Microenvironmental Behaviour of Nanotheranostic Systems for Controlled Oxidative Stress and Cancer Treatment
Abstract
1. Introduction
2. Au-Based Nanostructures
| Cell Line | NP Shape and Size | Synthesis Method | Effect |
|---|---|---|---|
| Human breast cancer cells (MDA-MB 231) | Spherical (25–30 nm) | Chemical reduction | Decrease in proliferation [41], caspase-3 activation and DNA fragmentation [42,43]. |
| Human keratinocyte cell line (HaCaT) | Nanorods (30 nm) | Citrate reduction | Au NPs caused necrosis and apoptosis, leading to cell death [44,45]. |
| Human liver cell line (HL7702 cells) | Spherical (5–100 nm) | Citrate reduction | Decrease in cytosolic GSH, depolarisation of transmembrane potential in the mitochondria, followed by apoptosis and death [46]. |
| Human lung adenocarcinoma (A549) cells | Spherical (30 nm) | Citrate reduction | Upregulation of apoptotic genes (including bax, bak and caspase-3), induction of apoptosis through depletion of ATP [47]. |
| Human leukaemia (HL-60) | Spherical (30, 50, 90 nm) | Commercial (CymitQuı’mica) | Membrane damage, reduction in GSH and generation of ROS, increase in cell mortality [48]. |
| MRC human foetal lung fibroblast cell | Unknown shape (20 nm) | Citrate reduction | Increase in oxidative stress, downregulation of cell cycle gene, inhibition of cell proliferation and DNA damage [27,49]. |
| Human foetal osteoblast (hFOB 1.19) | Spherical (38–60 nm) | Citrate-based reduction | Cell membrane penetration, ultrastructural changes and cell death [50]. |
| Human osteosarcoma cells (MG63) | Spherical (38–60 nm, 6.3 nm), nanorods (18, 39 nm), stars (215 nm) | Citrate-based reduction, chemical reduction-based immobilisation method | Increase in reactive oxygen species, disruption of membrane potential of mitochondrion, apoptosis and cell death [27,50,51]. |
| Human osteosarcoma cells 143B | Spherical (6.3 nm), nanorods (18, 39 nm), stars (215 nm) | Chemical reduction-based immobilisation method | Nanostars caused higher cytotoxicity, apoptosis and cell death than nanorods and nanospheres [51,52]. |
| Human colorectal adenocarcinoma cells (HT29) | Unknown shape (15 nm) | Institute of biochemistry & physiology of plants & microorganisms, Russian academy of sciences. | Cytotoxic and anti-cohesive effects, multicellular spheroid formation, reduced proliferation, apoptosis and necrosis [31,53]. |
| Human A549 cell line | Unknown (39–45 nm) | Commercial (Sigma-Aldrich, St. Louis, MO, USA | Growth inhibition, apoptosis and autophagy, DNA damage [54]. |
| Human HepG2 cells | Spherical (20 nm) | Citrate reduction | Size-dependent toxicity, comet tail intensity and tail moment were observed [55,56]. |
| Human colorectal adenocarcinoma cells (HT29) | Unknown shape (50–100 nm) | Photonic Technology (IPHT Jena, Germany) | Inhibition of cell proliferation and angiogenesis, reduction in cell viability [57,58]. |
3. Silver Nanoparticles
| Cell Line | Shape and Size | Synthesis Method | Effect |
|---|---|---|---|
| Human mesenchymal stem cells | Spherical (<50 nm) | Commercial (Sigma-Aldrich, Steinheim, Germany) | Alteration at the chromosomal level, cytotoxicity and genotoxicity, oxidative stress and DNA damage [69,70]. |
| Human hepatoblastoma (HepG2) cell line | Spherical (28–60 nm) | Citrate and polyvinyl alcohol-coated NPs | Increase in ROS production, depletion of GSH, oxidative stress and cytotoxicity [59,71]. |
| Human macrophages | Generation of ROS and increase in oxidative stress, induction of heme oxygenase I, DNA damage [72]. | ||
| Human neuroblastoma (SH-SY5Y) cell line | Spherical (30 nm) | Commercial (Shanghai Huzheng nano technology Co., Ltd., Shanghai, China) | Increased levels of phosphatase and tensin homolog, higher mitochondrial Ca2+ uptake, Ca2+ disrupts homeostasis and triggers apoptotic cell death [73]. |
| Human umbilical vein endothelial cells (HUVECs) | Spherical (65 nm) | Reduction by citrate (Turkevich method) | Damages the cell membrane, oxidative stress and apoptosis resulted in dysfunction of cell [74]. |
| Human embryonic kidney (HEK293T) cells | Spherical (60 nm) | Commercial (Sigma-Aldrich, Shanghai, China) | Cellular structural changes, downregulation of anti-apoptosis Bcl2-t and Bclw genes, upregulation of pro-apoptosis Bid gene, decreased cell viability and increased DNA damage [75]. |
| Human breast cancer cell line (MDA-MB 231) | Spherical (20 nm) | Green synthesis (bacterium, B. funiculus) | Increased ROS generation and caspase-3 activation cause cellular apoptosis, oxidative stress and cytotoxicity [76]. |
| Mouse MC3T3-E1 cell line | Spherical (100 nm, 50 nm) | Commercial (InkTec Co. (Asan, Korea), chemical reduction | Increased ROS generation, LDH release and expression of stress-related genes (ho-1 and MMP-3) and cellular apoptosis [77]. |
| Rat PC12 cell line | Spherical (100 nm, 50 nm) | Commercial (InkTec Co. (Asan, Korea), chemical reduction | Intracellular ROS generation, lactate dehydrogenase release, changes in cell morphology and induced necrotic cell death [77]. |
| Rat alveolar macrophage cell line | Spherical (15, 30, 55 nm) | Commercial (NovaCentrix Co., Austin, Texas, USA) | Mitochondrion depolarisation induced cytotoxicity, apoptosis and DNA damage [78]. |
| Human Chang liver cells | Spherical (100, 28–30 nm) | Commercial (Sigma-Aldrich, St. Louis, MO, USA) | Protein carbonylation, lipid membrane peroxidation and DNA break [79]. |
| Human keratinocyte (HaCaT) cell line | Spherical (25–50 nm) | Citrate reduction method and NP functionalisation | Long-lasting antiproliferative effect, even with a brief time contact with NPs. Permeation can damage the skin [80]. |
4. Manganese Oxide Nanostructures
| Cell Line | NP Size | Synthesis Method | Cell |
|---|---|---|---|
| Human breast cancer epithelial (MCF-7) cell line | MnO2, nano-flake (10–20 nm) | Hydrothermal processing | Oxidative stress mediated toxicity via p53 pathway, apoptosis induced by oxidative stress and dependent on dose [95]. |
| Rat dopaminergic (N27) cells | Mn, unknown shape (25 nm) | Commercial (Quantum Sphere Pty Ltd., Dural NSW, Australia) | ROS generation, caspase-mediated proteolytic cleavage of proapoptotic protein kinase Cδ (PKCδ), loss of TH-positive dopaminergic neurons and induced autophagy [89]. |
| Human fibrosarcoma epithelial (HT1080) cell line | MnO2, nano-flake (10–20 nm) | Hydrothermal processing | Higher NP internalisations, apoptosis induced by oxidative stress. Upregulation of pro-apoptotic genes and downregulation of anti-apoptotic genes [95]. |
| PC-12 cells | Mn, cubic (40 nm) | Commercial (Nanotechnology, Inc, Austin, TX, USA) | Increased concentration of ROS, depletion of dopamine (DA) and its metabolites caused cellular toxicity [96]. |
| Human lung epithelial (A549) cells | Mn3O4, unknown shape (10–20 nm) | Flame pyrolysis method | High NP internalisations, ROS generation induced oxidative stress and toxicity [14]. |
