Memory Effects in Nanolaminates of Hafnium and Iron Oxide Films Structured by Atomic Layer Deposition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Growth and Structure
3.2. Magnetization Behavior
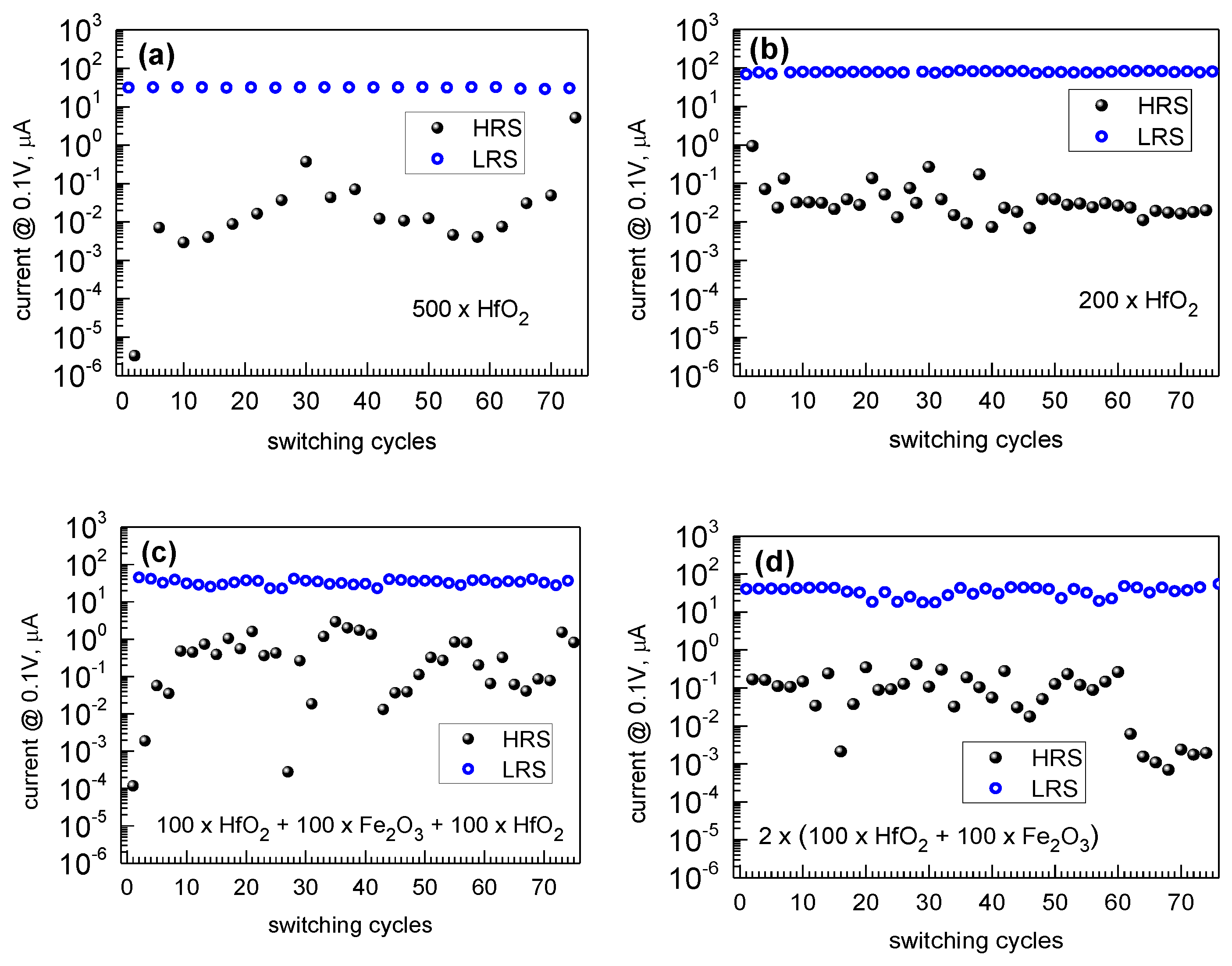
3.3. Electrical Behavior
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wolf, S.A.; Awschalom, D.D.; Buhrman, R.A.; Daughton, J.M.; von Molnár, S.; Roukes, M.L.; Chtchelkanova, A.Y.; Treger, D.M. Spintronics: A spin-based electronics vision for the future. Science 2001, 294, 1488–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waser, R.; Aono, M. Nanoionics-based resistive switching memories. Nat. Mater. 2007, 6, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Su, Y.-K. Conductive filaments controlled ferromagnetism in Co-doped ZnO resistive switching memory device. Jpn. J. Appl. Phys. 2019, 58, SBBI01. [Google Scholar] [CrossRef]
- Du, F.; Li, Y.; Li, X.; Yang, J.; Bai, Y.; Quan, Z.; Liu, C.; Xu, X. Resistive switching and its modulating ferromagnetism and magnetoresistance of a ZnO:Co/SiO2:Co film. J. Magn. Magn. Mater. 2019, 489, 165445. [Google Scholar] [CrossRef]
- Xie, J.; Ren, S.; Bu, J.; Cheng, B.; Liu, W.; Liu, L.; Zhou, G.; Qin, H.; Hu, J. Resistive switching and ferromagnetism modulation in copper oxide film on Nb:SrTiO3 substrate. J. Magn. Magn. Mater. 2018, 465, 295–299. [Google Scholar] [CrossRef]
- Ren, S.; Zhu, G.; Xie, J.; Bu, J.; Qin, H.; Hu, J. Resistive switching and electrical control of ferromagnetism in a Ag/HfO2/Nb:SrTiO3/Ag resistive random access memory (RRAM) device at room temperature. J. Phys. Condens. Matter 2016, 28, 056001. [Google Scholar] [CrossRef] [PubMed]
- Slesazeck, S.; Mikolajick, T. Nanoscale resistive switching memory devices: A review. Nanotechnology 2019, 30, 352003. [Google Scholar] [CrossRef] [PubMed]
- Chen, A. A review of emerging non-volatile memory (NVM) technologies and applications. Solid-State Electr. 2016, 125, 25–38. [Google Scholar] [CrossRef]
- Jasmin, A.C. Filamentary model in resistive switching materials. AIP Conf. Proc. 2017, 1901, 060004. [Google Scholar] [CrossRef]
- Waser, R. Redox-based resistive switching memories. J. Nanosci. Nanotech. 2012, 12, 7628–7640. [Google Scholar] [CrossRef] [Green Version]
