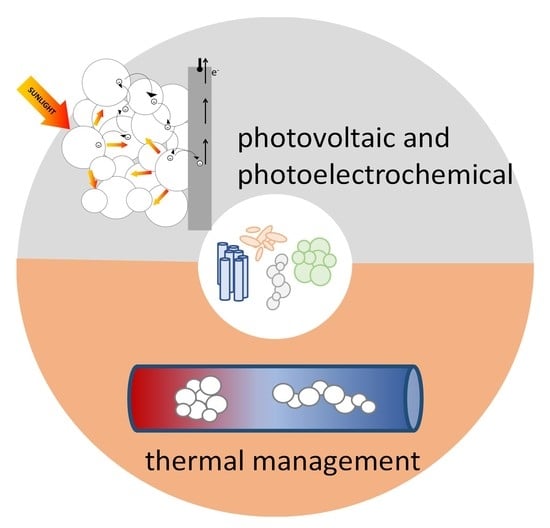
On the Morphology of Nanostructured TiO2 for Energy Applications: The Shape of the Ubiquitous Nanomaterial
Abstract
1. Introduction
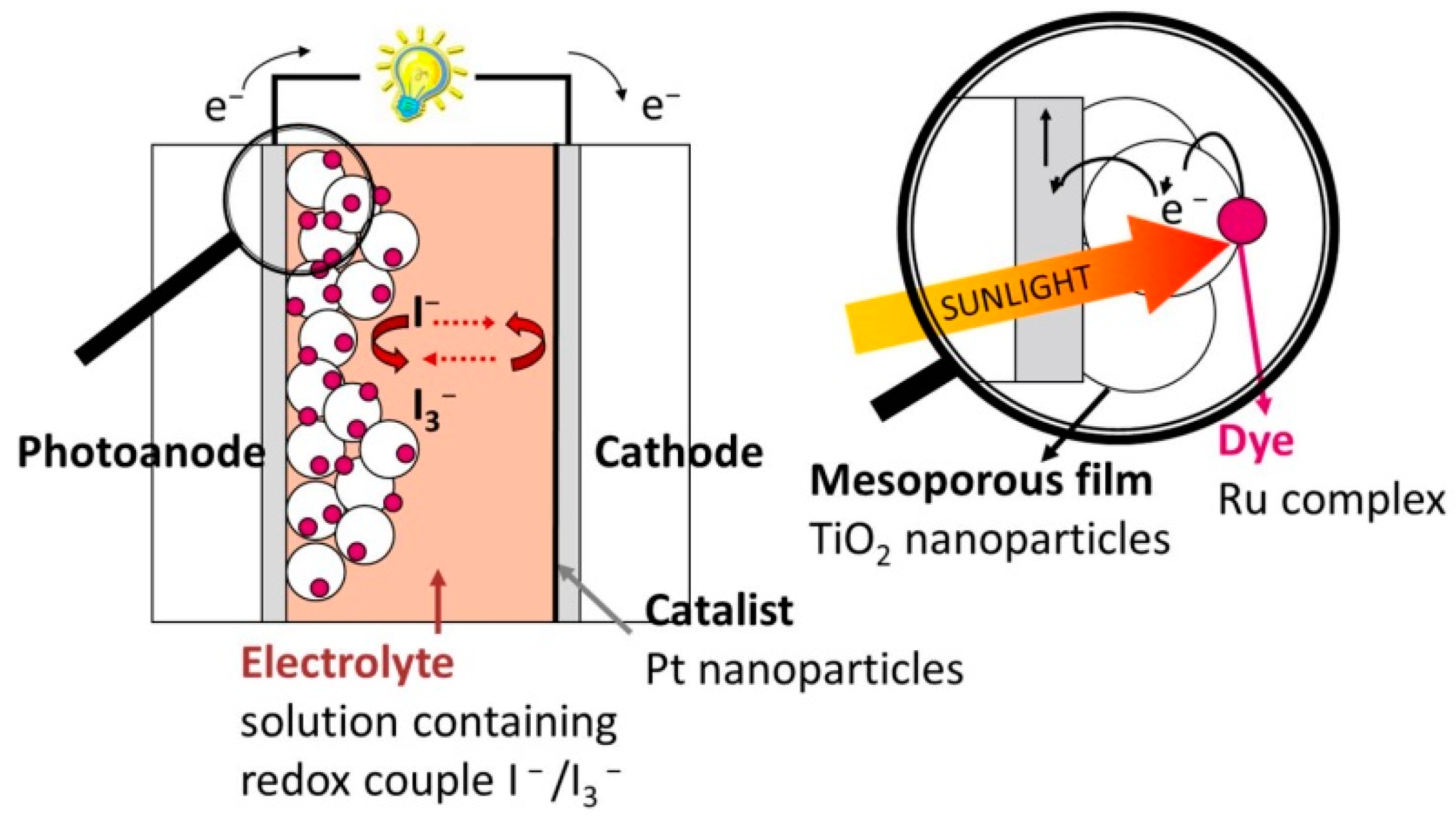
2. Photovoltaic Energy Conversion
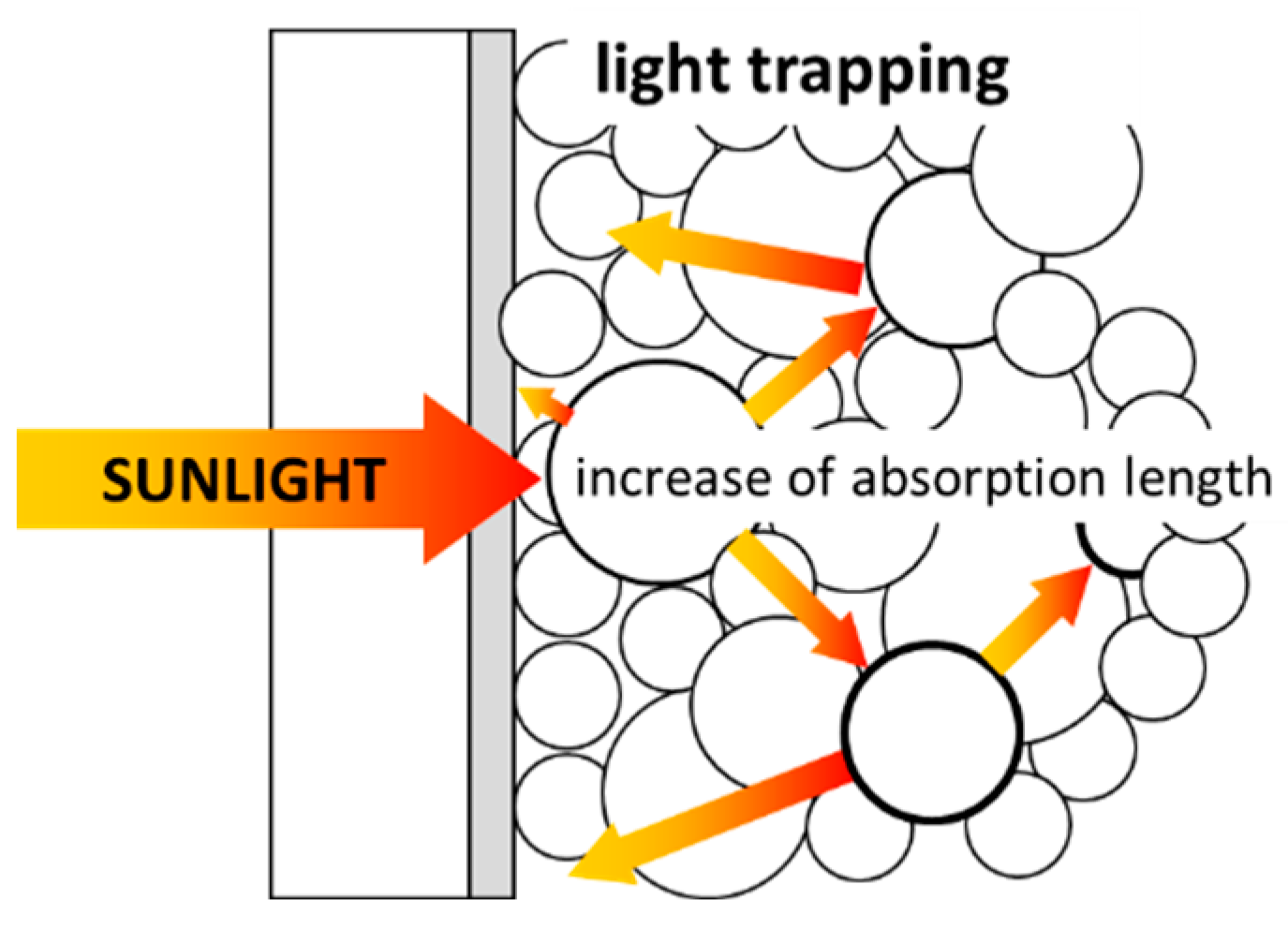
2.1. Light Management
2.2. Transport Properties
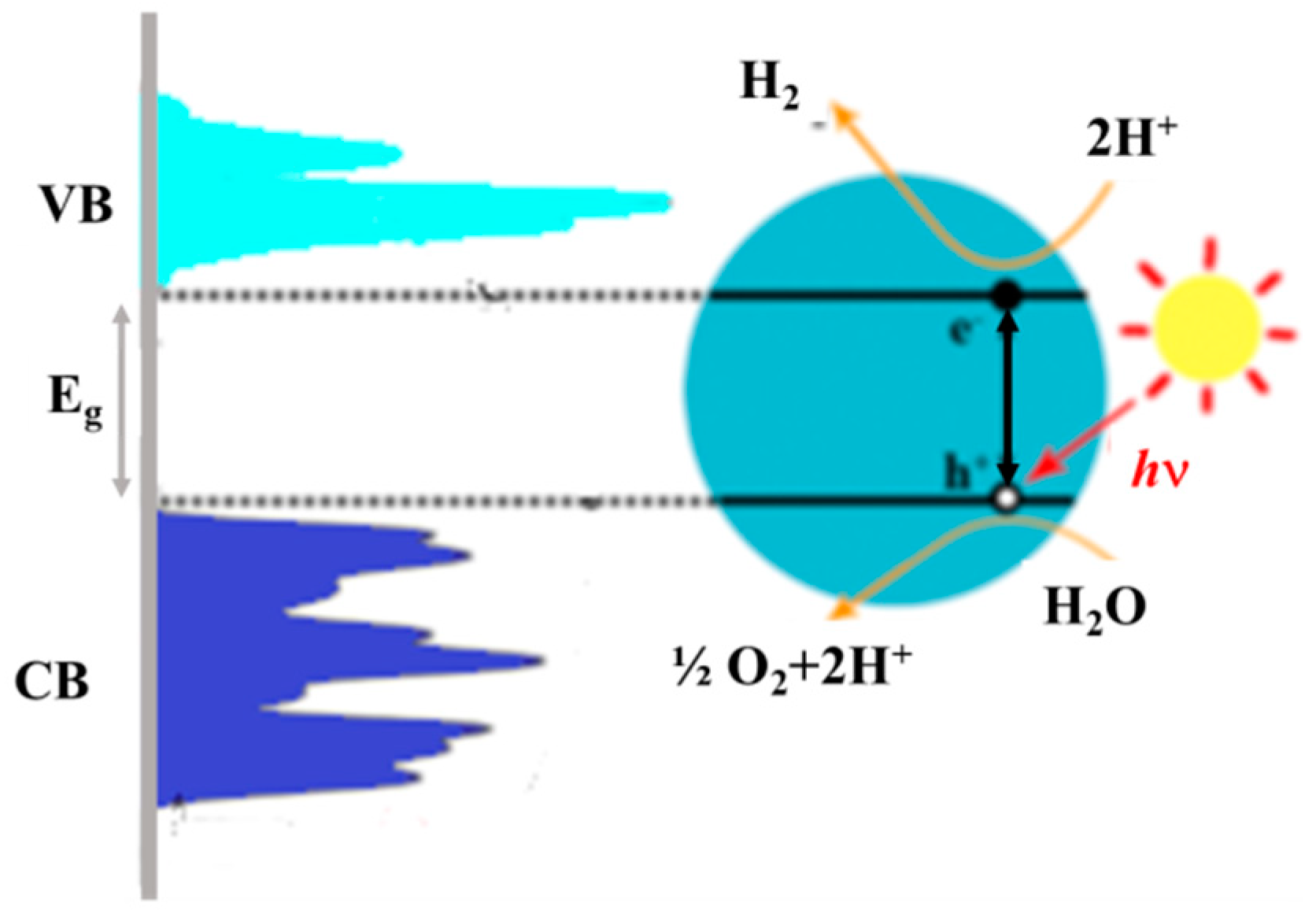
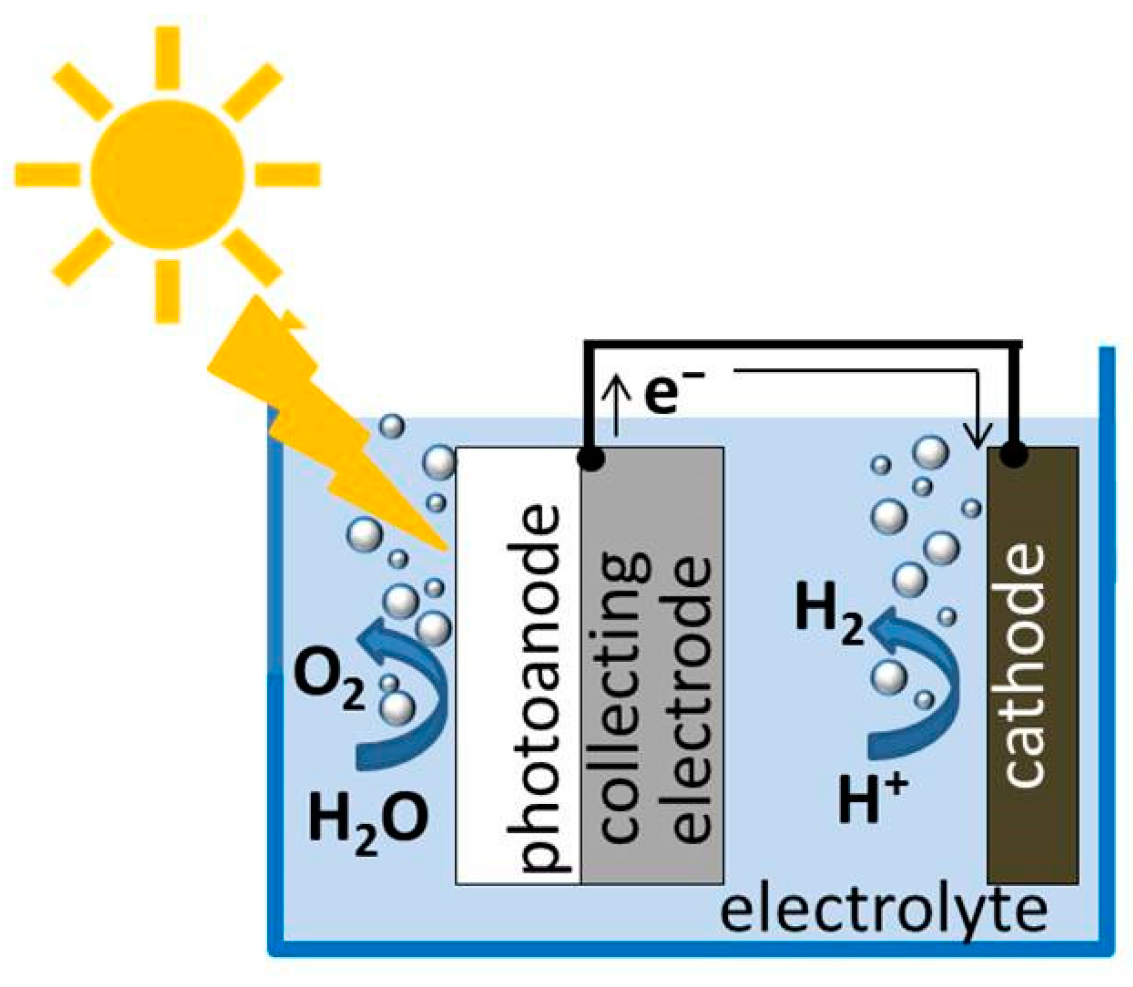
3. Photoelectrochemical Water Splitting
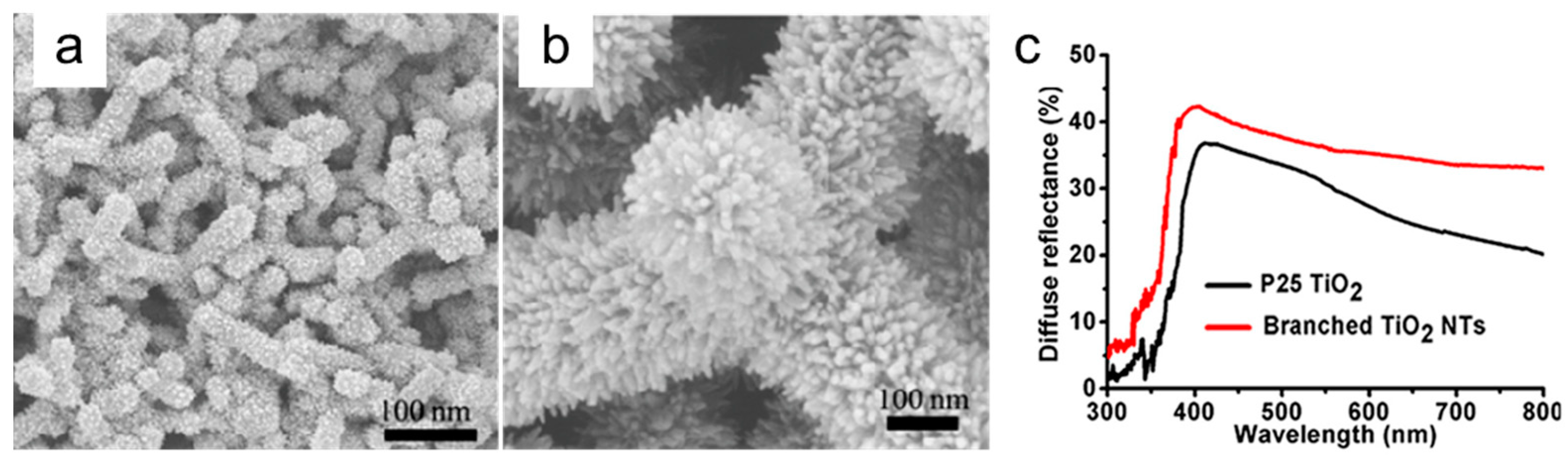
3.1. Light Management
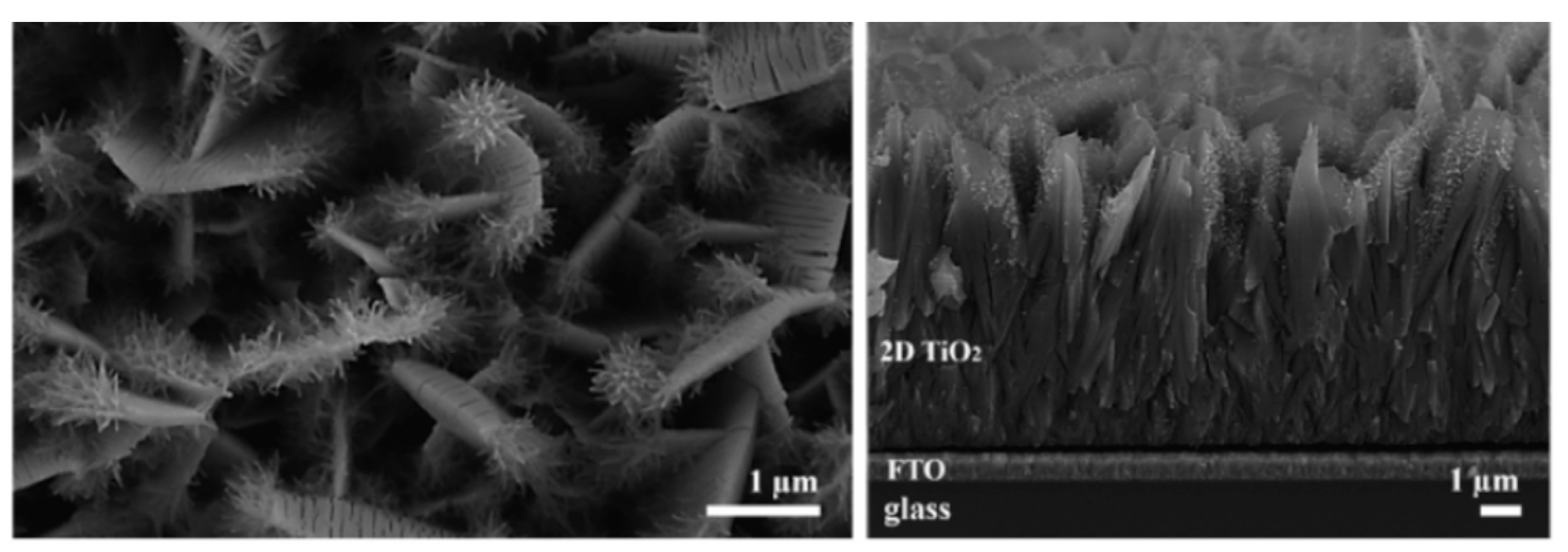
3.2. Surface Reactivity and Electron Transport
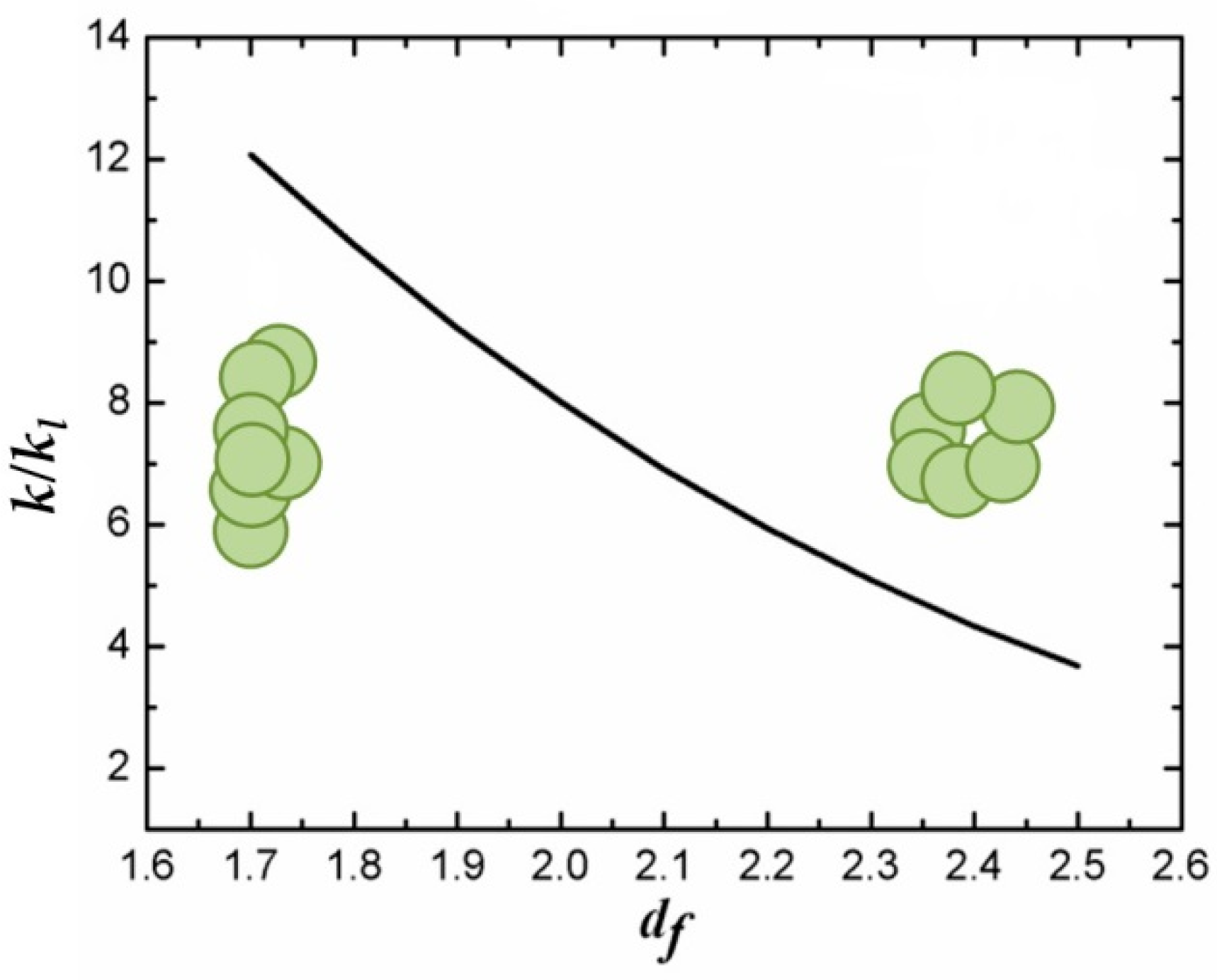
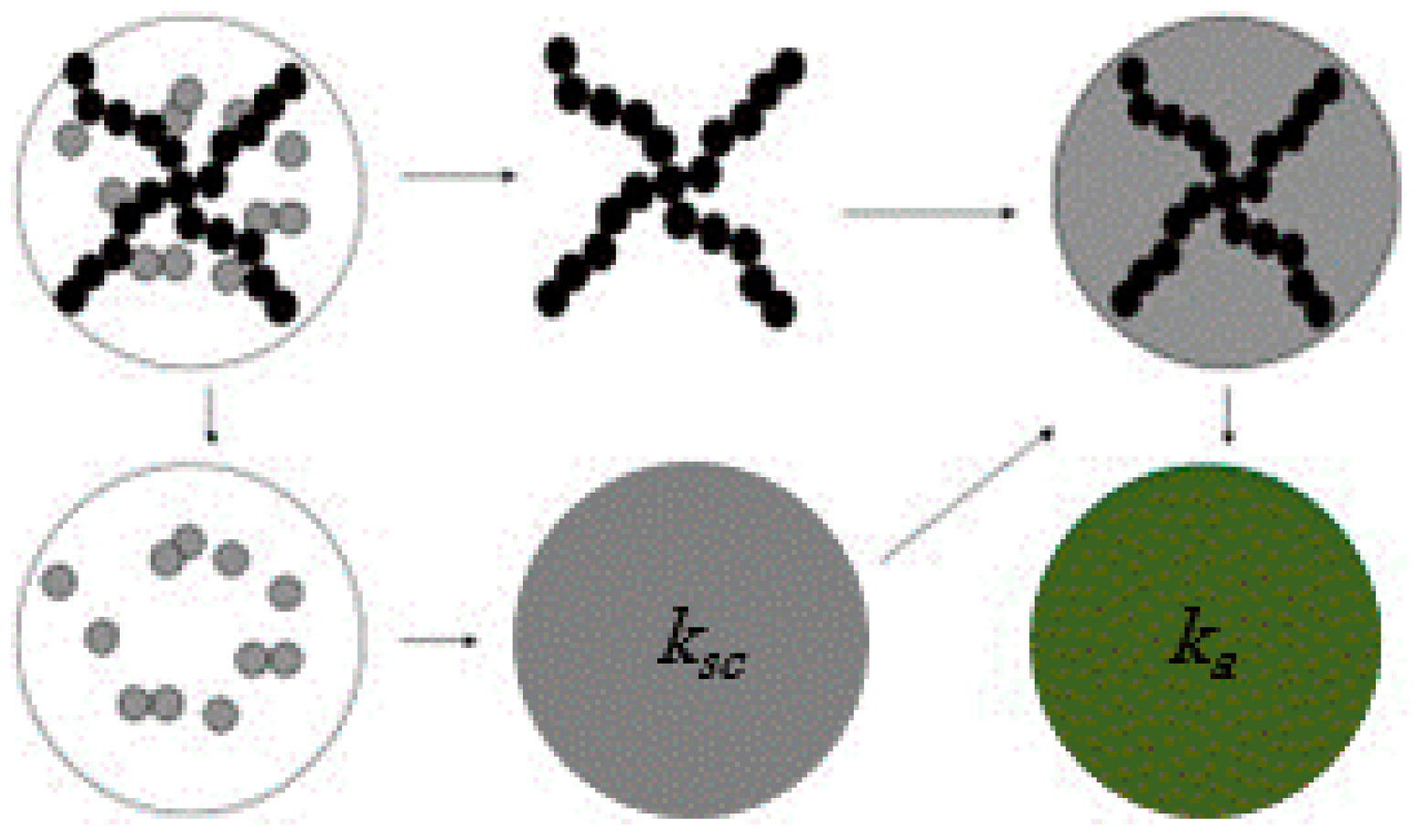
4. Thermal Energy Management by Nanofluids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Akakuru, O.U.; Iqbal, Z.M.; Wu, A. TiO2 Nanoparticles: Properties and applications. In TiO2 Nanoparticles; John Wiley & Sons, Ltd.: New York, NY, USA, 2020; pp. 1–66. [Google Scholar] [CrossRef]
- Lan, Y.; Cook, L.; Lisfi, A. New Applications of TiO2 Nanoparticles in Renewable Energy. In Titanium Dioxide Nanoparticles: Characterizations, Properties and Syntheses; NOVA Science Publisher: Hauppauge, NY, USA, 2017; p. 241. [Google Scholar]
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced Research Trends in Dye-Sensitized Solar Cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Zafar, M. Titanium Dioxide Nanostructures as Efficient Photocatalyst: Progress, Challenges and Perspective. Int. J. Energy Res. 2021, 45, 3569–3589. [Google Scholar] [CrossRef]
- Madian, M.; Eychmüller, A.; Giebeler, L. Current Advances in TiO2-Based Nanostructure Electrodes for High Performance Lithium Ion Batteries. Batteries 2018, 4, 7. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Bertel, L.; Miranda, D.A.; García-Martín, J.M. Nanostructured Titanium Dioxide Surfaces for Electrochemical Biosensing. Sensors 2021, 21, 6167. [Google Scholar] [CrossRef]
- Amri, F.; Septiani, N.L.W.; Rezki, M.; Iqbal, M.; Yamauchi, Y.; Golberg, D.; Kaneti, Y.V.; Yuliarto, B. Mesoporous TiO2-Based Architectures as Promising Sensing Materials towards next-Generation Biosensing Applications. J. Mater. Chem. B 2021, 9, 1189–1207. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.U.; Zhao, C.; Jiang, H.; Wang, X. Biomedical Applications of Nano-Titania in Theranostics and Photodynamic Therapy. Biomater. Sci. 2015, 4, 40–54. [Google Scholar] [CrossRef]
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A Review of Its Properties and Conflicting Trends. Chem. Eng. J. 2020, 389, 123918. [Google Scholar] [CrossRef]
- Zhu, T.; Gao, S.-P. The Stability, Electronic Structure, and Optical Property of TiO2 Polymorphs. J. Phys. Chem. C 2014, 118, 11385–11396. [Google Scholar] [CrossRef]
- Low, I.-M.J. Rutile: Properties, Synthesis and Applications; Rutile: Properties, Synthesis and Applications. In Rutile: Properties, Synthesis and Applications; Rutile: Properties, Synthesis and Applications; NOVA Science Publisher: Hauppauge, NY, USA, 2020; p. 240. [Google Scholar]
- Manzoli, M.; Freyria, F.S.; Blangetti, N.; Bonelli, B. Brookite, a Sometimes under Evaluated TiO2 Polymorph. RSC Adv. 2022, 12, 3322–3334. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New Understanding of the Difference of Photocatalytic Activity among Anatase, Rutile and Brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Wang, X.; Wu, K.; He, X.; Zhang, R. Mesoporous Titanium Dioxide: Synthesis and Applications in Photocatalysis, Energy and Biology. Materials 2018, 11, 1910. [Google Scholar] [CrossRef]
- Gerischer, H.; Willig, F. Reaction of Excited Dye Molecules at Electrodes. In Physical and Chemical Applications of Dyestuffs; Topics in Current Chemistry; Schäfer, F.P., Gerischer, H., Willig, F., Meier, H., Jahnke, H., Schönborn, M., Zimmermann, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1976; pp. 31–84. [Google Scholar] [CrossRef]
- Gratzel, M. (Ed.) Energy Resources through Photochemistry and Catalysis, 1st ed.; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- O’Regan, B.; Grätzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Halme, J.; Vahermaa, P.; Miettunen, K.; Lund, P. Device Physics of Dye Solar Cells. Adv. Mater. 2010, 22, E210–E234. [Google Scholar] [CrossRef]
- Amadelli, R.; Argazzi, R.; Bignozzi, C.A.; Scandola, F. Design of Antenna-Sensitizer Polynuclear Complexes. Sensitization of Titanium Dioxide with [Ru(Bpy)2(CN)2]2Ru(Bpy(COO)2)22-. J. Am. Chem. Soc. 1990, 112, 7099–7103. [Google Scholar] [CrossRef]
- Ren, Y.; Flores-Díaz, N.; Zhang, D.; Cao, Y.; Decoppet, J.-D.; Fish, G.C.; Moser, J.-E.; Zakeeruddin, S.M.; Wang, P.; Hagfeldt, A. Grätzel, M., Blue Photosensitizer with Copper(II/I) Redox Mediator for Efficient and Stable Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2020, 30, 2004804. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-Sensitized Solar Cells for Efficient Power Generation under Ambient Lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Saeed, M.A.; Kang, H.C.; Yoo, K.; Asiam, F.K.; Lee, J.-J.; Shim, J.W. Cosensitization of metal-based dyes for high-performance dye-sensitized photovoltaics under ambient lighting conditions. Dye Pigment. 2021, 194, 109624. [Google Scholar] [CrossRef]
- Saeed, M.A.; Yoo, K.; Kang, H.C.; Shim, J.W.; Lee, J.-J. Recent developments in dye-sensitized photovoltaic cells under ambient illumination. Dye Pigment. 2021, 194, 109626. [Google Scholar] [CrossRef]
- Luque, A.; Hegedus, S. (Eds.) Handbook of Photovoltaic Science and Engineering, 2nd ed.; Wiley: Hoboken, NJ, USA, 2003; pp. 154–196. [Google Scholar]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Redfield, D. Multiple-pass Thin-film Silicon Solar Cell. Appl. Phys. Lett. 1974, 25, 647–648. [Google Scholar] [CrossRef]
- Yablonovitch, E. Statistical Ray Optics. JOSA 1982, 72, 899–907. [Google Scholar] [CrossRef]
- Zhou, D.; Biswas, R. Photonic Crystal Enhanced Light-Trapping in Thin Film Solar Cells. J. Appl. Phys. 2008, 103, 093102. [Google Scholar] [CrossRef]
- Usami, A. Theoretical study of application of multiple scattering of light to a dye-sensitized nanocrystalline photoelectrichemical cell. Chem. Physi. Lett. 1997, 277, 105–108. [Google Scholar] [CrossRef]
- Usami, A. Theoretical Simulations of Optical Confinement in Dye-Sensitized Nanocrystalline Solar Cells. Sol. Energy Mater. Sol. Cells 2000, 64, 73–83. [Google Scholar] [CrossRef]
- Shigenori, T. Performance Simulation for Dye-Sensitized Solar Cells: Toward High Efficiency and Solid State. Jpn. J. Appl. Phys. 2001, 40, 97–107. [Google Scholar]
- Rothenberger, G.; Comte, P.; Grätzel, M. A contribution to the optical design of dye-sensitized nanocrystalline solar cells. Sol. Energy Mater. Sol. Cells 1999, 58, 321–336. [Google Scholar] [CrossRef]
- Gagliardi, S.; Falconieri, M. Experimental Determination of the Light-Trapping-Induced Absorption Enhancement Factor in DSSC Photoanodes. Beilstein J. Nanotechnol. 2015, 6, 886–892. [Google Scholar] [CrossRef]
- Ito, S.; Zakeeruddin, S.M.; Humphry-Baker, R.; Liska, P.; Charvet, R.; Comte, P.; Nazeeruddin, M.K.; Péchy, P.; Takata, M.; Miura, H.; et al. High-Efficiency Organic-Dye- Sensitized Solar Cells Controlled by Nanocrystalline-TiO2 Electrode Thickness. Adv. Mater. 2006, 18, 1202–1205. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Shi, D.; Zhang, J.; Wang, M.; Jing, X.; Humphry-Baker, R.; Wang, P.; Zakeeruddin, S.M.; Grätzel, M. Enhance the Optical Absorptivity of Nanocrystalline TiO2 Film with High Molar Extinction Coefficient Ruthenium Sensitizers for High Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2008, 130, 10720–10728. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Islam, A.; Komiya, R.; Koide, N.; Han, L. Conversion Efficiency of 10.8% by a Dye-Sensitized Solar Cell Using a TiO2 Electrode with High Haze. Appl. Phys. Lett. 2006, 88, 223505. [Google Scholar] [CrossRef]
