Two-Dimensional Perovskite (PEA)2PbI4 Two-Color Blue-Green Photodetector
Abstract
:1. Introduction
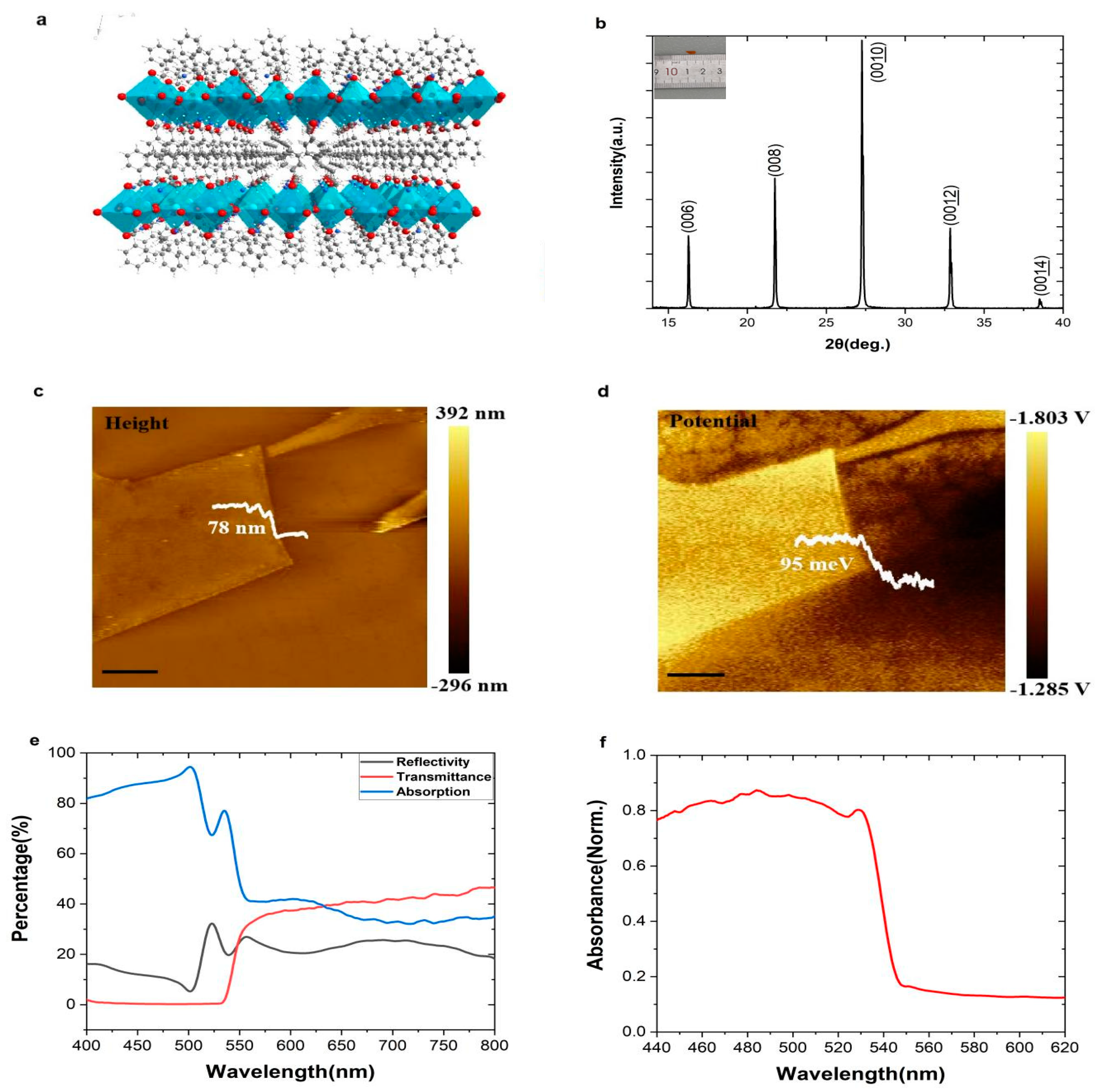
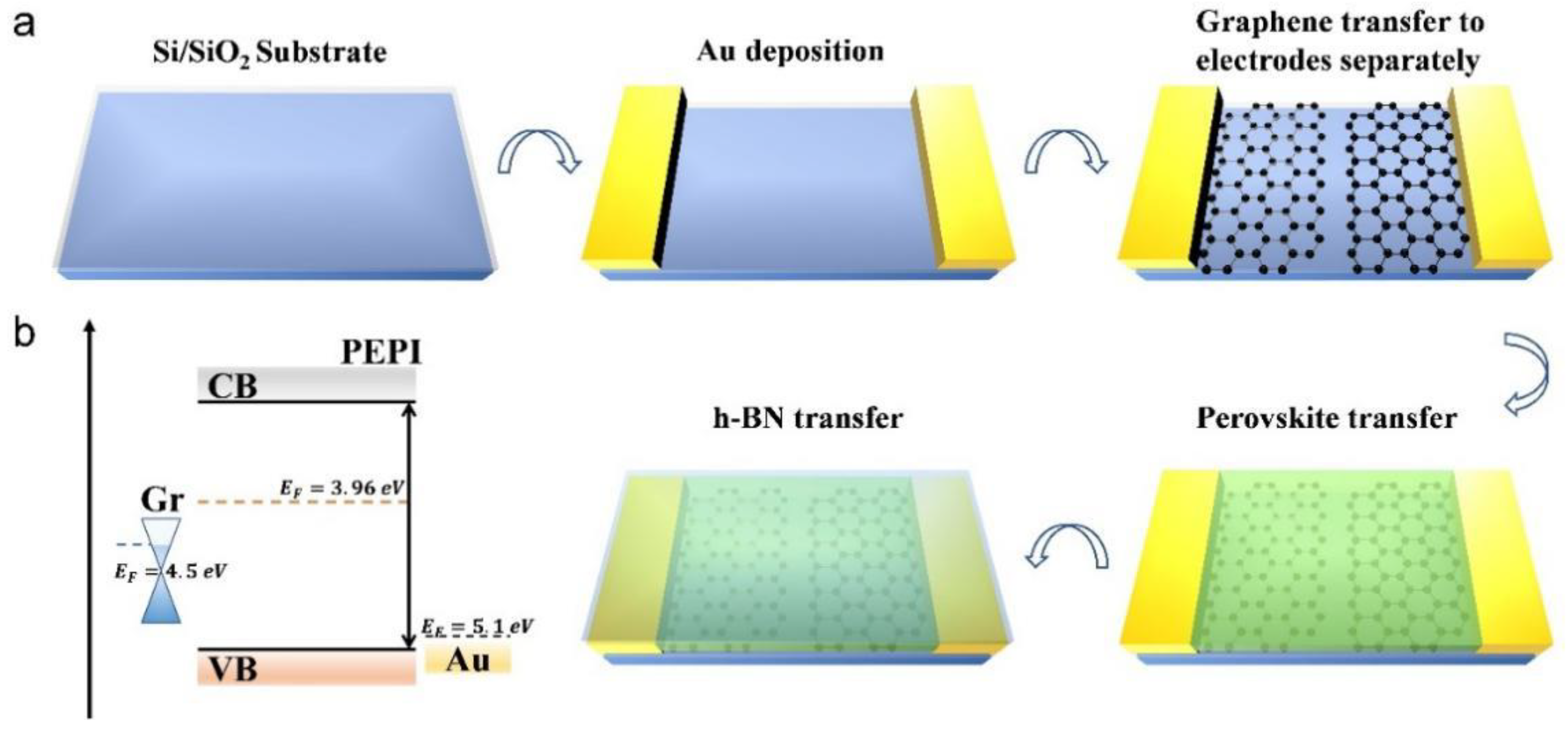
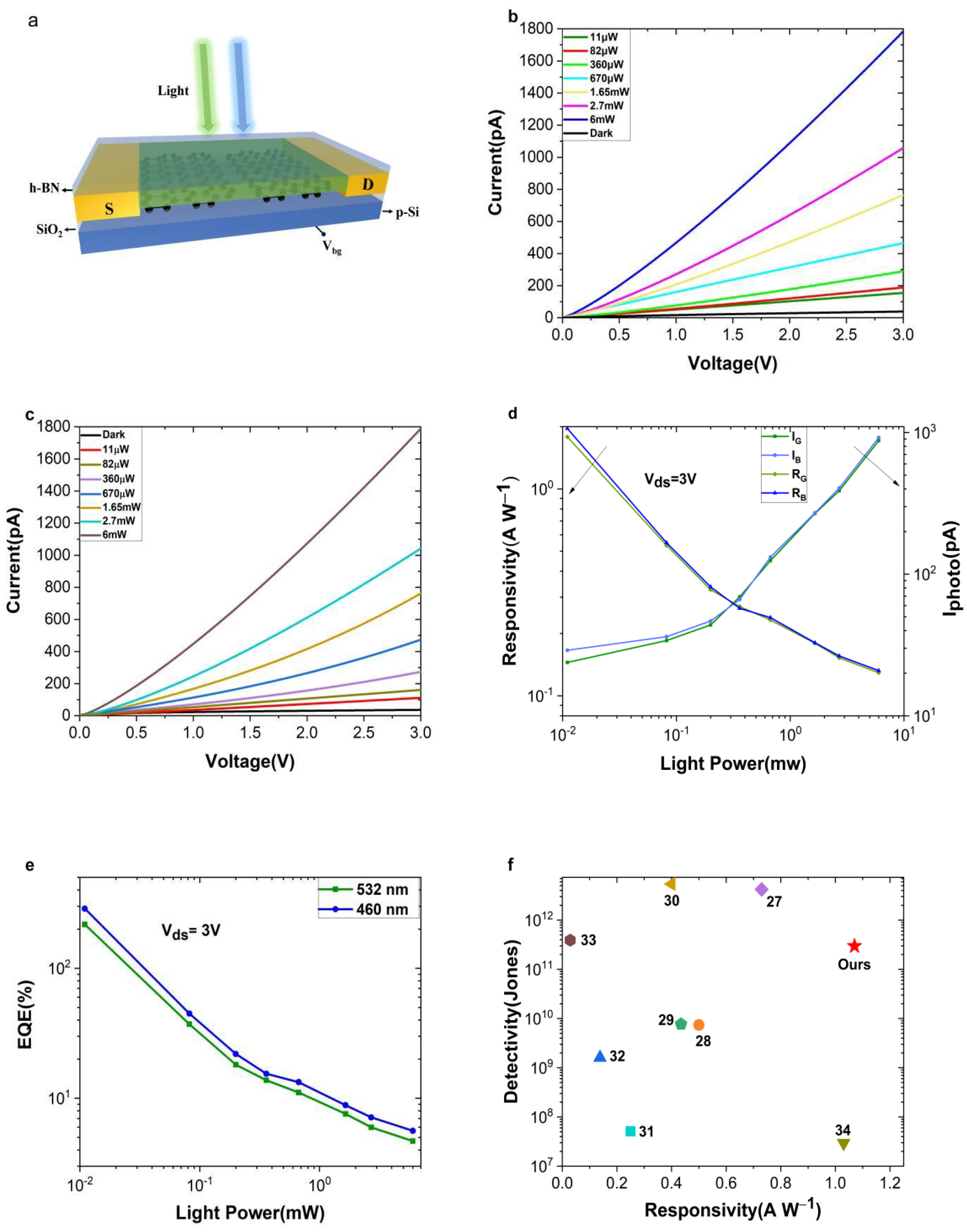
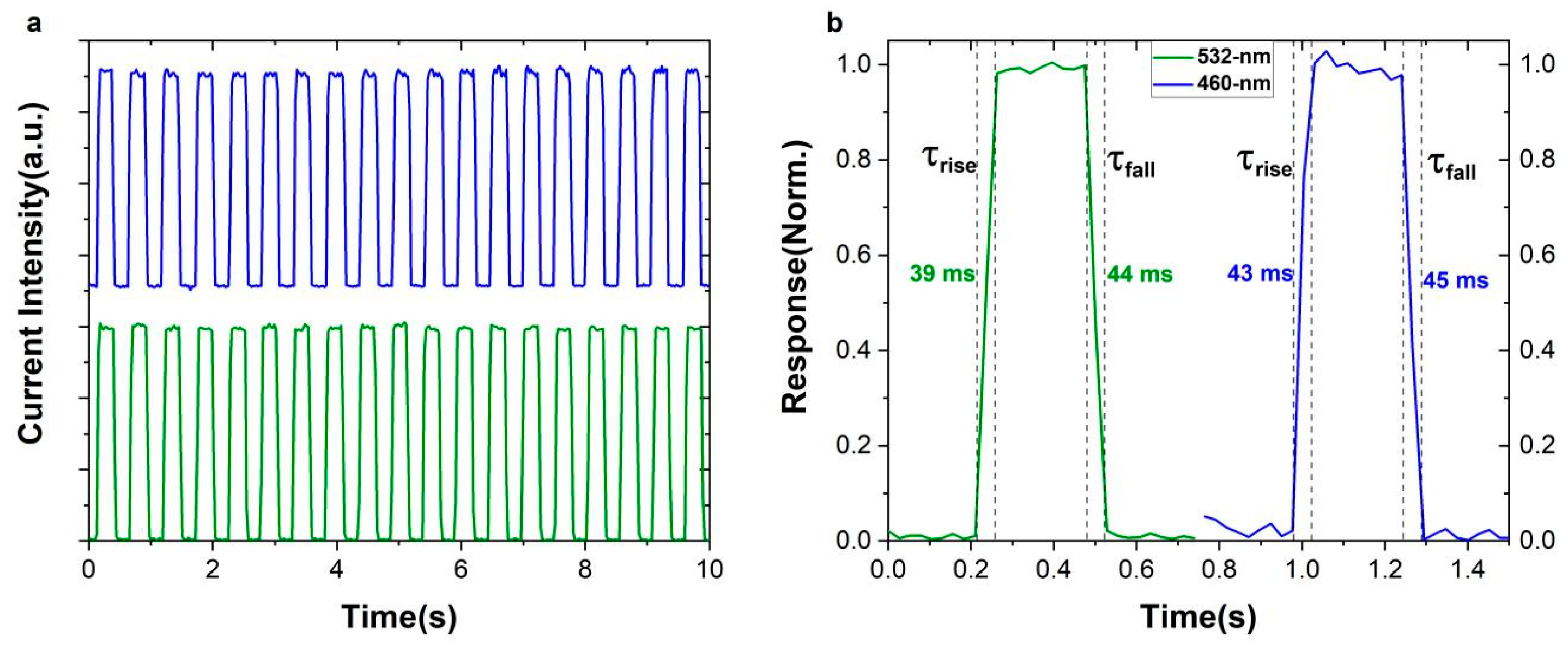
2. Result and Discussion
3. Conclusions
4. Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duntley, S.Q. Light in the Sea. J. Opt. Soc. Am. 1963, 53, 214–233. [Google Scholar] [CrossRef]
- Chuang, S.L.; Peyghambarian, N.; Koch, S. Physics of optoelectronic devices. Phys. Today 1996, 49, 62. [Google Scholar] [CrossRef] [Green Version]
- Headrick, R.; Freitag, L. Growth of underwater communication technology in the US Navy. IEEE Commun. Mag. 2009, 47, 80–82. [Google Scholar] [CrossRef]
- Pope, R.M.; Weidemann, A.D.; Fry, E.S. Integrating cavity absorption meter measurements of dissolved sub-stances and suspended particles in ocean water. Dyn. Atmos. Ocean. 2000, 31, 307–320. [Google Scholar] [CrossRef]
- Pepin, C.M.; Dautet, H.; Bergeron, M.; Cadorette, J.; Beaudoin, J.F.; Jacques-Bédard, X.; Couture, M.; Lecomte, R. New UV-enhanced, ultra-low noise silicon avalanche photodiode for radiation detec-tion and medical imaging. In IEEE Nuclear Science Symposium & Medical Imaging Conference; IEEE: Manhattan, NY, USA, 2010. [Google Scholar]
- Wang, Y.; Yang, D.; Zhou, X.; Alshehri, S.M.; Ahamad, T.; Vadim, A.; Ma, D. Vapour-assisted multi-functional perovskite thin films for solar cells and photodetectors. J. Mater. Chem. C 2016, 4, 7415. [Google Scholar] [CrossRef]