| Human intestinal epithelial (Caco2) cells | Mn3O4, unknown shape (10–20 nm) | Flame pyrolysis method | Increased ROS generation caused oxidative stress and hence apoptosis and cell membrane damage [14,97]. |
| Human neuroblastoma (SH-SY5Y) cell line | MnO2, round shape (40 nm) | Commercial (Research Nanomaterials, Inc., Houston, Texas, USA) | ROS generation induced oxidative stress, apoptosis (caspase-3 activation), PS translocation and fragmentation of chromosomes [98]. |
| PC-12/rat pheochromocytoma cells | Elemental Mn, irregular shape (20 nm) | Unknown | Significant dopaminergic, neurotoxicity, upregulation of genes involved in DA metabolism and PA pathogenesis [87,99]. |
| Rat lung epithelium (CCL-149) cell line | Mn3O4, sphere shape (30 nm) | Flame spray method | Redox disturbance, ROS generation, LDL release and cellular apoptosis [91]. |
| Mouse fibroblast (L929) cells | MnO, irregular shape (15–25 nm) | High temperature pyrolysis | Activation of p53, increase in the bax and a decrease in bcl-2, leading to G2/M phase arrest, increase in caspase-3 activity and apoptosis [100]. |
| Human cervical carcinoma (HeLa) cells | MnO, irregular shape (15–25 nm) | High temperature pyrolysis | ROS generation, loss of cell/cell contact between neighbouring cells, cytoplasm retraction, shrinkage of nuclei and multinucleated giant cells; cell death was observed [100]. |
5. Iron Oxide Nanostructures
- Ferroptosis
- Oxytosis
- Intercellular iron accumulation as a mediator of cell death
- Extracellular iron accumulation as a mediator of cell death
| Cell Line | NP Size | Synthesis Method | Effect |
|---|---|---|---|
| Human hepatocyte (HL-7702) cell lines | Fe3O4, unknown shape (50 nm) | Commercial (Colorobbia Consulting-Cericol, Vinci, Italy) | Induction of apoptosis and autophagy, nuclear condensation and chromosomal DNA fragmentation were observed [129]. |
| Human hepatoma HepG2 cells | Fe2O3, spherical (50 nm) | Commercial | Mitochondrial apoptosis through activation of loop phosphorylation, release of cytochrome c from the mitochondria, decrease in Bcl-2 protein expression, PARP activation and caspase cascades, ROS generation and DNA damage [130]. |
| Human lung (BEAS-2B) cells | Fe3O4, Fe2O3 irregular shape (˂100 nm) | Commercial (Sigma-Aldrich, St. Louis, USA) | Increased ROS generation and oxidative stress, mitochondrion and DNA damage [131]. |
| Human cerebral endothelial cells (HCECs) | Fe3O4, unknown shape (9 nm) | Commercial (PlasmaChem GmbH, Berlin, Germany) | Overexpression of cathepsin D accelerated apoptosis, ROS generation transported into lysosomes interfering with the lysosomal hydrolases, cathepsins D and B, and induced oxidative stress and, hence, autophagy [132]. |
| Human lung cancer (A549) cell line | Fe3O4, Fe2O3 irregular shape (˂100 nm) | Commercial (Sigma-Aldrich, St. Louis, MO, USA) | ROS generation, increased oxidative stress, cellular apoptosis and DNA damage [131]. |
| Lung cancer (HCC827) cell line | Iron oxide, unknown shape (NPs (73 nm) | Co-precipitation method and NP conjugation | Reduced EGFR phosphorylation, increased γH2AX foci and induced apoptosis, which resulted in suppression of tumour growth [133]. |
| Chinese hamster ovary (H9T3) cell lines | Fe2O3, hexagonal shape, (20–30 nm) | Harvard Versatile Engineered Nanomaterial Generation System (VENGES) | Cellular apoptosis and double-stranded DNA breaks [134]. |
| Human fibrosarcoma (HT-1080) cells | Irregular and spherical shapes, Fe3O4 (10–150 nm) | Massart’s method, and NP coating | Increased ROS generation caused oxidative stress and lipid peroxidation. Oxidative damage induced DNA damage [135]. |
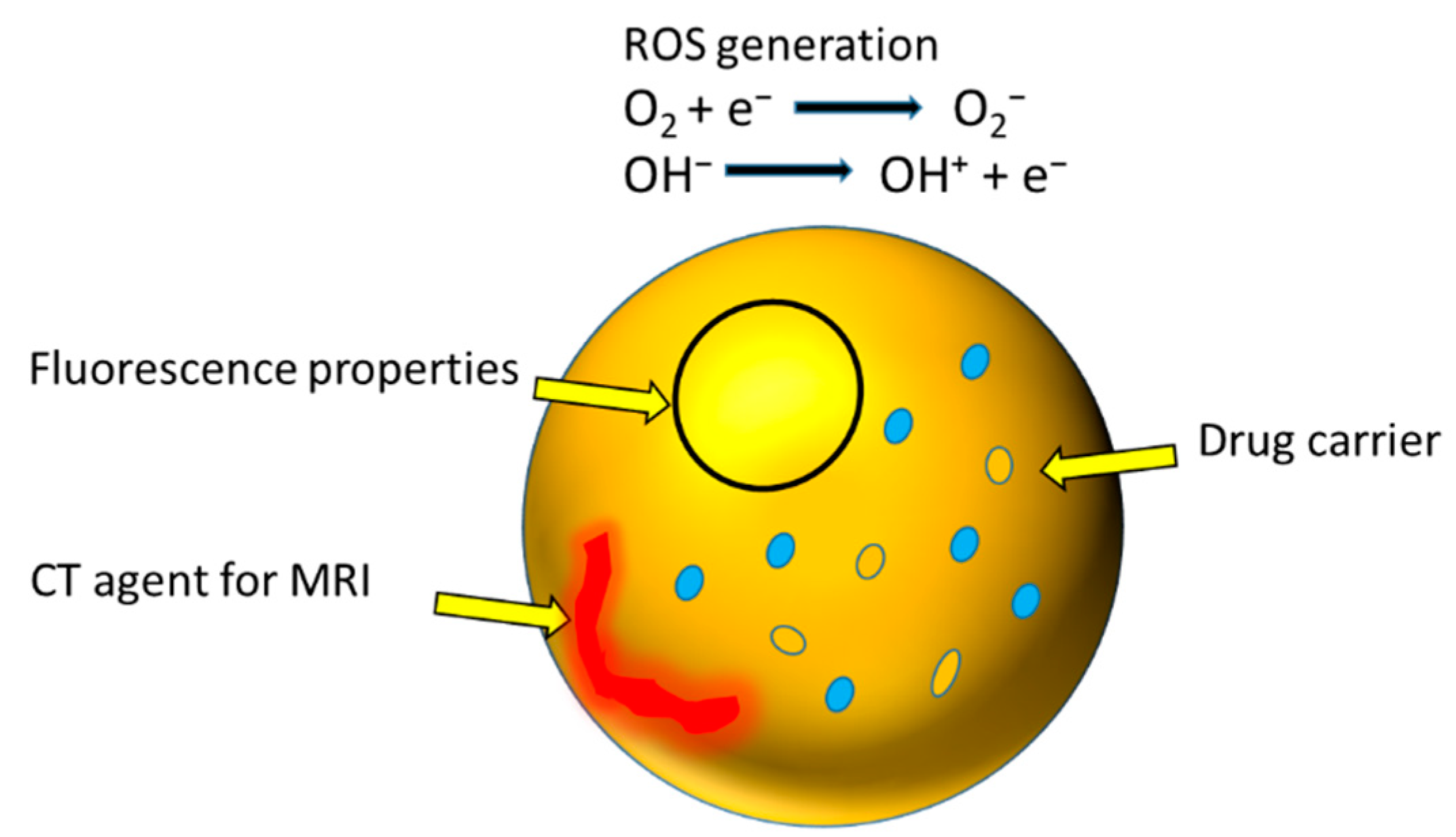
6. Recently Developed Theranostic Nanostructures
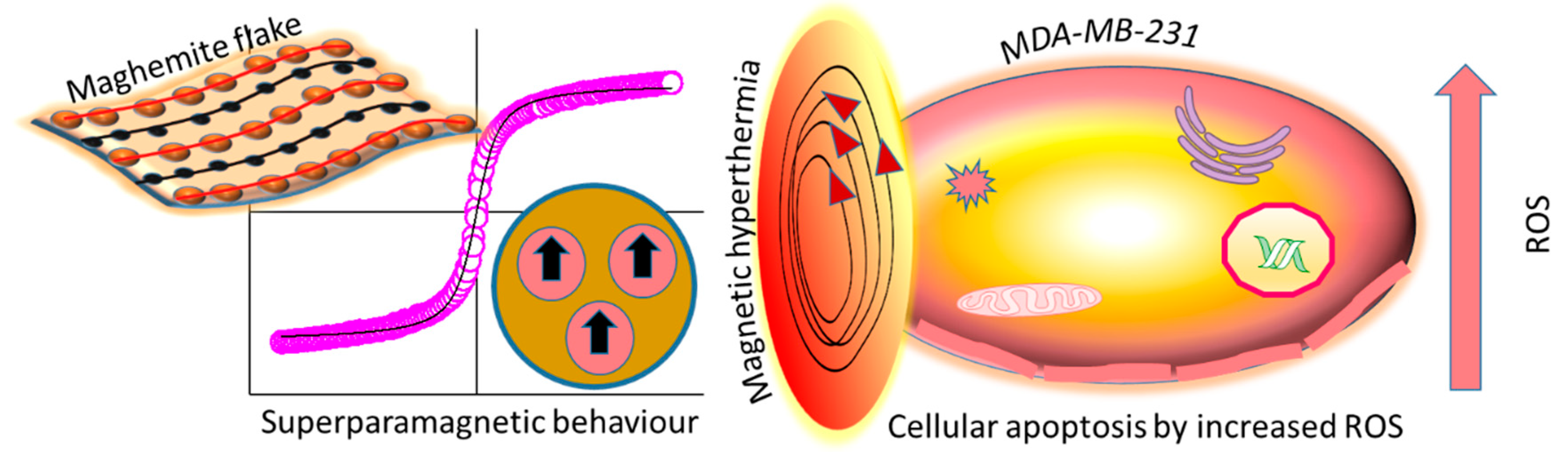
7. Maghemite Nano- and Quantum Structures
| Cell Line | NP Size and Shape | Synthesis Method | Effect |
|---|---|---|---|
| He La cells | γ-Fe2O3, spherical shape (<20 nm) | Microwave-assisted hydrothermal | No significant toxicity, generation of ROS, enhanced oxidative stress, application as multimodal (ROS and hyperthermia) anticancer therapy [138]. |
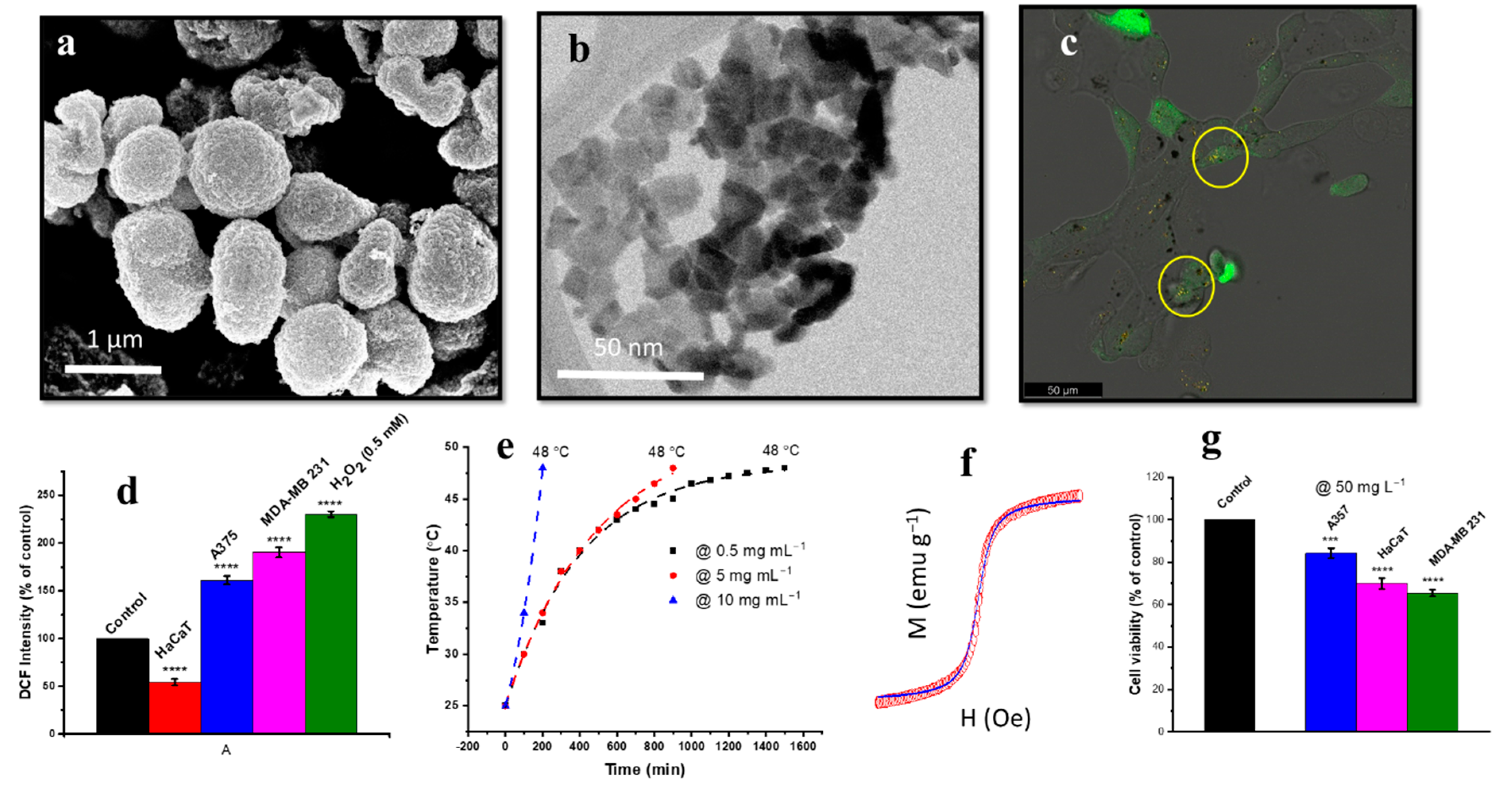
| Human breast cancer line (MDA-MB 231) | γ-Fe2O3, 2D flakes (4–10 nm) | Hydrothermal processing and calcination | ROS generation, apoptosis by magnetic hyperthermia therapy, higher cellular internalisation of flakes [141]. |
| Human keratinocyte cell line (HaCaT) | γ-Fe2O3, flakes (4–10 nm) | Hydrothermal processing and calcination | Less γ- Fe2O3 flake uptake by HaCaT cells than malignant (MDA-MB 231) cells, less apoptosis by magnetic hyperthermia therapy [141]. |
| Human melanoma cell line (A375), | γ-Fe2O3, 2D flakes (4–10 nm) | Hydrothermal processing and calcination | ROS generation, higher γ-Fe2O3 flake uptake by A375 cells. Apoptosis due to ROS augmentation and magnetic hyperthermia therapy [141]. |
| Human prostate cancer (PC3) cell line | γ-Fe2O3, nanosphere (12.5 nm) | Co-precipitation method | Lower NP uptake, lower toxicity than Fe3O4 NPs [150]. |
8. Indium Tin Oxide (ITO) Nanostructure in the Biological Field
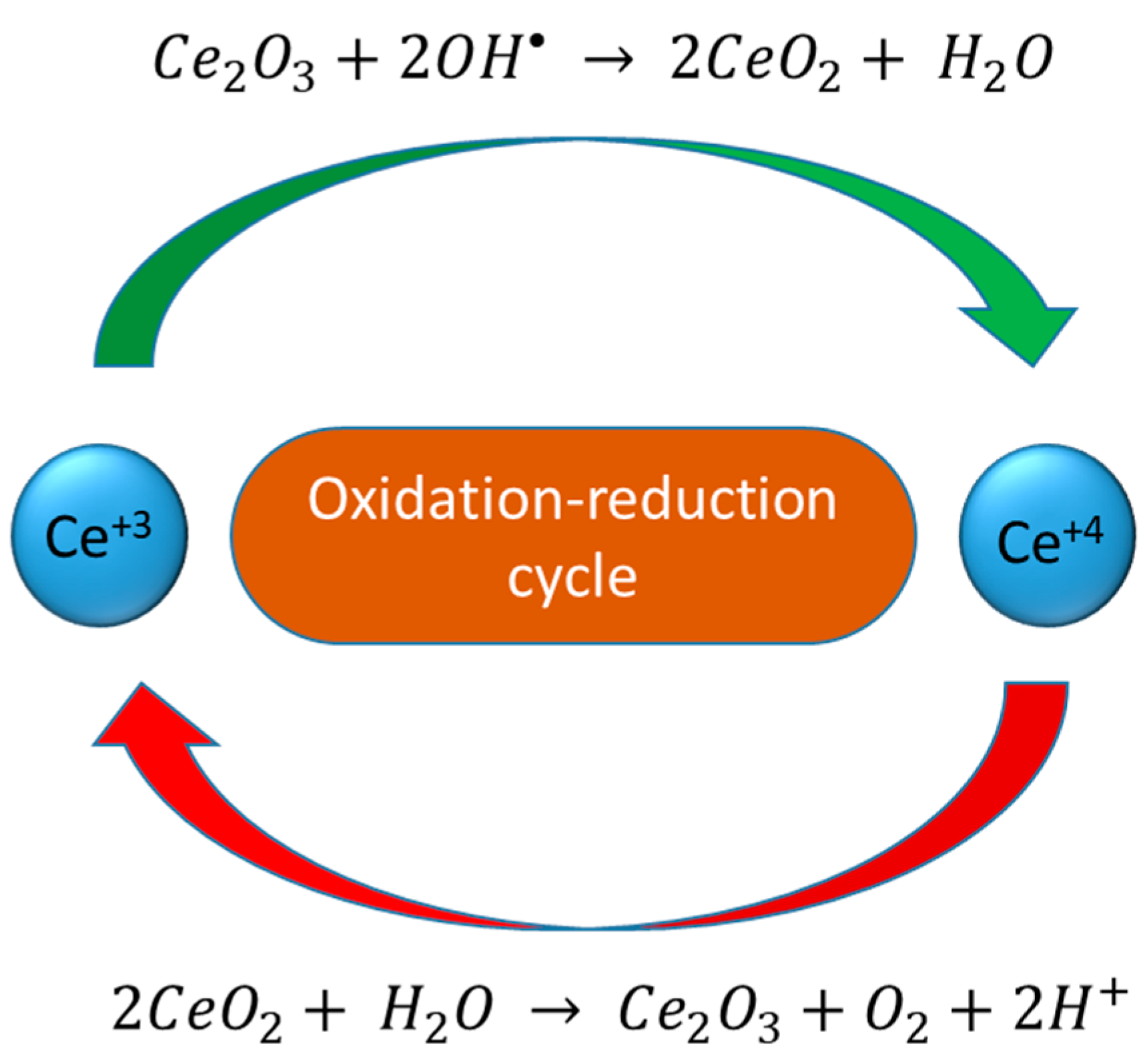
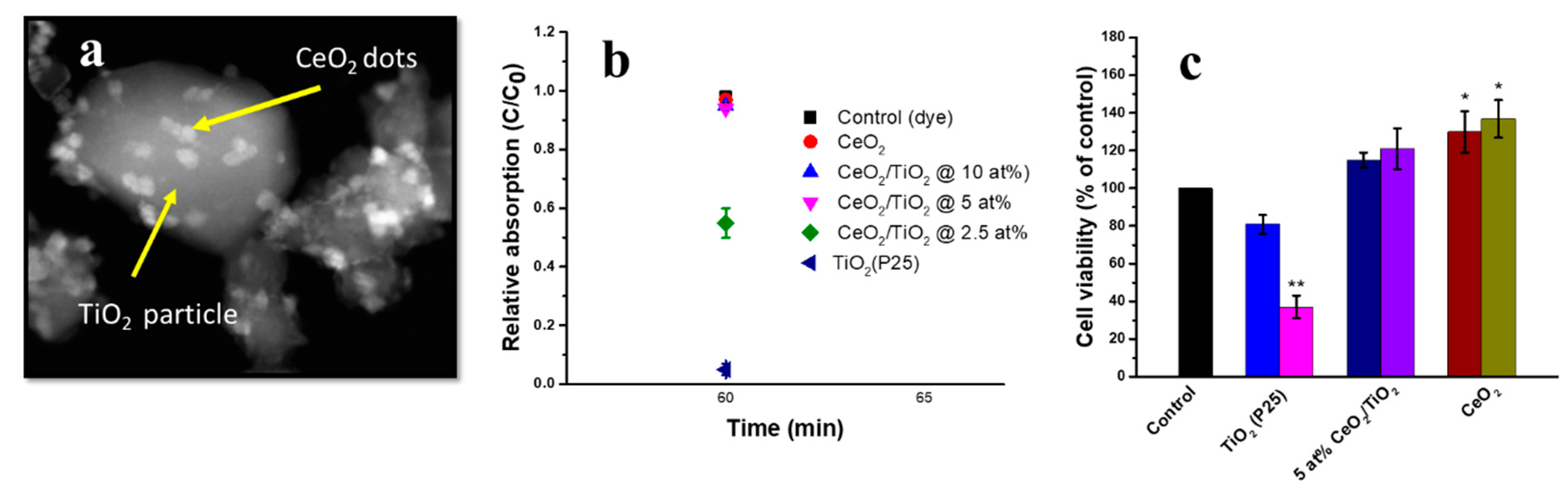
9. Doped Cerium and Nanodot-Encrusted Structures
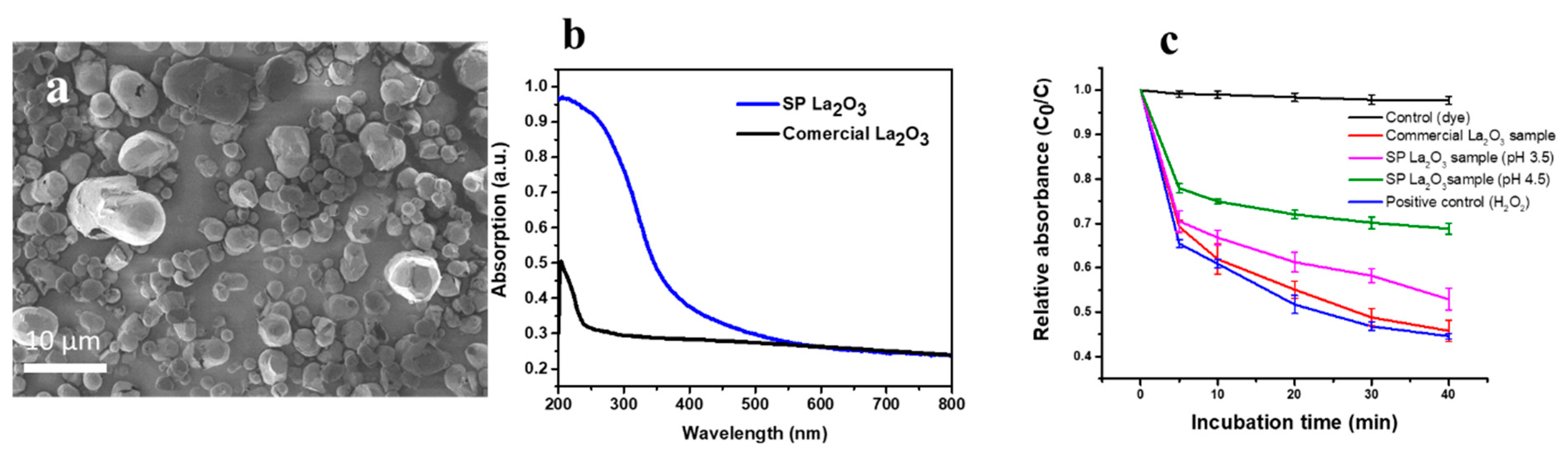
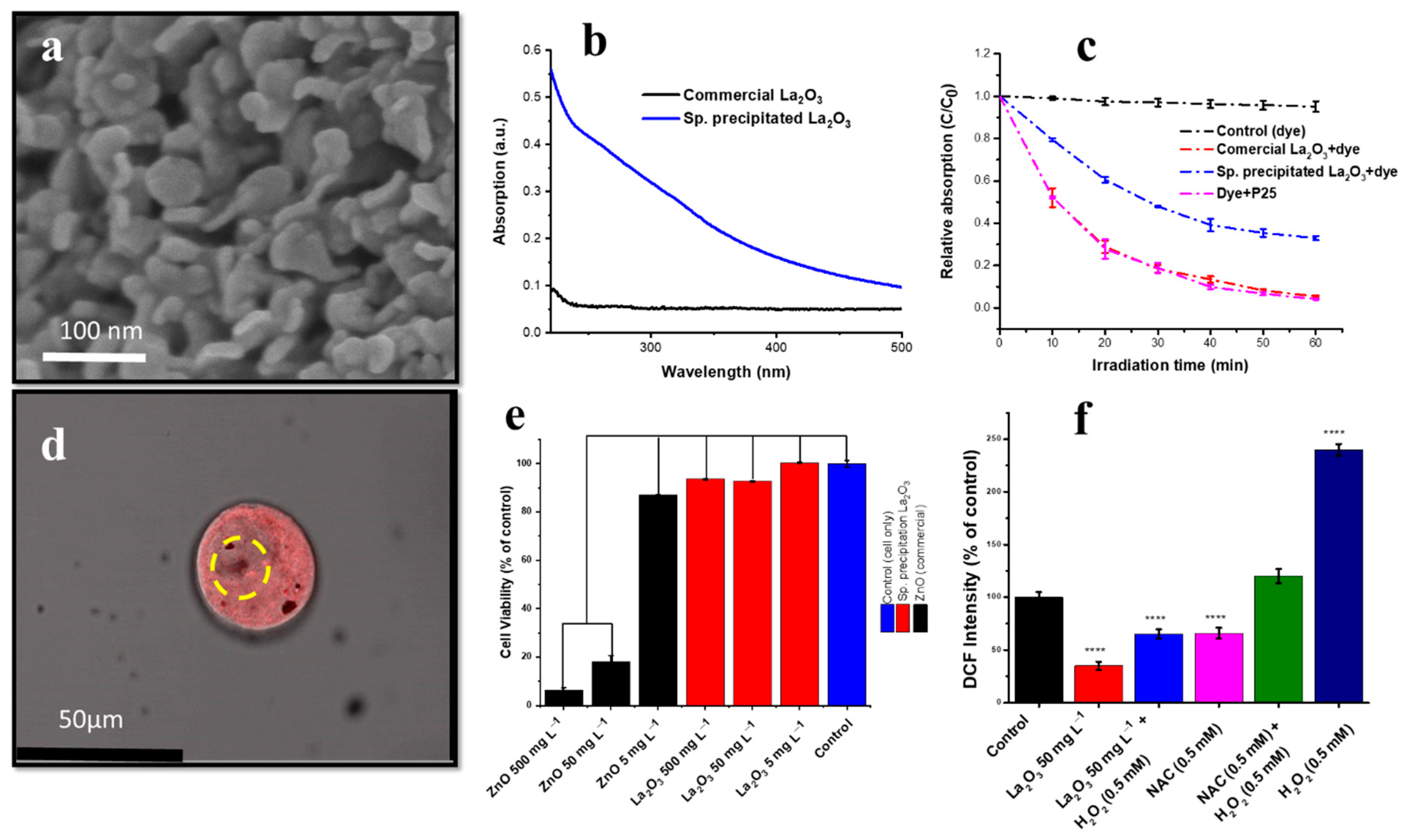
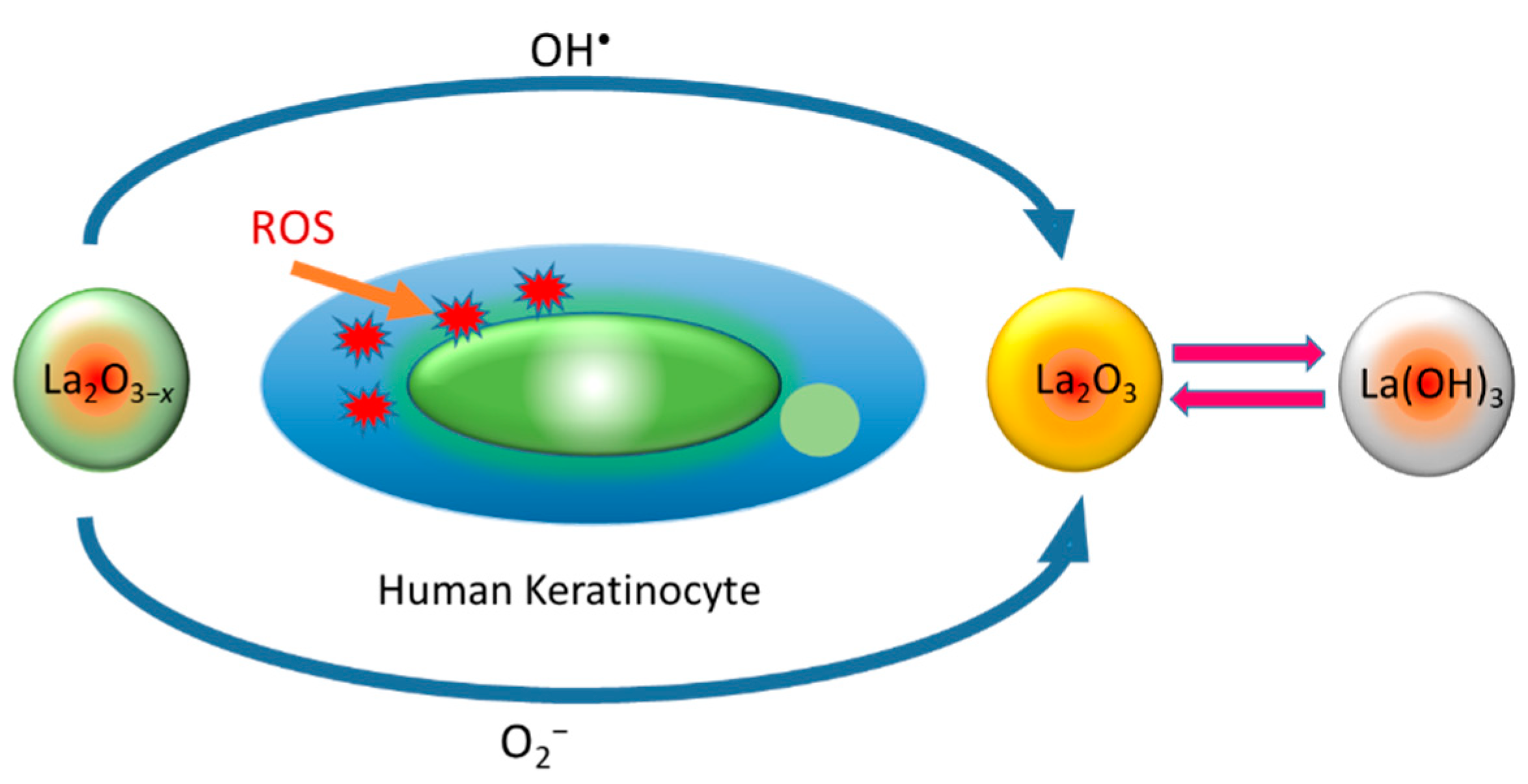
10. Oxygen-Deficient Lanthanum Oxide
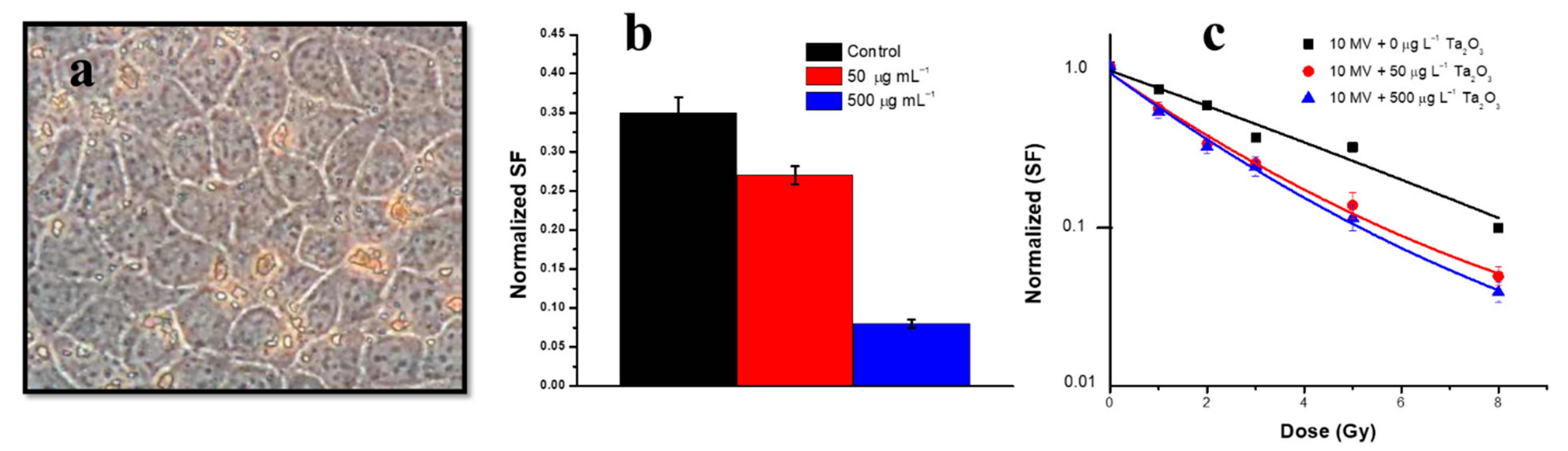
11. Tantalum Oxide Nanostructures
| Cell Line | NP Size and Shape | Synthesis Method | Effect |
|---|---|---|---|
| HEK 293 cell line | TaOx nanocrystals (9–12 nm) | Sol–gel | No significant cytosolic dissolution under cytosolic and lysosomal conditions, high in vitro cell viability [194]. |
| RAW 264.7 macrophage cells | TaOx nanocrystals (9–12) nm | Sol–gel | No significant cell toxicity, and produced effective CT contrast [194]. |
| RAW 264.7 macrophage cells | TaOx NPs (5–15 nm) | Micro-emulsion | Maintained high in vitro cell viability, effective in vitro fluorescence and CT contrast [195]. |
| Rodent brain cells (9L gliosarcoma cancer cells) | Ta2O3, irregular (50–70 nm) | Precipitation and calcination | Non-toxic over a wide range of concentrations. cell death in the localised radiation therapy and due to radiosensitivity of 9L cells [191]. |
| Madin–Darby Canine Kidney (MDCK) | Ta2O3, irregular (50–70 nm) | Precipitation and calcination | Non-toxic over a wide range of concentrations [191]. |
| Mammalian (HeLa) cell line | TaOx core shell structure (~10 nm) | Aqueous sol–gel method-based reverse-micelles assembly | Less toxicity and inflammation than commonly used adhesive CA-Lp. Multifunctional X-ray fluorescence and CT properties [196]. |
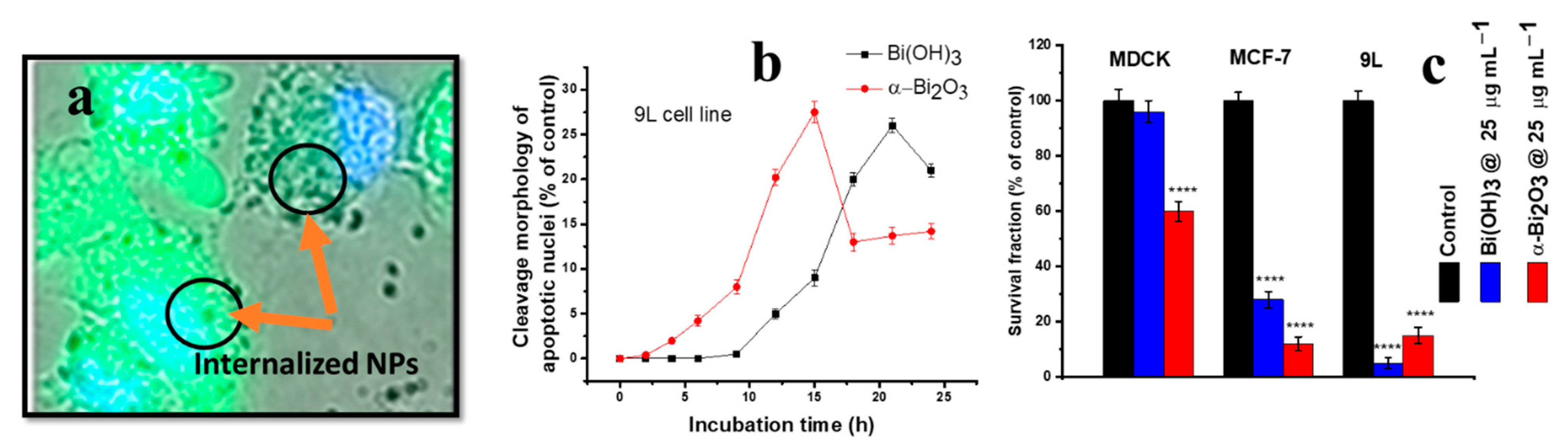
12. Bismuth Oxide and Hydroxide Nanostructures
13. Magnesium Oxide Nanostructure
14. Elimination of Nanoparticles from the Body after Treatment
