Improving Photoelectrochemical Activity of Magnetron-Sputtered Double-Layer Tungsten Trioxide Photoanodes by Irradiation with Intense Pulsed Ion Beams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. PEC Characterization
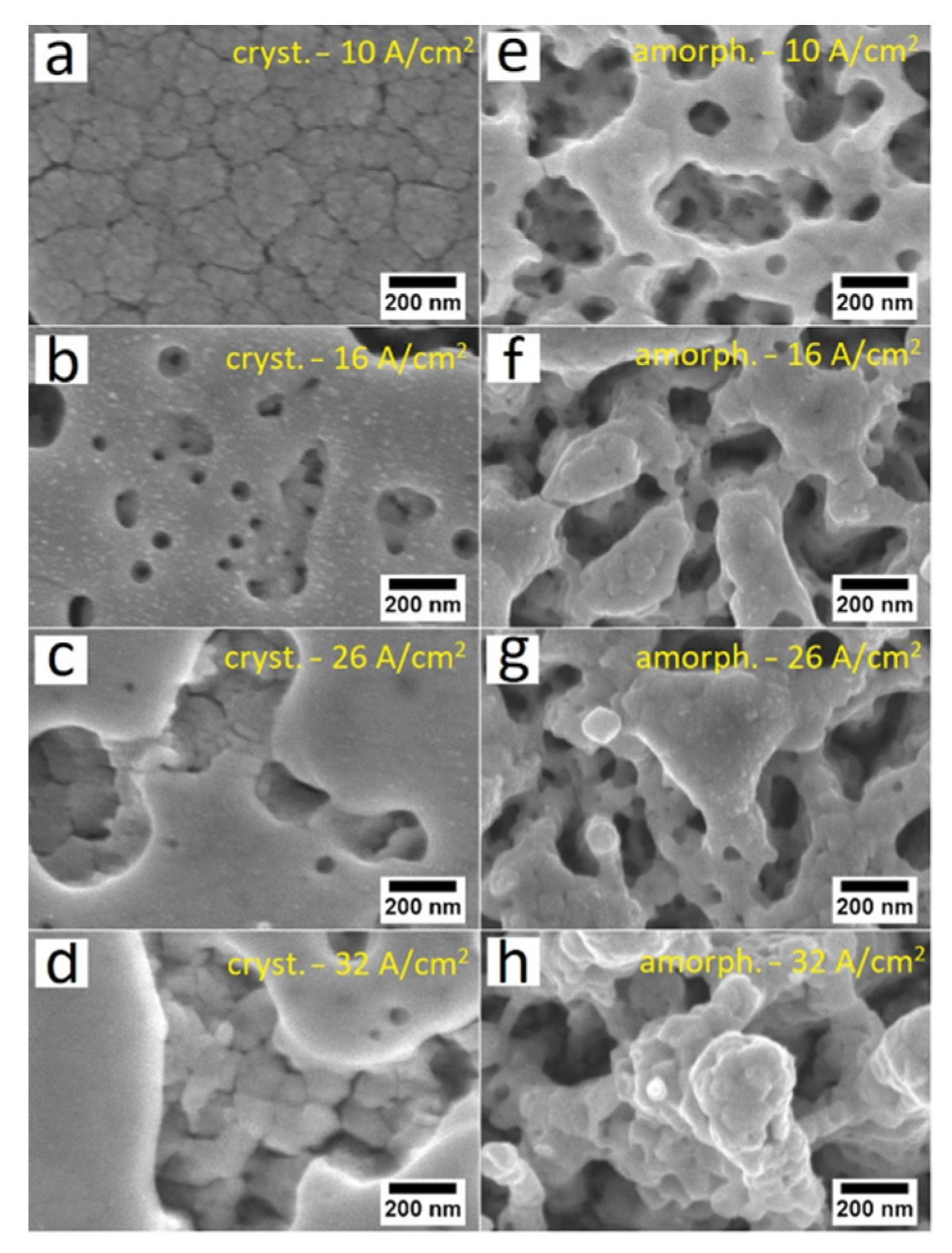
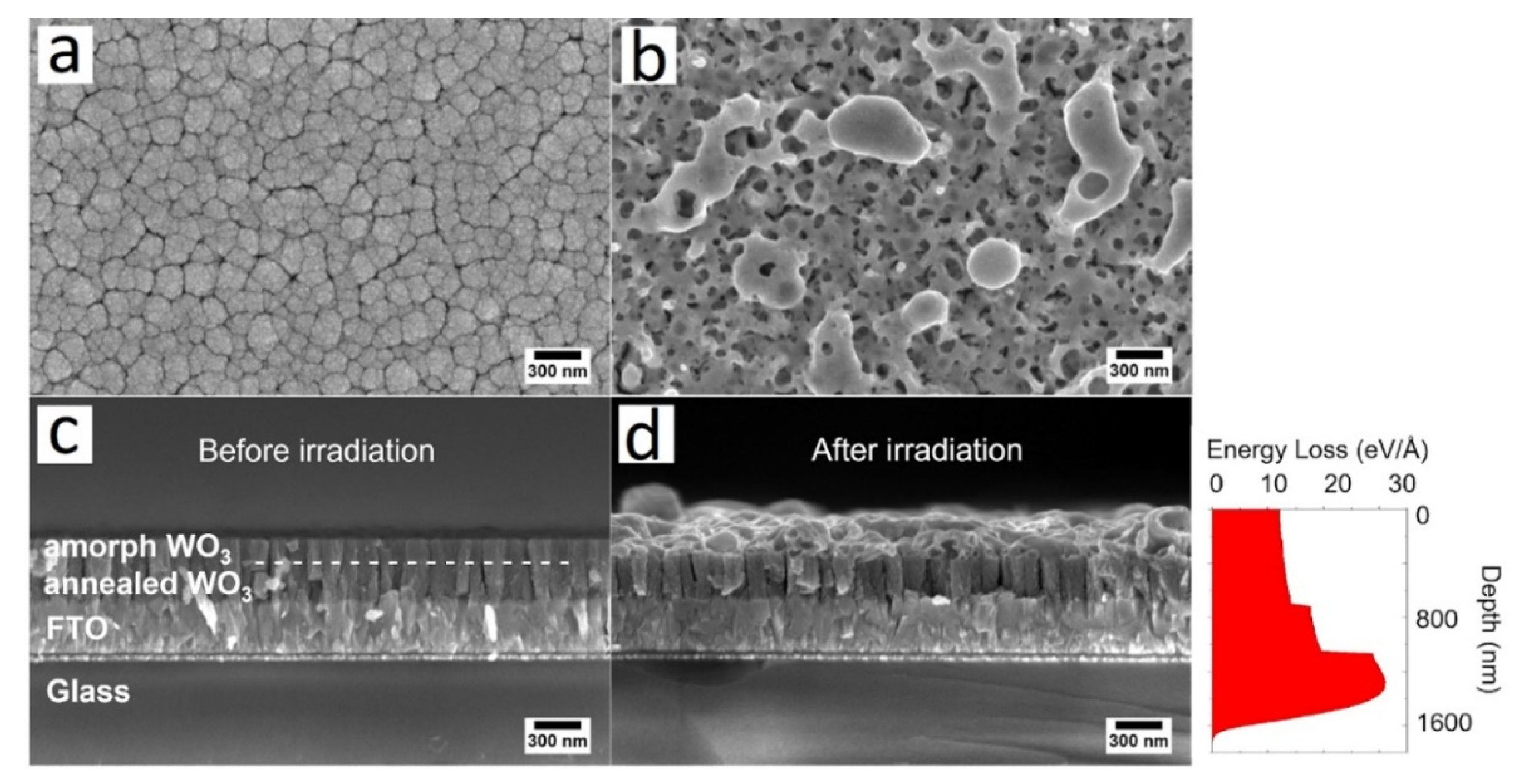
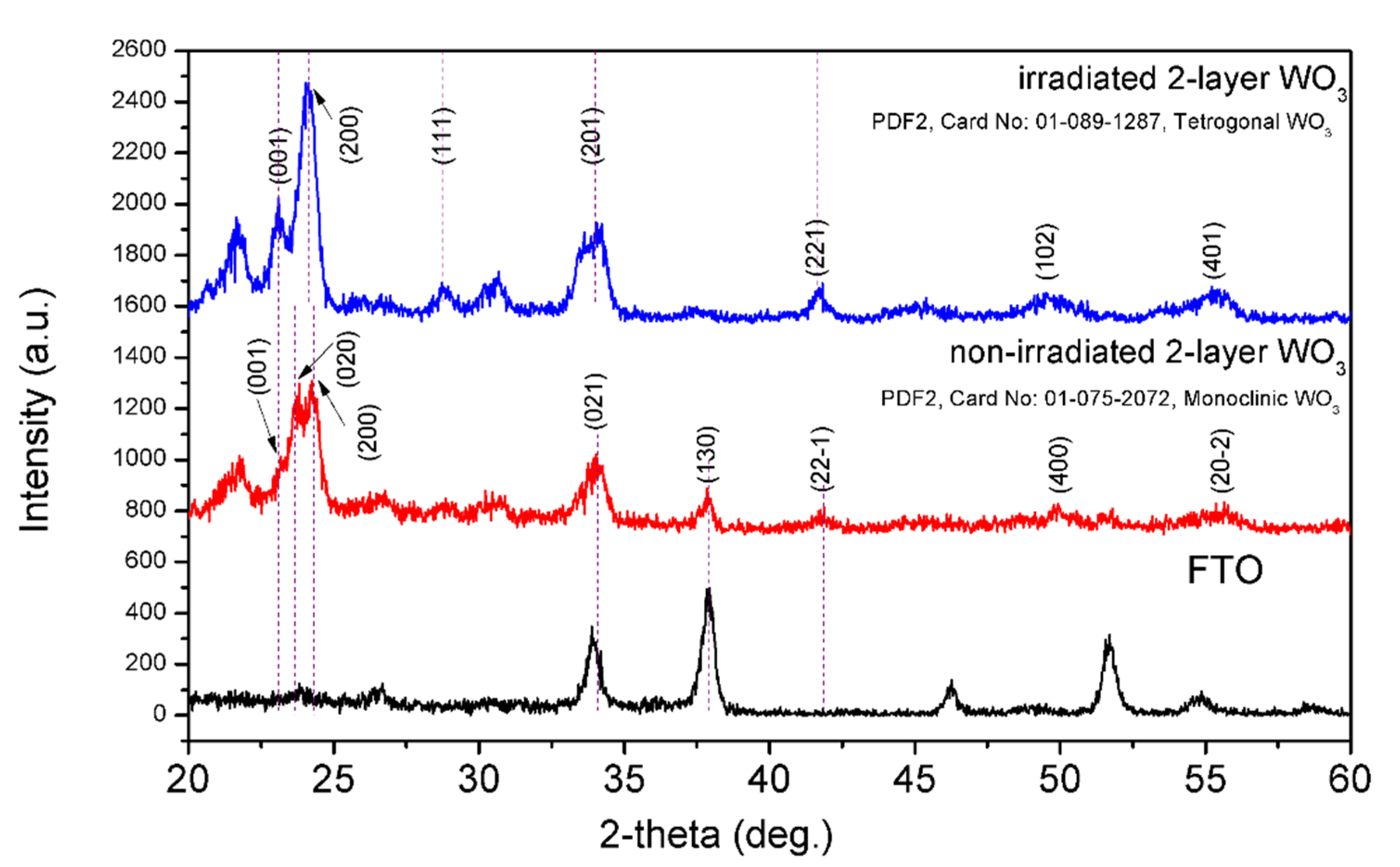
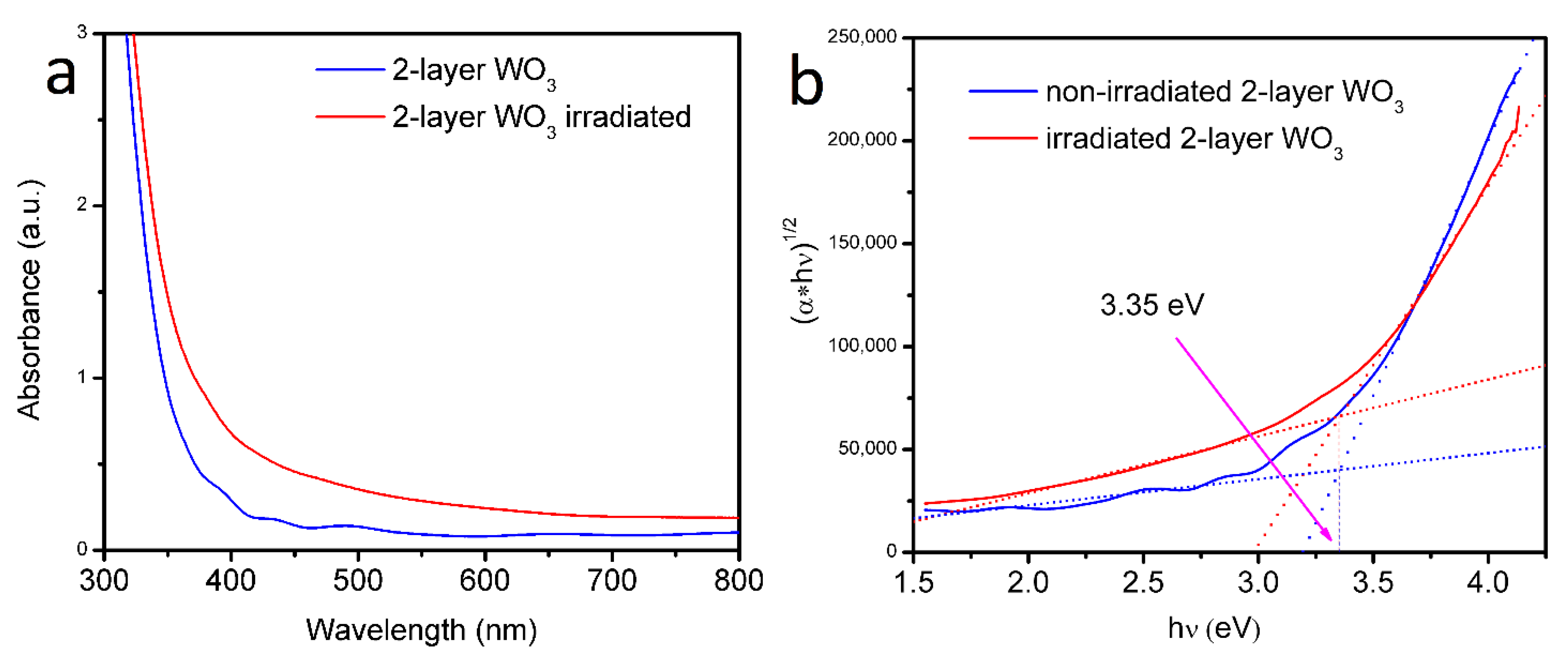
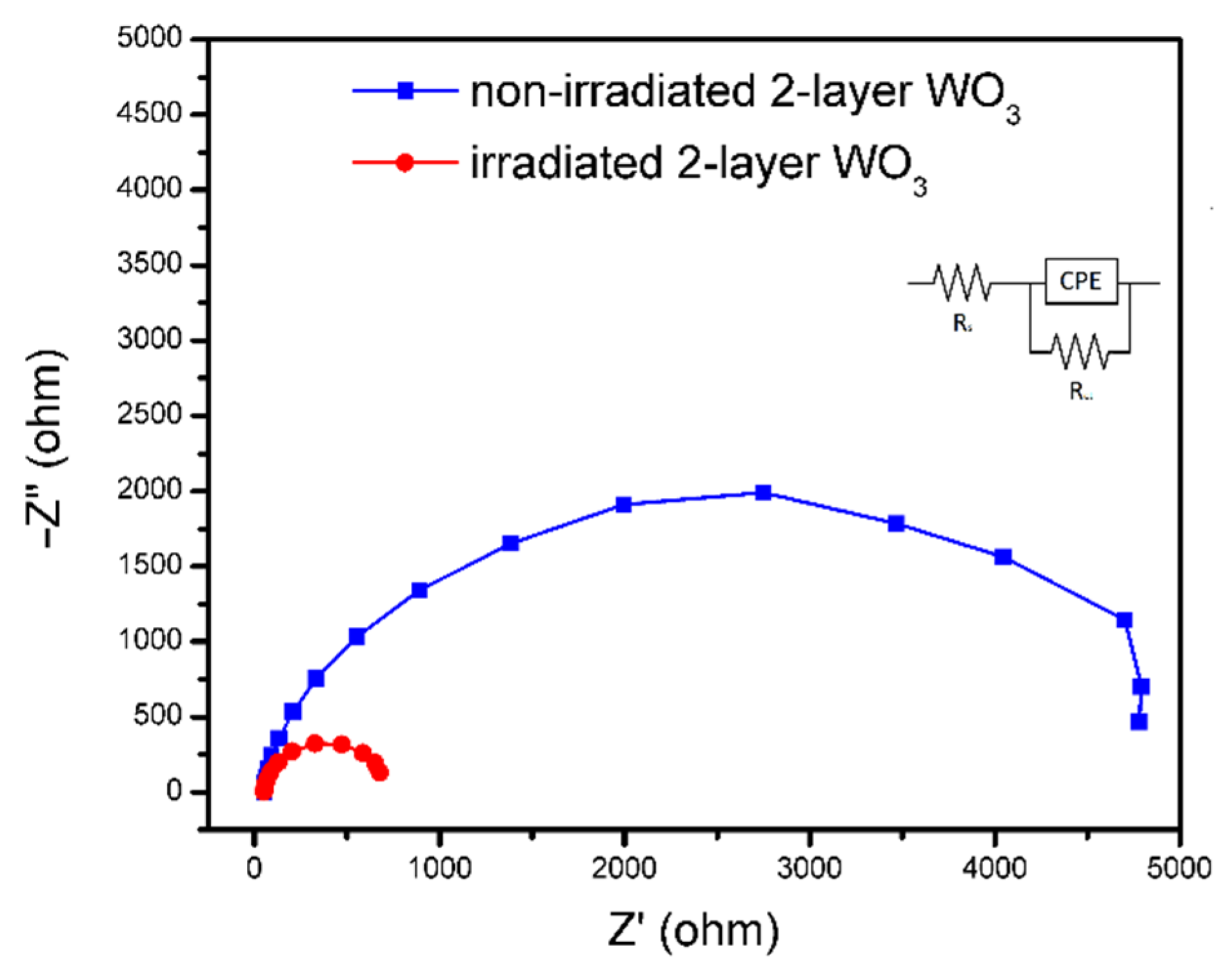
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sazali, N. Emerging Technologies by Hydrogen: A Review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Ng, K.H.; Lai, S.Y.; Cheng, C.K.; Cheng, Y.W.; Chong, C.C. Photocatalytic Water Splitting for Solving Energy Crisis: Myth, Fact or Busted? Chem. Eng. J. 2021, 417, 128847. [Google Scholar] [CrossRef]
- Jia, J.; Seitz, L.C.; Benck, J.D.; Huo, Y.; Chen, Y.; Ng, J.W.D.; Bilir, T.; Harris, J.S.; Jaramillo, T.F. Solar Water Splitting by Photovoltaic-Electrolysis with a Solar-to-Hydrogen Efficiency over 30%. Nat. Commun. 2016, 7, 13237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamedkhair, A.K.; Drmosh, Q.A.; Qamar, M.; Yamani, Z.H. Tuning Structural Properties of WO3 Thin Films for Photoelectrocatalytic Water Oxidation. Catalysts 2021, 11, 381. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Zdorovets, M. V Study of the Photocatalytic Activity of Irradiated WO3 Microparticles. Appl. Phys. A 2020, 126, 638. [Google Scholar] [CrossRef]