- Gálvez, F.E.; Kemppainen, E.; Míguez, H.; Halme, J. Effect of Diffuse Light Scattering Designs on the Efficiency of Dye Solar Cells: An Integral Optical and Electrical Description. J. Phys. Chem. C 2012, 116, 11426–11433. [Google Scholar] [CrossRef]
- Agrios, A.G.; Cesar, I.; Comte, P.; Nazeeruddin, M.K.; Grätzel, M. Nanostructured Composite Films for Dye-Sensitized Solar Cells by Electrostatic Layer-by-Layer Deposition. Chem. Mater. 2006, 18, 5395–5397. [Google Scholar] [CrossRef]
- Lee, K.-M.; Suryanarayanan, V.; Ho, K.-C. The Influence of Surface Morphology of TiO2 Coating on the Performance of Dye-Sensitized Solar Cells. Sol. Energy Mater. Sol. Cells 2006, 90, 2398–2404. [Google Scholar] [CrossRef]
- Huang, F.; Chen, D.; Zhang, X.L.; Caruso, R.A.; Cheng, Y.-B. Dual-Function Scattering Layer of Submicrometer-Sized Mesoporous TiO2 Beads for High-Efficiency Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2010, 20, 1301–1305. [Google Scholar] [CrossRef]
- Koo, H.-J.; Kim, Y.J.; Lee, Y.H.; Lee, W.I.; Kim, K.; Park, N.-G. Nano-Embossed Hollow Spherical TiO2 as Bifunctional Material for High-Efficiency Dye-Sensitized Solar Cells. Adv. Mater. 2008, 20, 195–199. [Google Scholar] [CrossRef]
- Shao, W.; Gu, F.; Gai, L.; Li, C. Planar Scattering from Hierarchical Anatase TiO2 Nanoplates with Variable Shells to Improve Light Harvesting in Dye-Sensitized Solar Cells. Chem. Commun. 2011, 47, 5046–5048. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, F.; Li, J.; Dong, H.; Li, Q.; Jiang, K.; Xu, D. Monodisperse TiO2 Hierarchical Hollow Spheres Assembled by Nanospindles for Dye-Sensitized Solar Cells. J. Mater. Chem. 2012, 22, 11665–11671. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Dong, H.; Zhu, F.; Gao, S.; Jiang, K.; Fu, L.; Zhang, J.; Xu, D. Hierarchical TiO2 Microspheres Comprised of Anatase Nanospindles for Improved Electron Transport in Dye-Sensitized Solar Cells. Nanoscale 2012, 5, 324–330. [Google Scholar] [CrossRef]
- Chuangchote, S.; Sagawa, T.; Yoshikawa, S. Efficient Dye-Sensitized Solar Cells Using Electrospun TiO2 Nanofibers as a Light Harvesting Layer. Appl. Phys. Lett. 2008, 93, 033310. [Google Scholar] [CrossRef]
- Ghadiri, E.; Taghavinia, N.; Zakeeruddin, S.M.; Grätzel, M.; Moser, J.-E. Enhanced Electron Collection Efficiency in Dye-Sensitized Solar Cells Based on Nanostructured TiO2 Hollow Fibers. Nano Lett. 2010, 10, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chen, W.; Yang, S. Double-Layered Photoanodes from Variable-Size Anatase TiO2 Nanospindles: A Candidate for High-Efficiency Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2010, 49, 3675–3679. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.S.; Jose, R.; Shengyuan, Y.; Ramakrishna, S. A simple recipe for an efficient TiO2 nanofiber-based dye-sensitized solar cell. J. Colloid Interface Sci. 2011, 353, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced Charge-Collection Efficiencies and Light Scattering in Dye-Sensitized Solar Cells Using Oriented TiO2 Nanotubes Arrays. Nano Lett. 2007, 7, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Kubo, T.; Nishikitani, Y. Electrophoretically Deposited TiO2 Nanotube Light-Scattering Layers of Dye-Sensitized Solar Cells. Jpn. J. Appl. Phys. 2008, 47, 6610. [Google Scholar] [CrossRef]
- Liu, Z.; Misra, M. Dye-Sensitized Photovoltaic Wires Using Highly Ordered TiO2 Nanotube Arrays. ACS Nano 2010, 4, 2196–2200. [Google Scholar] [CrossRef] [PubMed]
- So, S.; Kriesch, A.; Peschel, U.; Schmuki, P. Conical-Shaped Titania Nanotubes for Optimized Light Management in DSSCs Reach Back-Side Illumination Efficiencies > 8%. J. Mater. Chem. A 2015, 3, 12603–12608. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Huang, F.; Chen, D.; Cheng, Y.-B.; Caruso, R.A. Thin Films of Dendritic Anatase Titania Nanowires Enable Effective Hole-Blocking and Efficient Light-Harvesting for High-Performance Mesoscopic Perovskite Solar Cells. Adv. Funct. Mater. 2015, 25, 3264–3272. [Google Scholar] [CrossRef]
- Jang, S.; Yoon, J.; Ha, K.; Kim, M.-C.; Kim, D.H.; Kim, S.M.; Kang, S.M.; Park, S.J.; Jung, H.S.; Choi, M. Facile fabrication of three-dimensional TiO2 structures for highly efficient perovskite solar cells. Nano Energy 2016, 22, 499–506. [Google Scholar] [CrossRef]
- Khan, J.; Rahman, N.U.; Khan, W.U.; Wang, Y.; Fu, S.; Ahmed, G.; Akhtar, M.N.; Wu, M. Hollow 3D TiO2 sub-microspheres as an electron transporting layer for highly efficient perovskite solar cells. Mater. Today Energy 2021, 19, 100614. [Google Scholar] [CrossRef]
- Ha, S.-J.; Heo, J.H.; Im, S.H.; Moon, J.H. Mesoscopic CH3NH3PbI3 Perovskite Solar Cells Using TiO2 Inverse Opal Electron-Conducting Scaffolds. J. Mater. Chem. A 2017, 5, 1972–1977. [Google Scholar] [CrossRef]
- Kang, S.M.; Jang, S.; Lee, J.-K.; Yoon, J.; Yoo, D.-E.; Lee, J.-W.; Choi, M.; Park, N.-G. Moth-Eye TiO2 Layer for Improving Light Harvesting Efficiency in Perovskite Solar Cells. Small 2016, 12, 2443–2449. [Google Scholar] [CrossRef]
- Moon, B.C.; Park, J.H.; Lee, D.K.; Tsvetkov, N.; Ock, I.; Choi, K.M.; Kang, J.K. Broadband Light Absorption and Efficient Charge Separation Using a Light Scattering Layer with Mixed Cavities for High-Performance Perovskite Photovoltaic Cells with Stability. Small 2017, 13, 1700418. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Nam, S.K.; Jung, K.; Moon, J.H. 2D Photonic Crystal Nanodisk Array as Electron Transport Layer for Highly Efficient Perovskite Solar Cells. Nano Energy 2019, 56, 365–372. [Google Scholar] [CrossRef]
- Haque, S.; Mendes, M.J.; Sanchez-Sobrado, O.; Águas, H.; Fortunato, E.; Martins, R. Photonic-Structured TiO2 for High-Efficiency, Flexible and Stable Perovskite Solar Cells. Nano Energy 2019, 59, 91–101. [Google Scholar] [CrossRef]
- Kim, D.I.; Lee, J.W.; Jeong, R.H.; Yu, J.-H.; Yang, J.W.; Nam, S.-H.; Boo, J.-H. Enhancing the Optical Properties Using Hemisphere TiO2 Photonic Crystal as the Electron Acceptor for Perovskite Solar Cell. Appl. Surf. Sci. 2019, 487, 409–415. [Google Scholar] [CrossRef]
- Zhong, J.-X.; Liao, J.-F.; Jiang, Y.; Wang, L.; Kuang, D.-B.; Wu, W.-Q. Synchronous Surface and Bulk Composition Management for Red-Shifted Light Absorption and Suppressed Interfacial Recombination in Perovskite Solar Cells. J. Mater. Chem. A 2020, 8, 9743–9752. [Google Scholar] [CrossRef]
- Schwarzburg, K.; Willig, F. Origin of Photovoltage and Photocurrent in the Nanoporous Dye-Sensitized Electrochemical Solar Cell. J. Phys. Chem. B 1999, 103, 5743–5746. [Google Scholar] [CrossRef]
- Solbrand, A.; Lindström, H.; Rensmo, H.; Hagfeldt, A.; Lindquist, S.-E.; Södergren, S. Electron Transport in the Nanostructured TiO2−Electrolyte System Studied with Time-Resolved Photocurrents. J. Phys. Chem. B 1997, 101, 2514–2518. [Google Scholar] [CrossRef]
- Koenenkamp, R.; Henninger, R.; Hoyer, P. Photocarrier Transport in Colloidal Titanium Dioxide Films. J. Phys. Chem. 1993, 97, 7328–7330. [Google Scholar] [CrossRef]
- Hoyer, P.; Weller, H. Potential-Dependent Electron Injection in Nanoporous Colloidal ZnO Films. J. Phys. Chem. 1995, 99, 14096–14100. [Google Scholar] [CrossRef]
- Cass, M.J.; Qiu, F.L.; Walker, A.B.; Fisher, A.C.; Peter, L.M. Influence of Grain Morphology on Electron Transport in Dye Sensitized Nanocrystalline Solar Cells. J. Phys. Chem. B 2003, 107, 113–119. [Google Scholar] [CrossRef]
- Enright, B.; Fitzmaurice, D. Spectroscopic Determination of Electron and Hole Effective Masses in a Nanocrystalline Semiconductor Film. J. Phys. Chem. 1996, 100, 1027–1035. [Google Scholar] [CrossRef]
- Benkstein, K.D.; Kopidakis, N.; van de Lagemaat, J.; Frank, A.J. Influence of the Percolation Network Geometry on Electron Transport in Dye-Sensitized Titanium Dioxide Solar Cells. J. Phys. Chem. B 2003, 107, 7759–7767. [Google Scholar] [CrossRef]