- Menzel, S.; Waters, M.; Marchewka, A.; Böttger, U.; Dittmann, R.; Waser, R. Origin of the ultra-nonlinear switching kinetics in oxide-based resistive switches. Adv. Funct. Mater. 2011, 21, 4487–4492. [Google Scholar] [CrossRef]
- Valov, I. Redox-based resistive switching memories (ReRAMs): Electrochemical systems at the atomic scale. Chem. Electro. Chem. 2014, 1, 26–36. [Google Scholar] [CrossRef]
- Valov, I. Interfacial interactions and their impact on redox-based resistive switching memories (ReRAMs). Semicond. Sci. Technol. 2017, 32, 093006. [Google Scholar] [CrossRef]
- Ielmini, D.; Bruchhaus, R.; Waser, R. Thermochemical resistive switching: Materials, mechanisms, and scaling projections. Phase Trans. 2011, 84, 570–602. [Google Scholar] [CrossRef]
- Waser, R. Electrochemical and thermochemical memories. In Proceedings of the 2008 IEEE International Electron Devices Meeting, San Francisco, CA, USA, 15–17 December 2008. [Google Scholar] [CrossRef]
- Bersuker, G.; Gilmer, D.C.; Veksler, D.; Kirsch, P.; Vandelli, L.; Padovani, A.; Larcher, L.; McKenna, K.; Shluger, A.; Iglesias, V.; et al. Metal oxide resistive memory switching mechanism based on conductive filament properties. J. Appl. Phys. 2011, 110, 124518. [Google Scholar] [CrossRef]
- Lin, K.-L.; Hou, T.-H.; Shieh, J.; Lin, J.-H.; Chou, C.-T.; Lee, Y.-J. Electrode dependence of filament formation in HfO2 resistive-switching memory. J. Appl. Phys. 2011, 109, 084104. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, D.; Roldán, A.M.; González, M.B.; Jiménez-Molinos, F.; Campabadal, F.; Roldán, J.B. Influence of magnetic field on the operation of TiN/Ti/HfO2/W resistive memories. Microel. Eng. 2019, 215, 110983. [Google Scholar] [CrossRef] [Green Version]
- Hong, N.H. Magnetism due to defects/oxygen vacancies in HfO2 thin films. Phys. Stat. Sol. c 2007, 4, 1270–1275. [Google Scholar] [CrossRef]
- Tereshchenko, O.E.; Golyashov, V.A.; Eremeev, S.V.; Maurin, I.; Bakulin, A.V.; Kulkova, S.E.; Aksenov, M.S.; Preobrazhenskii, V.V.; Putyato, M.A.; Semyagin, B.R.; et al. Ferromagnetic HfO2/Si/GaAs interface for spin-polarimetry applications. Appl. Phys. Lett. 2015, 107, 123506. [Google Scholar] [CrossRef]