- Shandilya, P.; Sambyal, S.; Sharma, R.; Mandyal, P.; Fang, B. Properties, Optimized Morphologies, and Advanced Strategies for Photocatalytic Applications of WO3 Based Photocatalysts. J. Hazard. Mater. 2022, 428, 128218. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, J.; Liu, H.; Murugadoss, V.; Zu, G.; Che, H.; Lai, C.; Li, H.; Ding, T.; Gao, Q.; et al. Tungsten Oxide Nanostructures and Nanocomposites for Photoelectrochemical Water Splitting. Nanoscale 2019, 11, 18968–18994. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Noh, Y.-G.; Seo, H. Engineering Band Edge Properties of WO3 with Respect to Photoelectrochemical Water Splitting Potentials via a Generalized Doping Protocol of First-Row Transition Metal Ions. Appl. Surf. Sci. 2020, 509, 145253. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible Light Driven Type II Heterostructures and Their Enhanced Photocatalysis Properties: A Review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Zhang, J.; Balogun, M.-S.; Wang, P.; Tong, Y.; Huang, Y. Charge Relays via Dual Carbon-Actions on Nanostructured BiVO4 for High Performance Photoelectrochemical Water Splitting. Adv. Funct. Mater. 2022, 32, 2112738. [Google Scholar] [CrossRef]
- Zych, M.; Syrek, K.; Pisarek, M.; Sulka, G.D. Anodic WO3 Layers Sensitized with Hematite Operating under the Visible Light Spectrum. J. Power Sources 2022, 541, 231656. [Google Scholar] [CrossRef]
- Markhabayeva, A.A.; Moniruddin, M.; Dupre, R.; Abdullin, K.A.; Nuraje, N. Designing of WO3@Co3O4 Heterostructures to Enhance Photoelectrochemical Performances. J. Phys. Chem. A 2020, 124, 486–491. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, S.; Chen, Y.; Gaskov, A.M. Facile Morphology Control of WO3 Nanostructure Arrays with Enhanced Photoelectrochemical Performance. Appl. Surf. Sci. 2017, 403, 274–281. [Google Scholar] [CrossRef]