- van de Lagemaat, J.; Benkstein, K.D.; Frank, A.J. Relation between Particle Coordination Number and Porosity in Nanoparticle Films: Implications to Dye-Sensitized Solar Cells. J. Phys. Chem. B 2001, 105, 12433–12436. [Google Scholar] [CrossRef]
- Cao, F.; Oskam, G.; Meyer, G.J.; Searson, P.C. Electron Transport in Porous Nanocrystalline TiO2 Photoelectrochemical Cells. J. Phys. Chem. 1996, 100, 17021–17027. [Google Scholar] [CrossRef]
- Dloczik, L.; Ileperuma, O.; Lauermann, I.; Peter, L.M.; Ponomarev, E.A.; Redmond, G.; Shaw, N.J.; Uhlendorf, I. Dynamic Response of Dye-Sensitized Nanocrystalline Solar Cells: Characterization by Intensity-Modulated Photocurrent Spectroscopy. J. Phys. Chem. B 1997, 101, 10281–10289. [Google Scholar] [CrossRef]
- van der Zanden, B.; Goossens, A. The Nature of Electron Migration in Dye-Sensitized Nanostructured TiO2. J. Phys. Chem. B 2000, 104, 7171–7178. [Google Scholar] [CrossRef]
- Anta, J.A.; Dittrich, T.; Bisquert, J. Dynamics of Charge Separation and Trap-Limited Electron Transport in TiO2. J. Phys. Chem. C 2007, 111, 13997–14000. [Google Scholar] [CrossRef]
- van de Lagemaat, J.; Park, N.-G.; Frank, A.J. Influence of Electrical Potential Distribution, Charge Transport, and Recombination on the Photopotential and Photocurrent Conversion Efficiency of Dye-Sensitized Nanocrystalline TiO2 Solar Cells: A Study by Electrical Impedance and Optical Modulation Techniques. J. Phys. Chem. B 2000, 104, 2044–2052. [Google Scholar] [CrossRef]
- Kopidakis, N.; Schiff, E.A.; Park, N.-G.; van de Lagemaat, J.; Frank, A.J. Ambipolar Diffusion of Photocarriers in Electrolyte-Filled, Nanoporous TiO2. J. Phys. Chem. B 2000, 104, 3930–3936. [Google Scholar] [CrossRef]
- Solbrand, A.; Henningsson, A.; Södergren, S.; Lindström, H.; Hagfeldt, A.; Lindquist, S.-E. Charge Transport Properties in Dye-Sensitized Nanostructured TiO2 Thin Film Electrodes Studied by Photoinduced Current Transients. J. Phys. Chem. B 1999, 103, 1078–1083. [Google Scholar] [CrossRef]
- de Jongh, P.E.; Vanmaekelbergh, D. Trap-Limited Electronic Transport in Assemblies of Nanometer-Size TiO2 Particles. Phys. Rev. Lett. 1996, 77, 3427–3430. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.C.; Peter, L.M.; Ponomarev, E.A.; Walker, A.B.; Wijayantha, K.G.U. Intensity Dependence of the Back Reaction and Transport of Electrons in Dye-Sensitized Nanocrystalline TiO2 Solar Cells. J. Phys. Chem. B 2000, 104, 949–958. [Google Scholar] [CrossRef]
- Könenkamp, R. Carrier Transport in Nanoporous TiO2 Films. Phys. Rev. B 2000, 61, 11057–11064. [Google Scholar] [CrossRef]
- Nelson, J. Continuous-Time Random-Walk Model of Electron Transport in Nanocrystalline TiO2 Electrodes. Phys. Rev. B 1999, 59, 15374–15380. [Google Scholar] [CrossRef]
- Vanmaekelbergh, D.; de Jongh, P.E. Electron Transport in Disordered Semiconductors Studied by a Small Harmonic Modulation of the Steady State. Phys. Rev. B 2000, 61, 4699–4704. [Google Scholar] [CrossRef]
- van de Lagemaat, J.; Frank, A.J. Nonthermalized Electron Transport in Dye-Sensitized Nanocrystalline TiO2 Films: Transient Photocurrent and Random-Walk Modeling Studies. J. Phys. Chem. B 2001, 105, 11194–11205. [Google Scholar] [CrossRef]
- Falconieri, M.; Duva, G.; Gagliardi, S. On the Response Time of Dye-Sensitized Solar Cells to Pulsed Monochromatic Illumination. J. Phys. Appl. Phys. 2014, 47, 495102. [Google Scholar] [CrossRef]
- Bisquert, J.; Garcia-Belmonte, G.; Fabregat-Santiago, F.; Ferriols, N.S.; Bogdanoff, P.; Pereira, E.C. Doubling Exponent Models for the Analysis of Porous Film Electrodes by Impedance. Relaxation of TiO2 Nanoporous in Aqueous Solution. J. Phys. Chem. B 2000, 104, 2287–2298. [Google Scholar] [CrossRef]
- Shao, F.; Sun, J.; Gao, L.; Yang, S.; Luo, J. Growth of Various TiO2 Nanostructures for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2011, 115, 1819–1823. [Google Scholar] [CrossRef]
- Bakhshayesh, A.M.; Mohammadi, M.R.; Dadar, H.; Fray, D.J. Improved efficiency of dye-sensitized solar cells aided by corn-like TiO2 nanowires as the light scattering layer. Electrochim. Acta 2012, 90, 302–308. [Google Scholar] [CrossRef]
- Varghese, O.K.; Paulose, M.; Grimes, C.A. Long Vertically Aligned Titania Nanotubes on Transparent Conducting Oxide for Highly Efficient Solar Cells. Nat. Nanotechnol. 2009, 4, 592–597. [Google Scholar] [CrossRef]
- Paulose, M.; Shankar, K.; Varghese, O.K.; Mor, G.K.; Grimes, C.A. Application of Highly-Ordered TiO2 Nanotube-Arrays in Heterojunction Dye-Sensitized Solar Cells. J. Phys. Appl. Phys. 2006, 39, 2498–2503. [Google Scholar] [CrossRef]
- Zhong, P.; Que, W.; Zhang, J.; Jia, Q.; Wang, W.; Liao, Y.; Hu, X. Charge transport and recombination in dye-sensitized solar cells based on hybrid films of TiO2 particles/TiO2 nanotubes. J. Alloys Compd. 2011, 509, 7808–7813. [Google Scholar] [CrossRef]
- Villanueva-Cab, J.; Jang, S.-R.; Halverson, A.F.; Zhu, K.; Frank, A.J. Trap-Free Transport in Ordered and Disordered TiO2 Nanostructures. Nano Lett. 2014, 14, 2305–2309. [Google Scholar] [CrossRef]
- Qu, J.; Li, G.R.; Gao, X.P. One-Dimensional Hierarchical Titania for Fast Reaction Kinetics of Photoanode Materials of Dye-Sensitized Solar Cells. Energy Environ. Sci. 2010, 3, 2003. [Google Scholar] [CrossRef]
- Sauvage, F.; Di Fonzo, F.; Li Bassi, A.; Casari, C.S.; Russo, V.; Divitini, G.; Ducati, C.; Bottani, C.E.; Comte, P.; Graetzel, M. Hierarchical TiO2 Photoanode for Dye-Sensitized Solar Cells. Nano Lett. 2010, 10, 2562–2567. [Google Scholar] [CrossRef]
- Chen, W.; Chen, H.; Xu, G.; Xue, R.; Wang, S.; Li, Y.; Li, Y. Precise Control of Crystal Growth for Highly Efficient CsPbI2Br Perovskite Solar Cells. Joule 2019, 3, 191–204. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Lei, B.-X.; Rao, H.-S.; Xu, Y.-F.; Wang, Y.-F.; Su, C.-Y.; Kuang, D.-B. Hydrothermal Fabrication of Hierarchically Anatase TiO2 Nanowire Arrays on FTO Glass for Dye-Sensitized Solar Cells. Sci. Rep. 2013, 3, 1352. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential Deposition as a Route to High-Performance Perovskite-Sensitized Solar Cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Yadav, P.; Turren-Cruz, S.-H.; Prochowicz, D.; Tavakoli, M.M.; Pandey, K.; Zakeeruddin, S.M.; Grätzel, M.; Hagfeldt, A.; Saliba, M. Elucidation of Charge Recombination and Accumulation Mechanism in Mixed Perovskite Solar Cells. J. Phys. Chem. C 2018, 122, 15149–15154. [Google Scholar] [CrossRef]
- Shao, J.; Yang, S.; Lei, L.; Cao, Q.; Yu, Y.; Liu, Y. Pore Size Dependent Hysteresis Elimination in Perovskite Solar Cells Based on Highly Porous TiO2 Films with Widely Tunable Pores of 15–34 nm. Chem. Mater. 2016, 28, 7134–7144. [Google Scholar] [CrossRef]
- Leijtens, T.; Lauber, B.; Eperon, G.E.; Stranks, S.D.; Snaith, H.J. The Importance of Perovskite Pore Filling in Organometal Mixed Halide Sensitized TiO2-Based Solar Cells. J. Phys. Chem. Lett. 2014, 5, 1096–1102. [Google Scholar] [CrossRef]
- Yang, Y.; Ri, K.; Mei, A.; Liu, L.; Hu, M.; Liu, T.; Li, X.; Han, H. The Size Effect of TiO2 Nanoparticles on a Printable Mesoscopic Perovskite Solar Cell. J. Mater. Chem. A 2015, 3, 9103–9107. [Google Scholar] [CrossRef]
- Tanaka, Y.; Lim, S.L.; Goh, W.P.; Jiang, C.; Tee, S.Y.; Ye, T.; Li, X.; Nguyen, K.H.; Lee, C.J.J.; Ding, N.; et al. Fabrication of Mesoporous Titania Nanoparticles with Controlled Porosity and Connectivity for Studying the Photovoltaic Properties in Perovskite Solar Cells. Chem. Nano. Mat. 2018, 4, 394–400. [Google Scholar] [CrossRef]
- Sanchez, R.S.; Gonzalez-Pedro, V.; Lee, J.-W.; Park, N.-G.; Kang, Y.S.; Mora-Sero, I.; Bisquert, J. Slow Dynamic Processes in Lead Halide Perovskite Solar Cells. Characteristic Times and Hysteresis. J. Phys. Chem. Lett. 2014, 5, 2357–2363. [Google Scholar] [CrossRef]