- Xu, W.; Hu, Q.; Bai, S.; Bao, C.; Miao, Y.; Yuan, Z.; Borzda, T.; Barker, A.J.; Tyukalova, E.; Hu, Z. Rational molecular passivation for high-performance perovskite light-emitting diodes. Nat. Photonics 2019, 13, 418. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Hu, J.; Huang, B.; Sun, M.; Dong, B.; Yan, C.H. A Eu3+–Eu2+ ion redox shuttle imparts operational durability to Pb-I perovskite solar cells. Science 2019, 363, 265–270. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X. Lead halide perov-skite nanowire lasers with low lasing thresholds and high-quality factors. Nat. Mater. 2015, 14, 636. [Google Scholar] [CrossRef]
- Righetto, M.; Meggiolaro, D.; Rizzo, A.; Sorrentino, R.; He, Z.; Meneghesso, G.; Sum, T.C.; Gatti, T.; Lamberti, F. Coupling halide perovskites with different materials: From doping to nanocomposites, beyond photovoltaics. Prog. Mater. Sci. 2020, 110, 100639. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Haque, M.A.; Savoie, M.; Abdelhady, A.L.; Cho, N.; Dursun, I.; Buttner, U.; Alarousu, E.; Wu, T.; Bakr, O.M. Perovskite photodetectors operating in both narrowband and broadband regimes. Adv. Mater. 2016, 28, 8144. [Google Scholar] [CrossRef]
