A Mini Review of Antibacterial Properties of Al2O3 Nanoparticles
Abstract
1. Introduction
2. Literature Review
2.1. Process of Searching Articles
2.2. Ways of Synthesis and Possible Methods for Improving AlOxNP Properties
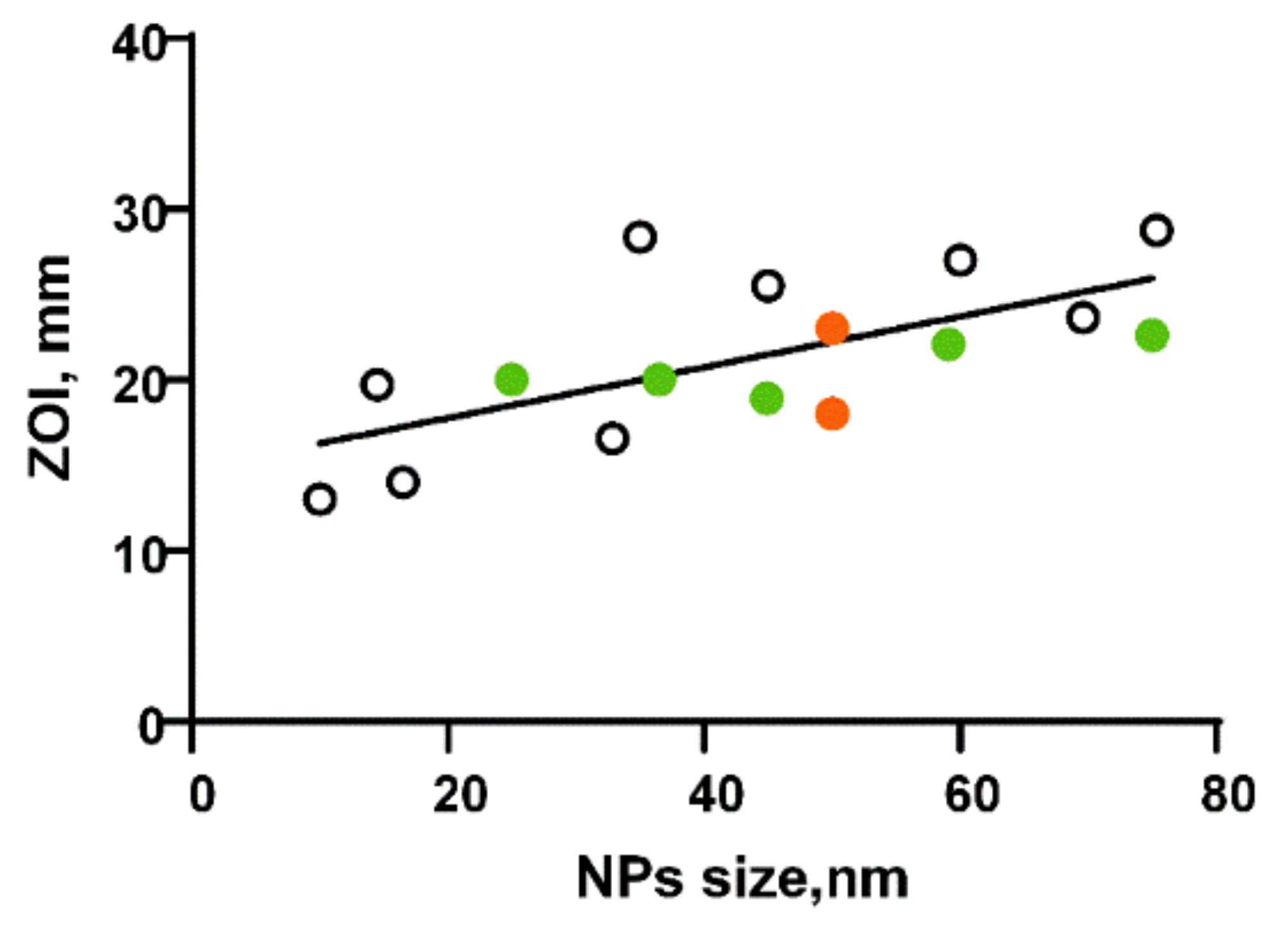
2.3. Peculiarities of Action of Aluminum Oxide Nanoparticles against Bacterial Cells
| № | Synthesis Method | Composition | Size, nm and Method | Shape | Concentration | Medium, Conditions | Type of Organism | Bio. Effect | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Microwave assisted synthesis using Prunus × yedoensis leaf extract (PYLE) for recovery | Al2O3, different pH | 50–100 (FE-SEM) | Sph, hexag | 50, 75, 100 μg/mL | NA, 37 °C, 24 h | S. aureus, E. coli | BS | [19] |
| 2 | Commercially available product by Sigma Aldrich (St. Louis, MO, USA; CAS Number 1344-28-1). | Al2O3 | <50 (Supplier’s data) 39 (SEM) | Sph | 3, 6, 12, 24, 48, 96, 192 mg/L | - | Scenedesmus sp., Chlorella sp. | AS | [57] |
| 3 | Chemical precipitation using algae extract L. majucula | Al2O3 | 36.42 (SEM) | Sph | - | 30 ± 2 °C, 24–48 h for bacteria; 37 ± 2 °C, 72–96 h for fungi | S. aureus, B. subtilis, K. pneumoniae, S. paratyphi, C. albicans u A. flavus | BS, FS | [40] |
| 4 | Microwave heating using mushroom extract Colletotrichum sp. | Al2O3 | 39 ± 35 (NTA) | Sph | MIC: 400 ± 1.08 mg/mL for S. typhi; 300 ± 2.36 mg/mL for C. violaceum; 1000 ± 1.1 mg/mL for L. monocytogenes; 250 ± 0.65 mg/mL for A. flavus; 150 ± 2.77 mg/mL for F. oxysporum | MHA, BHI, 37 °C, 24 h | S. typhi, F. oxysporum, A. flavus, C. violaceum, L.monocytogenes | BS | [34] |
| 5 | Commercially available product by Aldrich (St. Louis, MO, USA; CAS Number 1344-28-1) | Al2O3 | ~180 (SEM, DLS) | - | 10–1000 μg/L | LB, 30 °C | E. coli | BS | [49] |
| 6 | Commercially available product by Aldrich (St. Louis, MO, USA) | Chitosan coated Al2O3- NPs films | <50 (SEM) | Sph | 0.05, 0.1 g/mL | MHB, 37 °C, 24 h | S. aureus, P. aeruginosa, S. epidermidis, | BS | [45] |
| 7 | Microemulsion method | Al2O3 | 30–60 (SEM) | - | MIC: 10 μg/mL | - | S. typhi, V. cholerae, K. pneumoniae | BS | [32] |
| 8 | Commercially available product by (Sigma-Aldrich) | Al2O3 | <50 (Supplier’s data) | Sph | 0.5 mg/L | 26 °C, 16 h | P. putida | - | [51] |
| 9 | Commercially available product by Aldrich (MERCK, Darmstadt, Germany) | Al2O3 | <100 (TEM) | Rod, irregular, scaly | 100 µg/mL | TSB, 37 °C, 24 h | E. coli, S. aureus, P. aeruginosa, C. albicans | BS | [54] |
| 10 | Commercially available product by Sigma Aldrich (St. Louis, MO, USA; CAS Number 1344-28-1) | Al2O3 | 9–182 (HR-TEM, SEM) | Sph | 250, 500, 1000, 2000 μg/mL MIC: 1700–3400 μg/mL | LB, 37 °C, 16 h | multidrug-resistant strains of S. aureus (MRSA, MSSA, MRCoNS) | BS | [52] |
| 11 | Co-precipitation | Al2O3 | 35 (SEM) | Irregular sph. | 10, 20, 30, 40, 50 mg/mL MIC: 4 mg/mL for E. coli; 8 mg/mL for P. vulgaris; 6 mg/mL for S. mutans, 4 mg/mL for S. aureus | NA, SY, BHI, 37 °C, 24 h | S. aureus, S. mutans, E. coli, P. vulgaris | BS | [58] |
| 12 | Commercially available product (HiMedia Laboratories, India) | Al2O3 | 13.5 ± 2.3 (TEM) | Sph | 0.25, 0.5, 1 mg/L | NA, NB, 24 h | P. aeruginosa, B. altitudinis | BS | [59] |
| 13 | Commercially available product by Shenzhen Crystal Material Chemical Co., Ltd. (Shenzhen, China) | Al2O3 | 40 (SEM) | - | 0.05–2.0 g/L | 30 °C, 24 h | B. subtilis | BC | [60] |
| 14 | Solution combustion synthesis | α-Al2O3 | 5–30 (HR-TEM, FE-SEM) | flakes-like | 5, 500 mg/50 mL; 1000 mg/150 mL | 37 °C, 36 h | K. aerogenes, E. coli, P. desmolyticum, S. aureus | BS | [36] |
| 15 | Microwave assisted synthesis using leaf extracts Cymbopogon citratus | Al2O3 | 9–180 (HR-TEM); 50 (AFM) 34,5 (DLS) | Sph | 1600–3200 µg/mL | MHA, 37 °C, 24 h | multi-drug resistant P. aeruginosa | BC | [38] |