- Prochowicz, D.; Tavakoli, M.M.; Wolska-Pietkiewicz, M.; Jędrzejewska, M.; Trivedi, S.; Kumar, M.; Zakeeruddin, S.M.; Lewiński, J.; Graetzel, M.; Yadav, P. Suppressing Recombination in Perovskite Solar Cells via Surface Engineering of TiO2 ETL. Sol. Energy 2020, 197, 50–57. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, J.-W.; Yantara, N.; Boix, P.P.; Kulkarni, S.A.; Mhaisalkar, S.; Grätzel, M.; Park, N.-G. High Efficiency Solid-State Sensitized Solar Cell-Based on Submicrometer Rutile TiO2 Nanorod and CH3NH3PbI3 Perovskite Sensitizer. Nano Lett. 2013, 13, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, J.; Baker, J.; Hou, Y.; Guan, D.; Chen, J.; Yuan, C. Enhanced Photovoltaic Performance of Perovskite CH 3 NH 3 PbI 3 Solar Cells with Freestanding TiO2 Nanotube Array Films. Chem. Commun. 2014, 50, 6368–6371. [Google Scholar] [CrossRef] [PubMed]
- Salazar, R.; Altomare, M.; Lee, K.; Tripathy, J.; Kirchgeorg, R.; Nguyen, N.T.; Mokhtar, M.; Alshehri, A.; Al-Thabaiti, S.A.; Schmuki, P. Use of Anodic TiO2 Nanotube Layers as Mesoporous Scaffolds for Fabricating CH3NH3PbI3 Perovskite-Based Solid-State Solar Cells. Chem. Electro. Chem. 2015, 2, 824–828. [Google Scholar] [CrossRef]
- Lan, Z.; Xu, X.; Zhang, X.; Tang, J.; Zhang, L.; He, X.; Wu, J. Low-Temperature Solution-Processed Efficient Electron-Transporting Layers Based on BF4−-Capped TiO2 Nanorods for High-Performance Planar Perovskite Solar Cells. J. Mater. Chem. C 2018, 6, 334–341. [Google Scholar] [CrossRef]
- Wu, S.; Chen, C.; Wang, J.; Xiao, J.; Peng, T. Controllable Preparation of Rutile TiO2 Nanorod Array for Enhanced Photovoltaic Performance of Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 1649–1657. [Google Scholar] [CrossRef]
- Dharani, S.; Mulmudi, H.K.; Yantara, N.; Trang, P.T.T.; Park, N.G.; Graetzel, M.; Mhaisalkar, S.; Mathews, N.; Boix, P.P. High Efficiency Electrospun TiO2 Nanofiber Based Hybrid Organic–Inorganic Perovskite Solar Cell. Nanoscale 2014, 6, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Ke, W.; Wang, J.; Liu, Q.; Wan, J.; Yang, G.; Fang, G. Perovskite solar cell based on network nanoporous layer consisted of TiO2 nanowires and its interface optimization. J. Power Source 2015, 290, 144–152. [Google Scholar] [CrossRef]
- Mahmood, K.; Swain, B.S.; Amassian, A. Core–Shell Heterostructured Metal Oxide Arrays Enable Superior Light-Harvesting and Hysteresis-Free Mesoscopic Perovskite Solar Cells. Nanoscale 2015, 7, 12812–12819. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Q.; Wang, L. 3D Branched Nanowire-Coated Macroporous Titania Thin Films for Efficient Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1804356. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Xu, Y.-F.; Liao, J.-F.; Wang, L.; Kuang, D.-B. Branched Titania Nanostructures for Efficient Energy Conversion and Storage: A Review on Design Strategies, Structural Merits and Multifunctionalities. Nano Energy 2019, 62, 791–809. [Google Scholar] [CrossRef]
- Brown, T.M.; De Rossi, F.; Di Giacomo, F.; Mincuzzi, G.; Zardetto, V.; Reale, A.; Di Carlo, A. Progress in flexible dye solar cell materials, processes and devices. J. Mater. Chem. A 2014, 2, 10788–10817. [Google Scholar] [CrossRef]
- Li, G.; Sheng, L.; Li, T.; Hu, J.; Li, P.; Wang, K. Engineering flexible dye-sensitized solar cells for portable electronics. Solar Energy 2019, 177, 80–98. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Direct contact of selective charge extraction layers enables high-efficiency molecular photovoltaics. Joule 2018, 2, 1108–1117. [Google Scholar] [CrossRef]
- Wang, B.; Shen, S.; Mao, S.S. Black TiO2 for Solar Hydrogen Conversion. J. Materiomics 2017, 3, 96–111. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting-The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A Review and Recent Developments in Photocatalytic Water-Splitting Using TiO2 for Hydrogen Production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B. Efficient Photochemical Water Splitting by a Chemically Modified N-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef]
- Kasani, S.; Curtin, K.; Wu, N. A Review of 2D and 3D Plasmonic Nanostructure Array Patterns: Fabrication, Light Management and Sensing Applications. Nanophotonics 2019, 8, 2065–2089. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Schmuki, P.; Kment, S.; Zboril, R. Nanostar Morphology of Plasmonic Particles Strongly Enhances Photoelectrochemical Water Splitting of TiO2 Nanorods with Superior Incident Photon-to-Current Conversion Efficiency in Visible/near-Infrared Region. Electrochim. Acta 2018, 260, 212–220. [Google Scholar] [CrossRef]
- Sun, X.; Liu, H.; Dong, J.; Wei, J.; Zhang, Y. Preparation and Characterization of Ce/N-Co doped TiO2 Particles for Production of H2 by Photocatalytic Splitting Water Under Visible Light. Catal. Lett. 2010, 135, 219–225. [Google Scholar] [CrossRef]
- Youngblood, W.J.; Lee, S.-H.A.; Maeda, K.; Mallouk, T.E. Visible Light Water Splitting Using Dye-Sensitized Oxide Semiconductors. Acc. Chem. Res. 2009, 42, 1966–1973. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes Based on TiO2 and α-Fe2O3 for Solar Water Splitting-Superior Role of 1D Nanoarchitectures and of Combined Heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Bocarsly, A.B.; Fan, F.R.F.; Walton, E.G.; Wrighton, M.S. The Concept of Fermi Level Pinning at Semiconductor/Liquid Junctions. Consequences for Energy Conversion Efficiency and Selection of Useful Solution Redox Couples in Solar Devices. J. Am. Chem. Soc. 1980, 102, 3671–3677. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Babu, V.J.; Ramakrishna, S. Photoelectrode nanomaterials for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2017, 42, 11078–11109. [Google Scholar] [CrossRef]
- Kapilashrami, M.; Zhang, Y.; Liu, Y.S.; Hagfeldt, A.; Guo, J. Probing the optical property and electronic structure of TiO2 nanomaterials for renewable energy applications. Chem. Rev. 2014, 114, 9662–9707. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting–materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef]
- Chen, Z.; Jaramillo, T.F.; Deutsch, T.G.; Kleiman-Shwarsctein, A.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating Materials Development for Photoelectrochemical Hydrogen Production: Standards for Methods, Definitions, and Reporting Protocols. J. Mater. Res. 2010, 25, 3–16. [Google Scholar] [CrossRef]
- Ibadurrohman, M.; Hellgardt, K. Morphological Modification of TiO2 Thin Films as Highly Efficient Photoanodes for Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2015, 7, 9088–9097. [Google Scholar] [CrossRef]
- Chen, Z.; Deutsch, T.G.; Dinh, H.N.; Domen, K.; Emery, K.; Forman, A.J.; Gaillard, N.; Garland, R.; Heske, C.; Jaramillo, T.F.; et al. Efficiency Definitions in the Field of PEC. In Photoelectrochemical Water Splitting: Standards, Experimental Methods, and Protocols; Springer Briefs in Energy; Chen, Z., Dinh, H.N., Miller, E., Eds.; Springer: New York, NY, USA, 2013; pp. 7–16. [Google Scholar] [CrossRef]
- Cai, J.; Shen, J.; Zhang, X.; Ng, Y.H.; Huang, J.; Guo, W.; Lin, C.; Lai, Y. Light-Driven Sustainable Hydrogen Production Utilizing TiO2 Nanostructures: A Review. Small Methods 2019, 3, 1800184. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Boukai, A.; Yang, P. High Density N-Si/n-TiO2 Core/Shell Nanowire Arrays with Enhanced Photoactivity. Nano Lett. 2009, 9, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.M. Factors Affecting the Efficiency of a Water Splitting Photocatalyst: A Perspective. Renew. Sustain. Energy Rev. 2017, 71, 585–601. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic Nanostructures for Photoelectrochemical and Photocatalytic Water Splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Leng, W.H.; Barnes, P.R.F.; Juozapavicius, M.; O’Regan, B.C.; Durrant, J.R. Electron Diffusion Length in Mesoporous Nanocrystalline TiO2 Photoelectrodes during Water Oxidation. J. Phys. Chem. Lett. 2010, 1, 967–972. [Google Scholar] [CrossRef]
