Graphene-Based Ion-Selective Field-Effect Transistor for Sodium Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Graphene Synthesis
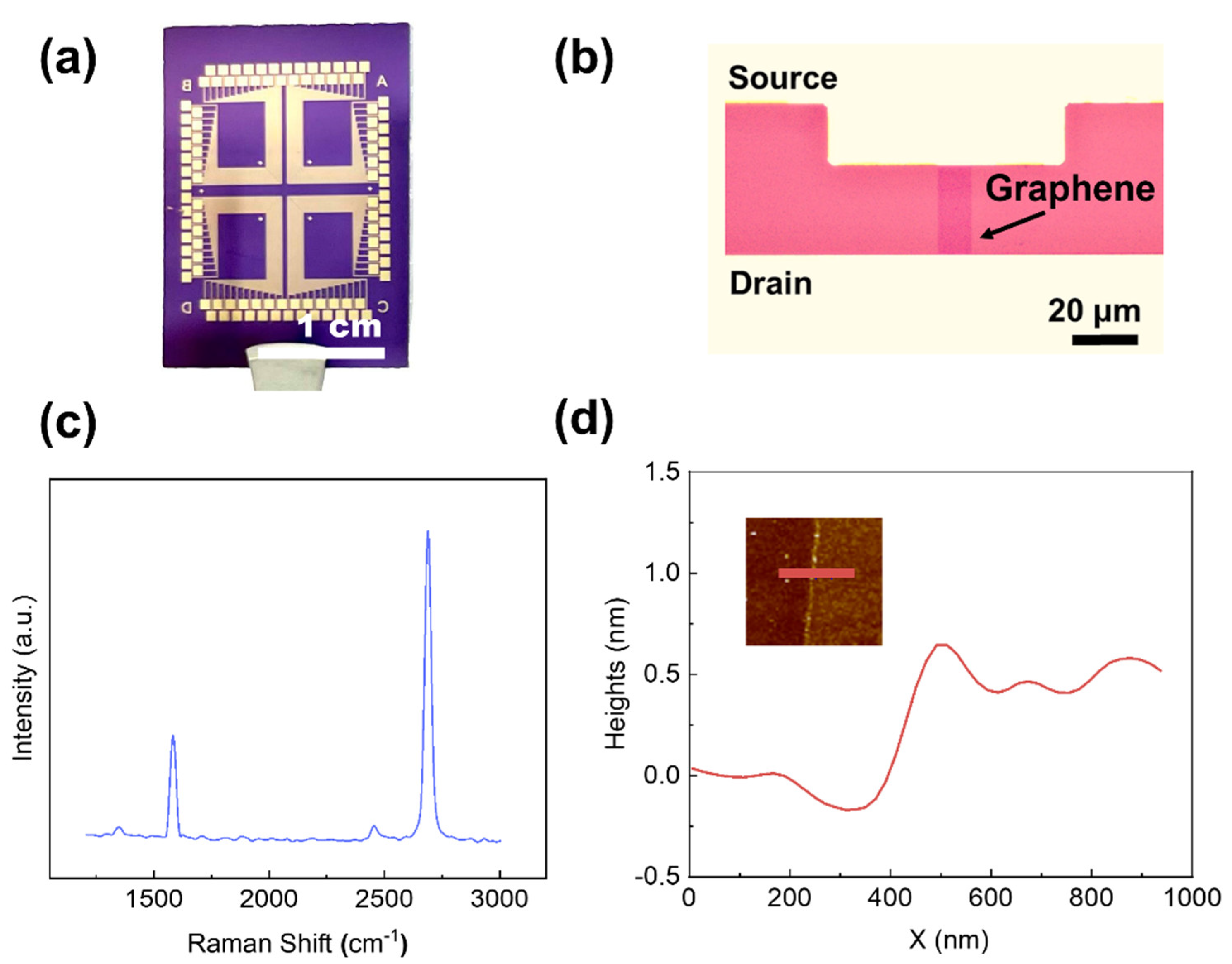
2.2. GFET Sensor Array Fabrication
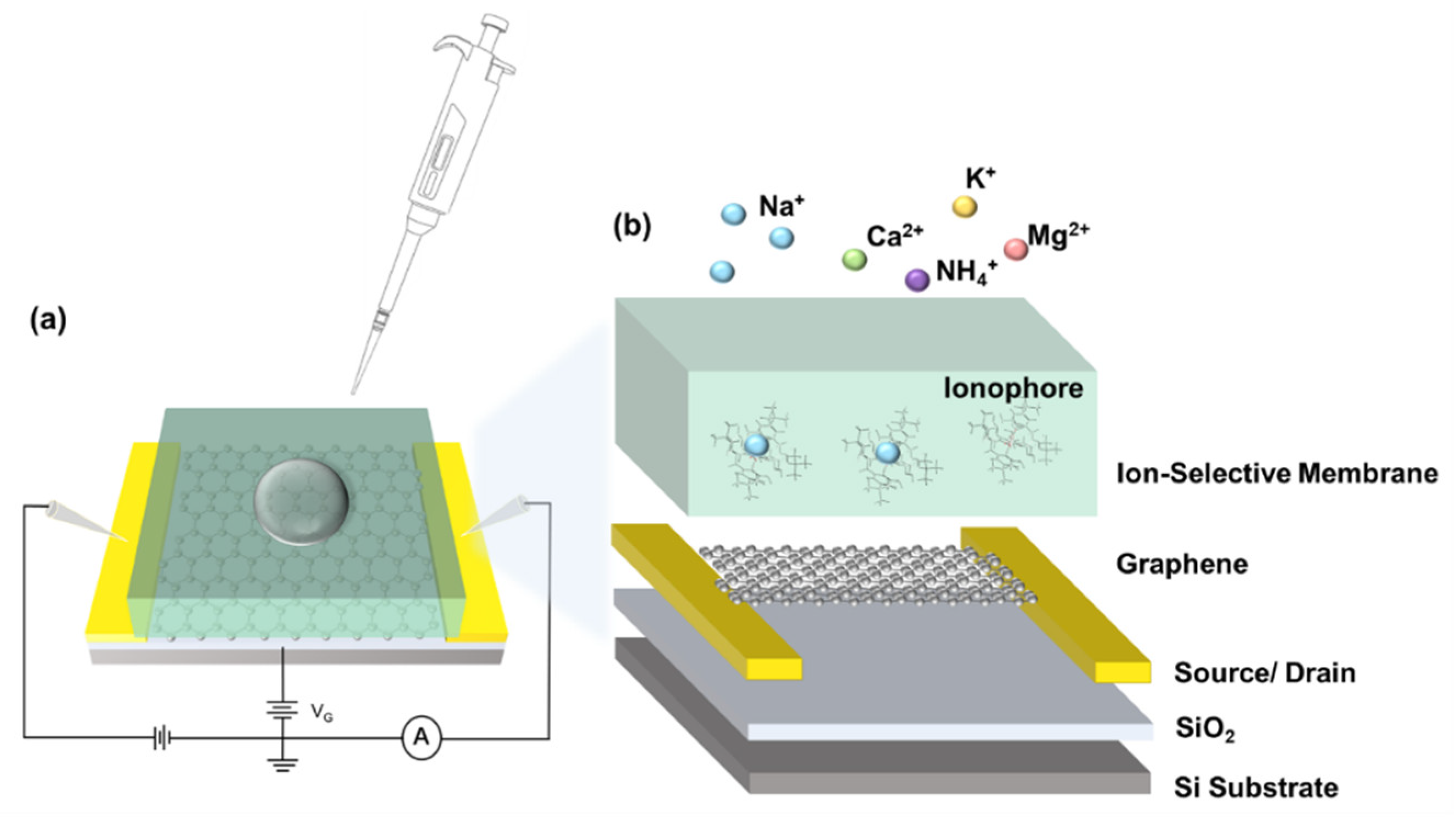
2.3. Ionophore Membrane Preparation
2.4. Material Characterization
2.5. Solution Preparation
2.6. Electrical Measurement
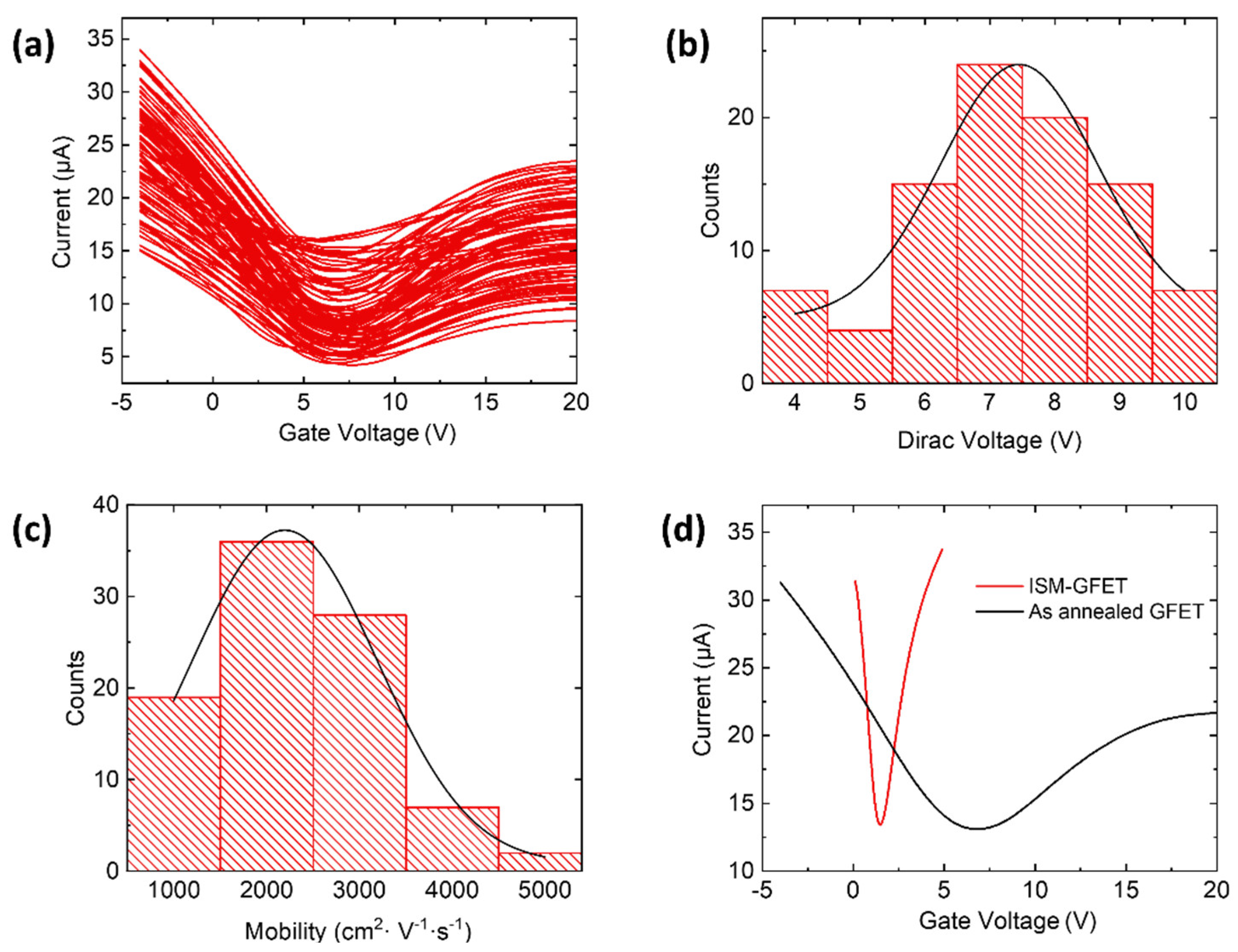
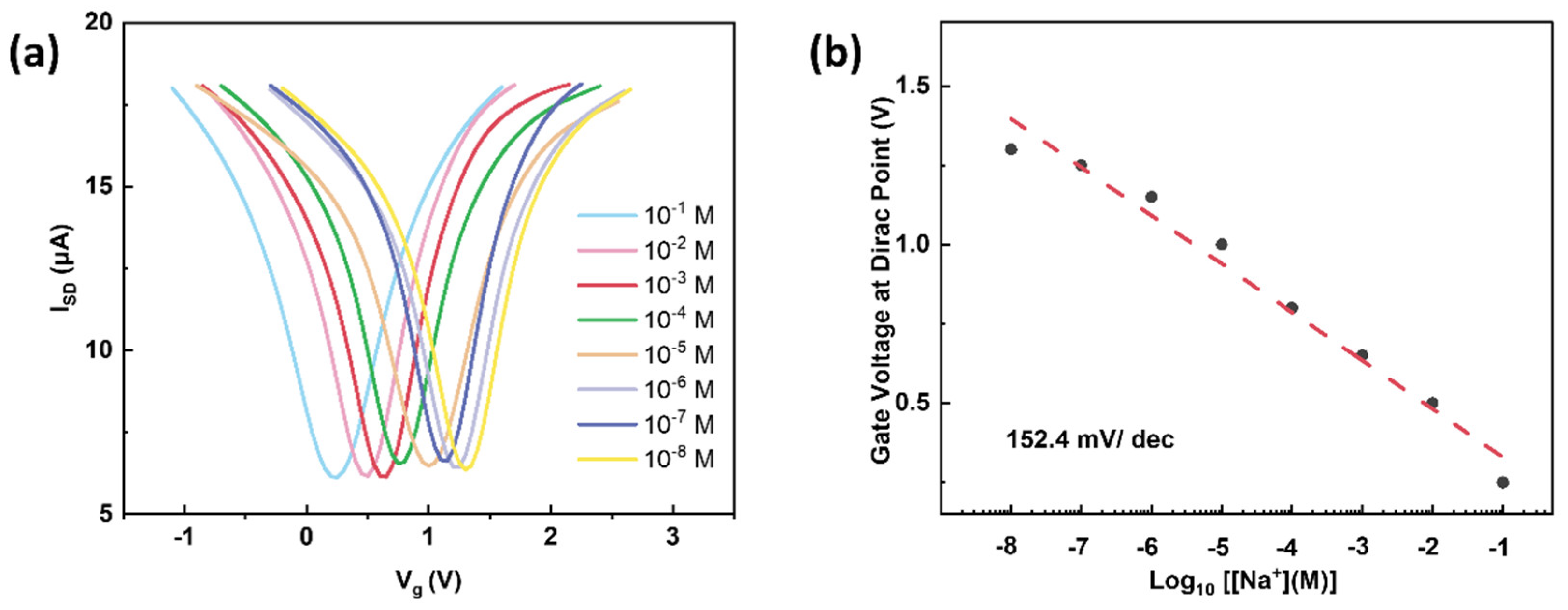
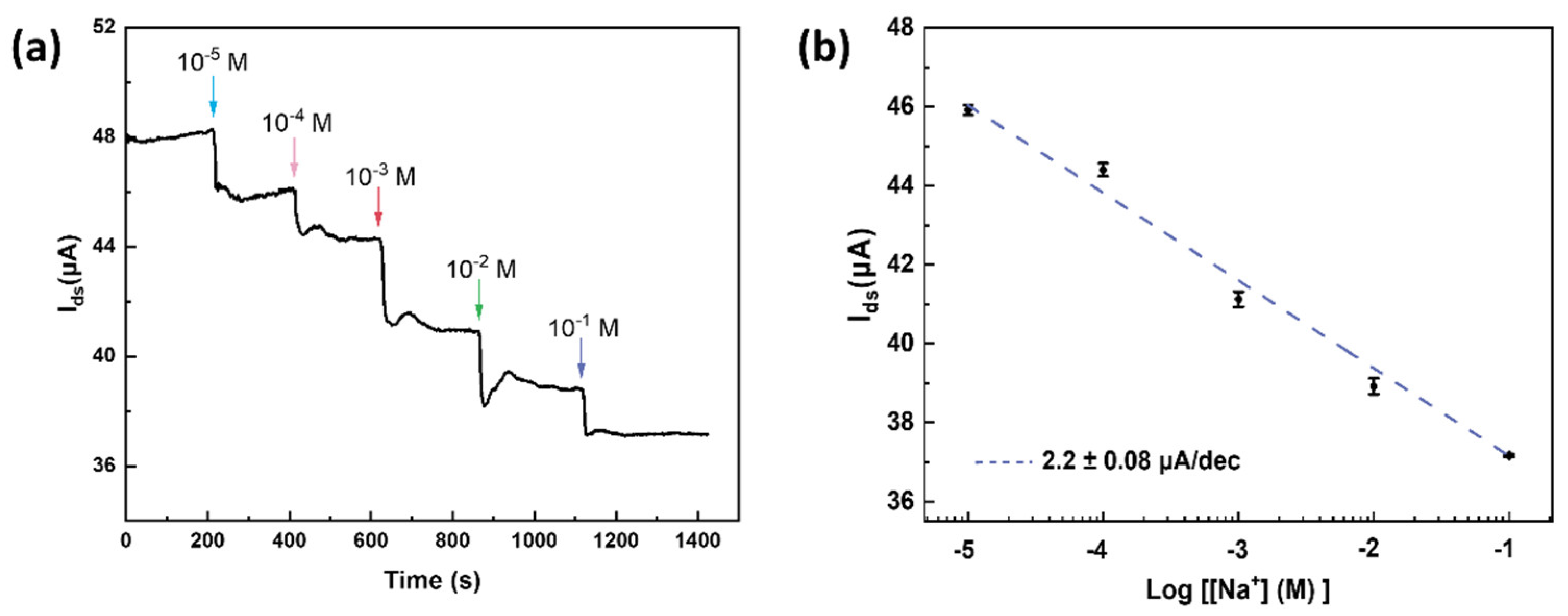
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lerch, M.M.; Hansen, M.J.; van Dam, G.M.; Szymanski, W.; Feringa, B.L. Emerging Targets in Photopharmacology. Angew. Chem. Int. Edit. 2016, 55, 10978–10999. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.M.; Padfield, P.L.; Seckl, J.R. Disorders of sodium balance. BMJ 2006, 332, 702–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.J.; MacGregor, G.A. Plasma sodium and hypertension. Kidney Int. 2004, 66, 2454–2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thulborn, K.; Lui, E.; Guntin, J.; Jamil, S.; Sun, Z.; Claiborne, T.C.; Atkinson, I.C. Quantitative sodium MRI of the human brain at 9.4 T provides assessment of tissue sodium concentration and cell volume fraction during normal aging. NMR Biomed. 