- Singh, R. Unexpected magnetism in nanomaterials. J. Mag. Mag. Mater. 2013, 346, 58–73. [Google Scholar] [CrossRef]
- Daub, M.; Bachmann, J.; Jing, J.; Knez, M.; Gösele, U.; Barth, S.; Mathur, S.; Escrig, J.; Altbir, D.; Nielsch, K. Ferromagnetic nanostructures by atomic layer deposition: From thin films towards core-shell nanotubes. ECS Trans. 2007, 11, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.R.; Baker, J.G.; Bent, S.F. The influence of ozone: Superstoichiometric oxygen in atomic layer deposition of Fe2O3 using tert-butylferrocene and O3. Adv. Mater. Interfaces 2020, 7, 2000318. [Google Scholar] [CrossRef]
- Márquez, P.; Alburquenque, D.; Celis, F.; Freire, R.M.; Escrig, J. Structural, morphological and magnetic properties of iron oxide thin films obtained by atomic layer deposition as a function of their thickness. J. Magn. Magn. Mater. 2021, 530, 167914. [Google Scholar] [CrossRef]
- Štefanić, G.; Musić, S. Thermal behavior of the amorphous precursors of the Fe2O3-HfO2 system. Thermochim. Acta 2001, 373, 59–67. [Google Scholar] [CrossRef]
- Rao, M.S.R.; Kundaliya, D.C.; Dhar, S.; Cardoso, C.A.; Curtin, A.; Welz, S.J.; Erni, R.; Browning, N.D.; Lofland, S.E.; Metting, C.J.; et al. Search for magnetism in Co and Fe doped HfO2 thin films for potential spintronic applications. Mater. Res. Soc. Proc. 2004, 830, 262–275. [Google Scholar] [CrossRef]
- Kukli, K.; Kemell, M.; Castán, H.; Dueñas, S.; Seemen, H.; Rähn, M.; Link, J.; Stern, R.; Ritala, M.; Leskelä, M. Atomic layer deposition and properties of HfO2-Al2O3 nanolaminates. ECS J. Solid State Sci. Tech. 2018, 7, P501–P508. [Google Scholar] [CrossRef] [Green Version]
- Kalam, K.; Seemen, H.; Mikkor, M.; Ritslaid, P.; Stern, R.; Dueñas, S.; Castán, H.; Tamm, A.; Kukli, K. Electric and magnetic properties of atomic layer deposited ZrO2-HfO2 thin films. ECS J. Solid State Sci. Technol. 2018, 7, N117–N122. [Google Scholar] [CrossRef]
- Ossorio, O.G.; Dueñas, S.; Castán, H.; Tamm, A.; Kalam, K.; Seemen, H.; Kukli, K. Resistive switching properties of atomic layer deposited ZrO2-HfO2 thin films. In Proceedings of the 2018 Spanish Conference on Electron Devices (CDE), Salamanca, Spain, 14–16 November 2018. [Google Scholar] [CrossRef]