- Fang, Y.; Lee, W.C.; Canciani, G.E.; Draper, T.C.; Al-Bawi, Z.F.; Bedi, J.S.; Perry, C.C.; Chen, Q. Thickness Control in Electrophoretic Deposition of WO3 Nanofiber Thin Films for Solar Water Splitting. Mater. Sci. Eng. B 2015, 202, 39–45. [Google Scholar] [CrossRef]
- Chae, S.Y.; Lee, C.S.; Jung, H.; Joo, O.-S.; Min, B.K.; Kim, J.H.; Hwang, Y.J. Insight into Charge Separation in WO3/BiVO4 Heterojunction for Solar Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 19780–19790. [Google Scholar] [CrossRef]
- Su, J.; Zhang, T.; Wang, L. Engineered WO3 Nanorods for Conformal Growth of WO3/BiVO4 Core–Shell Heterojunction towards Efficient Photoelectrochemical Water Oxidation. J. Mater. Sci. Mater. Electron. 2017, 28, 4481–4491. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, J.; Ke, L.; Sun, X.W.; Demir, H.V. Morphology-Tailored Synthesis of Tungsten Trioxide (Hydrate) Thin Films and Their Photocatalytic Properties. ACS Appl. Mater. Interfaces 2011, 3, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Samuel, E.; Joshi, B.; Kim, M.-W.; Swihart, M.T.; Yoon, S.S. Morphology Engineering of Photoelectrodes for Efficient Photoelectrochemical Water Splitting. Nano Energy 2020, 72, 104648. [Google Scholar] [CrossRef]
- Liu, Y.; Wygant, B.R.; Mabayoje, O.; Lin, J.; Kawashima, K.; Kim, J.-H.; Li, W.; Li, J.; Mullins, C.B. Interface Engineering and Its Effect on WO3-Based Photoanode and Tandem Cell. ACS Appl. Mater. Interfaces 2018, 10, 12639–12650. [Google Scholar] [CrossRef]