2016, 29, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inglese, M.; Madelin, G.; Oesingmann, N.; Babb, J.; Wu, W.; Stoeckel, B.; Herbert, J.; Johnson, G. Brain tissue sodium concentration in multiple sclerosis: A sodium imaging study at 3 tesla. Brain 2010, 133, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Gerhalter, T.; Gast, L.V.; Marty, B.; Uder, M.; Carlier, P.G.; Nagel, A.M. Assessing the variability of 23Na MRI in skeletal muscle tissue: Reproducibility and repeatability of tissue sodium concentration measurements in the lower leg at 3 T. NMR Biomed. 2020, 33, e4279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titze, J.; Machnik, A. Sodium sensing in the interstitium and relationship to hypertension. Curr. Opin. Nephrol. Hypertens. 2010, 19, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Shirreffs, S. Markers of hydration status. J. Sports Med. Phys. Fit. 2000, 40, 80–84. [Google Scholar] [CrossRef]
- Yang, Y.; Mis, M.A.; Estacion, M.; Dib-Hajj, S.D.; Waxman, S.G. NaV1. 7 as a pharmacogenomic target for pain: Moving toward precision medicine. Trends Pharmacol. Sci. 2018, 39, 258–275. [Google Scholar] [CrossRef]
- Sun, W.; Lee, J.; Zhang, S.; Benyshek, C.; Dokmeci, M.R.; Khademhosseini, A. Engineering precision medicine. Adv. Sci. 2019, 6, 1801039. [Google Scholar] [CrossRef] [Green Version]
- Freiser, H. Ion-Selective Electrodes in Analytical Chemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Mikhelson, K. Ion-selective electrodes with sensitivity in strongly diluted solutions. J. Anal. Chem. 2010, 65, 112–116. [Google Scholar] [CrossRef]