- Chen, G.; Feng, J.; Gao, H.; Zhao, Y.; Pi, Y.; Jiang, X.; Wu, Y.; Jiang, L. Stable α-CsPbI3 perovskite nanowire arrays with preferential crystallographic orientation for highly sensitive photodetectors. Adv. Funct. Mater. 2019, 29, 1808741. [Google Scholar] [CrossRef]
- Li, S.X.; Zhang, G.P.; Xia, H.; Xu, Y.S.; Lv, C.; Sun, H.B. Template-confined growth of Ruddlesden–Popper Perov-skite micro-wire arrays for stable polarized photodetectors. Nanoscale 2019, 11, 18272. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Frisov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, B.; Hwang, I.; Cho, S.H.; Kim, E.H.; Cha, S.; Lee, J.; Kang, H.S.; Cho, S.M.; Choi, H.; Park, C. Sol-vent-assisted gel printing for micropatterning thin organic-inorganic hybrid Perovskite Films. ACS Nano. 2016, 10, 9026–9035. [Google Scholar] [CrossRef]
- Doganov, R.A.; O’Farrell, E.C.; Koenig, S.P.; Taniguchi, T.; Neto, A.H.C.; Özyilmaz, B. Transport properties of pristine few-layer black phosphorus by van der Waals passivation in an inert atmosphere. Nat. Commun. 2015, 6, 6647. [Google Scholar] [CrossRef]
- Panchal, V.; Pearce, R.; Yakimova, R.; Tzalenchuk, A.; Kazakova, O. Standardization of surface potential meas-urements of graphene domains. Sci. Rep. 2013, 3, 2597. [Google Scholar] [CrossRef] [Green Version]
- Leng, K.; Wang, L.; Shao, Y.; Abdelwahab, I.; Grinblat, G.; Verzhbitskiy, I.; Li, R.; Cai, Y.; Chi, X.; Fu, W.; et al. Electron tunneling at the molecularly thin 2D perovskite and graphene van der waals interface. Nat. Commun. 2020, 11, 5483. [Google Scholar]
