Practical Approaches to Apply Ultra-Thick Graphite Anode to High-Energy Lithium-Ion Battery: Carbonization and 3-Dimensionalization
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Electrode Preparation
2.2. Carbonization and Laser Structuring
2.3. Cell Assembly and Electrochemical Analysis
2.4. Electrode and Material Characterizations
3. Results and Discussion
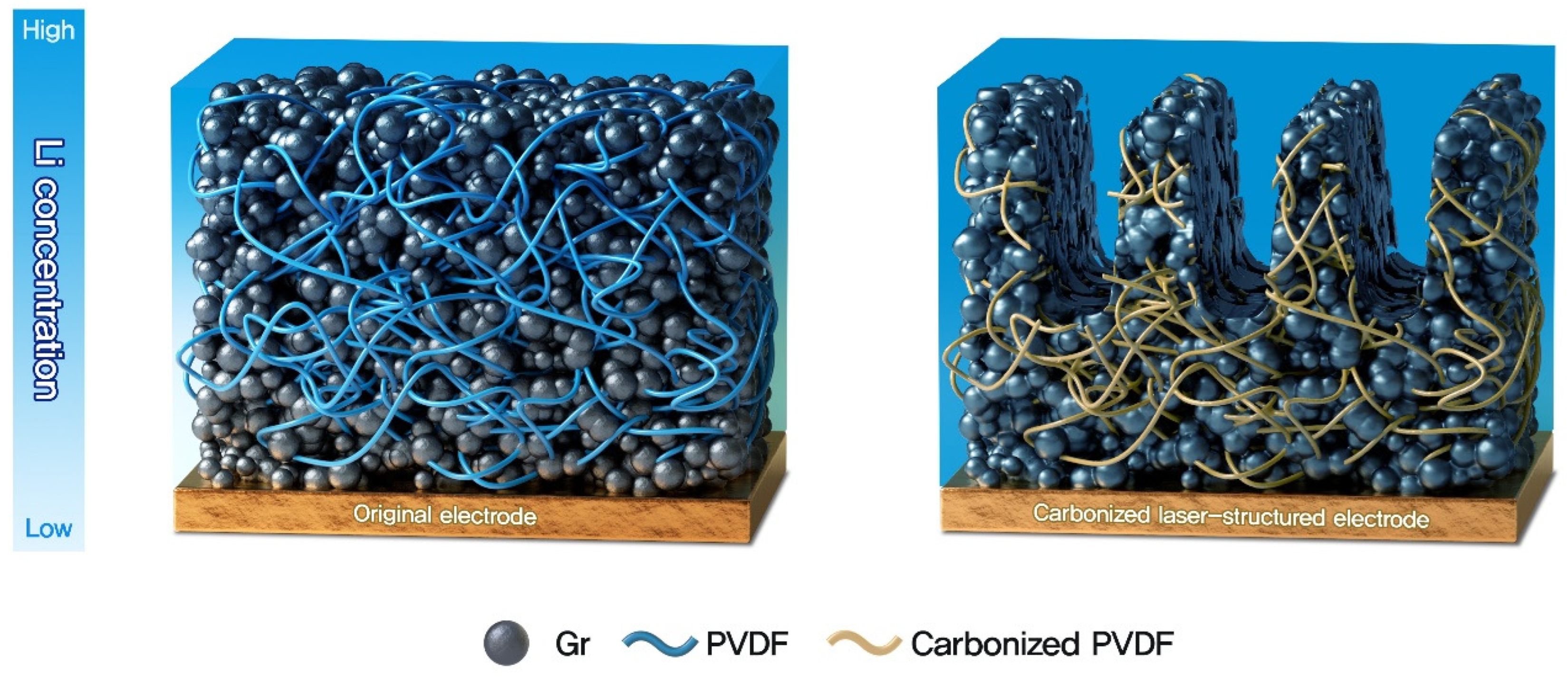
3.1. Physicochemical Characteristics of the Electrode
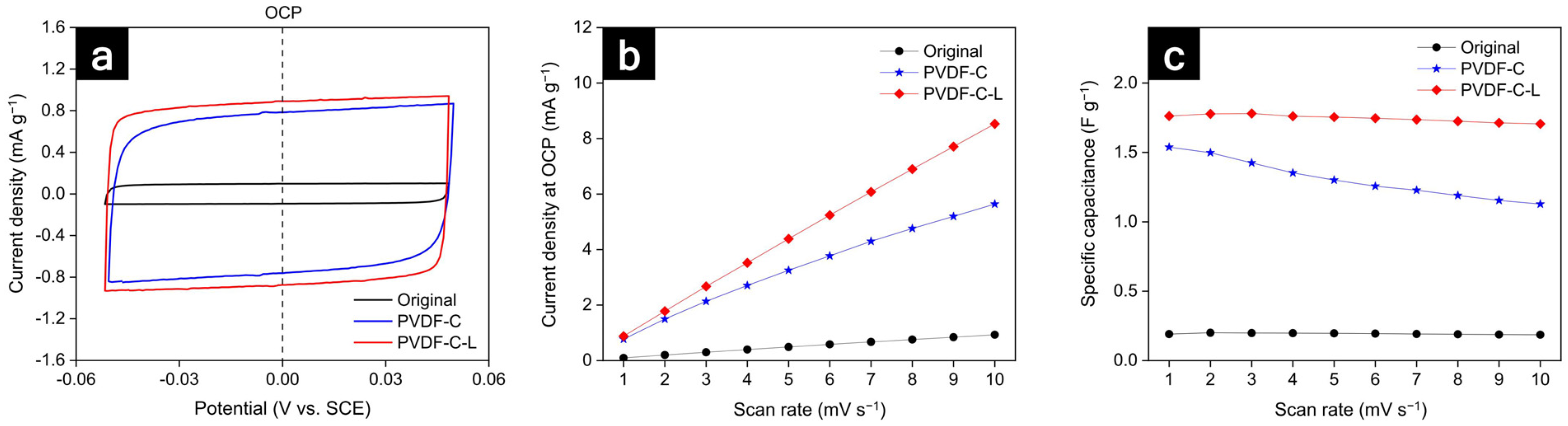
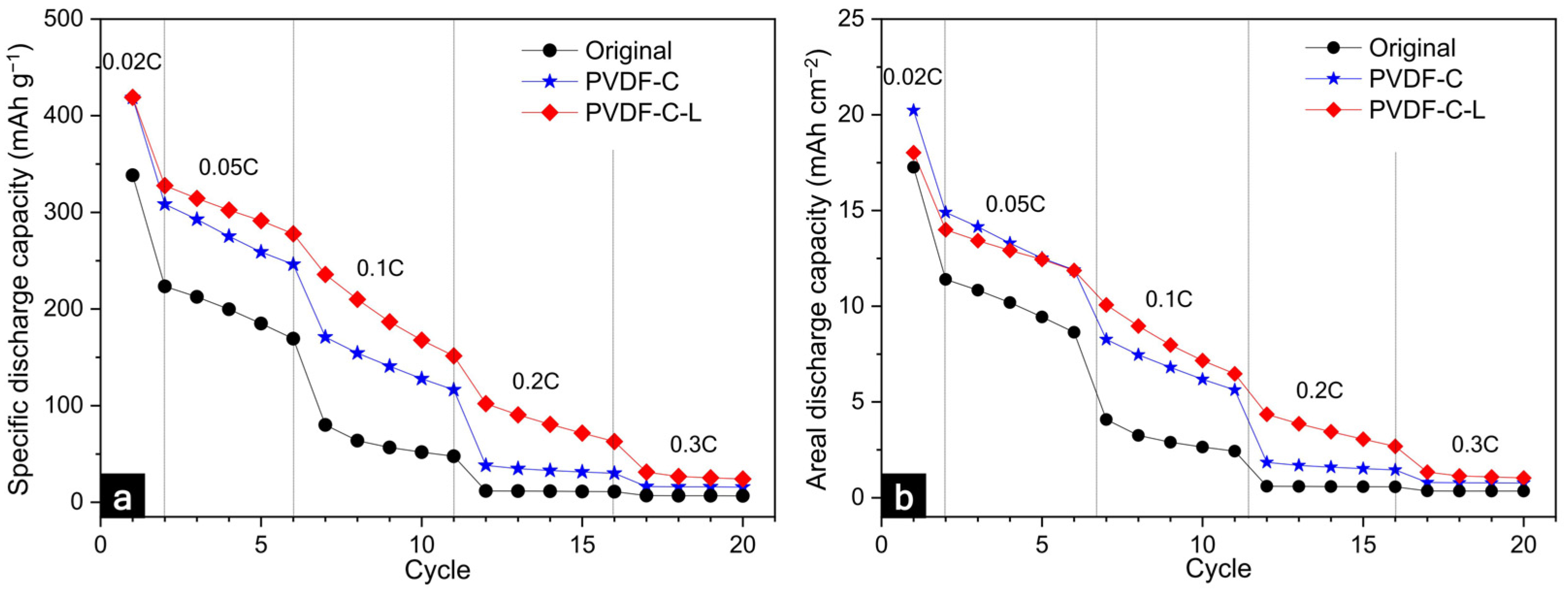
3.2. Electrochemical Characteristics of the Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Kang, K.; Meng, Y.S.; Bréger, J.; Grey, C.P.; Ceder, G. Electrodes with High Power and High Capacity for Rechargeable Lithium Batteries. Science 2006, 311, 977–980. [Google Scholar] [CrossRef]
- Tran, T.D.; Feikert, J.H.; Song, X.; Kinoshita, K. Commercial Carbonaceous Materials as Lithium Intercalation Anodes. J. Electrochem. Soc. 1995, 142, 3297–3302. [Google Scholar] [CrossRef]
- Leng, Y.; Ge, S.; Marple, D.; Yang, X.-G.; Bauer, C.; Lamp, P.; Wang, C.-Y. Electrochemical cycle-life characterization of high energy lithium-ion cells with thick Li(Ni0.6Mn0.2Co0.2)O2 and graphite electrodes. J. Electrochem. Soc. 2017, 164, A1037. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.; Erdonmez, C.K.; Marinis, T.F.; Bjune, C.K.; Dudney, N.J.; Xu, F.; Wartena, R.; Chiang, Y.-M. Ultrahigh-Energy-Density Microbatteries Enabled by New Electrode Architecture and Micropackaging Design. Adv. Mater. 2010, 22, E139–E144. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Song, H.; Jang, I.; Lee, J.; Um, J.; Bae, S.; Kim, J.; Jeong, S.; Kim, H.-J. Three-dimensionalization via control of la-ser-structuring parameters for high energy and high power lithium-ion battery under various operating conditions. J. Energy Chem. 2022, 64, 93–102. [Google Scholar] [CrossRef]