| 16 | Commercially available product: γ- Al2O3 Sigma-Aldrich (St. Louis, MO, USA); α-Al2O3 Sisco Research Laboratories Pvt. Ltd. | α-Al2O3; γ- Al2O3 | 20–30 (α- Al2O3), 13 (γ- Al2O3) (Supplier’s data); 280 ± 13 (α- Al2O3), 256 ± 19 (γ- Al2O3) (DLS) | - | 0.05, 0.5, 1, 5, µg/mL | 25 °C, 30 min | B. licheniformis | BS | [56] |
| 17 | Commercially available product by: γ-Al2O3 Sigma-Aldrich (St. Louis, MO, USA) | Al2O3 | <50 (Supplier’s data); 51 ± 8, 87 ± 11, 20 ± 13 (NTA) | - | 1, 5, 10 g/L | LB, 30 °C, 48 h for B. cereus; 37 °C for P. stutzeri | B. cereus, P. stutzeri | - | [48] |
| 18 | “Green method” using leaf extract Cymbopogon citratus | Al2O3 | 34.5 (XRD); 58.5 (HR-TEM) | Sph | 0–1500 µg/mL MIC: 250–500 µg/mL for Candida spp; | BHI, 28 °C, 48 h | C. albicans, C. parapsilosis, C. tropicalis, C. glabrata; fluconazole resistant C. albicans, C. dubliniensis; fluconazole susceptible C. albicans, C. dubliniensis | FS | [42] |
| 19 | Commercially available product by Aldrich (St. Louis, MO, USA; CAS Number 1344-28-1) | Al2O3 | 10–70 (TEM); 78 ± 9 (DLS) | Sph | 50, 500, 1000 µg/L | - | Scenedesmus sp., Chlorella sp. | BS | [61] |
| 20 | Commercially available product by Dr. Karl Martin of NovaCentrix, Austin, TX, USA (Product code: M1056, M1049-D; purity: >90%) | Al2O3 | 30 & 40 (TEM) | Sph | 0.02, 0.04, 0.075, 0.15, 0.30, 0.60, 1.25 and 2.5 mg/plate | NB, 37 °C, 48 h | S. typhimurium | - | [62] |
| 21 | Gas-phase condensation during laser evaporation of a solid target | Al2O3 | <10 (TEM) | - | 0–1 μg/mL | LB, 37 °C, 24–120 h | multi-drug resistant A. baumanii | BS | [53] |
| 22 | Commercially available product by Sigma-Aldrich (St. Louis, MO, USA; CAS Number 1344-28-1) | Al2O3 | <50 (Supplier’s data); 9–179 (TEM) | Sph | MIC: 1600–3200 μg /mL; MBC: 3200–6400 μg /mL | MHA, 37 °C, 24 h | multidrug-resistant clinical isolates of E. coli | BS, BC | [50] |
| 23 | Sol–gel synthesis | Chitosan/SiO2 nanocomposite with Al2O3 | - | Sph | - | 40 °C, 5 h | S. aureus, P. aeruginosa, C. albicans, A. niger | BS | [44] |
| 24 | Chemical precipitation using Urtica dioica as a reducing agent | Al2O3 | 10–13 (TEM) | Sph | 25, 50, 75 mg/mL | PDM, 25 ± 2 °C, 48 h | A. niger, M. piriformis | FS | [41] |
| 25 | Commercially available (Neutrino Co.) | Al2O3 coated by chitosan | 80 (Supplier’s data) | - | 0.025 mg/mL | NB, 37 °C, 24 h | S. aureus ATCC 6538 | BS | [46] |
| 26 | Chemical precipitation | γ-irradiated polyaniline (PANI)/ Al2O3 NPs composite | 17–19 (XDR) | - | 17 mg/mL | MHA, 37 °C, 24 h | E. coli, S. aureus | BS | [37] |
| 27 | Chemical synthesis | PANI–Al2O3 NPs composite | - | - | 5, 10 mg/mL | NA, 37 °C, 24 h | B. subtilis, E. coli | BS | [63] |
| 28 | Chemical synthesis, using aluminum waste | Al2O3 | 15–50 (XRD) | - | - | MHA, NB, 35 °C, 24–48 h | E. coli, S. typhimurium, P. aeruginosa, A. aquatilis, S. aureus, S. pneumonia, A. niger, A. flavus Penicillium sp. | BS | [64] |
| 29 | Commercially available product by: Sigma-Aldrich, USA (TiO2), XIYA REAGENT(Al2O3) | PLA/Al2O3 PLA/TiO2 -Al2O3 | 21 (TiO2), 30 (Al2O3) (Supplier’s data) | Sph | - | MHA, 37 °C, 24 h | P. aeruginosa, E. coli | BS | [47] |
| 30 | Laser ablation | Al2O3 | 10–60 (SEM) | Sph | 25, 50, 75, 100 µg/mL | MHA, 37 °C, 24 h | E. coli, P. aeruginosa, S. aureus | BS | [28] |
| 31 | Commercially available product by Zhejiang Hongsheng Material Technology Co., China | Al2O3 | 60 (Supplier’s data) | Sph | 20 mg/L | TSA, 30 °C, 24 h | B. subtilis, E. coli, P. fluorescens | BS | [65] |
| 32 | Ball milling method | Al2O3 | 100–200 (SEM) 50–60 (XRD) | Sph | MIC: 100µg | NA, 37 °C, 24 h | B. cereus, B. subtilis, K. pneumoniea, V. cholerae | BS | [30] |