- Liu, Y.C.; Ren, X.D.; Zhang, J.; Yang, Z.; Yang, D.; Yu, F.Y.; Sun, J.K.; Zhao, C.M.; Yao, Z.; Wang, B.; et al. 120 mm single crystalline perovskite and wafers: Towards viable applications. Sci. China Chem. 2017, 60, 1367–1376. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Yang, Z.; Yang, D.; Ren, X.; Xu, H.; Yang, Z.; Liu, S.F. 20-mm-large single-crystalline formamidinium-perovskite wafer for mass production of integrated photodetectors. Adv. Opt. Mater. 2016, 4, 1829–1837. [Google Scholar] [CrossRef]
- Ahmadi, M.; Wu, T.; Hu, B. A review on organic-inorganic halide perovskite photodetectors: Device engi-neering and fundamental physics. Adv. Mater. 2017, 29, 1605242. [Google Scholar] [CrossRef] [PubMed]
- Konstantatos, G.; Sargent, E.H. Nanostructured materials for photon detection. Nat. Nanotechnol. 2010, 5, 391–400. [Google Scholar] [CrossRef]
- Konstantatos, G.; Clifford, J.; Levina, L.; Sargent, H.E. Sensitive solution-processed visible-wavelength pho-todetectors. Nat. Photonics 2007, 1, 531–534. [Google Scholar] [CrossRef]
- Luo, J.; Li, S.; Wu, H.; Zhou, Y.; Li, Y.; Liu, J.; Li, J.; Li, K.; Yi, F.; Niu, G.; et al. Cs2AgInCl6 double perovskite single crys-tals: Parity forbidden transitions and their application for sensitive and fast UV photodetectors. ACS Photon. 2017, 5, 398–405. [Google Scholar] [CrossRef]