- Roselló-Márquez, G.; Fernández-Domene, R.M.; Sánchez-Tovar, R.; García-Antón, J. Influence of Annealing Conditions on the Photoelectrocatalytic Performance of WO3 Nanostructures. Sep. Purif. Technol. 2020, 238, 116417. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Yoo, I.-H.; Cho, I.-S.; Seo, H. Effect of Oxygen Vacancies on the Band Edge Properties of WO3 Producing Enhanced Photocurrents. Electrochim. Acta 2019, 296, 517–527. [Google Scholar] [CrossRef]
- Ma, S.; Zeng, L.; Tao, L.; Tang, C.Y.; Yuan, H.; Long, H.; Cheng, P.K.; Chai, Y.; Chen, C.; Fung, K.H.; et al. Enhanced Photocatalytic Activity of WS2 Film by Laser Drilling to Produce Porous WS2/WO3 Heterostructure. Sci. Rep. 2017, 7, 3125. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.-D.; Ren, F.; Wu, H.-Y.; Qin, W.-J.; Jiang, C.-Z. Formation of Tungsten Oxide Nanowires by Ion Irradiation and Vacuum Annealing. Nanotechnology 2018, 29, 155301. [Google Scholar] [CrossRef]
- Zheng, X.D. The Influence of Ion Implantation-Induced Oxygen Vacancy on Electrical Conductivity of WO3 Thin Films. Vacuum 2019, 165, 46–50. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Purans, J.; Popov, A.I.; Bocharov, D.; Chekhovska, A.; Jia, R. Ab Initio Computations of O and AO as Well as ReO2, WO2 and BO2-Terminated ReO3, WO3, BaTiO3, SrTiO3 and BaZrO3 (001) Surfaces. Symmetry 2022, 14, 1050. [Google Scholar] [CrossRef]
- Olejníček, J.; Hrubantová, A.; Volfová, L.; Dvořáková, M.; Kohout, M.; Tvarog, D.; Gedeon, O.; Wulff, H.; Hippler, R.; Hubička, Z. WO3 and WO3-x Thin Films Prepared by DC Hollow Cathode Discharge. Vacuum 2022, 195, 110679. [Google Scholar] [CrossRef]