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belyanina, I.; Kolovskaya, O.; Zamay, S.; Gargaun, A.; Zamay, T.; Kichkailo, A. Targeted magnetic nanotheranostics of cancer. Molecules 2017, 22, 975. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Mei, L.; Feng, S.-S. Nanotheranostics: Advanced nanomedicine for the integration of diagnosis and therapy. Nanomedicine 2014, 9, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics˗ application and further development of nanomedicine strategies for advanced theranostics. Theranostics 2014, 4, 660. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Copin, J.-C.; Gasche, Y.; Chan, P.H. Overexpression of copper/zinc superoxide dismutase does not prevent neonatal lethality in mutant mice that lack manganese superoxide dismutase. Free Radic. Biol. Med. 2000, 28, 1571–1576. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Shuvaev, V.V.; Christofidou-Solomidou, M.; Bhora, F.; Laude, K.; Cai, H.; Dikalov, S.; Arguiri, E.; Solomides, C.C.; Albelda, S.M.; Harrison, D.G. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J. Pharmacol. Exp. Ther. 2009, 331, 404–411. [Google Scholar] [CrossRef]
- Waddington, D.E.; Boele, T.; Maschmeyer, R.; Kuncic, Z.; Rosen, M.S. High-sensitivity in vivo contrast for ultra-low field magnetic resonance imaging using superparamagnetic iron oxide nanoparticles. Sci. Adv. 2020, 6, eabb0998. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef]
- Sneider, A.; VanDyke, D.; Paliwal, S.; Rai, P. Remotely triggered nano-theranostics for cancer applications. Nanotheranostics 2017, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Okur, N.Ü.; Karantas, I.D.; Okur, M.E.; Gündoğdu, E.A. Current update on nanoplatforms as therapeutic and diagnostic tools: A review for the materials used as nanotheranostics and imaging modalities. Asian J. Pharm. Sci. 2021, 16, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nakamura, M.; Sakamoto, W.; Yogo, T.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Ishimura, K. Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment. Theranostics 2013, 3, 366. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Titma, T.; Visnapuu, M.; Vija, H.; Kakinen, A.; Sihtmae, M.; Pokhrel, S.; Madler, L.; Heinlaan, M.; Kisand, V. Toxicity of 11 metal oxide nanoparticles to three mammalian cell types in vitro. Curr. Top. Med. Chem. 2015, 15, 1914–1929. [Google Scholar] [CrossRef]
- Paris, J.L.; Baeza, A.; Vallet-Regí, M. Overcoming the stability, toxicity, and biodegradation challenges of tumor stimuli-responsive inorganic nanoparticles for delivery of cancer therapeutics. Expert Opin. Drug Deliv. 2019, 16, 1095–1112. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Moore, T.L.; Urban, D.A.; Rodriguez-Lorenzo, L.; Milosevic, A.; Crippa, F.; Spuch-Calvar, M.; Balog, S.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle administration method in cell culture alters particle-cell interaction. Sci. Rep. 2019, 9, 900. [Google Scholar] [CrossRef]
- Lim, S.; Park, J.; Shim, M.K.; Um, W.; Yoon, H.Y.; Ryu, J.H.; Lim, D.-K.; Kim, K. Recent advances and challenges of repurposing nanoparticle-based drug delivery systems to enhance cancer immunotherapy. Theranostics 2019, 9, 7906. [Google Scholar] [CrossRef]
- Goldberg, M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef]
- Mazrad, Z.A.I.; Lee, K.; Chae, A.; In, I.; Lee, H.; Park, S.Y. Progress in internal/external stimuli responsive fluorescent carbon nanoparticles for theranostic and sensing applications. J. Mater. Chem. B 2018, 6, 1149–1178. [Google Scholar] [CrossRef] [PubMed]
- Goddard, Z.R.; Marín, M.J.; Russell, D.A.; Searcey, M. Active targeting of gold nanoparticles as cancer therapeutics. Chem. Soc. Rev. 2020, 49, 8774–8789. [Google Scholar] [CrossRef] [PubMed]
- Shuvaev, V.V.; Tliba, S.; Nakada, M.; Albelda, S.M.; Muzykantov, V.R. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J. Pharmacol. Exp. Ther. 2007, 323, 450–457. [Google Scholar] [CrossRef]
- Dziubla, T.D.; Shuvaev, V.V.; Hong, N.K.; Hawkins, B.J.; Madesh, M.; Takano, H.; Simone, E.; Nakada, M.T.; Fisher, A.; Albelda, S.M. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials 2008, 29, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, A.; Andersson, B.; Thurnherr, T.; Krug, H.; Scheynius, A.; Fadeel, B. Toxicology of engineered nanomaterials: Focus on biocompatibility, biodistribution and biodegradation. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2011, 1810, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Wilson, R. The use of gold nanoparticles in diagnostics and detection. Chem. Soc. Rev. 2008, 37, 2028–2045. [Google Scholar] [CrossRef]
- Xin, J.; Fu, L.; Wang, J.; Wang, S.; Zhang, L.; Zhang, Z.; Yao, C. Influence of parameters on the death pathway of gastric cells induced by gold nanosphere mediated phototherapy. Nanomaterials 2022, 12, 646. [Google Scholar] [CrossRef]
- Pavlovich, E.; Volkova, N.; Yakymchuk, E.; Perepelitsyna, O.; Sydorenko, M.; Goltsev, A. In vitro study of influence of Au nanoparticles on HT29 and SPEV cell lines. Nanoscale Res. Lett. 2017, 12, 494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loutfy, S.A.; Al-Ansary, N.A.; Abdel-Ghani, N.T.; Hamed, A.R.; Mohamed, M.B.; Craik, J.D.; Eldin, T.A.S.; Abdellah, A.M.; Hussein, Y.; Hasanin, M. Anti-proliferative activities of metallic nanoparticles in an in vitro breast cancer model. Asian Pac. J. Cancer Prev. 2015, 16, 6039–6046. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Hartono, D.; Ong, C.-N.; Bay, B.-H.; Yung, L.-Y.L. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of high aspect ratio cylindrical gold nanorods. J. Phys. Chem. B 2001, 105, 4065–4067. [Google Scholar] [CrossRef]
- Brundo, M.V.; Pecoraro, R.; Marino, F.; Salvaggio, A.; Tibullo, D.; Saccone, S.; Bramanti, V.; Buccheri, M.A.; Impellizzeri, G.; Scuderi, V. Toxicity evaluation of new engineered nanomaterials in zebrafish. Front. Physiol. 2016, 7, 130. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. The puzzle of toxicity of gold nanoparticles. The case-study of HeLa cells. Toxicol. Res. 2015, 4, 796–800. [Google Scholar] [CrossRef]
- Botha, T.L.; Elemike, E.E.; Horn, S.; Onwudiwe, D.C.; Giesy, J.P.; Wepener, V. Cytotoxicity of Ag, Au and Ag-Au bimetallic nanoparticles prepared using golden rod (Solidago canadensis) plant extract. Sci. Rep. 2019, 9, 4169. [Google Scholar] [CrossRef]
- Wu, Y.; Ali, M.R.; Chen, K.; Fang, N.; El-Sayed, M.A. Gold nanoparticles in biological optical imaging. Nano Today 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Gu, Y.-J.; Cheng, J.; Man, C.W.-Y.; Wong, W.-T.; Cheng, S.H. Gold-doxorubicin nanoconjugates for overcoming multidrug resistance. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 204–211. [Google Scholar] [CrossRef]
- Minai, L.; Yeheskely-Hayon, D.; Yelin, D. High levels of reactive oxygen species in gold nanoparticle-targeted cancer cells following femtosecond pulse irradiation. Sci. Rep. 2013, 3, srep02146. [Google Scholar] [CrossRef]