- Du, Z.; Wood, D.L., III; Daniel, C.; Kalnaus, S.; Li, J. Understanding limiting factors in thick electrode performance as applied to high energy density Li-ion batteries. J. Appl. Electrochem. 2017, 47, 405–415. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V.S. A comprehensive understanding of electrode thickness effects on the electrochemical performances of Li-ion battery cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- Patry, G.; Romagny, A.; Martinet, S.; Froelich, D. Cost modeling of lithium-ion battery cells for automotive applications. Energy Sci. Eng. 2014, 3, 71–82. [Google Scholar] [CrossRef]
- Zhu, P.; Han, J.; Pfleging, W. Characterization and Laser Structuring of Aqueous Processed Li(Ni0.6Mn0.2Co0.2)O2 Thick-Film Cathodes for Lithium-Ion Batteries. Nanomaterials 2021, 11, 1840. [Google Scholar] [CrossRef]
- Song, Z.; Zhu, P.; Pfleging, W.; Sun, J. Electrochemical Performance of Thick-Film Li(Ni0.6Mn0.2Co0.2)O2 Cathode with Hierarchic Structures and Laser Ablation. Nanomaterials 2021, 11, 2962. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaiser, J.; Hahn, H. Thick Electrodes for High Energy Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A1196–A1201. [Google Scholar] [CrossRef]
- Lu, W.; Jansen, A.; Dees, D.; Nelson, P.; Veselka, N.R.; Henriksen, G. High-energy electrode investigation for plug-in hybrid electric vehicles. J. Power Sources 2011, 196, 1537–1540. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Trask, S.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B.; et al. Optimizing Areal Capacities through Understanding the Limitations of Lithium-Ion Electrodes. J. Electrochem. Soc. 2015, 163, A138–A149. [Google Scholar] [CrossRef]
- Fongy, C.; Jouanneau, S.; Guyomard, D.; Badot, J.C.; Lestriez, B. Electronic and ionic wirings versus the insertion reaction contributions to the polarization in LiFePO4 composite electrodes. J. Electrochem. Soc. 2010, 157, A1347. [Google Scholar] [CrossRef]
- Fongy, C.; Gaillot, A.-C.; Jouanneau, S.; Guyomard, D.; Lestriez, B. Ionic vs electronic power limitations and analysis of the fraction of wired grains in LiFePO4 composite electrodes. J. Electrochem. Soc. 2010, 157, A885. [Google Scholar] [CrossRef]