- Bao, C.; Chen, Z.; Fang, Y.; Wei, H.; Deng, Y.; Huang, J. Low-noise and large-linear-dynamic-range photodetec-tors based on hybrid-perovskite thin-single-crystals. Adv. Mater. 2017, 29, 1703209. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, H.; Zhang, Y.; Zhao, K.; Yang, Z.; Yuan, Y.; Liu, S.F. Surface-tension-controlled crystallization for high-quality 2D perovskite single crystals for ultrahigh photodetection-science direct. Matter 2019, 1, 465–480. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhu, M.; Du, M.; Lv, Z.; Zhang, L.; Li, Y.; Yang, Y.; Yang, T.; Li, X.; Wang, K.; et al. High detectivity graphene-silicon heterojunction photodetector. Small 2016, 12, 595. [Google Scholar] [CrossRef]
- Yu, T.; Wang, F.; Xu, Y.; Ma, L.; Pi, X.; Yang, D. Graphene coupled with silicon quantum dots for high-performance bulk-silicon-based Schottky junction photodetectors. Adv. Mater. 2016, 28, 4912. [Google Scholar] [CrossRef]
- An, X.; Liu, F.; Jung, Y.J.; Kar, S. Tunable graphene-silicon heterojunctions for ultrasensitive photodetection. Nano Lett. 2013, 13, 909. [Google Scholar] [CrossRef]
- Xiang, D.; Han, C.; Hu, Z.; Lei, B.; Liu, Y.; Wang, L.; Hu, W.P.; Chen, W. Surface Transfer Doping-Induced, High-Performance Graphene/Silicon Schottky Junction-Based, Self-Powered Photodetector. Small 2015, 11, 4829–4836. [Google Scholar] [CrossRef]