- Lee, J.; Chatterjee, D.K.; Lee, M.H.; Krishnan, S. Gold nanoparticles in breast cancer treatment: Promise and potential pitfalls. Cancer Lett. 2014, 347, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Surapaneni, S.K.; Bashir, S.; Tikoo, K. Gold nanoparticles-induced cytotoxicity in triple negative breast cancer involves different epigenetic alterations depending upon the surface charge. Sci. Rep. 2018, 8, 12295. [Google Scholar] [CrossRef] [PubMed]
- Coulter, J.A.; Jain, S.; Butterworth, K.T.; Taggart, L.E.; Dickson, G.R.; McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Trainor, C.; Hounsell, A.R. Cell type-dependent uptake, localization, and cytotoxicity of 1.9 nm gold nanoparticles. Int. J. Nanomed. 2012, 7, 2673. [Google Scholar] [CrossRef] [PubMed]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Schrand, A.M.; Miller, J.M.; Hutchison, J.; Schlager, J.J.; Hussain, S.M. Surface charge of gold nanoparticles mediates mechanism of toxicity. Nanoscale 2011, 3, 410–420. [Google Scholar] [CrossRef]
- Wang, S.; Lu, W.; Tovmachenko, O.; Rai, U.S.; Yu, H.; Ray, P.C. Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem. Phys. Lett. 2008, 463, 145–149. [Google Scholar] [CrossRef]
- Gao, W.; Xu, K.; Ji, L.; Tang, B. Effect of gold nanoparticles on glutathione depletion-induced hydrogen peroxide generation and apoptosis in HL7702 cells. Toxicol. Lett. 2011, 205, 86–95. [Google Scholar] [CrossRef]
- Choi, S.Y.; Jeong, S.; Jang, S.H.; Park, J.; Park, J.H.; Ock, K.S.; Lee, S.Y.; Joo, S.-W. In vitro toxicity of serum protein-adsorbed citrate-reduced gold nanoparticles in human lung adenocarcinoma cells. Toxicol. Vitr. 2012, 26, 229–237. [Google Scholar] [CrossRef]
- Mateo, D.; Morales, P.; Ávalos, A.; Haza, A.I. Oxidative stress contributes to gold nanoparticle-induced cytotoxicity in human tumor cells. Toxicol. Mech. Methods 2014, 24, 161–172. [Google Scholar] [CrossRef]
- Li, J.J.; Zou, L.; Hartono, D.; Ong, C.N.; Bay, B.H.; Lanry Yung, L.Y. Gold nanoparticles induce oxidative damage in lung fibroblasts in vitro. Adv. Mater. 2008, 20, 138–142. [Google Scholar] [CrossRef]
- Chakraborty, A.; Das, A.; Raha, S.; Barui, A. Size-dependent apoptotic activity of gold nanoparticles on osteosarcoma cells correlated with SERS signal. J. Photochem. Photobiol. B Biol. Biol. 2020, 203, 111778. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz–Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J. Mater. Sci. Mater. Med. 2019, 30, 22. [Google Scholar] [CrossRef] [PubMed]
- Steckiewicz, K.P.; Inkielewicz-Stepniak, I. Modified nanoparticles as potential agents in bone diseases: Cancer and implant-related complications. Nanomaterials 2020, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.; Shahbazi-Gahrouei, D.; Abbasian, M.; Fesharaki, M.; Baharlouei, A.; Arab-Bafrani, Z. Gold nanoparticles in combination with megavoltage radiation energy increased radiosensitization and apoptosis in colon cancer HT-29 cells. Int. J. Radiat. Biol. 2017, 93, 315–323. [Google Scholar] [CrossRef]
- Liang, R.-Y.; Tu, H.-F.; Tan, X.; Yeh, Y.-S.; Chueh, P.J.; Chuang, S.-M. A gene signature for gold nanoparticle-exposed human cell lines. Toxicol. Res. 2015, 4, 365–375. [Google Scholar] [CrossRef]
- Kojić, V.; Djan, I.; Bogdanović, V.; Borišev, I.; Djordjević, A.; Ivković-Kapicl, T.; Jakimov, D. The effect of gold naiioparticles and irradiation on healthy and tumor human lung cells. Int. J. Radiat. Res. 2019, 17, 569–578. [Google Scholar]
- Fraga, S.; Faria, H.; Soares, M.E.; Duarte, J.A.; Soares, L.; Pereira, E.; Costa-Pereira, C.; Teixeira, J.P.; de Lourdes Bastos, M.; Carmo, H. Influence of the surface coating on the cytotoxicity, genotoxicity and uptake of gold nanoparticles in human HepG2 cells. J. Appl. Toxicol. 2013, 33, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Bai Aswathanarayan, J.; Rai Vittal, R.; Muddegowda, U. Anticancer activity of metal nanoparticles and their peptide conjugates against human colon adenorectal carcinoma cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1444–1451. [Google Scholar] [CrossRef]
- Schneider, T.; Westermann, M.; Glei, M. In vitro uptake and toxicity studies of metal nanoparticles and metal oxide nanoparticles in human HT29 cells. Arch. Toxicol. 2017, 91, 3517–3527. [Google Scholar] [CrossRef]
- Vrček, I.V.; Žuntar, I.; Petlevski, R.; Pavičić, I.; Dutour Sikirić, M.; Ćurlin, M.; Goessler, W. Comparison of in vitro toxicity of silver ions and silver nanoparticles on human hepatoma cells. Environ. Toxicol. 2016, 31, 679–692. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Shih, Y.-S.; Chen, Z.-Y.; Cheng, F.-Y.; Lu, J.-Y.; Wu, Y.-H.; Wang, Y.-J. Toxic Effects and Mechanisms of Silver and Zinc Oxide Nanoparticles on Zebrafish Embryos in Aquatic Ecosystems. Nanomaterials 2022, 12, 717. [Google Scholar] [CrossRef]
- Ishida, T. Antibacterial mechanism of Ag+ ions for bacteriolyses of bacterial cell walls via peptidoglycan autolysins, and DNA damages. MOJ Toxicol 2018, 4, 345–350. [Google Scholar] [CrossRef]
- Park, E.-J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. Vitr. 2010, 24, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Boca, S.C.; Potara, M.; Gabudean, A.-M.; Juhem, A.; Baldeck, P.L.; Astilean, S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett. 2011, 311, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Otari, S.; Patil, R.; Nadaf, N.; Ghosh, S.; Pawar, S. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Mater. Lett. 2012, 72, 92–94. [Google Scholar] [CrossRef]
- Rathod, V.; Banu, A.; Ranganath, E. Biosynthesis of highly stabilized silver nanoparticles by Rhizopus stolonifer and their anti-fungal efficacy. Int. J. Cur. Biomed. Phar. Res. 2012, 2, 241–245. [Google Scholar]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Plackal Adimuriyil George, B.; Kumar, N.; Abrahamse, H.; Ray, S.S. Apoptotic efficacy of multifaceted biosynthesized silver nanoparticles on human adenocarcinoma cells. Sci. Rep. 2018, 8, 14368. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Hummel, S.; Technau, A.; Froelich, K.; Ginzkey, C.; Koehler, C.; Hagen, R.; Kleinsasser, N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. 2011, 201, 27–33. [Google Scholar] [CrossRef]
- Kittler, S.; Greulich, C.; Diendorf, J.; Koller, M.; Epple, M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Kawata, K.; Osawa, M.; Okabe, S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environ. Sci. Technol. 2009, 43, 6046–6051. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.; Tentschert, J.; Jungnickel, H.; Graf, P.; Mantion, A.; Draude, F.; Plendl, J.; Goetz, M.; Galla, S.; Mašić, A. Toxicity of silver nanoparticles in human macrophages: Uptake, intracellular distribution and cellular responses. J. Phys. Conf. Ser. 2011, 304, 012030. [Google Scholar] [CrossRef]