- Chang, C.-C.; Her, L.-J.; Su, H.-K.; Hsu, S.-H.; Yen, Y.T. Effects of Dispersant on the Conductive Carbon for LiFePO4 Cathode. J. Electrochem. Soc. 2011, 158, A481–A486. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Demortière, A.; Fleutot, B.; Delobel, B.; Delacourt, C.; Cooper, S.J. The electrode tortuosity factor: Why the conventional tortuosity factor is not well suited for quantifying transport in porous Li-ion battery electrodes and what to use instead. npj Comput. Mater. 2020, 6, 123. [Google Scholar] [CrossRef]
- Lee, B.-S.; Wu, Z.; Petrova, V.; Xing, X.; Lim, H.-D.; Liu, H.; Liu, P. Analysis of Rate-Limiting Factors in Thick Electrodes for Electric Vehicle Applications. J. Electrochem. Soc. 2018, 165, A525–A533. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Drews, A.; Chandrasekaran, R.; Miller, T.; Sakamoto, J. Improving Li-ion battery charge rate acceptance through highly ordered hierarchical electrode design. Ionics 2018, 24, 2935–2943. [Google Scholar] [CrossRef]
- Nisar, U.; Amin, R.; Essehli, R.; Shakoor, R.; Kahraman, R.; Kim, D.K.; Khaleel, M.A.; Belharouak, I. Extreme fast charging characteristics of zirconia modified LiNi0.5Mn1.5O4 cathode for lithium ion batteries. J. Power Sources 2018, 396, 774–781. [Google Scholar] [CrossRef]
- Zheng, Y.; Yin, D.; Seifert, H.J.; Pfleging, W. Investigation of Fast-Charging and Degradation Processes in 3D Silicon–Graphite Anodes. Nanomaterials 2021, 12, 140. [Google Scholar] [CrossRef]
- Javed, M.S.; Shah, S.S.A.; Hussain, S.; Tan, S.; Mai, W. Mesoporous manganese-selenide microflowers with enhanced electrochemical performance as a flexible symmetric 1.8 V supercapacitor. Chem. Eng. J. 2019, 382, 122814. [Google Scholar] [CrossRef]
- Javed, M.S.; Lei, H.; Wang, Z.; Liu, B.-T.; Cai, X.; Mai, W. 2D V2O5 nanosheets as a binder-free high-energy cathode for ultrafast aqueous and flexible Zn-ion batteries. Nano Energy 2020, 70, 104573. [Google Scholar] [CrossRef]
- Zheng, Y.; Seifert, H.; Shi, H.; Zhang, Y.; Kübel, C.; Pfleging, W. 3D silicon/graphite composite electrodes for high-energy lithium-ion batteries. Electrochim. Acta 2019, 317, 502–508. [Google Scholar] [CrossRef]