- Kukli, K.; Kemell, M.; Castán, H.; Dueñas, S.; Link, J.; Stern, R.; Heikkilä, M.J.; Jõgiaas, T.; Kozlova, J.; Rähn, M.; et al. Magnetic properties and resistive switching in mixture films and nanolaminates consisting of iron and silicon oxides grown by atomic layer deposition. J. Vac. Sci. Technol. A 2020, 38, 042405. [Google Scholar] [CrossRef]
- Arroval, T.; Aarik, L.; Rammula, R.; Kruusla, V.; Aarik, J. Effect of substrate-enhanced and inhibited growth on atomic layer deposition and properties of aluminum–titanium oxide films. Thin Solid Films 2016, 600, 119–125. [Google Scholar] [CrossRef]
- Granneman, E.; Fischer, P.; Pierreux, D.; Terhorst, H.; Zagwijn, P. Batch ALD: Characteristics, comparison with single wafer ALD, and examples. Surf. Coat. Technol. 2017, 201, 8899–8907. [Google Scholar] [CrossRef]
- Zagwijn, P.; Verweij, W.; Pierreux, D.; Adjeroud, N.; Bankras, R.; Oosterlaken, E.; Snijders, G.J.; van den Hout, M.; Fischer, P.; Wilhelm, R.; et al. Novel batch titanium nitride CVD process for advanced metal electrodes. ECS Transact. 2008, 13, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Aarik, L.; Arroval, T.; Mändar, H.; Rammula, R.; Aarik, J. Influence of oxygen precursors on atomic layer deposition of HfO2 and hafnium-titanium oxide films: Comparison of O3- and H2O-based processes. Appl. Surf. Sci. 2020, 530, 147229. [Google Scholar] [CrossRef]
- Kalam, K.; Seemen, H.; Ritslaid, P.; Rähn, M.; Tamm, A.; Kukli, K.; Kasikov, A.; Link, J.; Stern, R.; Dueñas, S.; et al. Atomic layer deposition and properties of ZrO2/Fe2O3 thin films. Beilstein J. Nanotechnol. 2018, 9, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, D.W.; Frank, M.M.; Guha, S. Absence of magnetism in hafnium oxide films. Appl. Phys. Lett. 2005, 87, 252502. [Google Scholar] [CrossRef]
- Venkatesan, M.; Fitzgerald, C.B.; Coey, J.M.D. Unexpected magnetism in a dielectric oxide. Nature 2004, 430, 630. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.-H.; Miao, X.-S. Oxygen vacancy chain and conductive filament formation in hafnia. J. Appl. Phys. 2018, 123, 161505. [Google Scholar] [CrossRef]