- Li, L.; Cui, J.; Liu, Z.; Zhou, X.; Li, Z.; Yu, Y.; Jia, Y.; Zuo, D.; Wu, Y. Silver nanoparticles induce SH-SY5Y cell apoptosis via endoplasmic reticulum-and mitochondrial pathways that lengthen endoplasmic reticulum-mitochondria contact sites and alter inositol-3-phosphate receptor function. Toxicol. Lett. 2018, 285, 156–167. [Google Scholar] [CrossRef]
- Shi, J.; Sun, X.; Lin, Y.; Zou, X.; Li, Z.; Liao, Y.; Du, M.; Zhang, H. Endothelial cell injury and dysfunction induced by silver nanoparticles through oxidative stress via IKK/NF-κB pathways. Biomaterials 2014, 35, 6657–6666. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, C.; Tang, M.; Yang, Z.; Jia, W.; Ma, Y.; Jia, P.; Pei, D.; Wang, H. Nanotoxicity of silver nanoparticles on HEK293T cells: A combined study using biomechanical and biological techniques. ACS Omega 2018, 3, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.-H. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed. Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, M.; Park, H.S.; Shin, U.S.; Gong, M.S.; Kim, H.W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed. Mater. Res. Part A 2012, 100, 1033–1043. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; K. Braydich-Stolle, L.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef]
- Zanette, C.; Pelin, M.; Crosera, M.; Adami, G.; Bovenzi, M.; Larese, F.F.; Florio, C. Silver nanoparticles exert a long-lasting antiproliferative effect on human keratinocyte HaCaT cell line. Toxicol. Vitr. 2011, 25, 1053–1060. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Zink, J.I.; Nel, A.E. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano 2008, 2, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.J.; Avliyakulov, N.K.; Boontheung, P.; Loo, J.A.; Nel, A.E. Pro-oxidative DEP chemicals induce heat shock proteins and an unfolding protein response in a bronchial epithelial cell line as determined by DIGE analysis. Proteomics 2007, 7, 3906–3918. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, R.; George, S.; Li, M.; Pokhrel, S.; Ji, Z.; France, B.; Xia, T.; Suarez, E.; Rallo, R.; Mädler, L. No time to lose—high throughput screening to assess nanomaterial safety. Nanoscale 2011, 3, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Jiang, X.; Wang, L.; Yang, M.; Liu, Y.-N. Biomimetic mineralization guided one-pot preparation of gold clusters anchored two-dimensional MnO2 nanosheets for fluorometric/magnetic bimodal sensing. Anal. Chem. 2018, 90, 2926–2932. [Google Scholar] [CrossRef]
- Boppana, V.B.R.; Jiao, F. Nanostructured MnO2: An efficient and robust water oxidation catalyst. Chem. Commun. 2011, 47, 8973–8975. [Google Scholar] [CrossRef]
- Pfalzer, A.C.; Bowman, A.B. Relationships between essential manganese biology and manganese toxicity in neurological disease. Curr. Environ. Health Rep. 2017, 4, 223–228. [Google Scholar] [CrossRef]
- Sobańska, Z.; Roszak, J.; Kowalczyk, K.; Stępnik, M. Applications and Biological Activity of Nanoparticles of Manganese and Manganese Oxides in In Vitro and In Vivo Models. Nanomaterials 2021, 11, 1084. [Google Scholar] [CrossRef]
- Srnec, M.; Aquilante, F.; Ryde, U.; Rulisek, L. Reaction mechanism of manganese superoxide dismutase studied by combined quantum and molecular mechanical calculations and multiconfigurational methods. J. Phys. Chem. B 2009, 113, 6074–6086. [Google Scholar] [CrossRef]
- Ngwa, H.A.; Kanthasamy, A.; Gu, Y.; Fang, N.; Anantharam, V.; Kanthasamy, A.G. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol. Appl. Pharmacol. 2011, 256, 227–240. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, S.H.; Na, H.B.; An, K.; Hyeon, T.; Seo, T.S. In vitro cytotoxicity screening of water-dispersible metal oxide nanoparticles in human cell lines. Bioproc. Biosyst. Eng. 2010, 33, 21–30. [Google Scholar] [CrossRef]
- Frick, R.; Müller-Edenborn, B.; Schlicker, A.; Rothen-Rutishauser, B.; Raemy, D.O.; Günther, D.; Hattendorf, B.; Stark, W.; Beck-Schimmer, B. Comparison of manganese oxide nanoparticles and manganese sulfate with regard to oxidative stress, uptake and apoptosis in alveolar epithelial cells. Toxicol. Lett. 2011, 205, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Im, G.H.; Kim, S.M.; Lee, D.-G.; Lee, W.J.; Lee, J.H.; Lee, I.S. Fe3O4/MnO hybrid nanocrystals as a dual contrast agent for both T1-and T2-weighted liver MRI. Biomaterials 2013, 34, 2069–2076. [Google Scholar] [CrossRef]
- Dong, K.; Liu, Z.; Liu, J.; Huang, S.; Li, Z.; Yuan, Q.; Ren, J.; Qu, X. Biocompatible and high-performance amino acids-capped MnWO4 nanocasting as a novel non-lanthanide contrast agent for X-ray computed tomography and T 1-weighted magnetic resonance imaging. Nanoscale 2014, 6, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Alhadlaq, H.A.; Akhtar, M.J.; Ahamed, M. Different cytotoxic and apoptotic responses of MCF-7 and HT1080 cells to MnO2 nanoparticles are based on similar mode of action. Toxicology 2019, 411, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Javorina, A.K.; Schrand, A.M.; Duhart, H.M.; Ali, S.F.; Schlager, J.J. The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol. Sci. 2006, 92, 456–463. [Google Scholar] [CrossRef]
- Titma, T.; Shimmo, R.; Siigur, J.; Kahru, A. Toxicity of antimony, copper, cobalt, manganese, titanium and zinc oxide nanoparticles for the alveolar and intestinal epithelial barrier cells in vitro. Cytotechnology 2016, 68, 2363–2377. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Alkahtani, S. Oxidative stress-induced DNA damage by manganese dioxide nanoparticles in human neuronal cells. Biomed. Res. Int. 2017, 2017, 5478790. [Google Scholar] [CrossRef]
- Wang, J.; Rahman, M.F.; Duhart, H.M.; Newport, G.D.; Patterson, T.A.; Murdock, R.C.; Hussain, S.M.; Schlager, J.J.; Ali, S.F. Expression changes of dopaminergic system-related genes in PC12 cells induced by manganese, silver, or copper nanoparticles. Neurotoxicology 2009, 30, 926–933. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, Z.; Wang, J.; Sun, J.; Liu, W.; Sun, Y.; Kong, B.; Yang, H.; Yang, S. In depth analysis of apoptosis induced by silica coated manganese oxide nanoparticles in vitro. J. Hazard. Mater. 2015, 283, 519–528. [Google Scholar] [CrossRef]
- Smith, M.R.; Fernandes, J.; Go, Y.-M.; Jones, D.P. Redox dynamics of manganese as a mitochondrial life-death switch. Biochem. Biophys. Res. Commun. 2017, 482, 388–398. [Google Scholar] [CrossRef]
- Wang, Y.-X.J. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J. Gastroenterol. 2015, 21, 13400. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.P.; Reis, C.P.; Robalo, T.T.; Melo Jorge, M.; Ferreira, P.; Gonçalves, J.; Hajalilou, A.; Cruz, M.M. Assisted Synthesis of Coated Iron Oxide Nanoparticles for Magnetic Hyperthermia. Nanomaterials 2022, 12, 1870. [Google Scholar] [CrossRef] [PubMed]