- Kraft, L.; Habedank, J.B.; Frank, A.; Rheinfeld, A.; Jossen, A. Modeling and Simulation of Pore Morphology Modifications using Laser-Structured Graphite Anodes in Lithium-Ion Batteries. J. Electrochem. Soc. 2019, 167, 013506. [Google Scholar] [CrossRef]
- Park, J.; Hyeon, S.; Jeong, S.; Kim, H.-J. Performance enhancement of Li-ion battery by laser structuring of thick electrode with low porosity. J. Ind. Eng. Chem. 2018, 70, 178–185. [Google Scholar] [CrossRef]
- Yi, X.; Yu, W.-J.; Tsiamtsouri, M.A.; Zhang, F.; He, W.; Dai, Q.; Hu, S.; Tong, H.; Zheng, J.; Zhang, B.; et al. Highly conductive C-Si@G nanocomposite as a high-performance anode material for Li-ion batteries. Electrochim. Acta 2018, 295, 719–725. [Google Scholar] [CrossRef]
- Dong, Z.; Gu, H.; Du, W.; Feng, Z.; Zhang, C.; Jiang, Y.; Zhu, T.; Chen, G.; Chen, J.; Liu, Y.; et al. Si/Ti3SiC2 composite anode with enhanced elastic modulus and high electronic conductivity for lithium-ion batteries. J. Power Sources 2019, 431, 55–62. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Q.; Pan, J.; Xu, J.; Liu, Y.; Al-Shroofy, M.; Cheng, Y.-T. Low-Temperature Treated Lignin as Both Binder and Conductive Additive for Silicon Nanoparticle Composite Electrodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 32341–32348. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-J.; Park, S.-J. Hydrogen Storage Behaviors of Porous Carbons Derived from Poly(vinylidene fluoride). J. Nanosci. Nanotechnol. 2017, 17, 8075–8080. [Google Scholar] [CrossRef]
- Bisquert, J.; Compte, A. Theory of the electrochemical impedance of anomalous diffusion. J. Electroanal. Chem. 2001, 499, 112–120. [Google Scholar] [CrossRef]
- Diard, J.-P.; Montella, C. Diffusion-trapping impedance under restricted linear diffusion conditions. J. Electroanal. Chem. 2003, 557, 19–36. [Google Scholar] [CrossRef]
- Thorat, I.V.; Stephenson, D.E.; Zacharias, N.A.; Zaghib, K.; Harb, J.N.; Wheeler, D.R. Quantifying tortuosity in porous Li-ion battery materials. J. Power Sources 2009, 188, 592–600. [Google Scholar] [CrossRef]