- Dai, Y.; Pan, Z.; Wang, F.; Li, X. Oxygen vacancy effects in HfO2-based resistive switching memory: First principle study. AIP Adv. 2016, 6, 085209. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Rath, C. Oxygen vacancy mediated stabilization of cubic phase at room temperature and resistive switching effect in Sm- and Dy-doped HfO2 thin film. Phys. Stat. Sol. A 2020, 217, 1900756. [Google Scholar] [CrossRef]
- Zhao, X.; Vanderbilt, D. First-principles study of structural, vibrational, and lattice dielectric properties of hafnium oxide. Phys. Rev. B 2002, 65, 233106. [Google Scholar] [CrossRef] [Green Version]
- Kukli, K.; Ihanus, J.; Ritala, M.; Leskelä, M. Tailoring the dielectric properties of HfO2–Ta2O5 nanolaminates. Appl. Phys. Lett. 1996, 68, 3737–3739. [Google Scholar] [CrossRef]
- Chang, S.H.; Lee, J.S.; Chae, S.C.; Lee, S.B.; Liu, C.; Kahng, B.; Kim, D.-W.; Noh, T.W. Occurrence of both unipolar memory and threshold resistance switching in a NiO film. Phys. Rev. Lett. 2009, 102, 026801. [Google Scholar] [CrossRef] [PubMed]
- Baek, I.G.; Lee, M.S.; Seo, S.; Lee, M.J.; Seo, D.H.; Suh, D.-S.; Park, J.C.; Park, S.O.; Kim, H.S.; Yoo, I.K.; et al. Highly scalable nonvolatile resistive memory using simple binary oxide driven by asymmetric unipolar voltage pulses. In Proceedings of the IEDM Technical Digest. IEEE International Electron Devices Meeting, San Francisco, CA, USA, 13–15 December 2004. [Google Scholar] [CrossRef]
- Nardi, F.; Ielmini, D.; Cagli, C.; Spiga, S.; Fanciulli, M.; Goux, L.; Wouters, D.J. Sub-10 µA reset in NiO-based resistive switching memory (RRAM) cells. In Proceedings of the 2010 IEEE International Memory Workshop, Seoul, Korea, 16–19 May 2010. [Google Scholar] [CrossRef]
- Ielmini, D.; Spiga, S.; Nardi, F.; Cagli, C.; Lamperti, A.; Cianci, E.; Fanciulli, M. Scaling analysis of submicrometer nickel-oxide-based resistive switching memory devices. J. Appl. Phys. 2011, 109, 034506. [Google Scholar] [CrossRef]
- Chudnovskii, F.A.; Odynets, L.L.; Pergament, A.L.; Stefanovich, G.B. Electroforming and switching in oxides of transition metals: The role of metal–insulator transition in the switching mechanism. J. Solid State Chem. 1996, 122, 95–99. [Google Scholar] [CrossRef]