| 33 | Chemical precipitation | γ-Al2O3 folic acidacid (FA) | 23,5 (Al2O3) & 33 (FA-Al2O3) (TEM) | Rod | - | - | P. aeruginosa, B. subtilis | BS | [39] |
| 34 | - | Al2O3–Ag composite | 100–200 (TEM) | Sph | 1, 10, 30, and 50 wt.%. | LB for E. coli, BHI for S. epidermidis, 37 °C | E. coli, S. epidermidis | BS | [43] |
| 35 | Commercially available product (Degussa) | Al2O3 | 11 (TEM) | Sph | 50, 100, 500 mg/L | TSM, 29 °C, for C. metallidurans; LB, 37 °C for E. coli | C. metallidurans, E. coli | BC | [66] |
| 36 | Laser ablation | Al2O3 /borosiloxane composite | 45 (DLS) | Sph | 0.001–0.1 w.% | LB, 37 °C, 24 h | E. coli | BS | [29] |
| 37 | Commercially available product by Sigma–Aldrich (St. Louis, MO, USA) | Al2O3 | 50 (Supplier’s data, TEM) | Rod | 1000 mg/L | YEPD, 30 °C, 10 h | S. cerevisiae | FS | [67] |
2.3.1. Electrostatic Interaction between AlOxNPs and Bacterial Cells
2.3.2. ROS-Release
2.4. Genotoxic Action of AlOxNPs
2.5. AlOxNP Action on Microalgae of Water Reservoirs
2.6. Antimycotic Effect of AlOxNPs
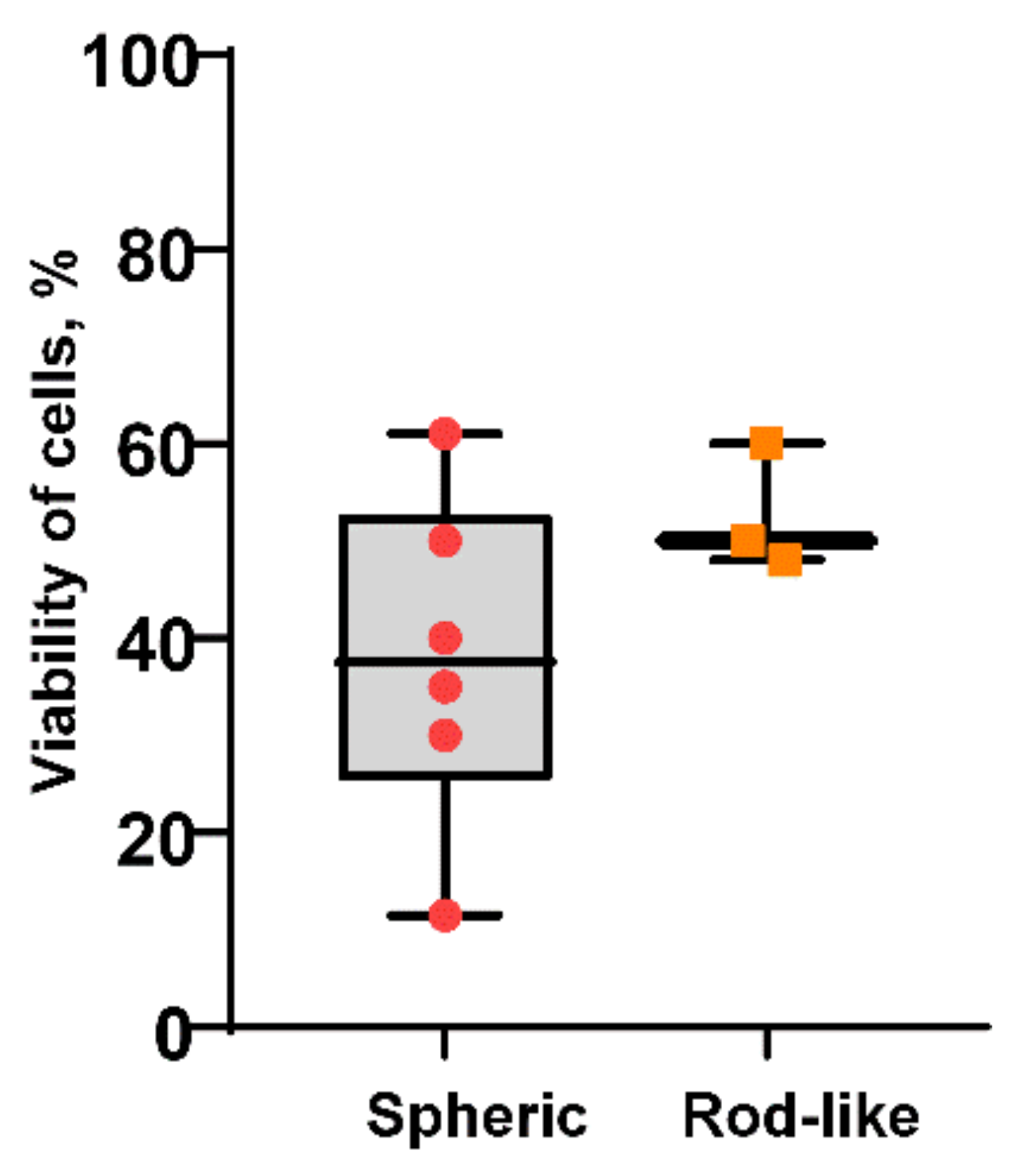
2.7. Cytotoxicity of AlOxNPs
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nano Metal Oxide Market Size, Trends & Analysis, by Product, by Application (Energy and Environment, Paints and Coatings, Medical and Personal Care, Electronics and Optics, Others), and by Distribution Channel (Offline Stores, Online Stores), Forecasts to 2027. Available online: https://www.reportsanddata.com/report-detail/nano-metal-oxide-market (accessed on 5 April 2022).
- Singh, S.; Gill, A.A.S.; Nlooto, M.; Karpoormath, R. Prostate cancer biomarkers detection using nanoparticles based electrochemical biosensors. Biosens. Bioelectron. 2019, 137, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, V.D.; Ramachandran, M.; Marotta, F.; Banerjee, A.; Sun, X.F.; Pathak, S. Comparative study on anti-proliferative potentials of zinc oxide and aluminium oxide nanoparticles in colon cancer cells. Acta Biomed. 2019, 90, 241–247. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic Nanoparticles and Composite Films for Antimicrobial Therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Akakuru, O.U.; Zheng, J.; Wu, A. Applications of Iron Oxide-Based Magnetic Nanoparticles in the Diagnosis and Treatment of Bacterial Infections. Front. Bioeng. Biotechnol. 2019, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Sundaramoorthy, N.S.; Nagarajan, S. Can Nanoparticles Help in the Battle against Drug-Resistant Bacterial Infections in “Post-Antibiotic Era”? In Antimicrobial Resistance; Springer: Berlin, Germany, 2022; pp. 175–213. [Google Scholar]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. Do Iron Oxide Nanoparticles Have Significant Antibacterial Properties? Antibiotics 2021, 10, 884. [Google Scholar] [CrossRef]
- Akhtar, S.; Shahzad, K.; Mushtaq, S.; Ali, I.; Rafe, M.H.; Fazal-ul-Karim, S.M. Antibacterial and antiviral potential of colloidal Titanium dioxide (TiO2) nanoparticles suitable for biological applications. Mater. Res. Express 2019, 6, 105409. [Google Scholar] [CrossRef]
- Shah, A.; Haq, S.; Rehman, W.; Waseem, M.; Shoukat, S.; Rehman, M.-U. Photocatalytic and antibacterial activities of paeonia emodi mediated silver oxide nanoparticles. Mater. Res. Express 2019, 6, 045045. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Abu-Elghait, M.; Ahmed, N.E.; Salem, S.S. Eco-friendly Mycogenic Synthesis of ZnO and CuO Nanoparticles for In Vitro Antibacterial, Antibiofilm, and Antifungal Applications. Biol. Trace Elem. Res. 2021, 199, 2788–2799. [Google Scholar] [CrossRef]