- Landesfeind, J.; Ebner, M.; Eldiven, A.; Wood, V.; Gasteiger, H. Tortuosity of Battery Electrodes: Validation of Impedance-Derived Values and Critical Comparison with 3D Tomography. J. Electrochem. Soc. 2018, 165, A469–A476. [Google Scholar] [CrossRef]
- Taer, E.; Agustino, A.; Farma, R.; Taslim, R.; Awitdrus, A.; Paiszal, M.; Ira, A.; Yardi, S.D.; Sari, Y.P.; Yusra, H. The relationship of surface area to cell capacitance for monolith carbon electrode from biomass materials for supercapacitor application. J. Phys. Conf. Ser. 2018, 1116, 032040. [Google Scholar] [CrossRef]
- Yoon, Y.; Yan, B.; Surendranath, Y. Suppressing Ion Transfer Enables Versatile Measurements of Electrochemical Surface Area for Intrinsic Activity Comparisons. J. Am. Chem. Soc. 2017, 140, 2397–2400. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Li, Y.; Feng, Y.; Cao, Y.; Cao, C.; Long, P.; Li, S.; Feng, W. Reduced graphene oxide doped predominantly with CF2 groups as a superior anode material for long-life lithium-ion batteries. Chem. Commun. 2018, 54, 2727–2730. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bettge, M.; Bareño, J.; Trask, S.E.; Abraham, D.P. Exploring Electrochemistry and Interface Characteristics of Lithium-Ion Cells with Li1.2Ni0.15Mn0.55Co0.1O2 Positive and Li4Ti5O12 Negative Electrodes. J. Electrochem. Soc. 2015, 162, A7049. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Meng, Y.; Li, X.; Guo, Y.; Xiao, D. Sodium storage in fluorine-rich mesoporous carbon fabricated by low-temperature carbonization of polyvinylidene fluoride with a silica template. RSC Adv. 2016, 6, 110850–110857. [Google Scholar] [CrossRef]
- Zuo, X.; Fan, C.; Xiao, X.; Liu, J.; Nan, J. High-voltage performance of LiCoO2/graphite batteries with methylene methanedisulfonate as electrolyte additive. J. Power Sources 2012, 219, 94–99. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Simple one-step synthesis of fluorine-doped carbon nanoparticles as potential alternative metal-free electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 9972–9981. [Google Scholar] [CrossRef]