- Casalino, M.; Sassi, U.; Goykhman, I.; Eiden, A.; Lidorikis, E.; Milana, S.; de Fazio, D.; Tomarchio, F.; Iodice, M.; Coppola, G.; et al. Vertically illuminated, resonant cavity enhanced, graphene-silicon Schottky photodetectors. ACS Nano. 2017, 11, 10955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhou, Q.; Luo, S.; Du, H.; Cao, Z.; Peng, X.; Feng, W.; Shen, J.; Wei, D. Infrared photodetector based on the photothermionic effect of graphene-nanowall/silicon heterojunction. ACS Appl. Mater. Interfaces 2019, 11, 17663. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Zhang, X.J.; Zhang, X.W.; Deng, W.; Jie, J.S. High-sensitivity and fast-response graphene/crystalline silicon Schottky junction-based near-IR photodetectors. IEEE Electron Device Lett. 2013, 34, 1337. [Google Scholar] [CrossRef]
- Yin, J.; Liu, L.; Zang, Y.; Ying, A.; Hui, W.; Jiang, S.; Kang, J. Engineered tunneling layer with enhanced impact ionization for detection im-provement in graphene/silicon heterojunction photodetectors. Light Sci. Appl. 2021, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, R.K.; Chinnadurai, D.; Selvaraj, A.R.; Rajendiran, R.; Senthil, K.; Prabakar, K. Revealing the self-degradation mechanisms in methylammonium lead iodide perovskites in dark and vacuum. Chem. Phys. Chem. 2018, 19, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, Y.; Juarez-Perez, E.J.; Ono, L.K.; Qi, Y. Accelerated degradation of methylammonium lead iodide perovskites induced by exposure to iodine vapor. Nat. Energy 2016, 2, 16195. [Google Scholar] [CrossRef]