- Radziun, E.; Dudkiewicz Wilczyńska, J.; Książek, I.; Nowak, K.; Anuszewska, E.L.; Kunicki, A.; Olszyna, A.; Ząbkowski, T. Assessment of the cytotoxicity of aluminium oxide nanoparticles on selected mammalian cells. Toxicol. In Vitro 2011, 25, 1694–1700. [Google Scholar] [CrossRef]
- Sliwinska, A.; Kwiatkowski, D.; Czarny, P.; Milczarek, J.; Toma, M.; Korycinska, A.; Szemraj, J.; Sliwinski, T. Genotoxicity and cytotoxicity of ZnO and Al2O3 nanoparticles. Toxicol. Mech. Methods 2015, 25, 176–183. [Google Scholar] [CrossRef]
- Trivedi, D.; Trivedi, M.K.; Branton, A.; Nayak, G.; Jana, S. Characterization of the biofield energy treated aluminium using PSA, PXRD, and TGA/DTG analytical techniques. Lett. Appl. NanoBioScience 2019, 8, 643–648. [Google Scholar]
- Paglia, G. Determination of the Structure of y-alumina Using Empirical and First Principle Calculations Combined with Supporting Experiments. Ph.D. Thesis, Curtin University, Perth, Australia, 2004. [Google Scholar]
- Jiao, W.Q.; Yue, M.B.; Wang, Y.M.; He, M.-Y. Synthesis of morphology-controlled mesoporous transition aluminas derived from the decomposition of alumina hydrates. Microporous Mesoporous Mater. 2012, 147, 167–177. [Google Scholar] [CrossRef]
- Manikandan, V.; Jayanthi, P.; Priyadharsan, A.; Vijayaprathap, E.; Anbarasan, P.M.; Velmurugan, P. Green synthesis of pH-responsive Al2O3 nanoparticles: Application to rapid removal of nitrate ions with enhanced antibacterial activity. J. Photochem. Photobiol. A Chem. 2019, 371, 205–215. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Issaabadi, Z.; Sajadi, S.M. Green synthesis of Cu/Al2O3 nanoparticles as efficient and recyclable catalyst for reduction of 2,4-dinitrophenylhydrazine, Methylene blue and Congo red. Compos. Part B Eng. 2019, 166, 112–119. [Google Scholar] [CrossRef]
- Ezati, F.; Sepehr, E.; Ahmadi, F. The efficiency of nano-TiO2 and γ-Al2O3 in copper removal from aqueous solution by characterization and adsorption study. Sci. Rep. 2021, 11, 18831. [Google Scholar] [CrossRef]
- Saliani, M.; Honarbakhsh, A.; Zhiani, R.; Movahedifar, S.M.; Motavalizadehkakhky, A. Effects of GO/Al2O3 and Al2O3 Nanoparticles on Concrete Durability against High Temperature, Freeze-Thaw Cycles, and Acidic Environments. Adv. Civ. Eng. 2021, 2021, 4555802. [Google Scholar] [CrossRef]
- Kalneus, V.; Nemushchenko, D.; Larichkin, V.; Briutov, A. Research of Physical and Mechanical Properties of Fly Ash Ceramics with SiO2 and Al2O3 Nanoparticles as Functional Addition. In Proceedings of the Key Engineering Materials; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2021; pp. 528–535. [Google Scholar]
- Wu, F.; Ge, J.; Qin, Y.; Li, Z.; Li, Q. Research progress in applying nanomaterials in the field of functional textiles. Charact. Appl. Nanomater. 2022, 5, 52–58. [Google Scholar] [CrossRef]
- Devendiran, S.; Priya, A.K.; Sastikumar, D. Design of aluminium oxide (Al2O3) fiber optic gas sensor based on detection of refracted light in evanescent mode from the side-polished modified clad region. Sens. Actuators B Chem. 2022, 361, 131738. [Google Scholar] [CrossRef]
- Hassanpour, P.; Panahi, Y.; Ebrahimi-Kalan, A.; Akbarzadeh, A.; Davaran, S.; Nasibova, A.N.; Khalilov, R.; Kavetskyy, T. Biomedical applications of aluminium oxide nanoparticles. Micro Nano Lett. 2018, 13, 1227–1231. [Google Scholar] [CrossRef]
- Zahra, A.L.T.; Tammemi, Z. Nanoparticles of Alumina (Al2O3): An Overview and Their Applications in Medical Surgery. Nanomedicine 2021, 4, 1. [Google Scholar]
- Jwad, K.H.; Saleh, T.H.; Abd-Alhamza, B. Preparation of Aluminum Oxide Nanoparticles by Laser Ablation and a Study of Their Applications as Antibacterial and Wounds Healing Agent. Nano Biomed. Eng. 2019, 11, 313–319. [Google Scholar] [CrossRef]