- Andrade, T.S.; Sá, B.A.C.; Oliveira, A.T.; Bruziquesi, C.G.O.; Salomão, P.E.A.; Rodriguez, M.; Nogueira, F.G.E.; Alves de Oliveira, L.C.; Pereira, M.C. W:BiVO4-WO3-V2O5 Heterostructures Increase Light Absorption and Charge Transport in Photoanodes for Water Splitting. J. Environ. Chem. Eng. 2022, 10, 107278. [Google Scholar] [CrossRef]
- Prasad, U.; Young, J.L.; Johnson, J.C.; McGott, D.L.; Gu, H.; Garfunkel, E.; Kannan, A.M. Enhancing Interfacial Charge Transfer in a WO3/BiVO4 Photoanode Heterojunction through Gallium and Tungsten Co-Doping and a Sulfur Modified Bi2O3 Interfacial Layer. J. Mater. Chem. A 2021, 9, 16137–16149. [Google Scholar] [CrossRef]
- Choi, M.-J.; Kim, T.L.; Choi, K.S.; Sohn, W.; Lee, T.H.; Lee, S.A.; Park, H.; Jeong, S.Y.; Yang, J.W.; Lee, S.; et al. Controlled Band Offsets in Ultrathin Hematite for Enhancing the Photoelectrochemical Water Splitting Performance of Heterostructured Photoanodes. ACS Appl. Mater. Interfaces 2022, 14, 7788–7795. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, Z.; Tayebi, M.; Kolaei, M.; Lee, B.-K. Efficient and Stable Core-Shell α–Fe2O3/WS2/WOx Photoanode for Oxygen Evolution Reaction to Enhance Photoelectrochemical Water Splitting. Appl. Catal. B Environ. 2022, 313, 121447. [Google Scholar] [CrossRef]
- Seo, D.-B.; Dongquoc, V.; Jayarathna, R.A.; Lee, S.; Lee, J.-H.; Kim, E.-T. Rational Heterojunction Design of 1D WO3 Nanorods Decorated with Vertical 2D MoS2 Nanosheets for Enhanced Photoelectrochemical Performance. J. Alloys Compd. 2022, 911, 165090. [Google Scholar] [CrossRef]