- Qiu, Y.; Leung, S.-F.; Wei, Z.; Lin, Q.; Zheng, X.; Zhang, Y.; Fan, Z.; Yang, S. Enhanced Charge Collection for Splitting of Water Enabled by an Engineered Three-Dimensional Nanospike Array. J. Phys. Chem. C 2014, 118, 22465–22472. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, H.; Zhu, M.; Li, Y.; Li, W. Interfacial Charge Transport in 1D TiO2 Based Photoelectrodes for Photoelectrochemical Water Splitting. Small 2021, 17, 1903378. [Google Scholar] [CrossRef]
- Shankar, K.; Basham, J.I.; Allam, N.K.; Varghese, O.K.; Mor, G.K.; Feng, X.; Paulose, M.; Seabold, J.A.; Choi, K.-S.; Grimes, C.A. Recent Advances In the Use of TiO2 Nanotube and Nanowire Arrays for Oxidative Photoelectrochemistry. J. Phys. Chem. C 2009, 113, 6327–6359. [Google Scholar] [CrossRef]
- Ozkan, S.; Nguyen, N.T.; Mazare, A.; Schmuki, P. Optimized Spacing between TiO2 Nanotubes for Enhanced Light Harvesting and Charge Transfer. Chem. Electro. Chem. 2018, 5, 3183–3190. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Zuliani, A.; Ceresoli, D.; Martinazzo, R.; Selli, E. Exploiting the Photonic Crystal Properties of TiO2 Nanotube Arrays To Enhance Photocatalytic Hydrogen Production. ACS Catal. 2016, 6, 1345–1353. [Google Scholar] [CrossRef]
- Escudero, J.C.; Cervera-March, S.; Giménez, J.; Simarro, R. Preparation and Characterization of Pt(RuO2)/TiO2 Catalysts: Test in a Continuous Water Photolysis System. J. Catal. 1990, 123, 319–332. [Google Scholar] [CrossRef]
- Hartig, K.J.; Getoff, N.; Rumpelmayer, G.; Popkirov, G.; Kotchev, K.; Kanev, S.; Veziroğlu, T.N.; Getoff, N.; Weinzierl, P. (Eds.) Hydrogen Energy Progress VI; Pergamon Press: Oxford, UK, 1986; Volume 2, p. 546. [Google Scholar]
- Berardo, E.; Zwijnenburg, M.A. Modeling the Water Splitting Activity of a TiO2 Rutile Nanoparticle. J. Phys. Chem. C 2015, 119, 13384–13393. [Google Scholar] [CrossRef]
- Banno, H.; Kariya, B.; Isu, N.; Ogawa, M.; Miwa, S.; Sawada, K.; Tsuge, J.; Imaizumi, S.; Kato, H.; Tokutake, K.; et al. Effect of TiO2 Crystallite Diameter on Photocatalytic Water Splitting Rate. Green Sustain. Chem. 2014, 4, 87–94. [Google Scholar] [CrossRef][Green Version]
- Hidalgo, D.; Messina, R.; Sacco, A.; Manfredi, D.; Vankova, S.; Garrone, E.; Saracco, G.; Hernández, S. Thick Mesoporous TiO2 Films through a Sol–Gel Method Involving a Non-Ionic Surfactant: Characterization and Enhanced Performance for Water Photo-Electrolysis. Int. J. Hydrogen Energy 2014, 39, 21512–21522. [Google Scholar] [CrossRef]
- Modesto-Lopez, L.B.; Biswas, P. Role of the Effective Electrical Conductivity of Nanosuspensions in the Generation of TiO2 Agglomerates with Electrospray. J. Aerosol Sci. 2010, 41, 790–804. [Google Scholar] [CrossRef]
- Hartmann, P.; Lee, D.-K.; Smarsly, B.M.; Janek, J. Mesoporous TiO2: Comparison of Classical Sol−Gel and Nanoparticle Based Photoelectrodes for the Water Splitting Reaction. ACS Nano 2010, 4, 3147–3154. [Google Scholar] [CrossRef]
- Paulose, M.; Varghese, O.K.; Shankar, K.; Mor, G.K.; Grimes, C.A. Photoelectrochemical Properties of Highly-Ordered Titania Nanotube-Arrays. MRS Online Proc. Libr. (OPL) 2004, 837, N3.13. [Google Scholar] [CrossRef]
- Fujimoto, M.; Kado, T.; Takashima, W.; Kaneto, K.; Hayase, S. Dye-Sensitized Solar Cells Fabricated by Electrospray Coating Using TiO2 Nanocrystal Dispersion Solution. J. Electrochem. Soc. 2006, 153, A826. [Google Scholar] [CrossRef]
- Liu, M.; de Leon Snapp, N.; Park, H. Water Photolysis with a Cross-Linked Titanium Dioxide Nanowire Anode. Chem. Sci. 2011, 2, 80–87. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, P. Optimization of Photoelectrochemical Water Splitting Performance on Hierarchical TiO2 Nanotube Arrays. Energy Environ. Sci. 2012, 5, 6506. [Google Scholar] [CrossRef]
- Nozik, A.J.; Miller, J. Introduction to Solar Photon Conversion. Chem. Rev. 2010, 110, 6443–6445. [Google Scholar] [CrossRef]
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a Photocatalyst for Water Splitting—An Experimental and Theoretical Review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef]
- Thimsen, E.; Rastgar, N.; Biswas, P. Nanostructured TiO2 Films with Controlled Morphology Synthesized in a Single Step Process: Performance of Dye-Sensitized Solar Cells and Photo Watersplitting. J. Phys. Chem. C 2008, 112, 4134–4140. [Google Scholar] [CrossRef]
- Regonini, D.; Clemens, F.J. Anodized TiO2 Nanotubes: Effect of Anodizing Time on Film Length, Morphology and Photoelectrochemical Properties. Mater. Lett. 2015, 142, 97–101. [Google Scholar] [CrossRef]
- Palmas, S.; Polcaro, A.M.; Ruiz, J.R.; Da Pozzo, A.; Mascia, M.; Vacca, A. TiO2 Photoanodes for Electrically Enhanced Water Splitting. Int. J. Hydrogen Energy 2010, 35, 6561–6570. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A Review on Highly Ordered, Vertically Oriented TiO2 Nanotube Arrays: Fabrication, Material Properties, and Solar Energy Applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Sun, P.; Lu, S.; Wang, L.; Wang, C.; Liu, Y. Photoelectrochemical Water Splitting with Rutile TiO2 Nanowires Array: Synergistic Effect of Hydrogen Treatment and Surface Modification with Anatase Nanoparticles. Electrochim. Acta 2014, 130, 290–295. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Hahn, C.; Liu, B.; Yang, P. Photoelectrochemical Properties of TiO2 Nanowire Arrays: A Study of the Dependence on Length and Atomic Layer Deposition Coating. ACS Nano 2012, 6, 5060–5069. [Google Scholar] [CrossRef]
- Mao, Y.; Ning, C.; Zhang, N.; Hu, Y.; Li, M.; Yang, H.; Chen, S.; Su, S.; Liang, E. Enhancing Photoelectrochemical Performance of TiO2 Nanowires through a Facile Acid Treatment Method. J. Electrochem. Soc. 2018, 165, H799. [Google Scholar] [CrossRef]
- Mali, M.G.; An, S.; Liou, M.; Al-Deyab, S.S.; Yoon, S.S. Photoelectrochemical solar water splitting using electrospun TiO2 nanofibers. Appl. Surf. Sci. 2015, 328, 109–114. [Google Scholar] [CrossRef]
- Li, W.; Bai, Y.; Zhuang, W.; Chan, K.-Y.; Liu, C.; Yang, Z.; Feng, X.; Lu, X. Highly Crystalline Mesoporous TiO2(B) Nanofibers. J. Phys. Chem. C 2014, 118, 3049–3055. [Google Scholar] [CrossRef]
- Ongaro, M.; Mardegan, A.; Stortini, A.M.; Signoretto, M.; Ugo, P. Arrays of Templated TiO2 Nanofibres as Improved Photoanodes for Water Splitting under Visible Light. Nanotechnology 2015, 26, 165402. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, A.; Smith, W.A.; Kuykendall, T.R.; Zhao, Y.; Zhang, J.Z. Photoelectrochemical Water Splitting Using Dense and Aligned TiO2 Nanorod Arrays. Small 2009, 5, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Fàbrega, C.; Andreu, T.; Tarancón, A.; Flox, C.; Morata, A.; Calvo-Barrio, L.; Morante, J.R. Optimization of Surface Charge Transfer Processes on Rutile TiO2 Nanorods Photoanodes for Water Splitting. Int. J. Hydrogen Energy 2013, 38, 2979–2985. [Google Scholar] [CrossRef]
- Paulose, M.; Shankar, K.; Yoriya, S.; Prakasam, H.E.; Varghese, O.K.; Mor, G.K.; Latempa, T.A.; Fitzgerald, A.; Grimes, C.A. Anodic Growth of Highly Ordered TiO2 Nanotube Arrays to 134 mm in Length. J. Phys. Chem. B 2006, 110, 16179–16184. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Enhanced Photocleavage of Water Using Titania Nanotube Arrays. Nano Lett. 2005, 5, 191–195. [Google Scholar] [CrossRef]
- Liang, S.; He, J.; Sun, Z.; Liu, Q.; Jiang, Y.; Cheng, H.; He, B.; Xie, Z.; Wei, S. Improving Photoelectrochemical Water Splitting Activity of TiO2 Nanotube Arrays by Tuning Geometrical Parameters. J. Phys. Chem. C 2012, 116, 9049–9053. [Google Scholar] [CrossRef]