- Astashev, M.; Sarimov, R.; Serov, D.; Matveeva, T.; Simakin, A.; Ignatenko, D.; Burmistrov, D.; Smirnova, V.; Kurilov, A.; Mashchenko, V. Antibacterial behavior of organosilicon composite with nano aluminum oxide without influencing animal cells. React. Funct. Polym. 2022, 170, 105143. [Google Scholar] [CrossRef]
- Geoprincy, G.; Gandhi, N.; Renganathan, S. Novel antibacterial effects of alumina nanoparticles on Bacillus cereus and Bacillus subtilis in comparison with antibiotics. Int. J. Pharm. Pharm. Sci. 2012, 4, 544–548. [Google Scholar]
- Mohamad, S.N.S.; Mahmed, N.; Halin, D.S.C.; Razak, K.A.; Norizan, M.N.; Mohamad, I.S. Synthesis of alumina nanoparticles by sol-gel method and their applications in the removal of copper ions (Cu2+) from the solution. In Proceedings of the Electronic Packaging Interconnect Technology Symposium 2019, Penang, Malaysia, 24–25 November 2019; p. 012034. [Google Scholar]
- Francis, A.P.; Babu, G.J.; Lavanya, M.; Vidhya, K.S.; Devasena, T. Toxicity studies of aluminium oxide nanoparticles in cell lines. Int. J. Nanotechnol. Appl. 2011, 5, 99–107. [Google Scholar]
- Sutradhar, P.; Debnath, N.; Saha, M. Microwave-assisted rapid synthesis of alumina nanoparticles using tea, coffee and triphala extracts. Adv. Manuf. 2013, 1, 357–361. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Pandit, R.; Gade, A.; Derita, M.; Zachino, S.; Rai, M. Colletotrichum sp.-mediated synthesis of sulphur and aluminium oxide nanoparticles and its in vitro activity against selected food-borne pathogens. LWT-Food Sci. Technol. 2017, 81, 188–194. [Google Scholar] [CrossRef]
- Chu, T.P.M.; Nguyen, N.T.; Vu, T.L.; Dao, T.H.; Dinh, L.C.; Nguyen, H.L.; Hoang, T.H.; Le, T.S.; Pham, T.D. Synthesis, Characterization, and Modification of Alumina Nanoparticles for Cationic Dye Removal. Materials 2019, 12, 450. [Google Scholar] [CrossRef]
- Prashanth, P.; Raveendra, R.; Hari Krishna, R.; Ananda, S.; Bhagya, N.; Nagabhushana, B.; Lingaraju, K.; Raja Naika, H. Synthesis, characterizations, antibacterial and photoluminescence studies of solution combustion-derived α-Al2O3 nanoparticles. J. Asian Ceram. Soc. 2015, 3, 345–351. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Rajakarthihan, S. Antimicrobial study on gamma-irradiated polyaniline–aluminum oxide (PANI–Al2O3) nanoparticles. Int. Nano Lett. 2020, 10, 97–110. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Alzohairy, M.A.; Jalal, M.; Ali, S.G.; Pal, R.; Musarrat, J. Green synthesis of Al2O3 nanoparticles and their bactericidal potential against clinical isolates of multi-drug resistant Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2015, 31, 153–164. [Google Scholar] [CrossRef]
- Arunarajeswari, P.; Mathavan, T.; Jeyaseelan, S.C.; Divya, A.; Benial, A.M.F. Anionic acid functionalized mesoporous γ-Al2O3 nanorods: Preparation, physicochemical and biological characterizations. Chem. Data Collect. 2022, 37, 100819. [Google Scholar] [CrossRef]
- Manogar, P.; Esther Morvinyabesh, J.; Ramesh, P.; Dayana Jeyaleela, G.; Amalan, V.; Ajarem, J.S.; Allam, A.A.; Seong Khim, J.; Vijayakumar, N. Biosynthesis and antimicrobial activity of aluminium oxide nanoparticles using Lyngbya majuscula extract. Mater. Lett. 2022, 311, 131569. [Google Scholar] [CrossRef]
- Devi, H.S.; Boda, M.A.; Rubab, S.; Parveen, S.; Wani, A.H.; Shah, M.A. Chapter Thirteen–Biosynthesis and antifungal activities of CuO and Al2O3 nanoparticles. In Comprehensive Analytical Chemistry; Verma, S.K., Das, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 94, pp. 533–546. [Google Scholar]
- Jalal, M.; Ansari, M.A.; Shukla, A.K.; Ali, S.G.; Khan, H.M.; Pal, R.; Alam, J.; Cameotra, S.S. Green synthesis and antifungal activity of Al2O3 NPs against fluconazole-resistant Candida spp isolated from a tertiary care hospital. RSC Adv. 2016, 6, 107577–107590. [Google Scholar] [CrossRef]