- Mohammad, B.; Jaoude, M.A.; Kumar, V.; Al Homouz, D.M.; Nahla, H.A.; Al-Qutayri, M.; Christoforou, N. State of the art of metal oxide memristor devices. Nanotechnol. Rev. 2016, 5, 311–329. [Google Scholar] [CrossRef]
- Traoré, B.; Xue, K.-H.; Vianello, E.; Molas, G.; Blaise, P.; De Salvo, B.; Padovani, A.; Pirrotta, O.; Larcher, L.; Fonseca, L.R.C.; et al. Investigation of the role of electrodes on the retention performance of HfOx based RRAM cells by experiments, atomistic simulations and device physical modeling. In Proceedings of the 2013 IEEE International Reliability Physics Symposium (IRPS), Monterey, CA, USA, 14–18 April 2013. [Google Scholar] [CrossRef]
- Traoré, B.; Blaise, P.; Vianello, E.; Perniola, L.; De Salvo, B.; Nishi, Y. HfO2 based RRAM: Electrode effects, Ti/HfO2 interface, charge injection, and oxygen (O) defects diffusion through experiment and Ab Initio calculations. IEEE Transact. Electron Dev. 2016, 63, 360–368. [Google Scholar] [CrossRef]
- Wang, X.P.; Chen, Y.Y.; Pantisano, L.; Goux, L.; Jurczak, M.; Groeseneken, G.; Wouters, D.J. Effect of anodic interface layers on the unipolar switching of HfO2-based resistive RAM. In Proceedings of the 2010 International Symposium on VLSI Technology, System and Application, Hsinchu, Taiwan, 26–28 April 2010. [Google Scholar] [CrossRef]
- Goux, L.; Czarnecki, P.; Chen, Y.Y.; Pantisano, L.; Wang, X.P.; Degraeve, R.; Govoreanu, B.; Jurczak, M.; Wouters, D.J.; Altimime, L. Evidences of oxygen-mediated resistive-switching mechanism in TiN\HfO2\Pt cells. Appl. Phys. Lett. 2010, 97, 243509. [Google Scholar] [CrossRef]
- Goux, L.; Chen, Y.-Y.; Pantisano, L.; Wang, X.-P.; Groeseneken, G.; Jurczak, M.; Wouters, D.J. On the gradual unipolar and bipolar resistive switching of TiN\HfO2\Pt memory systems. Electrochem. Solid-State Lett. 2010, 13, G54–G56. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chen, Y.S.; Chen, P.S.; Wu, T.Y.; Chen, F.; Wang, C.C.; Tzeng, P.J.; Tsai, M.-J.; Lien, C. Low power and nanosecond switching in robust hafnium oxide resistive memory with a thin Ti cap. IEEE Electron. Dev. Lett. 2010, 31, 44–46. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lee, H.Y.; Chen, P.S.; Gu, P.Y.; Chen, C.W.; Lin, W.P.; Liu, W.H.; Hsu, Y.Y.; Sheu, S.S.; Chiang, P.C.; et al. Highly scalable hafnium oxide memory with improvements of resistive distribution and read disturb immunity. In Proceedings of the 2009 IEEE International Electron Devices Meeting (IEDM), Baltimore, MD, USA, 7–9 December 2009. [Google Scholar] [CrossRef]
- Bertaud, T.; Walczyk, D.; Walczyk, C.; Kubotsch, S.; Sowinska, M.; Schroeder, T.; Wenger, C.; Vallée, C.; Gonon, P.; Mannequin, C.; et al. Resistive switching of HfO2-based metal–insulator–metal diodes: Impact of the top electrode material. Thin Solid Films 2012, 520, 4551–4555. [Google Scholar] [CrossRef]
- Walczyk, C.; Wenger, C.; Sohal, R.; Lukosius, M.; Fox, A.; Dąbrowski, J.; Wolansky, D.; Tillack, B.; Müssig, H.-J.; Schroeder, T. Pulse-induced low-power resistive switching in HfO2 metal-insulator-metal diodes for nonvolatile memory applications. J. Appl. Phys. 2009, 105, 114103. [Google Scholar] [CrossRef]
- Inoue, I.H.; Yasuda, S.; Akinaga, H.; Takagi, H. Nonpolar resistance switching of metal/binary-transition-metal oxides/metal sandwiches: Homogeneous/inhomogeneous transition of current distribution. Phys. Rev. B 2008, 77, 035105. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.C.; Christopher, J.E.; Coleman, R.V. Conduction phenomena in thin layers of iron oxide. Phys. Rev. 1969, 84, 565–573. [Google Scholar] [CrossRef]
- Zimmers, A.; Aigouy, L.; Mortier, M.; Sharoni, A.; Wang, S.; West, K.G.; Ramirez, J.G.; Schuller, I.K. Role of thermal heating on the voltage induced insulator-metal transition in VO2. Phys. Rev. Lett. 2013, 110, 056601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Hosseini, S.R.; Bonyani, M.; Leonardi, S.G.; Bonavita, A.; Neri, G. Synthesis and characterization of mesoporous α-Fe2O3 nanoparticles and investigation of electrical properties of fabricated thick films. Process. Appl. Ceram. 2016, 10, 209–217. [Google Scholar] [CrossRef]
- Engel, J.; Tuller, H.L. The electrical conductivity of thin film donor doped hematite: From insulator to semiconductor by defect modulation. Phys. Chem. Chem. Phys. 2014, 16, 11374. [Google Scholar] [CrossRef] [PubMed]