- Ennaceri, H.; Fischer, K.; Hanus, K.; Chemseddine, A.; Prager, A.; Griebel, J.; Kühnert, M.; Schulze, A.; Abel, B. Effect of Morphology on the Photoelectrochemical Activity of TiO2 Self-Organized Nanotube Arrays. Catalysts 2020, 10, 279. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Aljaber, A.S.; AlQaradawi, S.Y.; Allam, N.K. TiO2 Nanotubes with Ultrathin Walls for Enhanced Water Splitting. Chem. Commun. 2015, 51, 12617–12620. [Google Scholar] [CrossRef]
- Kim, H.S.; Ahn, K.-S.; Kang, S.H. Enhancing Photoelectrochemical Water Splitting Performance of TiO2 nanotube Arrays by Controlling Morphological Properties. Electron. Mater. Lett. 2014, 10, 345–349. [Google Scholar] [CrossRef]
- Xu, X.; Fang, X.; Zhai, T.; Zeng, H.; Liu, B.; Hu, X.; Bando, Y.; Golberg, D. Tube-in-Tube TiO2 Nanotubes with Porous Walls: Fabrication, Formation Mechanism, and Photocatalytic Properties. Small 2011, 7, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Adán, C.; Marugán, J.; Sánchez, E.; Pablos, C.; Van Grieken, R. Understanding the effect of morphology on the photocatalytic activity of TiO2 nanotube array electrodes. Electrochim. Acta 2016, 191, 521–529. [Google Scholar] [CrossRef]
- Nishanthi, S.T.; Subramanian, E.; Sundarakannan, B.; Pathinettam Padiyan, D. An insight into the influence of morphology on the photoelectrochemical activity of TiO2 nanotube arrays. Sol. Energy Mater. Sol. Cells 2015, 132, 204–209. [Google Scholar] [CrossRef]
- Varghese, O.K.; Paulose, M.; Shankar, K.; Mor, G.K.; Grimes, C.A. Water-Photolysis Properties of Micron-Length Highly-Ordered Titania Nanotube-Arrays. J. Nanosci. Nanotechnol. 2005, 5, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Mor, G.K.; Prakasam, H.E.; Yoriya, S.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Highly-Ordered TiO2 Nanotube Arrays up to 220 Μm in Length: Use in Water photoelectrolysis and Dye-Sensitized Solar Cells. Nanotechnology 2007, 18, 065707. [Google Scholar] [CrossRef]
- Kusior, A.; Wnuk, A.; Trenczek-Zajac, A.; Zakrzewska, K.; Radecka, M. TiO2 nanostructures for photoelectrochemical cells (PECs). Int. J. Hydrogen Energy 2015, 40, 4936–4944. [Google Scholar] [CrossRef]
- Butburee, T.; Bai, Y.; Wang, H.; Chen, H.; Wang, Z.; Liu, G.; Zou, J.; Khemthong, P.; Lu, G.Q.M.; Wang, L. 2D Porous TiO2 Single-Crystalline Nanostructure Demonstrating High Photo-Electrochemical Water Splitting Performance. Adv. Mater. 2018, 30, 1705666. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.S.; Chen, Z.; Forman, A.J.; Kim, D.R.; Rao, P.M.; Jaramillo, T.F.; Zheng, X. Branched TiO2 Nanorods for Photoelectrochemical Hydrogen Production. Nano Lett. 2011, 11, 4978–4984. [Google Scholar] [CrossRef]
- Zhang, R.; Shen, D.; Xu, M.; Feng, D.; Li, W.; Zheng, G.; Che, R.; Elzatahry, A.A.; Zhao, D. Ordered Macro-/Mesoporous Anatase Films with High Thermal Stability and Crystallinity for Photoelectrocatalytic Water-Splitting. Adv. Energy Mater. 2014, 4, 1301725. [Google Scholar] [CrossRef]
- Yang, J.S.; Liao, W.P.; Wu, J.J. Morphology and interfacial energetics controls for hierarchical anatase/rutile TiO2 nanostructured array for efficient photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2013, 5, 7425–7431. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.M.; Sajid, M.U.; Arshad, A. Heat transfer applications of TiO2 nanofluids. In Application of Titanium dioxide; Janus, M., Ed.; IntechOpen: London, UK, 2017; pp. 181–200. [Google Scholar] [CrossRef]
- Tawfik, M.M. Experimental Studies of Nanofluid Thermal Conductivity Enhancement and Applications: A Review. Renew. Sustain. Energy Rev. 2017, 75, 1239–1253. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, N.; Feng, Y.; Michaelides, E.E.; Żyła, G.; Jing, D.; Zhang, X.; Norris, P.M.; Markides, C.N.; Mahian, O. A Review of Recent Advances in Thermophysical Properties at the Nanoscale: From Solid State to Colloids. Phys. Rep. 2020, 843, 1–81. [Google Scholar] [CrossRef]
- Iqbal, S.M.; Arulprakasajothi, M.; Raja, N.D.; Bosko, S.M.J.; Teja, M.B. Investigation On The Thermal Behaviour Of Titanium Dioxide Nanofluid. Mater. Today Proc. 2018, 5, 20608–20613. [Google Scholar] [CrossRef]
- Gonçalves, I.; Souza, R.; Coutinho, G.; Miranda, J.; Moita, A.; Pereira, J.E.; Moreira, A.; Lima, R. Thermal Conductivity of Nanofluids: A Review on Prediction Models, Controversies and Challenges. Appl. Sci. 2021, 11, 2525. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Investigations of Thermal Conductivity and Viscosity of Nanofluids. Int. J. Therm. Sci. 2008, 47, 560–568. [Google Scholar] [CrossRef]
- Yang, L.; Hu, Y. Toward TiO2 Nanofluids—Part 2: Applications and Challenges. Nanoscale Res. Lett. 2017, 12, 446. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, E.C.; Wole-Osho, I.; Almanassra, I.W.; Abdullatif, Y.M.; Al-Ansari, T. An Updated Review of Nanofluids in Various Heat Transfer Devices. J. Therm. Anal. Calorim. 2021, 145, 2817–2872. [Google Scholar] [CrossRef]
- Elsaid, K.; Olabi, A.G.; Wilberforce, T.; Abdelkareem, M.A.; Sayed, E.T. Environmental Impacts of Nanofluids: A Review. Sci. Total Environ. 2021, 763, 144202. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.C. A Treatise on Electricity and Magnetism. Nature 1873, 7, 478–480. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Der Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Stroud, D. The Effective Medium Approximations: Some Recent Developments. Superlattices Microstruct. 1998, 23, 567–573. [Google Scholar] [CrossRef]
- Garnett, J.M. Colours in metal glasses and in metallic films. In Philosophical Transactions of the Royal Society of London; Series A; Containing Papers of a Mathematical or Physical Character; Royal Society: London, UK, 1904; Volume 203, pp. 385–420. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O.K. Thermal Conductivity of Heterogeneous Two-Component Systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Hasselman, D.P.H.; Lloyd, F.J. Effective Thermal Conductivity of Composites with Interfacial Thermal Barrier Resistance. J. Compos. Mater. 1987, 21, 508–515. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S.U.S. The Role of Interfacial Layers in the Enhanced Thermal Conductivity of Nanofluids: A Renovated Maxwell Model. J. Nanopart. Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Nan, C.-W.; Birringer, R.; Clarke, D.R.; Gleiter, H. Effective Thermal Conductivity of Particulate Composites with Interfacial Thermal Resistance. J. Appl. Phys. 1997, 81, 6692–6699. [Google Scholar] [CrossRef]
- Wilson, O.M.; Hu, X.; Cahill, D.G.; Braun, P.V. Colloidal Metal Particles as Probes of Nanoscale Thermal Transport in Fluids. Phys. Rev. B 2002, 66, 224301. [Google Scholar] [CrossRef]
- Huxtable, S.T.; Cahill, D.G.; Shenogin, S.; Xue, L.; Ozisik, R.; Barone, P.; Usrey, M.; Strano, M.S.; Siddons, G.; Shim, M.; et al. Interfacial Heat Flow in Carbon Nanotube Suspensions. Nat. Mater. 2003, 2, 731–734. [Google Scholar] [CrossRef]
- Pil Jang, S.; Choi, S.U.S. Effects of Various Parameters on Nanofluid Thermal Conductivity. J. Heat Transf. 2006, 129, 617–623. [Google Scholar] [CrossRef]
- Jang, S.P.; Choi, S.U.S. Role of Brownian Motion in the Enhanced Thermal Conductivity of Nanofluids. Appl. Phys. Lett. 2004, 84, 4316–4318. [Google Scholar] [CrossRef]
- Koo, J.; Kleinstreuer, C. A new thermal conductivity model for nanofluids. J. Nanopart. Res. 2004, 6, 577–588. [Google Scholar] [CrossRef]
- Shukla, R.K.; Dhir, V.K. Numerical Study of the Effective Thermal Conductivity of Nanofluids. In American Society of Mechanical Engineers Digital Collection; American Society of Mechanical Engineers: New York, NY, USA, 2009; pp. 449–457. [Google Scholar] [CrossRef]
- Shukla, R.K.; Dhir, V.K. Effect of Brownian Motion on Thermal Conductivity of Nanofluids. J. Heat Transf. 2008, 130, 042406. [Google Scholar] [CrossRef]