- Bala, T.; Armstrong, G.; Laffir, F.; Thornton, R. Titania–silver and alumina–silver composite nanoparticles: Novel, versatile synthesis, reaction mechanism and potential antimicrobial application. J. Colloid Interface Sci. 2011, 356, 395–403. [Google Scholar] [CrossRef]
- El Nahrawy, A.M.; Abou Hammad, A.B.; Abdel-Aziz, M.S.; Wassel, A.R. Spectroscopic and Antimicrobial Activity of Hybrid Chitosan/Silica Membranes doped with Al2O3 Nanoparticles. Silicon 2019, 11, 1677–1685. [Google Scholar] [CrossRef]
- Abdel-Naby, A.S.; Nabil, S.; Aldulaijan, S.; Ababutain, I.M.; Alghamdi, A.I.; Almubayedh, S.; Khalil, K.D. Synthesis, Characterization of Chitosan-Aluminum Oxide Nanocomposite for Green Synthesis of Annulated Imidazopyrazol Thione Derivatives. Polymers 2021, 13, 1160. [Google Scholar] [CrossRef]
- Khajeh Mehrizi, M.; Mashroteh, H.; Nabizadeh Moghadam Noghabi, N. Effect of Chitosan, Aluminum Oxide and Silver Nanoparticles on Antibacterial, Deodorizing and Moisture Absorption Properties of Nonwoven Polyester Fabrics for Use in Medical Textiles. Med. Lab. J. 2016, 10, 46–52. [Google Scholar] [CrossRef][Green Version]
- Yakdoumi, F.Z.; Hadj-Hamou, A.S. Effectiveness assessment of TiO2-Al2O3 nano-mixture as a filler material for improvement of packaging performance of PLA nanocomposite films. J. Polym. Eng. 2020, 40, 848–858. [Google Scholar] [CrossRef]
- Fajardo, C.; Saccà, M.L.; Costa, G.; Nande, M.; Martin, M. Impact of Ag and Al2O3 nanoparticles on soil organisms: In Vitro and soil experiments. Sci. Total Environ. 2014, 473–474, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, I.M.; Chowdhury, B.; Chandrasekaran, N.; Mukherjee, A. Antimicrobial sensitivity of Escherichia coli to alumina nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Khan, H.; Khan, A.; Cameotra, S.S.; Saquib, Q.; Musarrat, J. Interaction of Al2O3 nanoparticles with E scherichia coli and their cell envelope biomolecules. J. Appl. Microbiol. 2014, 116, 772–783. [Google Scholar] [CrossRef]
- Doskocz, N.; Affek, K.; Załęska-Radziwiłł, M. Effects of aluminium oxide nanoparticles on bacterial growth. E3S Web Conf. 2017, 17, 00019. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Pal, R.; Cameotra, S.S. Antibacterial potential of Al2O3 nanoparticles against multidrug resistance strains of Staphylococcusaureus isolated from skin exudates. J. Nanopart. Res. 2013, 15, 1970. [Google Scholar] [CrossRef]
- Muzammil, S.; Khurshid, M.; Nawaz, I.; Siddique, M.H.; Zubair, M.; Nisar, M.A.; Imran, M.; Hayat, S. Aluminium oxide nanoparticles inhibit EPS production, adhesion and biofilm formation by multidrug resistant Acinetobacter baumannii. Biofouling 2020, 36, 492–504. [Google Scholar] [CrossRef]
- Sikora, P.; Augustyniak, A.; Cendrowski, K.; Nawrotek, P.; Mijowska, E. Antimicrobial Activity of Al2O3, CuO, Fe3O4, and ZnO Nanoparticles in Scope of Their Further Application in Cement-Based Building Materials. Nanomaterials 2018, 8, 212. [Google Scholar] [CrossRef]
- Manyasree, D.; Kiranmayi, P.; Venkata, R.K. Nanomaterial oxides as antimicrobal agents (Al2O3, CuO, Fe3O4, and ZnO): Comparative study. Indo Am. J. Pharm. Res. 2019, 9, 1852. [Google Scholar]
- Pakrashi, S.; Kumar, D.; Iswarya, V.; Bhuvaneshwari, M.; Chandrasekaran, N.; Mukherjee, A. A comparative ecotoxicity analysis of α- and γ-phase aluminium oxide nanoparticles towards a freshwater bacterial isolate Bacillus licheniformis. Bioprocess Biosyst. Eng. 2014, 37, 2415–2423. [Google Scholar] [CrossRef]
- Sadiq, I.M.; Pakrashi, S.; Chandrasekaran, N.; Mukherjee, A. Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp. J. Nanopart. Res. 2011, 13, 3287–3299. [Google Scholar] [CrossRef]