- Arnold, M.A.; Meyerhoff, M.E. Ion-selective electrodes. Anal. Chem. 1984, 56, 20–48. [Google Scholar] [CrossRef]
- De Marco, R.; Clarke, G.; Pejcic, B. Ion-selective electrode potentiometry in environmental analysis. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2007, 19, 1987–2001. [Google Scholar] [CrossRef]
- Yeung, K.K.; Huang, T.; Hua, Y.; Zhang, K.; Yuen, M.M.; Gao, Z. Recent advances in electrochemical sensors for wearable sweat monitoring: A review. IEEE Sens. J. 2021, 21, 14522–14539. [Google Scholar] [CrossRef]
- Bobacka, J.; Lindfors, T.; Mccarrick, M.; Ivaska, A.; Lewenstam, A. Single Piece All-Solid-State Ion-Selective Electrode. Anal. Chem. 1995, 67, 3819–3823. [Google Scholar] [CrossRef]
- Cammann, K. Bio-sensors based on ion-selective electrodes. Fresenius’ Z. Für Anal. Chem. 1977, 287, 1–9. [Google Scholar] [CrossRef]
- Ezzat, S.; Ahmed, M.A.; Amr, A.G.E.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Single-Piece All-Solid-State Potential Ion-Selective Electrodes Integrated with Molecularly Imprinted Polymers (MIPs) for Neutral 2,4-Dichlorophenol Assessment. Materials 2019, 12, 2924. [Google Scholar] [CrossRef] [Green Version]