- Coricovac, D.-E.; Moacă, E.-A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.-V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.-A. Biocompatible colloidal suspensions based on magnetic iron oxide nanoparticles: Synthesis, characterization and toxicological profile. Fron. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updat. 2004, 7, 97–110. [Google Scholar] [CrossRef]
- Raj, L.; Ide, T.; Gurkar, A.U.; Foley, M.; Schenone, M.; Li, X.; Tolliday, N.J.; Golub, T.R.; Carr, S.A.; Shamji, A.F. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011, 475, 231–234. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Guzman, M.L.; Rivella, S. Iron and reactive oxygen species: Friends or foes of cancer cells? Antioxid. Redox Signal. 2014, 20, 1917–1924. [Google Scholar] [CrossRef]
- Benhar, M.; Engelberg, D.; Levitzki, A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002, 3, 420–425. [Google Scholar] [CrossRef]
- Benassi, B.; Fanciulli, M.; Fiorentino, F.; Porrello, A.; Chiorino, G.; Loda, M.; Zupi, G.; Biroccio, A. c-Myc phosphorylation is required for cellular response to oxidative stress. Mol. Cell 2006, 21, 509–519. [Google Scholar] [CrossRef]
- Wu, X.-J.; Hua, X. Targeting ROS: Selective killing of cancer cells by a cruciferous vegetable derived pro-oxidant compound. Cancer Biol. Ther. 2007, 6, 646–647. [Google Scholar] [CrossRef]
- Trachootham, D.; Zhou, Y.; Zhang, H.; Demizu, Y.; Chen, Z.; Pelicano, H.; Chiao, P.J.; Achanta, G.; Arlinghaus, R.B.; Liu, J. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell 2006, 10, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Yagoda, N.; von Rechenberg, M.; Zaganjor, E.; Bauer, A.J.; Yang, W.S.; Fridman, D.J.; Wolpaw, A.J.; Smukste, I.; Peltier, J.M.; Boniface, J.J. RAS–RAF–MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 2007, 447, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Schubert, D.; Maher, P. Oxytosis: A novel form of programmed cell death. Curr. Top. Med. Chem. 2001, 1, 497–506. [Google Scholar] [PubMed]
- Volpe, J.J.; Kinney, H.C.; Jensen, F.E.; Rosenberg, P.A. The developing oligodendrocyte: Key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci. 2011, 29, 423–440. [Google Scholar] [CrossRef]
- Sato, H.; Tamba, M.; Okuno, S.; Sato, K.; Keino-Masu, K.; Masu, M.; Bannai, S. Distribution of cystine/glutamate exchange transporter, system xc−, in the mouse brain. J. Neurosci. 2002, 22, 8028–8033. [Google Scholar] [CrossRef]
- Silva-Gomes, S.; Santos, A.G.; Caldas, C.; Silva, C.M.; Neves, J.V.; Lopes, J.; Carneiro, F.; Rodrigues, P.N.; Duarte, T.L. Transcription factor NRF2 protects mice against dietary iron-induced liver injury by preventing hepatocytic cell death. J. Hepatol. 2014, 60, 354–361. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Kruer, M.C. The neuropathology of neurodegeneration with brain iron accumulation. Int. Rev. Neurobiol. 2013, 110, 165–194. [Google Scholar]
- Guzman, J.N.; Sanchez-Padilla, J.; Wokosin, D.; Kondapalli, J.; Ilijic, E.; Schumacker, P.T.; Surmeier, D.J. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 2010, 468, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.F.; Toth, R.; James, J.; Ganley, I.G. Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep. 2013, 14, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Rottkamp, C.A.; Raina, A.K.; Zhu, X.; Gaier, E.; Bush, A.I.; Atwood, C.S.; Chevion, M.; Perry, G.; Smith, M.A. Redox-active iron mediates amyloid-β toxicity. Free Radic. Biol. Med. 2001, 30, 447–450. [Google Scholar] [CrossRef]
- Fagerqvist, T.; Lindström, V.; Nordström, E.; Lord, A.; Tucker, S.M.; Su, X.; Sahlin, C.; Kasrayan, A.; Andersson, J.; Welander, H. Monoclonal antibodies selective for α-synuclein oligomers/protofibrils recognize brain pathology in Lewy body disorders and α-synuclein transgenic mice with the disease-causing A30P mutation. J. Neurochem. 2013, 126, 131–144. [Google Scholar] [CrossRef]
- Ashraf, A.; So, P.-W. Spotlight on ferroptosis: Iron-dependent cell death in Alzheimer’s disease. Front. Aging Neurosci. 2020, 12, 196. [Google Scholar] [CrossRef]
- Chen, Y.; Khan, R.S.; Cwanger, A.; Song, Y.; Steenstra, C.; Bang, S.; Cheah, J.H.; Dunaief, J.; Shindler, K.S.; Snyder, S.H. Dexras1, a small GTPase, is required for glutamate-NMDA neurotoxicity. J. Neurosci. 2013, 33, 3582–3587. [Google Scholar] [CrossRef]
- Brennan, A.M.; Suh, S.W.; Won, S.J.; Narasimhan, P.; Kauppinen, T.M.; Lee, H.; Edling, Y.; Chan, P.H.; Swanson, R.A. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat. Neurosci. 2009, 12, 857–863. [Google Scholar] [CrossRef]
- Cellai, F.; Munnia, A.; Viti, J.; Doumett, S.; Ravagli, C.; Ceni, E.; Mello, T.; Polvani, S.; Giese, R.W.; Baldi, G. Magnetic hyperthermia and oxidative damage to DNA of human hepatocarcinoma cells. Int. J. Mol. Sci. 2017, 18, 939. [Google Scholar] [CrossRef]
- Sadeghi, L.; Tanwir, F.; Babadi, V.Y. In vitro toxicity of iron oxide nanoparticle: Oxidative damages on Hep G2 cells. Exp. Toxicol. Pathol. 2015, 67, 197–203. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-dependent toxicity of metal oxide particles—a comparison between nano-and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Halamoda Kenzaoui, B.; Chapuis Bernasconi, C.; Guney-Ayra, S.; Juillerat-Jeanneret, L. Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. Biochem. J. 2012, 441, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Tam, J.; Roth, J.A.; Sokolov, K.; Ramesh, R. EGFR-targeted plasmonic magnetic nanoparticles suppress lung tumor growth by abrogating G2/M cell-cycle arrest and inducing DNA damage. Int. J. Nanomed. 2014, 9, 3825. [Google Scholar]
- Watson, C.; Ge, J.; Cohen, J.; Pyrgiotakis, G.; Engelward, B.P.; Demokritou, P. High-throughput screening platform for engineered nanoparticle-mediated genotoxicity using CometChip technology. ACS Nano 2014, 8, 2118–2133. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Lee, J.H.; Hong, S.C.; Lee, J.; Lee, J.; Han, D.-W. Difference between toxicities of iron oxide magnetic nanoparticles with various surface-functional groups against human normal fibroblasts and fibrosarcoma cells. Materials 2013, 6, 4689–4706. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional magnetic iron oxide nanoparticles: Diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. BMC Mater. 2019, 1, 2. [Google Scholar] [CrossRef]
- Kuchma, E.A.; Zolotukhin, P.V.; Belanova, A.A.; Soldatov, M.A.; Lastovina, T.A.; Kubrin, S.P.; Nikolsky, A.V.; Mirmikova, L.I.; Soldatov, A.V. Low toxic maghemite nanoparticles for theranostic applications. Int. J. Nanomed. 2017, 12, 6365. [Google Scholar] [CrossRef]
- Lee, J.H.; Ju, J.E.; Kim, B.I.; Pak, P.J.; Choi, E.K.; Lee, H.S.; Chung, N. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ. Toxicol. Chem. 2014, 33, 2759–2766. [Google Scholar] [CrossRef]
- Gaharwar, U.S.; Paulraj, R. Iron oxide nanoparticles induced oxidative damage in peripheral blood cells of rat. J. Biomed. Sci. Eng. 2015, 8, 274. [Google Scholar] [CrossRef]