- Han, H.S.; Park, W.; Hwang, S.W.; Kim, H.; Sim, Y.; Surendran, S.; Sim, U.; Cho, I.S. (020)-Textured Tungsten Trioxide Nanostructure with Enhanced Photoelectrochemical Activity. J. Catal. 2020, 389, 328–336. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, J.; Zu, G.; Che, H.; Lai, C.; Li, H.; Murugadoss, V.; Yan, C.; Fan, J.; Guo, Z. Sandwich Structured WO3 Nanoplatelets for Highly Efficient Photoelectrochemical Water Splitting. J. Mater. Chem. A 2019, 7, 26077–26088. [Google Scholar] [CrossRef]
- Le, H.V.; Pham, P.T.; Le, L.T.; Nguyen, A.D.; Tran, N.Q.; Tran, P.D. Fabrication of Tungsten Oxide Photoanode by Doctor Blade Technique and Investigation on Its Photocatalytic Operation Mechanism. Int. J. Hydrogen Energy 2021, 46, 22852–22863. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, H.; Cui, K.; Zhang, L.; Ge, S.; Yu, J. Reversible Electron Storage in Tandem Photoelectrochemical Cell for Light Driven Unassisted Overall Water Splitting. Appl. Catal. B Environ. 2020, 275, 119094. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Qiu, B. Enhanced Surface Charge Separation Induced by Ag Nanoparticles on WO3 Photoanode for Photoelectrochemical Water Splitting. Chem. Lett. 2020, 49, 741–744. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Balogun, M.-S.; Tong, Y.; Huang, Y. Oxygen Vacancy–Based Metal Oxides Photoanodes in Photoelectrochemical Water Splitting. Mater. Today Sustain. 2022, 18, 100118. [Google Scholar] [CrossRef]