- Philippe, B.; Jacobsson, T.J.; Correa-Baena, J.P.; Jena, N.K.; Banerjee, A.; Chakraborty, S.; Cappel, U.B.; Ahuja, R.; Hagfeldt, A.; Odelius, M.; et al. Valence level character in a mixed perovskite material and determination of the valence band maximum from photoelectron spectroscopy: Variation with photon energy. J. Phys. Chem. C 2017, 121, 26655–26666. [Google Scholar] [CrossRef]
- Hösel, M.; Sondergaard, R.R.; Jorgensen, M.; Krebs, F.C. Comparison of UV-curing, hotmelt, and pressure sensi-tive adhesive as roll-to-roll encapsulation methods for polymer solar cells. Adv. Eng. Mater. 2013, 15, 1068–1075. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, W.; Yin, Z.; Zhang, Y.; Deng, H.; Dai, N. Two-Dimensional Perovskite (PEA)2PbI4 Two-Color Blue-Green Photodetector. Nanomaterials 2022, 12, 2556. https://doi.org/10.3390/nano12152556
Dou W, Yin Z, Zhang Y, Deng H, Dai N. Two-Dimensional Perovskite (PEA)2PbI4 Two-Color Blue-Green Photodetector. Nanomaterials. 2022; 12(15):2556. https://doi.org/10.3390/nano12152556
Chicago/Turabian StyleDou, Wei, Ziwei Yin, Yi Zhang, Huiyong Deng, and Ning Dai. 2022. "Two-Dimensional Perovskite (PEA)2PbI4 Two-Color Blue-Green Photodetector" Nanomaterials 12, no. 15: 2556. https://doi.org/10.3390/nano12152556
APA StyleDou, W., Yin, Z., Zhang, Y., Deng, H., & Dai, N. (2022). Two-Dimensional Perovskite (PEA)2PbI4 Two-Color Blue-Green Photodetector. Nanomaterials, 12(15), 2556. https://doi.org/10.3390/nano12152556