- Kumar, D.H.; Patel, H.E.; Kumar, V.R.R.; Sundararajan, T.; Pradeep, T.; Das, S.K. Model for Heat Conduction in Nanofluids. Phys. Rev. Lett. 2004, 93, 144301. [Google Scholar] [CrossRef] [PubMed]
- Prasher, R.; Bhattacharya, P.; Phelan, P.E. Brownian-Motion-Based Convective-Conductive Model for the Effective Thermal Conductivity of Nanofluids. J. Heat Transf. 2005, 128, 588–595. [Google Scholar] [CrossRef]
- Evans, W.; Fish, J.; Keblinski, P. Role of Brownian Motion Hydrodynamics on Nanofluid Thermal Conductivity. Appl. Phys. Lett. 2006, 88, 093116. [Google Scholar] [CrossRef]
- Keblinski, P.; Eastman, J.A.; Cahill, D.G. Nanofluids for Thermal Transport. Mater. Today 2005, 8, 36–44. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Saha, S.K.; Yadav, A.; Phelan, P.E.; Prasher, R.S. Brownian Dynamics Simulation to Determine the Effective Thermal Conductivity of Nanofluids. J. Appl. Phys. 2004, 95, 6492–6494. [Google Scholar] [CrossRef]
- Wang, Z.L.; Tang, D.W.; Liu, S.; Zheng, X.H.; Araki, N. Thermal-Conductivity and Thermal-Diffusivity Measurements of Nanofluids by 3ω Method and Mechanism Analysis of Heat Transport. Int. J. Thermophys. 2007, 28, 1255–1268. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, H.; Fujii, M. Effective Thermal Conductivity and Thermal Diffusivity of Nanofluids Containing Spherical and Cylindrical Nanoparticles. Exp. Therm. Fluid Sci. 2007, 31, 593–599. [Google Scholar] [CrossRef]
- Turgut, A.; Tavman, I.; Chirtoc, M.; Schuchmann, H.P.; Sauter, C.; Tavman, S. Thermal Conductivity and Viscosity Measurements of Water-Based TiO2 Nanofluids. Int. J. Thermophys. 2009, 30, 1213–1226. [Google Scholar] [CrossRef]
- Rondino, F.; D’Amato, R.; Terranova, G.; Borsella, E.; Falconieri, M. Thermal Diffusivity Enhancement in Nanofluids Based on Pyrolytic Titania Nanopowders: Importance of Aggregate Morphology. J. Raman Spectrosc. 2014, 45, 528–532. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.; Mamat, R.; Usri, N. Thermal Conductivity Enhancement of TiO2 Nanofluid in Water and Ethylene Glycol (EG) Mixture. Indian J. Pure Appl. Phys. 2016, 54, 651–655. [Google Scholar]
- Ali, H.M.; Babar, H.; Shah, T.R.; Sajid, M.U.; Qasim, M.A.; Javed, S. Preparation Techniques of TiO2 Nanofluids and Challenges: A Review. Appl. Sci. 2018, 8, 587. [Google Scholar] [CrossRef]
- Coccia, G.; Tomassetti, S.; Di Nicola, G. Thermal Conductivity of Nanofluids: A Review of the Existing Correlations and a Scaled Semi-Empirical Equation. Renew. Sustain. Energy Rev. 2021, 151, 111573. [Google Scholar] [CrossRef]
- Zagabathuni, A.; Ghosh, S.; Pabi, S.K. The Difference in the Thermal Conductivity of Nanofluids Measured by Different Methods and Its Rationalization. Beilstein J. Nanotechnol. 2016, 7, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Qian, X.; Gu, X.; Jajja, S.; Yang, R. Measurement Techniques for Thermal Conductivity and Interfacial Thermal Conductance of Bulk and Thin Film Materials. J. Electron. Packag. 2016, 138, 040802. [Google Scholar] [CrossRef]
- Roetzel, W.; Prinzen, S.; Wandelt, M. Temperature Oscillation Technique for Determination of Local Convective Heat Transfer Coefficients without Fluid Temperature Measurement. Chem. Eng. Technol. 1993, 16, 89–93. [Google Scholar] [CrossRef]
- Gustafsson, S.E.; Karawacki, E.; Khan, M.N. Transient Hot-Strip Method for Simultaneously Measuring Thermal Conductivity and Thermal Diffusivity of Solids and Fluids. J. Phys. Appl. Phys. 1979, 12, 1411–1421. [Google Scholar] [CrossRef]
- Eichler, H.J.; Günter, P.; Pohl, D.W. (Eds.) Mechanisms of Grating Formation and Grating Materials. In Laser-Induced Dynamic Gratings; Springer Series in Optical Sciences; Springer: Berlin/Heidelberg, Germany, 1986; pp. 38–93. [Google Scholar] [CrossRef]
- Venerus, D.C.; Schieber, J.D.; Iddir, H.; Guzman, J.D.; Broerman, A.W. Measurement of Thermal Diffusivity in Polymer Melts Using Forced Rayleigh Light Scattering. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1069–1078. [Google Scholar] [CrossRef]
- Wang, B.-X.; Zhou, L.-P.; Peng, X.-F. A Fractal Model for Predicting the Effective Thermal Conductivity of Liquid with Suspension of Nanoparticles. Int. J. Heat Mass Transf. 2003, 46, 2665–2672. [Google Scholar] [CrossRef]
- Prasher, R.; Evans, W.; Meakin, P.; Fish, J.; Phelan, P.; Keblinski, P. Effect of aggregation on thermal conduction in colloidal nanofluids. Appl. Phys. Lett. 2006, 89, 143119. [Google Scholar] [CrossRef]
- Fan, J.; Wang, L. Review of Heat Conduction in Nanofluids. J. Heat Transf. 2011, 133, 097001. [Google Scholar] [CrossRef]
- Yu, C.-J.; Richter, A.G.; Datta, A.; Durbin, M.K.; Dutta, P. Molecular Layering in a Liquid on a Solid Substrate: An X-Ray Reflectivity Study. Phys. B Condens. Matter 2000, 283, 27–31. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S.U.S. The Role of Interfacial Layers in the Enhanced Thermal Conductivity of Nanofluids: A Renovated Hamilton–Crosser Model. J. Nanopart. Res. 2004, 6, 355–361. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, B.; Xu, P.; Zou, M. The Effective Thermal Conductivity of Nanofluids Based on the Nanolayer and the Aggregation of Nanoparticles. J. Phys. Appl. Phys. 2007, 40, 3164–3171. [Google Scholar] [CrossRef]
- Potanin, A.A.; De Rooij, R.; Van den Ende, D.; Mellema, J. Microrheological Modeling of Weakly Aggregated Dispersions. J. Chem. Phys. 1995, 102, 5845–5853. [Google Scholar] [CrossRef]
- Shih, W.-H.; Shih, W.Y.; Kim, S.-I.; Liu, J.; Aksay, I.A. Scaling Behavior of the Elastic Properties of Colloidal Gels. Phys. Rev. A 1990, 42, 4772–4779. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Walker, S.L.; Mylon, S.E. Aggregate Morphology of Nano-TiO2: Role of Primary Particle Size, Solution Chemistry, and Organic Matter. Environ. Sci. Process. Impacts 2013, 15, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Prasher, R.; Phelan, P.; Bhattacharya, P. Effect of Aggregation Kinetics on the Thermal Conductivity of Nanoscale Colloidal Solutions (Nanofluid). Nano Lett. 2006, 6, 1529–1534. [Google Scholar] [CrossRef]
- Kundan, L.; Mallick, S.S. Effect of Time Dependent Morphological Parameters of Nanoclusters on Perikinetic Heat Conduction and Induced Micro-Convection Mechanisms of Oxide Based Nanofluids. Exp. Heat Transf. 2018, 31, 251–274. [Google Scholar] [CrossRef]
- Gao, J.W.; Zheng, R.T.; Ohtani, H.; Zhu, D.S.; Chen, G. Experimental Investigation of Heat Conduction Mechanisms in Nanofluids. Clue on Clustering. Nano Lett. 2009, 9, 4128–4132. [Google Scholar] [CrossRef]
- Philip, J.; Shima, P.D.; Raj, B. Evidence for Enhanced Thermal Conduction through Percolating Structures in Nanofluids. Nanotechnology 2008, 19, 305706. [Google Scholar] [CrossRef]
- Jungblut, S.; Eychmüller, A. Modeling Nanoparticle Aggregation. In Chemical Modelling; RSC Publishing: London, UK, 2019; pp. 1–27. [Google Scholar] [CrossRef]
- Russel, W.B.; Saville, D.A.; Schowalter, W.R. Colloidal Dispersions; Cambridge University Press: New York, NY, USA, 1989. [Google Scholar]
- Sonawane, S.S.; Khedkar, R.S.; Wasewar, K.L. Effect of Sonication Time on Enhancement of Effective Thermal Conductivity of Nano TiO2 –Water, Ethylene Glycol, and Paraffin Oil Nanofluids and Models Comparisons. J. Exp. Nanosci. 2015, 10, 310–322. [Google Scholar] [CrossRef]
- Asadi, A.; Pourfattah, F.; Miklós Szilágyi, I.; Afrand, M.; Żyła, G.; Seon Ahn, H.; Wongwises, S.; Minh Nguyen, H.; Arabkoohsar, A.; Mahian, O. Effect of Sonication Characteristics on Stability, Thermophysical Properties, and Heat Transfer of Nanofluids: A Comprehensive Review. Ultrason. Sonochem. 2019, 58, 104701. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, A.; Metselaar, I.H. The Influence of Surfactant and Ultrasonic Processing on Improvement of Stability, Thermal Conductivity and Viscosity of Titania Nanofluid. Exp. Therm. Fluid Sci. 2013, 51, 1–9. [Google Scholar] [CrossRef]
