA | 55.2 | 0.39 | 9.04 | 121.8 |
| β-CD-rGOA-0.25 | 69.3 | 0.41 | 7.28 | 122.0 |
| β-CD-rGOA-0.5 | 163.5 | 0.68 | 5.93 | 128.4 |
| β-CD-rGOA-1 | 112.3 | 0.65 | 7.87 | 127.1 |
| Absorbent | tB (min) | tB,th (min) | QB,th (mg g−1) | KYN | τ (min) | R2 |
|---|---|---|---|---|---|---|
| IGGO | 1.16 | 0.89 | 7.1 | 3.0263 | 1.86 | 0.9901 |
| rGOA | 5.14 | 4.64 | 37.1 | 1.6239 | 6.45 | 0.9941 |
| β-CD-rGOA-0.25 | 5.78 | 5.64 | 45.2 | 1.5359 | 7.55 | 0.9934 |
| β-CD-rGOA-0.5 | 11.27 | 11.03 | 88.7 | 1.3434 | 13.22 | 0.9952 |
| β-CD-rGOA-1 | 8.00 | 7.62 | 61.2 | 1.6331 | 9.42 | 0.9938 |
| Temp. °C | tB,th min | QB,th mg g−1 | KYN | τ min | R2 |
|---|---|---|---|---|---|
| 0 | 14.56 | 111.8 | 1.5189 | 16.50 | 0.9977 |
| 10 | 12.94 | 96.3 | 1.7121 | 14.66 | 0.9978 |
| 15 | 11.80 | 91.5 | 1.8068 | 13.43 | 0.9975 |
| 25 | 10.78 | 82.7 | 1.7031 | 12.51 | 0.9963 |
| 30 | 9.95 | 75.5 | 1.8085 | 11.57 | 0.9989 |
| 35 | 9.18 | 74.4 | 1.9482 | 10.69 | 0.9974 |
| 45 | 7.59 | 61.54 | 1.6291 | 9.38 | 0.9941 |
| 55 | 5.60 | 45.96 | 1.5249 | 7.46 | 0.9938 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Hou, X.; Lv, S.; Ma, Z.; Ma, X. Efficient Removal of Siloxane from Biogas by Using β-Cyclodextrin-Modified Reduced Graphene Oxide Aerogels. Nanomaterials 2022, 12, 2643. https://doi.org/10.3390/nano12152643
Zheng Y, Hou X, Lv S, Ma Z, Ma X. Efficient Removal of Siloxane from Biogas by Using β-Cyclodextrin-Modified Reduced Graphene Oxide Aerogels. Nanomaterials. 2022; 12(15):2643. https://doi.org/10.3390/nano12152643
Chicago/Turabian StyleZheng, Yanhui, Xifeng Hou, Siqi Lv, Zichuan Ma, and Xiaolong Ma. 2022. "Efficient Removal of Siloxane from Biogas by Using β-Cyclodextrin-Modified Reduced Graphene Oxide Aerogels" Nanomaterials 12, no. 15: 2643. https://doi.org/10.3390/nano12152643
APA StyleZheng, Y., Hou, X., Lv, S., Ma, Z., & Ma, X. (2022). Efficient Removal of Siloxane from Biogas by Using β-Cyclodextrin-Modified Reduced Graphene Oxide Aerogels. Nanomaterials, 12(15), 2643. https://doi.org/10.3390/nano12152643