- Yang, B.; Miao, P.; Cui, J. Characteristics of Amorphous WO3 Thin Films as Anode Materials for Lithium-Ion Batteries. J. Mater. Sci. Mater. Electron. 2020, 31, 11071–11076. [Google Scholar] [CrossRef]
- Kaikanov, M.; Baigarin, K.; Tikhonov, A.; Urazbayev, A.; Kwan, J.W.; Henestroza, E.; Remnev, G.; Shubin, B.; Stepanov, A.; Shamanin, V.; et al. An Accelerator Facility for WDM, HEDP, and HIF Investigations in Nazarbayev University. J. Phys. Conf. Ser. 2016, 717, 12099. [Google Scholar] [CrossRef] [Green Version]
- Bondarenko, A.S.; Ragoisha, G.A.; Pomerantsev, A.L. Progress in Chemometrics Research; Pomerantsev, A.L., Ed.; Nova Science Publishers: New York, NY, USA, 2005; pp. 89–102. [Google Scholar]
- Popov, A.I.; Lushchik, A.; Shablonin, E.; Vasil’chenko, E.; Kotomin, E.A.; Moskina, A.M.; Kuzovkov, V.N. Comparison of the F-Type Center Thermal Annealing in Heavy-Ion and Neutron Irradiated Al2O3 Single Crystals. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 433, 93–97. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Valerini, D.; Hernández, S.; Di Benedetto, F.; Russo, N.; Saracco, G.; Rizzo, A. Sputtered WO3 Films for Water Splitting Applications. Mater. Sci. Semicond. Process. 2016, 42, 150–154. [Google Scholar] [CrossRef]
- Wang, X.; Wan, W.; Shen, S.; Wu, H.; Zhong, H.; Jiang, C.; Ren, F. Application of Ion Beam Technology in (Photo)Electrocatalytic Materials for Renewable Energy. Appl. Phys. Rev. 2020, 7, 41303. [Google Scholar] [CrossRef]