- Lv, P.; Zhao, H.; Wang, J.; Liu, X.; Zhang, T.; Xia, Q. Facile preparation and electrochemical properties of amorphous SiO2/C composite as anode material for lithium ion batteries. J. Power Sources 2013, 237, 291–294. [Google Scholar] [CrossRef]
- Zhou, N.; Wu, Y.; Li, Y.; Yang, J.; Zhou, Q.; Guo, Y.; Xia, M.; Zhou, Z. Interconnected structure Si@TiO2-B/CNTs composite anode applied for high-energy lithium-ion batteries. Appl. Surf. Sci. 2020, 500, 144026. [Google Scholar] [CrossRef]
- Landesfeind, J.; Hattendorff, J.; Ehrl, A.; Wall, W.A.; Gasteiger, H.A. Tortuosity Determination of Battery Electrodes and Separators by Impedance Spectroscopy. J. Electrochem. Soc. 2016, 163, A1373–A1387. [Google Scholar] [CrossRef]
- Kim, J.; Zhou, R.; Murakoshi, K.; Yasuda, S. Advantage of semi-ionic bonding in fluorine-doped carbon materials for the oxygen evolution reaction in alkaline media. RSC Adv. 2018, 8, 14152–14156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, T.; Chen, J.; Wang, C.; Qian, Y.; Lu, L. Facile synthesis of fluorine-doped graphene aerogel with rich semi-ionic C–F bonds for high-performance supercapacitor application. J. Mater. Sci. 2020, 55, 12103–12113. [Google Scholar] [CrossRef]
- Yan, Z.; Huang, D.; Fan, X.; Zheng, F.; Pan, Q.; Ma, Z.; Wang, H.; Huang, Y.; Li, Q. Fluorine-Doped Carbon Coated LiFePO3.938F0.062 Composites as Cathode Materials for High-Performance Lithium-Ion Batteries. Front. Mater. 2020, 6, 341. [Google Scholar] [CrossRef] [Green Version]
- Heubner, C.; Nikolowski, K.; Reuber, S.; Schneider, M.; Wolter, M.; Michaelis, A. Recent Insights into Rate Performance Limitations of Li-ion Batteries. Batter. Supercaps 2020, 4, 268–285. [Google Scholar] [CrossRef]
- Forouhi, A.R.; Bloomer, I. Optical dispersion relations for amorphous semiconductors and amorphous dielectrics. Phys. Rev. B 1986, 34, 7018–7026. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Erb, R.M.; Wang, J.; Wang, J.; Chiang, Y.-M. Fabrication of Low-Tortuosity Ultrahigh-Area-Capacity Battery Electrodes through Magnetic Alignment of Emulsion-Based Slurries. Adv. Energy Mater. 2019, 9, 1802472. [Google Scholar] [CrossRef]