- Rehman, Y.; Cheng, Z.; Wang, X.; Huang, X.-F.; Konstantinov, K. Theranostic two-dimensional superparamagnetic maghemite quantum structures for ROS-mediated cancer therapy. J. Mater. Chem. B 2021, 9, 5805–5817. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Mayo, J.; William, W.Y.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.; Shipley, H.J.; Kan, A.; Tomson, M. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 2006, 314, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Tufani, A.; Qureshi, A.; Niazi, J.H. Iron oxide nanoparticles based magnetic luminescent quantum dots (MQDs) synthesis and biomedical/biological applications: A review. Mater. Sci. Eng. C 2021, 118, 111545. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.L. Synthesis, properties, and applications of iron nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef]
- Huh, Y.-M.; Jun, Y.-w.; Song, H.-T.; Kim, S.; Choi, J.-s.; Lee, J.-H.; Yoon, S.; Kim, K.-S.; Shin, J.-S.; Suh, J.-S. In vivo magnetic resonance detection of cancer by using multifunctional magnetic nanocrystals. J. Am. Chem. Soc. 2005, 127, 12387–12391. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Peng, J.; Sun, S.-R.; Peng, C.-W.; Li, Y.; Pang, D.-W. Tapping the potential of quantum dots for personalized oncology: Current status and future perspectives. Nanomedicine 2012, 7, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Israel, L.L.; Lellouche, E.; Kenett, R.S.; Green, O.; Michaeli, S.; Lellouche, J.-P. Ce 3/4+ cation-functionalized maghemite nanoparticles towards siRNA-mediated gene silencing. J. Mater. Chem. B 2014, 2, 6215–6225. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, M.; Seydi, E.; Rahimi, S.; Tahmasebi, G.; Jahanbani, J.; Pourahmad, J. The selective toxicity of superparamagnetic iron oxide nanoparticles (SPIONs) on oral squamous cell carcinoma (OSCC) by targeting their mitochondria. J. Biochem. Mol. Toxicol. 2021, 35, e22769. [Google Scholar] [CrossRef]
- Chen, Z.; Yin, J.-J.; Zhou, Y.-T.; Zhang, Y.; Song, L.; Song, M.; Hu, S.; Gu, N. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano 2012, 6, 4001–4012. [Google Scholar] [CrossRef]
- Cabana, S.; Curcio, A.; Michel, A.; Wilhelm, C.; Abou-Hassan, A. Iron oxide mediated photothermal therapy in the second biological window: A comparative study between magnetite/maghemite nanospheres and nanoflowers. Nanomaterials 2020, 10, 1548. [Google Scholar] [CrossRef]
- López, M.; Frieiro, J.L.; Nuez-Martínez, M.; Pedemonte, M.; Palacio, F.; Teixidor, F. Nanostructure ITO and Get More of It. Better Performance at Lower Cost. Nanomaterials 2020, 10, 1974. [Google Scholar] [CrossRef] [PubMed]
- Eshaghi, A.; Graeli, A. Optical and electrical properties of indium tin oxide (ITO) nanostructured thin films deposited on polycarbonate substrates “thickness effect”. Optik 2014, 125, 1478–1481. [Google Scholar] [CrossRef]
- Vaishnav, V.; Patel, P.; Patel, N. Indium Tin Oxide thin film gas sensors for detection of ethanol vapours. Thin Solid Film. 2005, 490, 94–100. [Google Scholar] [CrossRef]
- Bowden, E.F.; Hawkridge, F.M.; Blount, H.N. Interfacial electrochemistry of cytochrome c at tin oxide, indium oxide, gold, and platinum electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1984, 161, 355–376. [Google Scholar] [CrossRef]
- Fu, Y.; Liang, F.; Tian, H.; Hu, J. Nonenzymatic glucose sensor based on ITO electrode modified with gold nanoparticles by ion implantation. Electrochim. Acta 2014, 120, 314–318. [Google Scholar] [CrossRef]
- Khan, M. Nanoparticles modified ITO based biosensor. J. Electron. Mater. 2017, 46, 2254–2268. [Google Scholar] [CrossRef]
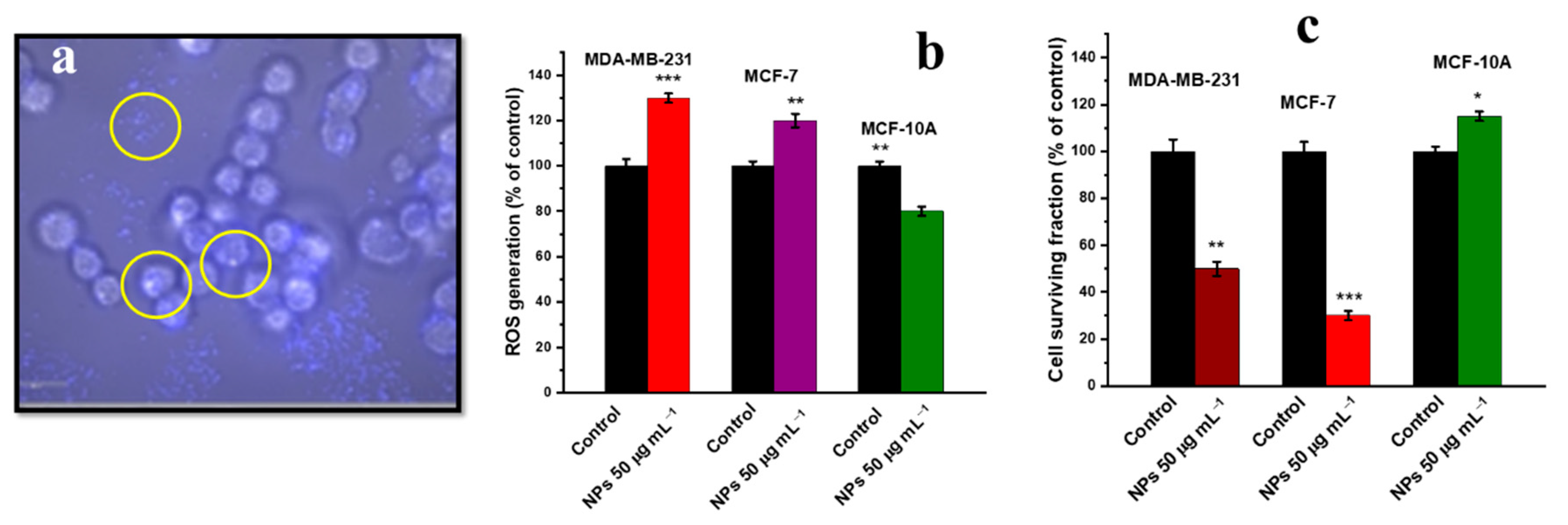
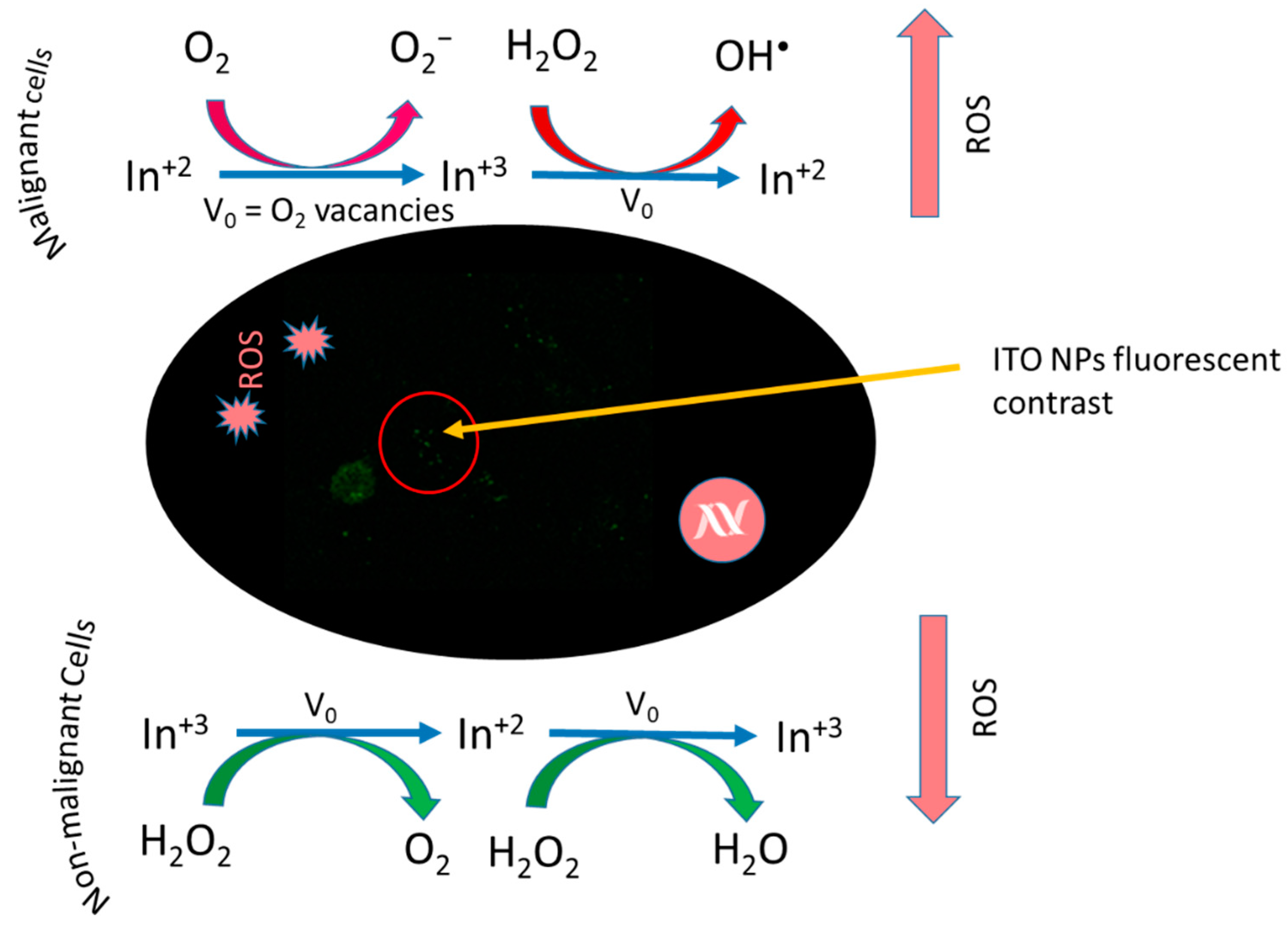
- Hsu, N.-S.; Tehei, M.; Hossain, M.S.; Rosenfeld, A.; Shiddiky, M.J.; Sluyter, R.; Dou, S.X.; Yamauchi, Y.; Konstantinov, K. Oxi-Redox Selective Breast Cancer Treatment: An In Vitro Study of Theranostic In-Based Oxide Nanoparticles for Controlled Generation or Prevention of Oxidative Stress. ACS Appl. Mater. Interfaces 2021, 13, 2204–2217. [Google Scholar] [CrossRef]
- Horiuchi, N. Light-emitting indium tin oxide. Nat. Photonics 2011, 5, 332. [Google Scholar] [CrossRef]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Graciani, J.; Mudiyanselage, K.; Xu, F.; Baber, A.E.; Evans, J.; Senanayake, S.D.; Stacchiola, D.J.; Liu, P.; Hrbek, J.; Sanz, J.F. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 2014, 345, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Fornasiero, P.; Dimonte, R.; Rao, G.R.; Kaspar, J.; Meriani, S.; Trovarelli, A.; Graziani, M. Rh-loaded CeO2-ZrO2 solid-solutions as highly efficient oxygen exchangers: Dependence of the reduction behavior and the oxygen storage capacity on the structural-properties. J. Catal. 1995, 151, 168–177. [Google Scholar] [CrossRef]
- Eriksson, P.; Tal, A.A.; Skallberg, A.; Brommesson, C.; Hu, Z.; Boyd, R.D.; Olovsson, W.; Fairley, N.; Abrikosov, I.A.; Zhang, X. Cerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancement. Sci. Rep. 2018, 8, 6999. [Google Scholar] [CrossRef] [PubMed]
- Tsamesidis, I.; Gkiliopoulos, D.; Pouroutzidou, G.K.; Lymperaki, E.; Papoulia, C.; Reybier, K.; Perio, P.; Paraskevopoulos, K.M.; Kontonasaki, E.; Theocharidou, A. Effect of artemisinin-loaded mesoporous cerium-doped calcium silicate nanopowder on cell proliferation of human periodontal ligament fibroblasts. Nanomaterials 2021, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, N.; Vitiello, G.; Di Girolamo, R.; Imbimbo, P.; Monti, D.M.; Tarallo, O.; Vergara, A.; Russo Krauss, I.; Paduano, L. Towards the development of antioxidant cerium oxide nanoparticles for biomedical applications: Controlling the properties by tuning synthesis conditions. Nanomaterials 2021, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Kim, C.K.; Kim, T.; Choi, I.Y.; Soh, M.; Kim, D.; Kim, Y.J.; Jang, H.; Yang, H.S.; Kim, J.Y.; Park, H.K. Rücktitelbild: Ceria Nanoparticles that can Protect against Ischemic Stroke (Angew. Chem. 44/2012). Angew. Chem. 2012, 124, 11334. [Google Scholar] [CrossRef]
- Banavar, S.; Deshpande, A.; Sur, S.; Andreescu, S. Ceria Nanoparticle Theranostics: Harnessing Antioxidant Properties in Biomedicine and Beyond. J. Phys. Mater. 2021, 4, 042003. [Google Scholar] [CrossRef]
- Ornatska, M.; Sharpe, E.; Andreescu, D.; Andreescu, S. Paper bioassay based on ceria nanoparticles as colorimetric probes. Anal. Chem. 2011, 83, 4273–4280. [Google Scholar] [CrossRef]
- Sharpe, E.; Frasco, T.; Andreescu, D.; Andreescu, S. Portable ceria nanoparticle-based assay for rapid detection of food antioxidants (NanoCerac). Analyst 2013, 138, 249–262. [Google Scholar] [CrossRef]
- Dong, H.; Du, S.-R.; Zheng, X.-Y.; Lyu, G.-M.; Sun, L.-D.; Li, L.-D.; Zhang, P.-Z.; Zhang, C.; Yan, C.-H. Lanthanide nanoparticles: From design toward bioimaging and therapy. Chem. Rev. 2015, 115, 10725–10815. [Google Scholar] [CrossRef]