- Manyasree, D.; Kiranmayi, P.; Kumar, R. Synthesis, characterization and antibacterial activity of aluminium oxide nanoparticles. Int. J. Pharm. Pharm. Sci. 2018, 10, 32–35. [Google Scholar]
- Bhuvaneshwari, M.; Bairoliya, S.; Parashar, A.; Chandrasekaran, N.; Mukherjee, A. Differential toxicity of Al2O3 particles on Gram-positive and Gram-negative sediment bacterial isolates from freshwater. Environ. Sci. Pollut. Res. 2016, 23, 12095–12106. [Google Scholar] [CrossRef]
- Mu, D.; Mu, X.; Xu, Z.; Du, Z.; Chen, G. Removing Bacillus subtilis from fermentation broth using alumina nanoparticles. Bioresour. Technol. 2015, 197, 508–511. [Google Scholar] [CrossRef]
- Pakrashi, S.; Dalai, S.; Ritika; Sneha, B.; Chandrasekaran, N.; Mukherjee, A. A temporal study on fate of Al2O3 nanoparticles in a fresh water microcosm at environmentally relevant low concentrations. Ecotoxicol. Environ. Saf. 2012, 84, 70–77. [Google Scholar] [CrossRef]
- Balasubramanyam, A.; Sailaja, N.; Mahboob, M.; Rahman, M.F.; Hussain, S.M.; Grover, P. In Vitro mutagenicity assessment of aluminium oxide nanomaterials using the Salmonella/microsome assay. Toxicol. Vitr. 2010, 24, 1871–1876. [Google Scholar] [CrossRef]
- Vyas, S.; Shukla, A.; Shivhare, S.; Upadhyay, N. Facile synthesis and characterization of polyaniline (PANI)–Aluminium oxide (Al2O3) nanocomposites by using chemical oxidative polymerization. AIP Conf. Proc. 2021, 2369, 020192. [Google Scholar]
- Baghdadi, A.M.; Saddiq, A.A.; Aissa, A.; Algamal, Y.; Khalil, N.M. Structural refinement and antimicrobial activity of aluminum oxide nanoparticles. J. Ceram. Soc. Jpn. 2022, 130, 257–263. [Google Scholar] [CrossRef]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef]
- Simon-Deckers, A.; Loo, S.; Mayne L’hermite, M.; Herlin-Boime, N.; Menguy, N.; Reynaud, C.; Gouget, B.; Carriere, M. Size-, composition- and shape-dependent toxicological impact of metal oxide nanoparticles and carbon nanotubes toward bacteria. Environ. Sci. Technol. 2009, 43, 8423–8429. [Google Scholar] [CrossRef]
- García-Saucedo, C.; Field, J.A.; Otero-Gonzalez, L.; Sierra-Álvarez, R. Low toxicity of HfO2, SiO2, Al2O3 and CeO2 nanoparticles to the yeast, Saccharomyces cerevisiae. J. Hazard. Mater. 2011, 192, 1572–1579. [Google Scholar] [CrossRef]
- Archibald, A.; Hancock, I.; Harwood, C. Cell wall structure, synthesis, and turnover. In Bacillus Subtilis and Other Gram-Positive Bacteria: Biochemistry, Physiology, and Molecular Genetics; John Wiley & Sons: Hoboken, NJ, USA, 1993; pp. 379–410. [Google Scholar]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.; Kim, T.; Kim, J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Mukha, I.P.; Eremenko, A.M.; Smirnova, N.P.; Mikhienkova, A.I.; Korchak, G.I.; Gorchev, V.F.; Chunikhin, A.Y. Antimicrobial activity of stable silver nanoparticles of a certain size. Appl. Biochem. Microbiol. 2013, 49, 199–206. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sadiq, I.M.; Prathna, T.; Chandrasekaran, N. Antimicrobial activity of aluminium oxide nanoparticles for potential clinical applications. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; FORMATEX: Badajoz, Spain, 2011; Volume 1, pp. 245–251. [Google Scholar]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Londono, S.C.; Hartnett, H.E.; Williams, L.B. Antibacterial Activity of Aluminum in Clay from the Colombian Amazon. Environ. Sci. Technol. 2017, 51, 2401–2408. [Google Scholar] [CrossRef]
- Zatta, P.; Kiss, T.; Suwalsky, M.; Berthon, G. Aluminium(III) as a promoter of cellular oxidation. Coord. Chem. Rev. 2002, 228, 271–284. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Quinlan, G.J.; Clark, I.; Halliwell, B. Aluminium salts accelerate peroxidation of membrane lipids stimulated by iron salts. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1985, 835, 441–447. [Google Scholar] [CrossRef]