- Cammann, K. Working with Ion-Selective Electrodes: Chemical Laboratory Practice; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Fu, W.; Jiang, L.; van Geest, E.P.; Lima, L.M.; Schneider, G.F. Sensing at the surface of graphene field-effect transistors. Adv. Mater. 2017, 29, 1603610. [Google Scholar] [CrossRef] [Green Version]
- Syu, Y.-C.; Hsu, W.-E.; Lin, C.-T. Field-effect transistor biosensing: Devices and clinical applications. ECS J. Solid State Sci. Technol. 2018, 7, Q3196. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef]
- Akinwande, D.; Huyghebaert, C.; Wang, C.-H.; Serna, M.I.; Goossens, S.; Li, L.-J.; Wong, H.-S.P.; Koppens, F.H. Graphene and two-dimensional materials for silicon technology. Nature 2019, 573, 507–518. [Google Scholar] [CrossRef]
- Cho, S.K.; Cho, W.J. Highly Sensitive and Selective Sodium Ion Sensor Based on Silicon Nanowire Dual Gate Field-Effect Transistor. Sensors 2021, 21, 4213. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Kim, S.K.; Kim, M. Ion-sensitive field-effect transistor for biological sensing. Sensors 2009, 9, 7111–7131. [Google Scholar] [CrossRef]
- Maehashi, K.; Sofue, Y.; Okamoto, S.; Ohno, Y.; Inoue, K.; Matsumoto, K. Selective ion sensors based on ionophore-modified graphene field-effect transistors. Sens. Actuators B Chem. 2013, 187, 45–49. [Google Scholar] [CrossRef]