- Wamkam, C.T.; Opoku, M.K.; Hong, H.; Smith, P. Effects of pH on heat transfer nanofluids containing ZrO2 and TiO2 nanoparticles. J. Appl. Phys. 2011, 109, 024305. [Google Scholar] [CrossRef]
- Khurana, D.; Subudhi, S. Effects of PH and Surfactant on the Forced Convection of Al2O3/Water and TiO2/Water Nanofluids. J. Therm. Sci. Eng. Appl. 2021, 13, 1–18. [Google Scholar] [CrossRef]
- Oliveira, L.R.; Silva, A.C.; Dantas, N.O.; Bandarra Filho, E.P. Thermophysical properties of TiO2-PVA/water nanofluids. Int. J. Heat Mass Transf. 2017, 1, 795–808. [Google Scholar] [CrossRef]
- Ouikhalfan, M.; Labihi, A.; Belaqziz, M.; Chehouani, H.; Benhamou, B.; Sarı, A.; Belfkira, A. Stability and Thermal Conductivity Enhancement of Aqueous Nanofluid Based on Surfactant-Modified TiO2. J. Dispers. Sci. Technol. 2020, 41, 374–382. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sarkar, I.; Behera, D.K.; Pal, S.K.; Chakraborty, S. Experimental Investigation on the Effect of Dispersant Addition on Thermal and Rheological Characteristics of TiO2 Nanofluid. Powder Technol. 2017, 307, 10–24. [Google Scholar] [CrossRef]
- Saleh, R.; Putra, N.; Wibowo, R.E.; Septiadi, W.N.; Prakoso, S.P. Titanium Dioxide Nanofluids for Heat Transfer Applications. Exp. Therm. Fluid Sci. 2014, 52, 19–29. [Google Scholar] [CrossRef]
- Xie, H.; Fujii, M.; Zhang, X. Effect of Interfacial Nanolayer on the Effective Thermal Conductivity of Nanoparticle-Fluid Mixture. Int. J. Heat Mass Transf. 2005, 48, 2926–2932. [Google Scholar] [CrossRef]
- Yu, W.; France, D.M.; Routbort, J.L.; Choi, S.U.S. Review and Comparison of Nanofluid Thermal Conductivity and Heat Transfer Enhancements. Heat Transf. Eng. 2008, 29, 432–460. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.U.-S.; Li, S.; Eastman, J.A. Measuring Thermal Conductivity of Fluids Containing Oxide Nanoparticles. J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Reynolds, O. An Experimental Investigation of the Circumstances Which Determine Whether the Motion of Water Shall Be Direct or Sinuous, and of the Law of Resistance in Parallel Channels. Philos. Trans. R. Soc. Lond. 1883, 174, 935–982. [Google Scholar]
- Rapp, B.E. (Ed.) Chapter 9—Fluids. In Microfluidics: Modelling, Mechanics and Mathematics; Micro and Nano Technologies; Elsevier: Oxford, UK, 2017; pp. 243–263. [Google Scholar] [CrossRef]
- Fedele, L.; Colla, L.; Bobbo, S. Viscosity and Thermal Conductivity Measurements of Water-Based Nanofluids Containing Titanium Oxide Nanoparticles. Int. J. Refrig. 2012, 35, 1359–1366. [Google Scholar] [CrossRef]
- Hojjat, M.; Etemad, S.; Bagheri, R.; Thibault, J. Thermal Conductivity of Non-Newtonian Nanofluids: Experimental Data and Modeling Using Neural Network. Int. J. Heat Mass Transf. 2011, 54, 1017–1023. [Google Scholar] [CrossRef]
- Batmunkh, M.; Tanshen, R.; Nine, J.; Myekhlai, M.; Choi, H.; Chung, H.; Jeong, H. Thermal Conductivity of TiO2 Nanoparticles Based Aqueous Nanofluids with an Addition of a Modified Silver Particle. Ind. Eng. Chem. Res. 2014, 53, 8445–8451. [Google Scholar] [CrossRef]
- Duangthongsuk, W.; Wongwises, S. Measurement of Temperature-Dependent Thermal Conductivity and Viscosity of TiO2-Water Nanofluids. Exp. Therm. Fluid Sci. 2009, 33, 706–714. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle Processing: Understanding and Controlling Aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef]
- Kamiya, H.; Gotoh, K.; Shimada, M.; Uchikoshi, T.; Otani, Y.; Fuji, M.; Matsusaka, S.; Matsuyama, T.; Tatami, J.; Higashitani, K.; et al. Characteristics and behavior of nanoparticles and its dispersion system. In Nanoparticle Technology Handbook; Hosokawa, M., Nogi, K., Naito, M., Yokoyama, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 113–176. [Google Scholar] [CrossRef]
- Fan, W.; Zhong, F. Effects of Macroparameters on the Thickness of an Interfacial Nanolayer of Al2O3– and– and TiO2–Water-Based Nanofluids. ACS Omega 2020, 5, 27972–27977. [Google Scholar] [CrossRef]
- Li, Z.H.; Gong, Y.J.; Pu, M.; Wu, D.; Sun, Y.H.; Wang, J.; Liu, Y.; Dong, B.Z. Determination of Interface Layer Thickness of a Pseudo Two-Phase System by Extension of the Debye Equation. J. Phys. Appl. Phys. 2001, 34, 2085–2088. [Google Scholar] [CrossRef]
- Berger, F.; Dékány, I. Variable Thickness of the Liquid Sorption Layers on Solid Surfaces. Colloids Surf. A Physicochem. Eng. Asp. 1998, 141, 305–317. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Suresh, S.; Srinivasan, R.; Bose, A.C. New Analytical Models to Investigate Thermal Conductivity of Nanofluids. J. Nanosci. Nanotechnol. 2009, 9, 533–538. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Enhanced Thermal Conductivity of TiO2—Water Based Nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Wei, W.; Cai, J.; Hu, X.; Han, Q.; Liu, S.; Zhou, Y. Fractal Analysis of the Effect of Particle Aggregation Distribution on Thermal Conductivity of Nanofluids. Phys. Lett. A 2016, 380, 2953–2956. [Google Scholar] [CrossRef]
- Gao, T.; Jelle, B.P. Thermal Conductivity of TiO2 Nanotubes. J. Phys. Chem. C 2013, 117, 1401–1408. [Google Scholar] [CrossRef]
- Ali, S.; Orell, O.; Kanerva, M.; Hannula, S.-P. Effect of Morphology and Crystal Structure on the Thermal Conductivity of Titania Nanotubes. Nanoscale Res. Lett. 2018, 13, 212. [Google Scholar] [CrossRef]
- Yang, L.; Wang, C.-Z.; Lin, S.; Chen, T.; Cao, Y.; Zhang, P.; Liu, X. Thermal Conductivity of TiO2 Nanotube: A Molecular Dynamics Study. J. Phys. Condens. Matter 2018, 31, 055302. [Google Scholar] [CrossRef]
- Ali, N.; Bahman, A.M.; Aljuwayhel, N.F.; Ebrahim, S.A.; Mukherjee, S.; Alsayegh, A. Carbon-Based Nanofluids and Their Advances towards Heat Transfer Applications—A Review. Nanomaterials 2021, 11, 1628. [Google Scholar] [CrossRef]
- Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Kleinerman, O.; Wang, X.; Ma, A.W.K.; Bengio, E.A.; ter Waarbeek, R.F.; de Jong, J.J.; Hoogerwerf, R.E.; et al. Strong, Light, Multifunctional Fibers of Carbon Nanotubes with Ultrahigh Conductivity. Science 2013, 339, 182–186. [Google Scholar] [CrossRef]
- Chen, H.; Witharana, S.; Jin, Y.; Kim, C.; Ding, Y. Predicting Thermal Conductivity of Liquid Suspensions of Nanoparticles (Nanofluids) Based on Rheology. Particuology 2009, 7, 151–157. [Google Scholar] [CrossRef]
- Chen, H.; Yang, W.; He, Y.; Ding, Y.; Zhang, L.; Tan, C.; Lapkin, A.A.; Bavykin, D.V. Heat Transfer and Flow Behaviour of Aqueous Suspensions of Titanate Nanotubes (Nanofluids). Powder Technol. 2008, 183, 63–72. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania nanotubes prepared by chemical processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
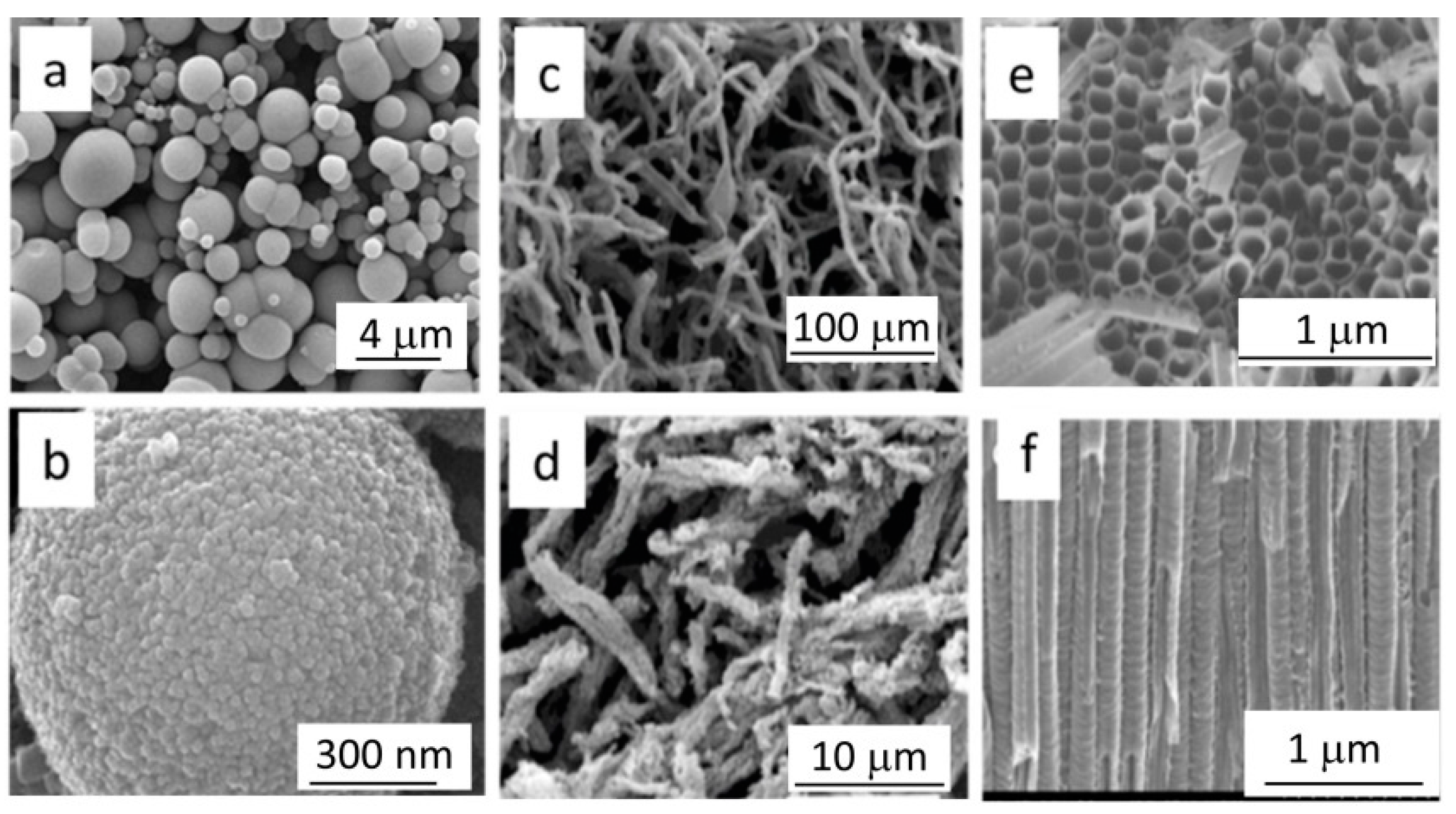
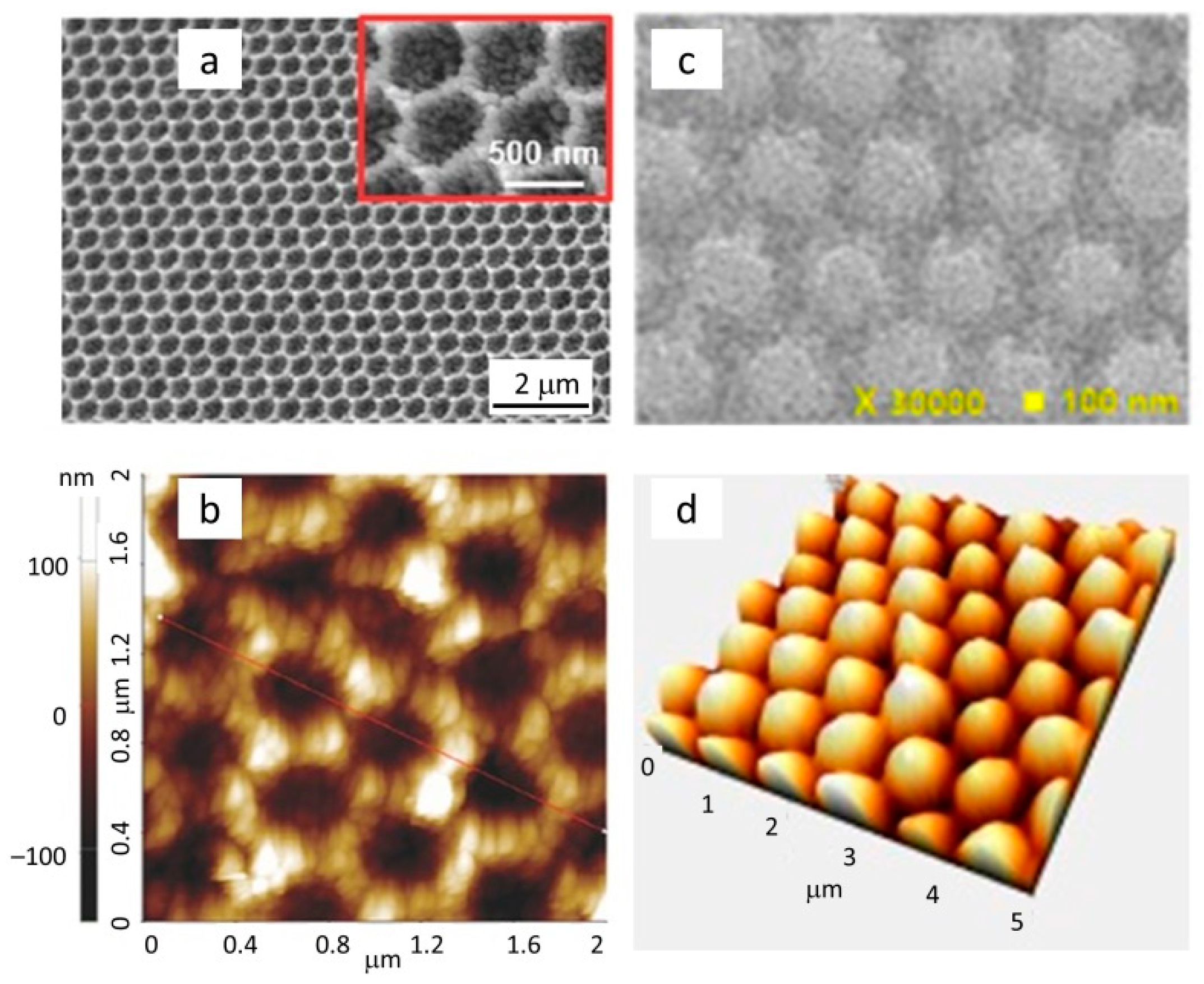
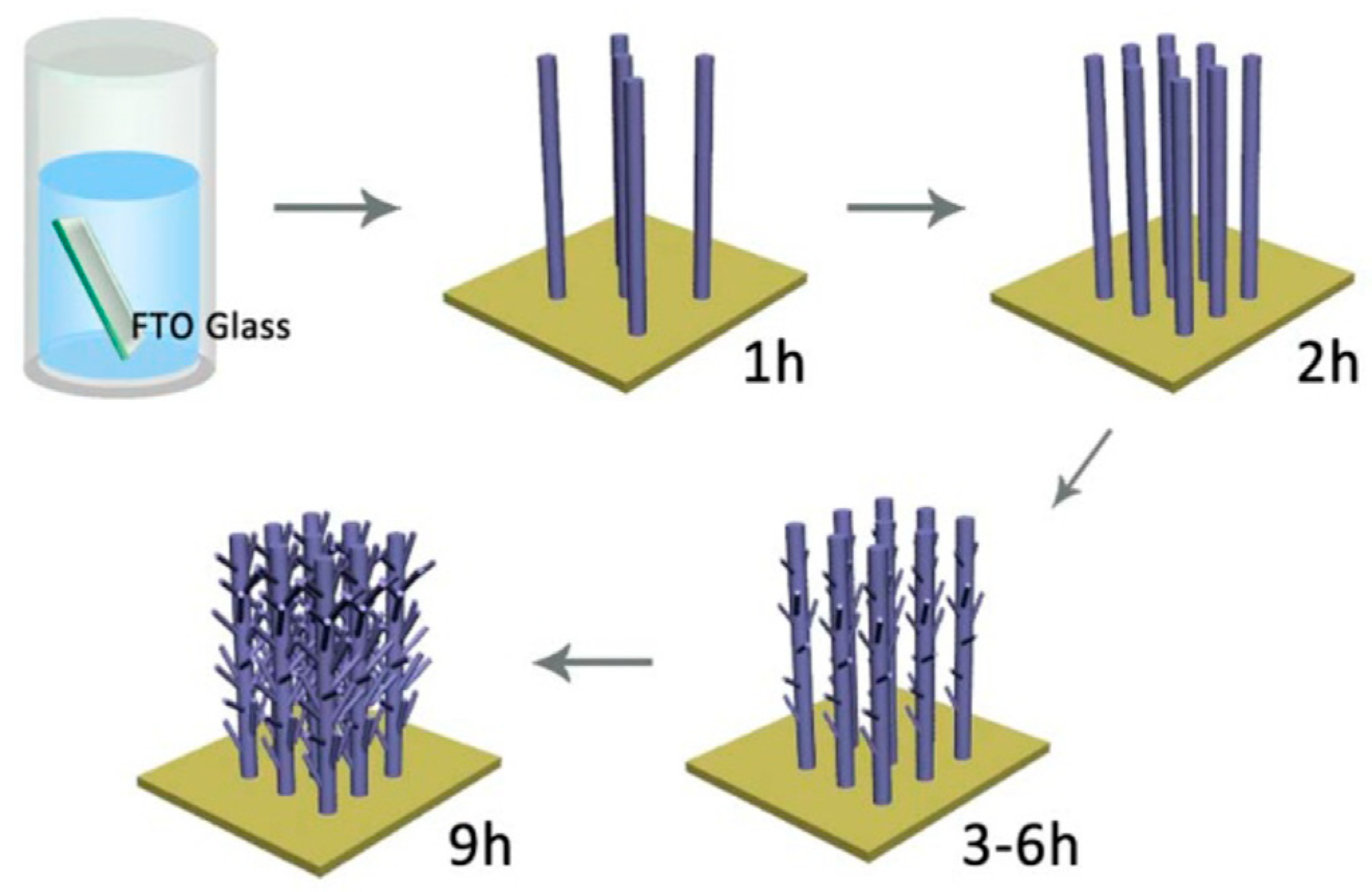

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliardi, S.; Rondino, F.; Paoletti, C.; Falconieri, M. On the Morphology of Nanostructured TiO2 for Energy Applications: The Shape of the Ubiquitous Nanomaterial. Nanomaterials 2022, 12, 2608. https://doi.org/10.3390/nano12152608
Gagliardi S, Rondino F, Paoletti C, Falconieri M. On the Morphology of Nanostructured TiO2 for Energy Applications: The Shape of the Ubiquitous Nanomaterial. Nanomaterials. 2022; 12(15):2608. https://doi.org/10.3390/nano12152608
Chicago/Turabian StyleGagliardi, Serena, Flaminia Rondino, Claudia Paoletti, and Mauro Falconieri. 2022. "On the Morphology of Nanostructured TiO2 for Energy Applications: The Shape of the Ubiquitous Nanomaterial" Nanomaterials 12, no. 15: 2608. https://doi.org/10.3390/nano12152608
APA StyleGagliardi, S., Rondino, F., Paoletti, C., & Falconieri, M. (2022). On the Morphology of Nanostructured TiO2 for Energy Applications: The Shape of the Ubiquitous Nanomaterial. Nanomaterials, 12(15), 2608. https://doi.org/10.3390/nano12152608