- Morrison, K.D.; Misra, R.; Williams, L.B. Unearthing the antibacterial mechanism of medicinal clay: A geochemical approach to combating antibiotic resistance. Sci. Rep. 2016, 6, 19043. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic. Biol. Med. 2011, 51, 1872–1881. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.; Dubey, R.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Schins, R.P.; Knaapen, A.M. Genotoxicity of poorly soluble particles. Inhal. Toxicol. 2007, 19, 189–198. [Google Scholar] [CrossRef]
- Lin, W.; Stayton, I.; Huang, Y.-W.; Zhou, X.-D.; Ma, Y. Cytotoxicity and cell membrane depolarization induced by aluminum oxide nanoparticles in human lung epithelial cells A549. Toxicol. Environ. Chem. 2008, 90, 983–996. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, W.; Fang, Y.; Yang, H.; Tian, L.; Li, K.; Lai, W.; Bian, L.; Lin, B.; Liu, X.; et al. Neurotoxicity of aluminum oxide nanoparticles and their mechanistic role in dopaminergic neuron injury involving p53-related pathways. J. Hazard. Mater. 2020, 392, 122312. [Google Scholar] [CrossRef]
- Mirshafa, A.; Nazari, M.; Jahani, D.; Shaki, F. Size-Dependent Neurotoxicity of Aluminum Oxide Particles: A Comparison Between Nano- and Micrometer Size on the Basis of Mitochondrial Oxidative Damage. Biol. Trace Elem. Res. 2018, 183, 261–269. [Google Scholar] [CrossRef]
- Dey, M.; Singh, R.K. Neurotoxic effects of aluminium exposure as a potential risk factor for Alzheimer’s disease. Pharmacol. Rep. 2022, 74, 439–450. [Google Scholar] [CrossRef]
- Raj, K.; Kaur, P.; Gupta, G.D.; Singh, S. Metals associated neurodegeneration in Parkinson’s disease: Insight to physiological, pathological mechanisms and management. Neurosci. Lett. 2021, 753, 135873. [Google Scholar] [CrossRef]
- Exley, C.; Clarkson, E. Aluminium in human brain tissue from donors without neurodegenerative disease: A comparison with Alzheimer’s disease, multiple sclerosis and autism. Sci. Rep. 2020, 10, 7770. [Google Scholar] [CrossRef]
- Crisponi, G.; Nurchi, V.M.; Bertolasi, V.; Remelli, M.; Faa, G. Chelating agents for human diseases related to aluminium overload. Coord. Chem. Rev. 2012, 256, 89–104. [Google Scholar] [CrossRef]
- Praticò, D.; Uryu, K.; Sung, S.; Tang, S.; Trojanowski, J.Q.; Lee, V.M.Y. Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. FASEB J. 2002, 16, 1138–1140. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gill, K.D. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: A review. Neurotoxicology 2014, 41, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Fulgenzi, A.; Vietti, D.; Ferrero, M.E. Aluminium involvement in neurotoxicity. BioMed Res. Int. 2014, 2014, 758323. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudkov, S.V.; Burmistrov, D.E.; Smirnova, V.V.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of Al2O3 Nanoparticles. Nanomaterials 2022, 12, 2635. https://doi.org/10.3390/nano12152635
Gudkov SV, Burmistrov DE, Smirnova VV, Semenova AA, Lisitsyn AB. A Mini Review of Antibacterial Properties of Al2O3 Nanoparticles. Nanomaterials. 2022; 12(15):2635. https://doi.org/10.3390/nano12152635
Chicago/Turabian StyleGudkov, Sergey V., Dmitriy E. Burmistrov, Veronika V. Smirnova, Anastasia A. Semenova, and Andrey B. Lisitsyn. 2022. "A Mini Review of Antibacterial Properties of Al2O3 Nanoparticles" Nanomaterials 12, no. 15: 2635. https://doi.org/10.3390/nano12152635
APA StyleGudkov, S. V., Burmistrov, D. E., Smirnova, V. V., Semenova, A. A., & Lisitsyn, A. B. (2022). A Mini Review of Antibacterial Properties of Al2O3 Nanoparticles. Nanomaterials, 12(15), 2635. https://doi.org/10.3390/nano12152635