- Lim, H.R.; Lee, S.M.; Mahmood, M.; Kwon, S.; Kim, Y.S.; Lee, Y.; Yeo, W.H. Development of Flexible Ion-Selective Electrodes for Saliva Sodium Detection. Sensors 2021, 21, 1642. [Google Scholar] [CrossRef] [PubMed]
- Spijkman, M.; Smits, E.C.P.; Cillessen, J.F.M.; Biscarini, F.; Blom, P.W.M.; de Leeuw, D.M. Beyond the Nernst-limit with dual-gate ZnO ion-sensitive field-effect transistors. Appl. Phys. Lett. 2011, 98, 6169. [Google Scholar] [CrossRef] [Green Version]
- Neto, A.C.; Guinea, F.; Peres, N.M.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Colombo, L.; Gellert, P.; Schwab, M.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Ovid’Ko, I. Mechanical properties of graphene. Rev. Adv. Mater. Sci. 2013, 34, 1–11. [Google Scholar]
- Gao, L.; Ren, W.; Xu, H.; Jin, L.; Wang, Z.; Ma, T.; Ma, L.-P.; Zhang, Z.; Fu, Q.; Peng, L.-M. Repeated growth and bubbling transfer of graphene with millimetre-size single-crystal grains using platinum. Nat. Commun. 2012, 3, 699. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdés-Ramírez, G.; Andrade, F.J.; Schöning, M.J.; Wang, J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xia, H.; Zauberman, J.; Tomaiuolo, M.; Ping, J.; Zhang, Q.; Ducos, P.; Ye, H.; Wang, S.; Yang, X. Detection of sub-fM DNA with target recycling and self-assembly amplification on graphene field-effect biosensors. Nano Lett. 2018, 18, 3509–3515. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morozov, S.; Novoselov, K.; Katsnelson, M.; Schedin, F.; Elias, D.C.; Jaszczak, J.A.; Geim, A. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Xia, J.; Ferry, D.K.; Tao, N. Dielectric screening enhanced performance in graphene FET. Nano Lett. 2009, 9, 2571–2574. [Google Scholar] [CrossRef] [PubMed]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-based ion-selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors. Chem. Rev. 1998, 98, 1593–1688. [Google Scholar] [CrossRef]
- Luboch, E.; Jeszke, M.; Szarmach, M.; Łukasik, N. New bis (azobenzocrown) s with dodecylmethylmalonyl linkers as ionophores for sodium selective potentiometric sensors. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 323–335. [Google Scholar] [CrossRef] [Green Version]
- Meric, I.; Han, M.Y.; Young, A.F.; Ozyilmaz, B.; Kim, P.; Shepard, K.L. Current saturation in zero-bandgap, top-gated graphene field-effect transistors. Nat. Nanotechnol. 2008, 3, 654–659. [Google Scholar] [CrossRef]
- Garcia-Cordero, E.; Bellando, F.; Zhang, J.; Wildhaber, F.; Longo, J.; Guérin, H.; Ionescu, A.M. Three-dimensional integrated ultra-low-volume passive microfluidics with ion-sensitive field-effect transistors for multiparameter wearable sweat analyzers. ACS Nano 2018, 12, 12646–12656. [Google Scholar] [CrossRef] [PubMed]