- Javed, M.S.; Shaheen, N.; Hussain, S.; Li, J.; Shah, S.S.A.; Abbas, Y.; Ahmad, M.A.; Raza, R.; Mai, W. An ultra-high energy density flexible asymmetric supercapacitor based on hierarchical fabric decorated with 2D bimetallic oxide nanosheets and MOF-derived porous carbon polyhedral. J. Mater. Chem. 2019, 7, 946–957. [Google Scholar] [CrossRef]
- Park, K.-Y.; Park, J.-W.; Seong, W.M.; Yoon, K.; Hwang, T.-H.; Ko, K.-H.; Han, J.-H.; Jaedong, Y.; Kang, K. Understanding capacity fading mechanism of thick electrodes for lithium-ion rechargeable batteries. J. Power Sources 2020, 468, 228369. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Ju, M.J.; Xu, J.; Choi, H.-J.; Seo, J.-M.; Kim, M.-J.; Choi, I.T.; Kim, H.M.; Kim, J.C.; Lee, J.-J.; et al. Edge-Fluorinated Graphene Nanoplatelets as High Performance Electrodes for Dye-Sensitized Solar Cells and Lithium Ion Batteries. Adv. Funct. Mater. 2015, 25, 1170–1179. [Google Scholar] [CrossRef]
- Park, J.; Jeon, C.; Kim, W.; Bong, S.-J.; Jeong, S.; Kim, H.-J. Challenges, laser processing and electrochemical characteristics on application of ultra-thick electrode for high-energy lithium-ion battery. J. Power Sources 2020, 482, 228948. [Google Scholar] [CrossRef]
- Orikasa, Y.; Gogyo, Y.; Yamashige, H.; Katayama, M.; Chen, K.; Mori, T.; Yamamoto, K.; Masese, T.; Inada, Y.; Ohta, T.; et al. Ionic Conduction in Lithium Ion Battery Composite Electrode Governs Cross-sectional Reaction Distribution. Sci. Rep. 2016, 6, 26382. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Chen, C.; Kirsch, D.; Hu, L. Thick Electrode Batteries: Principles, Opportunities, and Challenges. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Pfleging, W.; Pröll, J. A new approach for rapid electrolyte wetting in tape cast electrodes for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 14918–14926. [Google Scholar] [CrossRef]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