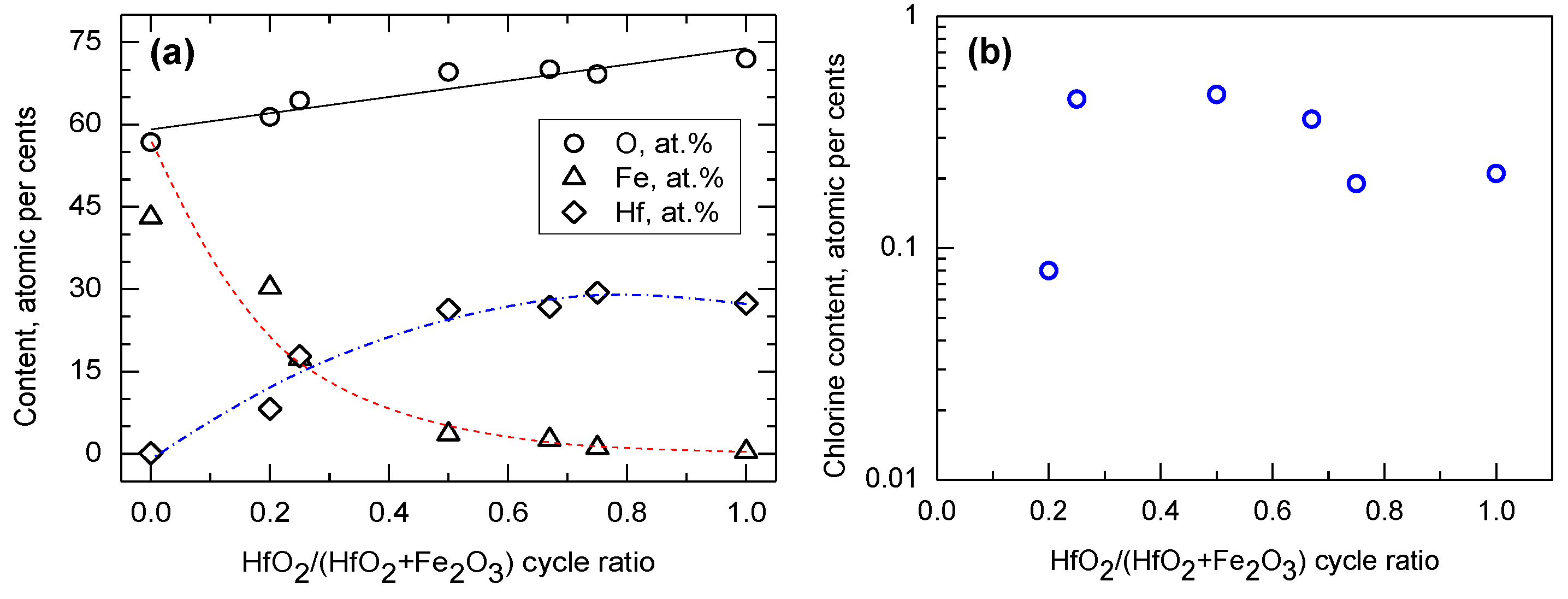
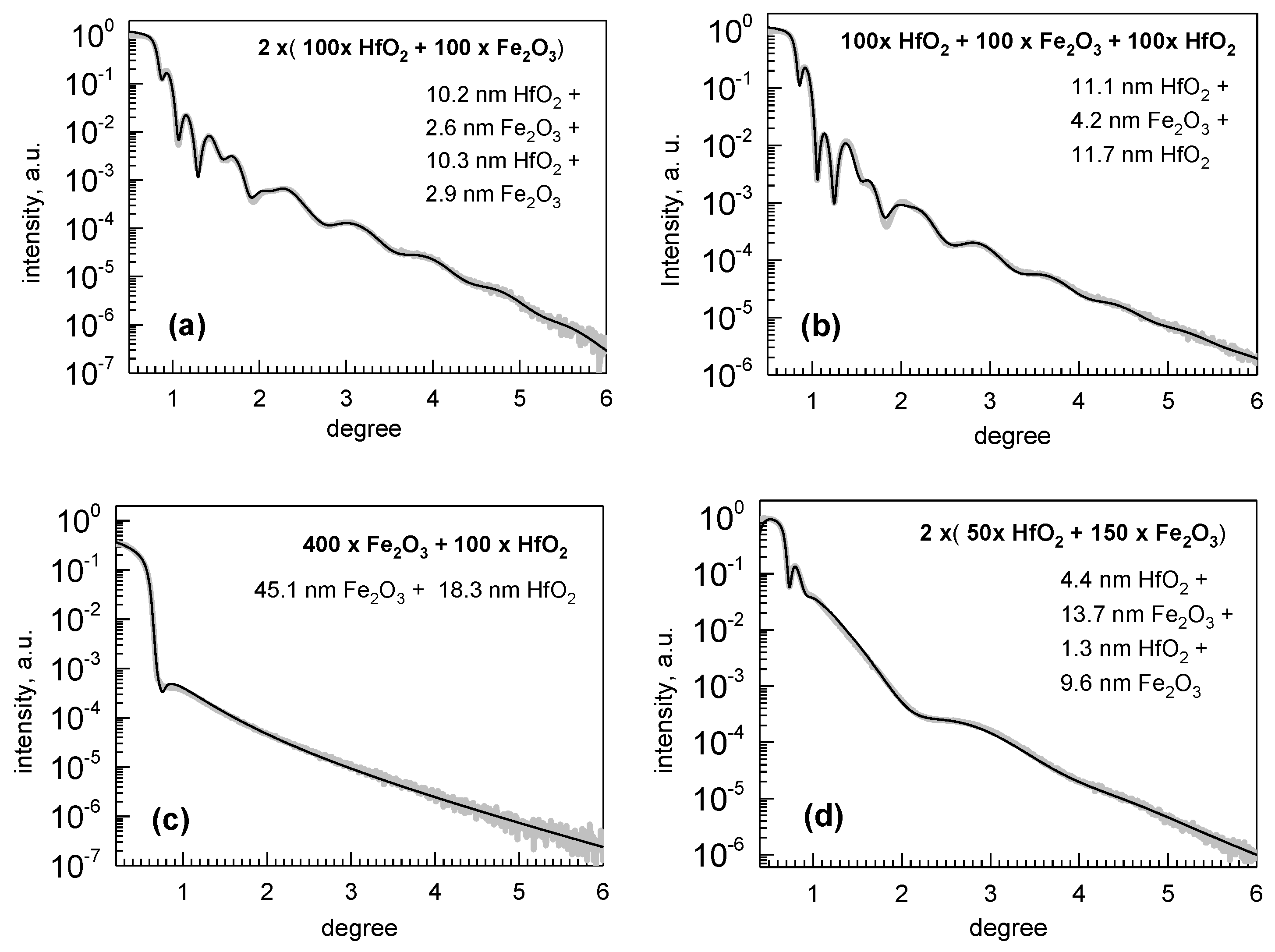

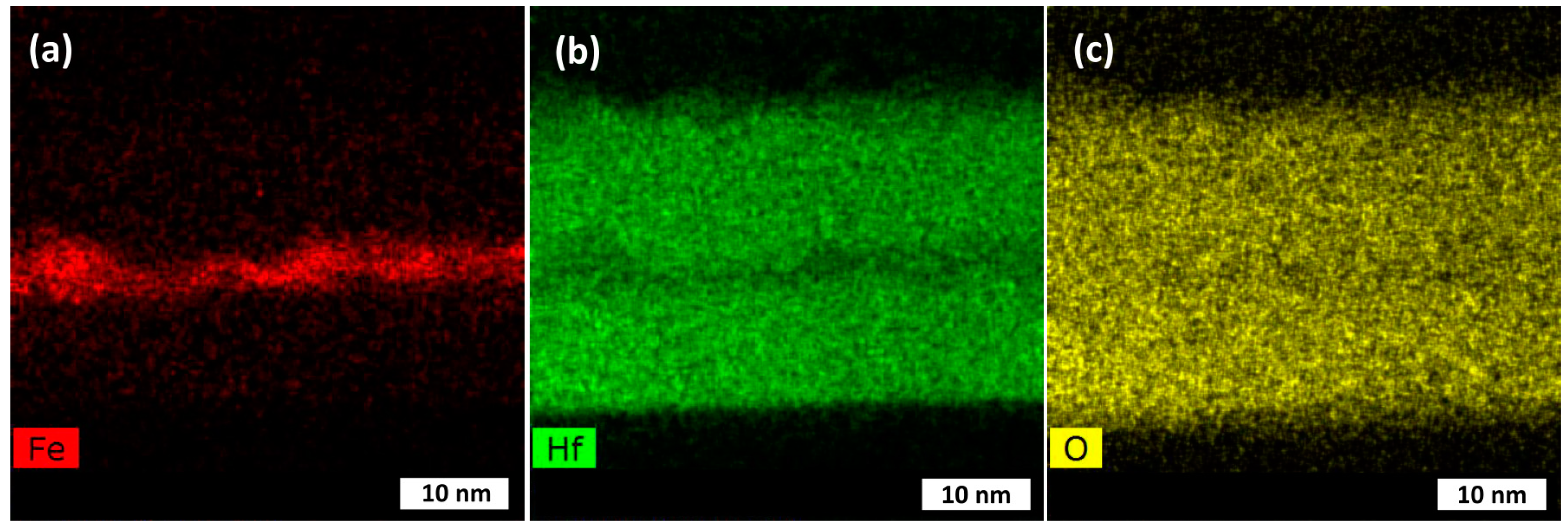
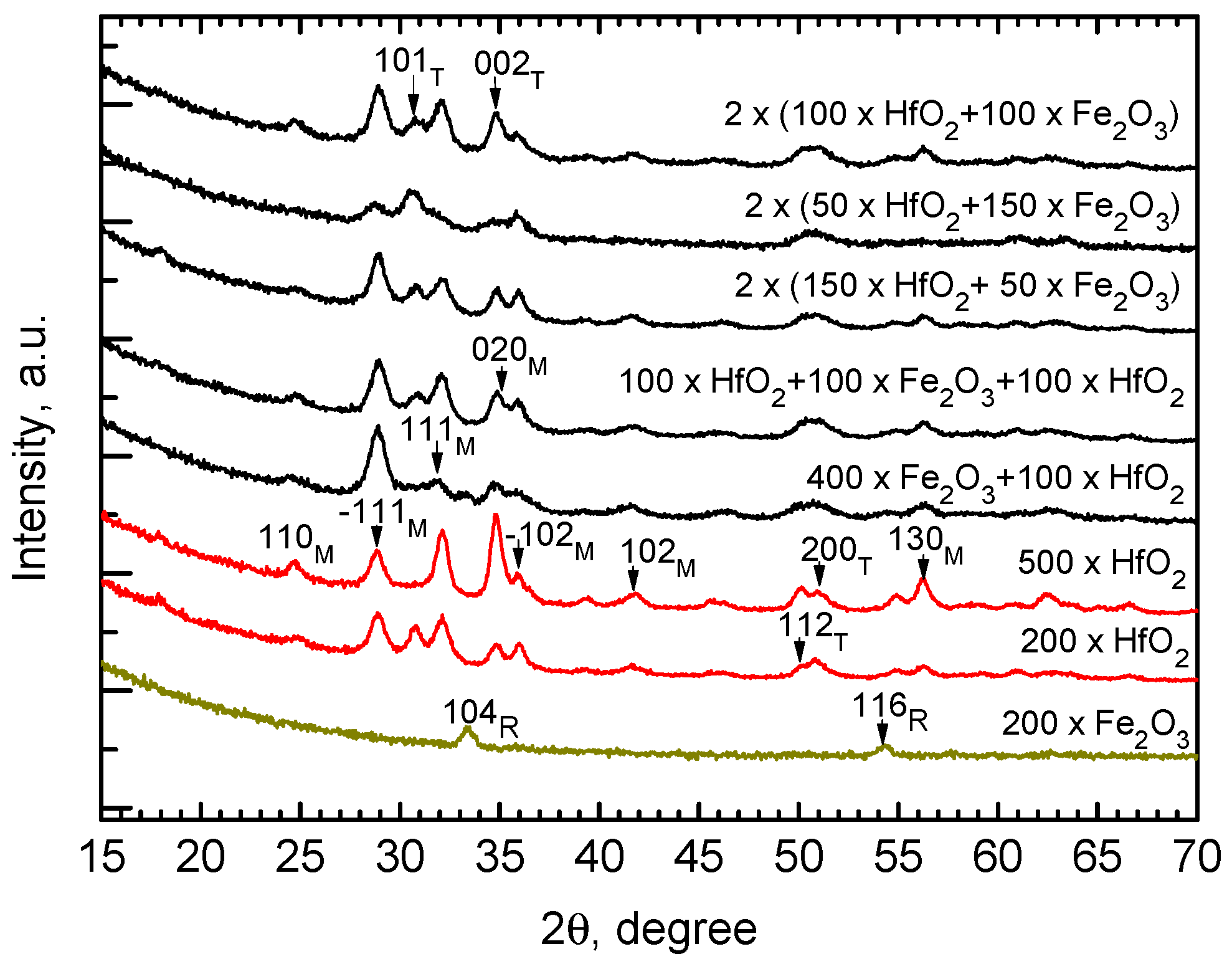
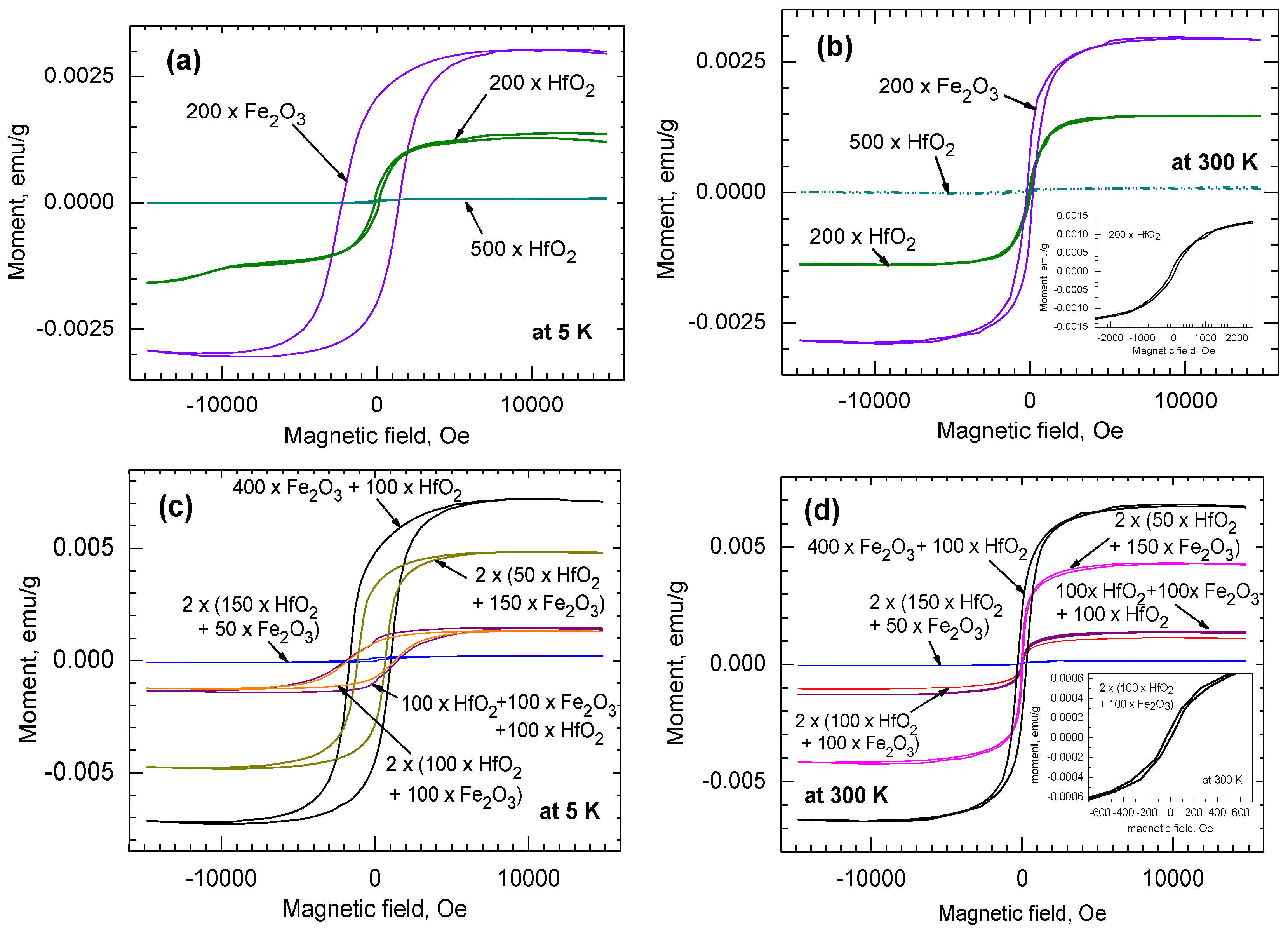
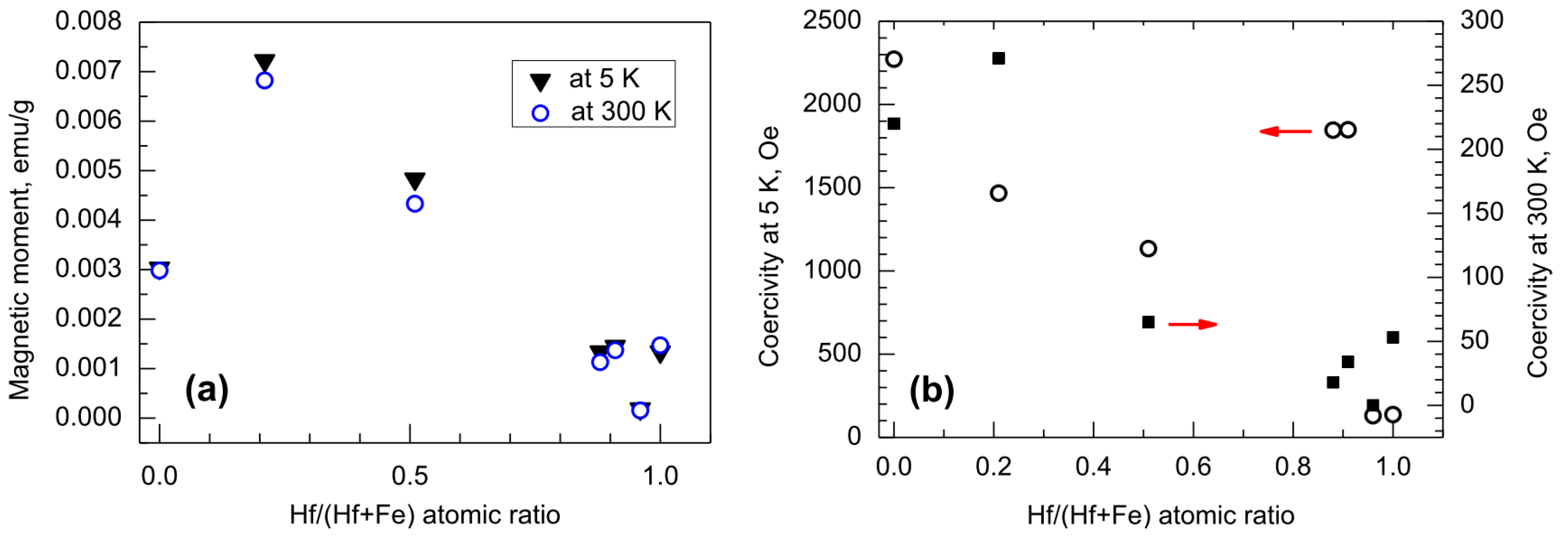

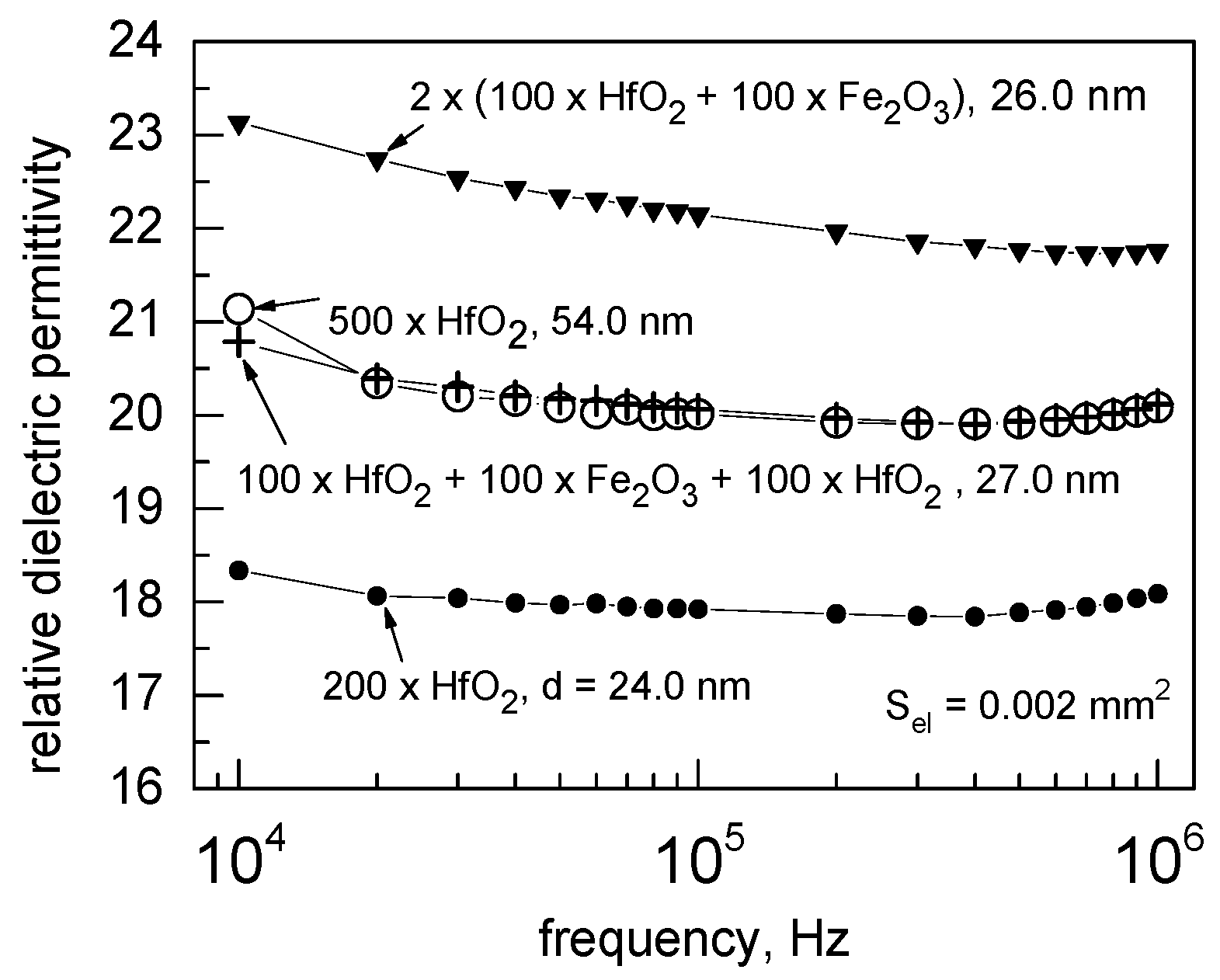
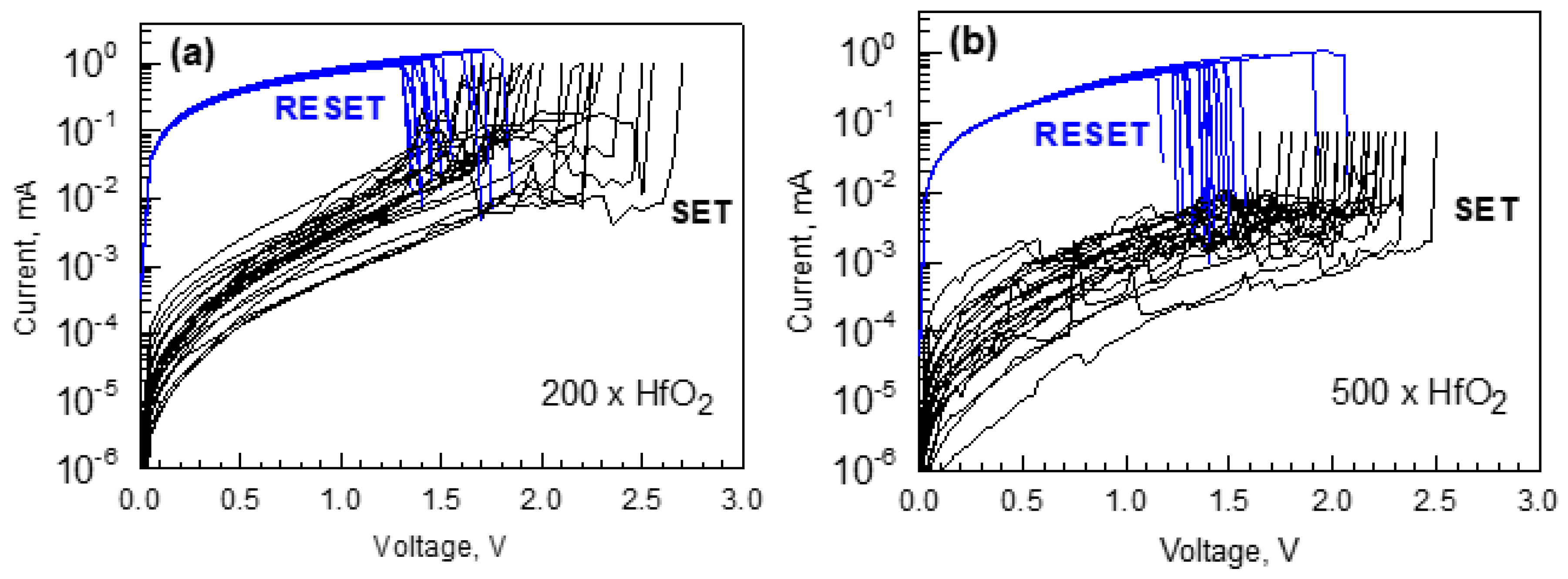

| Cycle Sequence | Cycle Ratio | ttotal, nm | Hf/(Hf + Fe) by XRF | Hf/(Hf + Fe) by EDS |
|---|---|---|---|---|
| 500 × HfO2 | 1 | 54 | 1 | 1 |
| 2 × (150 × HfO2 +50 × Fe2O3) | 0.75 | 23 | 0.96 (0.03) | 0.98 |
| 100 × HfO2 + 100 × Fe2O3 + 100 × HfO2 | 0.67 | 27 | 0.91 (0.04) | 0.95 |
| 2 × (100 × HfO2 +100 × Fe2O3) | 0.5 | 26 | 0.88 (0.02) | 0.91 |
| 2 × (50 × HfO2 +150 × Fe2O3) | 0.25 | 29 | 0.51 (0.01) | 0.49 |
| 400 × Fe2O3 +100 × HfO2 | 0.2 | 63 | 0.21 (0.01) | 0.24 |
| 500 × Fe2O3 | 0 | 37 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalam, K.; Otsus, M.; Kozlova, J.; Tarre, A.; Kasikov, A.; Rammula, R.; Link, J.; Stern, R.; Vinuesa, G.; Lendínez, J.M.; et al. Memory Effects in Nanolaminates of Hafnium and Iron Oxide Films Structured by Atomic Layer Deposition. Nanomaterials 2022, 12, 2593. https://doi.org/10.3390/nano12152593
Kalam K, Otsus M, Kozlova J, Tarre A, Kasikov A, Rammula R, Link J, Stern R, Vinuesa G, Lendínez JM, et al. Memory Effects in Nanolaminates of Hafnium and Iron Oxide Films Structured by Atomic Layer Deposition. Nanomaterials. 2022; 12(15):2593. https://doi.org/10.3390/nano12152593
Chicago/Turabian StyleKalam, Kristjan, Markus Otsus, Jekaterina Kozlova, Aivar Tarre, Aarne Kasikov, Raul Rammula, Joosep Link, Raivo Stern, Guillermo Vinuesa, José Miguel Lendínez, and et al. 2022. "Memory Effects in Nanolaminates of Hafnium and Iron Oxide Films Structured by Atomic Layer Deposition" Nanomaterials 12, no. 15: 2593. https://doi.org/10.3390/nano12152593
APA StyleKalam, K., Otsus, M., Kozlova, J., Tarre, A., Kasikov, A., Rammula, R., Link, J., Stern, R., Vinuesa, G., Lendínez, J. M., Dueñas, S., Castán, H., Tamm, A., & Kukli, K. (2022). Memory Effects in Nanolaminates of Hafnium and Iron Oxide Films Structured by Atomic Layer Deposition. Nanomaterials, 12(15), 2593. https://doi.org/10.3390/nano12152593