- Fakih, I.; Durnan, O.; Mahvash, F.; Napal, I.; Centeno, A.; Zurutuza, A.; Yargeau, V.; Szkopek, T. Selective ion sensing with high resolution large area graphene field effect transistor arrays. Nat. Commun. 2020, 11, 3226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rupakula, M.; Bellando, F.; Garcia Cordero, E.; Longo, J.; Wildhaber, F.; Herment, G.; Guerin, H.; Ionescu, A.M. Sweat biomarker sensor incorporating picowatt, three-dimensional extended metal gate ion sensitive field effect transistors. ACS Sens. 2019, 4, 2039–2047. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Yeung, K.K.; Li, J.; Sun, H.; Alam, M.M.; Gao, Z. Graphene-Based Ion-Selective Field-Effect Transistor for Sodium Sensing. Nanomaterials 2022, 12, 2620. https://doi.org/10.3390/nano12152620
Huang T, Yeung KK, Li J, Sun H, Alam MM, Gao Z. Graphene-Based Ion-Selective Field-Effect Transistor for Sodium Sensing. Nanomaterials. 2022; 12(15):2620. https://doi.org/10.3390/nano12152620
Chicago/Turabian StyleHuang, Ting, Kan Kan Yeung, Jingwei Li, Honglin Sun, Md Masruck Alam, and Zhaoli Gao. 2022. "Graphene-Based Ion-Selective Field-Effect Transistor for Sodium Sensing" Nanomaterials 12, no. 15: 2620. https://doi.org/10.3390/nano12152620
APA StyleHuang, T., Yeung, K. K., Li, J., Sun, H., Alam, M. M., & Gao, Z. (2022). Graphene-Based Ion-Selective Field-Effect Transistor for Sodium Sensing. Nanomaterials, 12(15), 2620. https://doi.org/10.3390/nano12152620