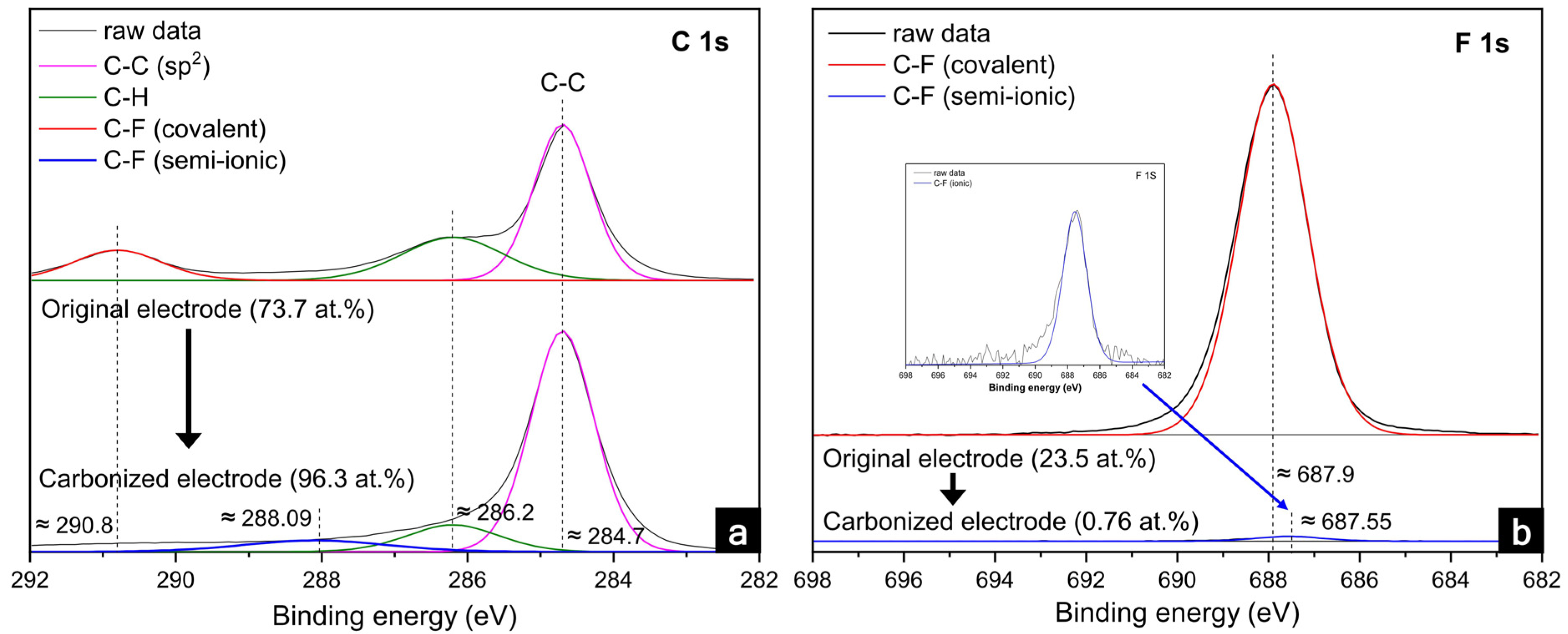
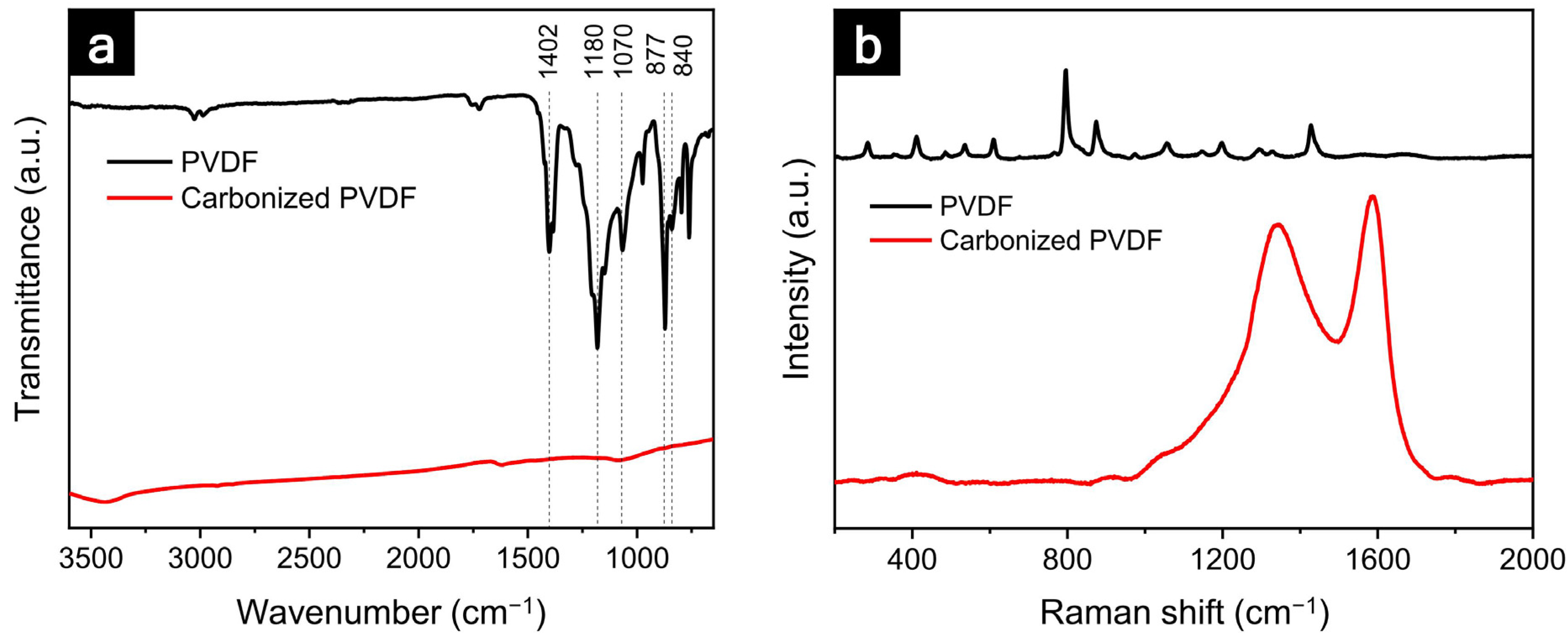

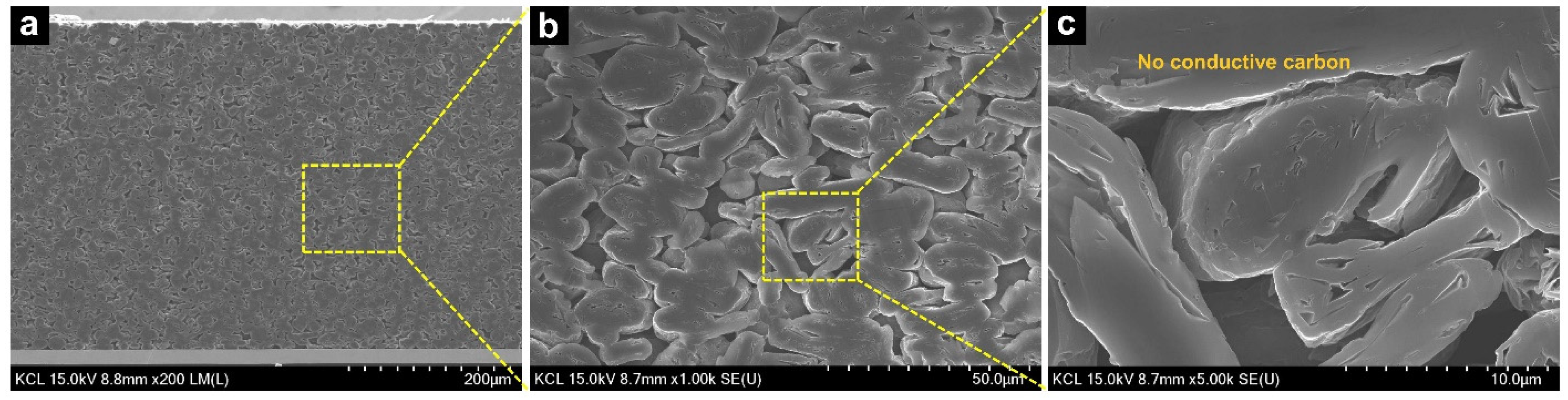
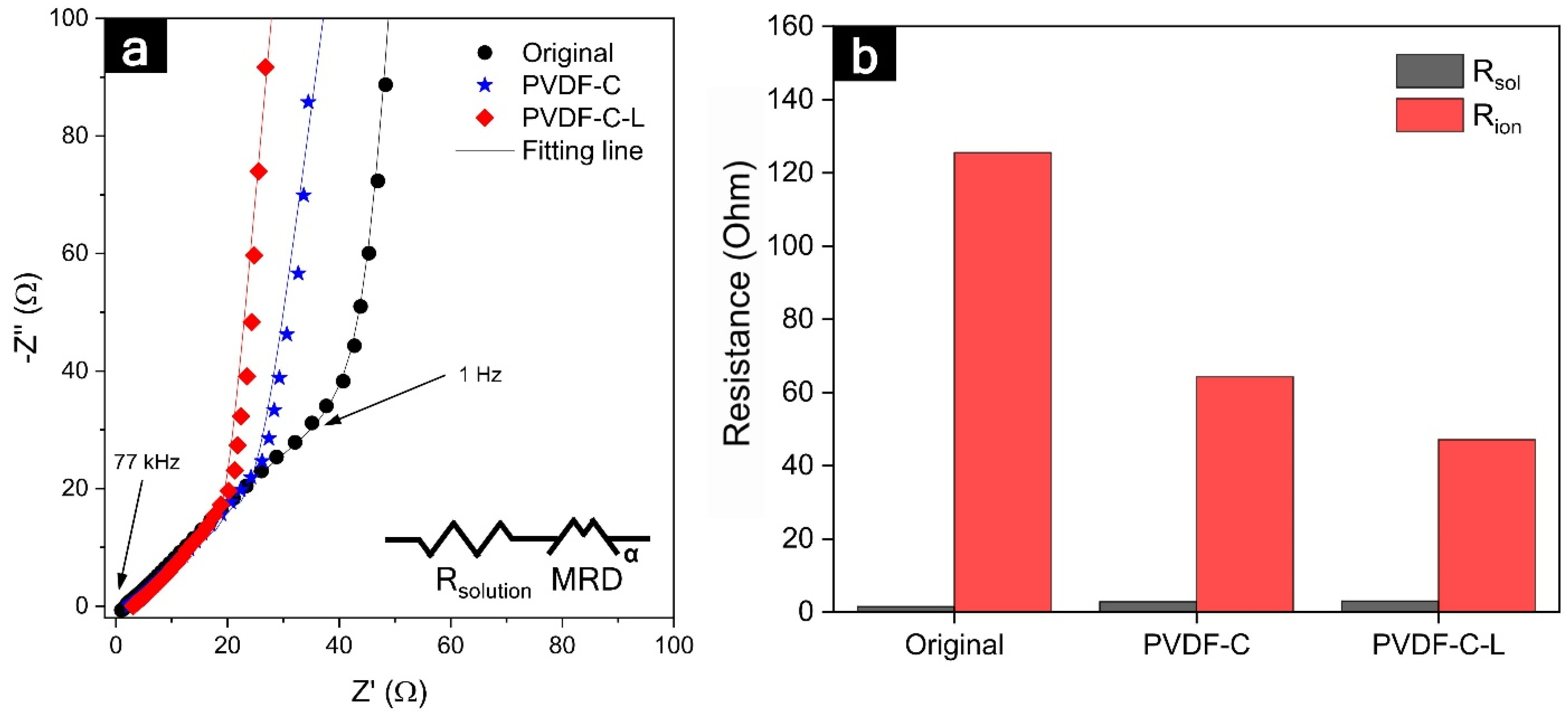
| Peak | Binding Energy (eV) | Assignment | Original (Fraction of Species, %) | PVDF-C (Fraction of Species, %) |
|---|---|---|---|---|
| C 1s | 284.7 | C–C (sp2) | 55.7 | 74.6 |
| 286.2 | C–H | 27.5 | 20.2 | |
| 288.09 | C–F (semi-ionic) | - | 5.2 | |
| 290.8 | C–F (covalent) | 16.8 | - | |
| F 1s | 687.55 | C–F (semi-ionic) | - | 100 |
| 687.9 | C–F (covalent) | 100 | - |
| Electrode Porosity (ε) | Rion | Electrode Thickness (t) | Tortuosity (τ) | |
|---|---|---|---|---|
| Original | 35% | 125.5 ohm | 414 µm | 6.8 |
| PVDF-C | 39% | 64.3 ohm | 413 µm | 3.9 |
| PVDF-C-L | 47% | 47.1 ohm | 421 µm | 3.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Suh, S.; Tamulevičius, S.; Kim, D.; Choi, D.; Jeong, S.; Kim, H.-J. Practical Approaches to Apply Ultra-Thick Graphite Anode to High-Energy Lithium-Ion Battery: Carbonization and 3-Dimensionalization. Nanomaterials 2022, 12, 2625. https://doi.org/10.3390/nano12152625
Park J, Suh S, Tamulevičius S, Kim D, Choi D, Jeong S, Kim H-J. Practical Approaches to Apply Ultra-Thick Graphite Anode to High-Energy Lithium-Ion Battery: Carbonization and 3-Dimensionalization. Nanomaterials. 2022; 12(15):2625. https://doi.org/10.3390/nano12152625
Chicago/Turabian StylePark, Junsu, Seokho Suh, Sigitas Tamulevičius, Daesoo Kim, Dongin Choi, Sungho Jeong, and Hyeong-Jin Kim. 2022. "Practical Approaches to Apply Ultra-Thick Graphite Anode to High-Energy Lithium-Ion Battery: Carbonization and 3-Dimensionalization" Nanomaterials 12, no. 15: 2625. https://doi.org/10.3390/nano12152625
APA StylePark, J., Suh, S., Tamulevičius, S., Kim, D., Choi, D., Jeong, S., & Kim, H.-J. (2022). Practical Approaches to Apply Ultra-Thick Graphite Anode to High-Energy Lithium-Ion Battery: Carbonization and 3-Dimensionalization. Nanomaterials, 12(15), 2625. https://doi.org/10.3390/nano12152625