- Stepanov, A.D.; Barnard, J.J.; Friedman, A.; Gilson, E.P.; Grote, D.P.; Ji, Q.; Kaganovich, I.D.; Persaud, A.; Seidl, P.A.; Schenkel, T. Optimizing Beam Transport in Rapidly Compressing Beams on the Neutralized Drift Compression Experiment-II. Matter Radiat. Extrem. 2018, 3, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Zhang, G.; Wang, G.; Zhu, G.; Zhou, W.; Wang, J.; Sun, B. Surface Morphology, Microstructure and Properties of as-Cast AZ31 Magnesium Alloy Irradiated by High Intensity Pulsed Ion Beams. Appl. Surf. Sci. 2014, 311, 567–573. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abduvalov, A.; Kaikanov, M.; Atabaev, T.S.; Tikhonov, A. Improving Photoelectrochemical Activity of Magnetron-Sputtered Double-Layer Tungsten Trioxide Photoanodes by Irradiation with Intense Pulsed Ion Beams. Nanomaterials 2022, 12, 2639. https://doi.org/10.3390/nano12152639
Abduvalov A, Kaikanov M, Atabaev TS, Tikhonov A. Improving Photoelectrochemical Activity of Magnetron-Sputtered Double-Layer Tungsten Trioxide Photoanodes by Irradiation with Intense Pulsed Ion Beams. Nanomaterials. 2022; 12(15):2639. https://doi.org/10.3390/nano12152639
Chicago/Turabian StyleAbduvalov, Alshyn, Marat Kaikanov, Timur Sh. Atabaev, and Alexander Tikhonov. 2022. "Improving Photoelectrochemical Activity of Magnetron-Sputtered Double-Layer Tungsten Trioxide Photoanodes by Irradiation with Intense Pulsed Ion Beams" Nanomaterials 12, no. 15: 2639. https://doi.org/10.3390/nano12152639
APA StyleAbduvalov, A., Kaikanov, M., Atabaev, T. S., & Tikhonov, A. (2022). Improving Photoelectrochemical Activity of Magnetron-Sputtered Double-Layer Tungsten Trioxide Photoanodes by Irradiation with Intense Pulsed Ion Beams. Nanomaterials, 12(15), 2639. https://doi.org/10.3390/nano12152639