- Chen, F.; Huang, P.; Zhu, Y.-J.; Wu, J.; Zhang, C.-L.; Cui, D.-X. The photoluminescence, drug delivery and imaging properties of multifunctional Eu3+/Gd3+ dual-doped hydroxyapatite nanorods. Biomaterials 2011, 32, 9031–9039. [Google Scholar] [CrossRef] [PubMed]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI contrast agents: Current challenges and new frontiers. Chem. Rev. 2018, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Dimri, M.C.; Khanduri, H.; Kooskora, H.; Subbi, J.; Heinmaa, I.; Mere, A.; Krustok, J.; Stern, R. Ferromagnetism in rare earth doped cerium oxide bulk samples. Phys. Status Solidi (A) 2012, 209, 353–358. [Google Scholar] [CrossRef]
- Morlando, A.; Borrás, M.C.; Rehman, Y.; Bakand, S.; Barker, P.; Sluyter, R.; Konstantinov, K. Development of CeO2 nanodot encrusted TiO2 nanoparticles with reduced photocatalytic activity and increased biocompatibility towards a human keratinocyte cell line. J. Mater. Chem. B 2020, 8, 4016–4028. [Google Scholar] [CrossRef]
- Cardillo, D.; Weiss, M.; Tehei, M.; Devers, T.; Rosenfeld, A.; Konstantinov, K. Multifunctional Fe2O3/CeO2 nanocomposites for free radical scavenging ultraviolet protection. RSC Adv. 2016, 6, 65397–65402. [Google Scholar] [CrossRef]
- Morlando, A.; McNamara, J.; Rehman, Y.; Sencadas, V.; Barker, P.J.; Konstantinov, K. Hydrothermal synthesis of rutile TiO2 nanorods and their decoration with CeO2 nanoparticles as low-photocatalytic active ingredients in UV filtering applications. J. Mater. Sci. 2020, 55, 8095–8108. [Google Scholar] [CrossRef]
- Hu, C.; Liu, H.; Dong, W.; Zhang, Y.; Bao, G.; Lao, C.; Wang, Z.L. La(OH)3 and La2O3 nanobelts—synthesis and physical properties. Adv. Mater. 2007, 19, 470–474. [Google Scholar] [CrossRef]
- Nowicki, W.; Rypka, G.; Kawałko, A.; Tolińska, A.; Kirszensztejn, P. Synthesis and characterization of SiO2–La2O3 gels obtained in a water-free environment. J. Mater. Sci. 2014, 49, 4416–4422. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Wang, G.; Lu, L.; Guo, Y.; Yang, L. Facile shape-controlled synthesis of lanthanum oxide with different hierarchical micro/nanostructures for antibacterial activity based on phosphate removal. RSC Adv. 2017, 7, 40965–40972. [Google Scholar] [CrossRef]
- Wang, K.; Wu, Y.; Li, H.; Li, M.; Guan, F.; Fan, H. A hybrid antioxidizing and antibacterial material based on Ag–La2O3 nanocomposites. J. Inorg. Biochem. 2014, 141, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Y.; Morlando, A.; Chaki Borras, M.; Sluyter, R.; Wang, X.; Huang, X.-F.; Konstantinov, K. Defect-Rich La2O3 Nanoparticles with Antioxidant Activity for Human Keratinocytes. ACS Appl. Nano Mater. 2021, 4, 6345–6356. [Google Scholar] [CrossRef]
- Fernandez-Garcia, S.; Jiang, L.; Tinoco, M.; Hungria, A.B.; Han, J.; Blanco, G.; Calvino, J.J.; Chen, X. Enhanced hydroxyl radical scavenging activity by doping lanthanum in ceria nanocubes. J. Phys. Chem. C 2016, 120, 1891–1901. [Google Scholar] [CrossRef]
- Hirosaki, N.; Ogata, S.; Kocer, C. Ab initio calculation of the crystal structure of the lanthanide Ln2O3 sesquioxides. J. Alloys Comp. 2003, 351, 31–34. [Google Scholar] [CrossRef]
- Hoekstra, H.R.; Gingerich, K.A. High-pressure B-type polymorphs of some rare-earth sesquioxides. Science 1964, 146, 1163–1164. [Google Scholar] [CrossRef]
- Zinkevich, M. Thermodynamics of rare earth sesquioxides. Prog. Mater. Sci. 2007, 52, 597–647. [Google Scholar] [CrossRef]
- Rehman, Y.; Copet, C.; Morlando, A.; Huang, X.-F.; Konstantinov, K. Investigation of ROS scavenging properties and in vitro cytotoxicity of oxygen-deficient La2O3-x nanostructure synthesized by spray pyrolysis method. J. Nanostructure Chem. 2020, 10, 347–361. [Google Scholar] [CrossRef]
- Liu, D.; Zheng, S.; Wang, X. Lanthanum regulates the reactive oxygen species in the roots of rice seedlings. Sci. Rep. 2016, 6, 31860. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Zhou, Q. Protective effect of rare earth against oxidative stress under ultraviolet-B radiation. Biol. Trace Ele. Res. 2009, 128, 82–93. [Google Scholar] [CrossRef]
- Koshevaya, E.D.; Krivoshapkina, E.F.; Krivoshapkin, P.V. Tantalum oxide nanoparticles as an advanced platform for cancer diagnostics: A review and perspective. J. Mater. Chem. B 2021, 9, 5008–5024. [Google Scholar] [CrossRef]
- Brown, R.; Tehei, M.; Oktaria, S.; Briggs, A.; Stewart, C.; Konstantinov, K.; Rosenfeld, A.; Corde, S.; Lerch, M. High-Z nanostructured ceramics in radiotherapy: First evidence of Ta2O5-induced dose enhancement on radioresistant cancer cells in an MV photon field. Part. Part. Syst. Charact. 2014, 31, 500–505. [Google Scholar] [CrossRef]
- Engels, E.; Lerch, M.; Tehei, M.; Konstantinov, K.; Guatelli, S.; Rosenfeld, A.; Corde, S. Synchrotron activation radiotherapy: Effects of dose-rate and energy spectra to tantalum oxide nanoparticles selective tumour cell radiosentization enhancement. J. Phys. Conf. Ser. 2017, 777, 012011. [Google Scholar] [CrossRef]
- Engels, E.; Corde, S.; McKinnon, S.; Incerti, S.; Konstantinov, K.; Rosenfeld, A.; Tehei, M.; Lerch, M.; Guatelli, S. Optimizing dose enhancement with Ta2O5 nanoparticles for synchrotron microbeam activated radiation therapy. Phys. Med. 2016, 32, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Hix, J.M.; Wiewiora, K.A.; Volk, M.C.; Kenyon, E.; Shuboni-Mulligan, D.D.; Blanco-Fernandez, B.; Kiupel, M.; Thomas, J.; Sempere, L.F. Tantalum oxide nanoparticles as versatile contrast agents for X-ray computed tomography. Nanoscale 2020, 12, 7720–7734. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.H.; Lee, N.; Kim, H.; Park, S.P.; Piao, Y.; Lee, J.; Jun, S.W.; Moon, W.K.; Choi, S.H.; Hyeon, T. Large-scale synthesis of bioinert tantalum oxide nanoparticles for X-ray computed tomography imaging and bimodal image-guided sentinel lymph node mapping. J. Am. Chem. Soc. 2011, 133, 5508–5515. [Google Scholar] [CrossRef]
- Shin, K.; Choi, J.W.; Ko, G.; Baik, S.; Kim, D.; Park, O.K.; Lee, K.; Cho, H.R.; Han, S.I.; Lee, S.H. Multifunctional nanoparticles as a tissue adhesive and an injectable marker for image-guided procedures. Nat. Commun. 2017, 8, 15807. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Naha, P.C.; Fayad, Z.A. Nanoparticle contrast agents for computed tomography: A focus on micelles. Contrast Media Mol. Imaging 2014, 9, 37–52. [Google Scholar] [CrossRef]
- Lusic, H.; Grinstaff, M.W. X-ray-computed tomography contrast agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Hu, Y.; Li, Z.; Fan, X.; Sun, Y.; Besenbacher, F.; Chen, C.; Yu, M. Biocompatible PEGylated bismuth nanocrystals:“All-in-one” theranostic agent with triple-modal imaging and efficient in vivo photothermal ablation of tumors. Biomaterials 2017, 141, 284–295. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Li, X.; Li, Y.; Li, C.; Wang, X.; Wang, J.; Guan, S.; Xu, Y.; Deng, G. A biocompatible theranostic agent based on stable bismuth nanoparticles for X-ray computed tomography/magnetic resonance imaging-guided enhanced chemo/photothermal/chemodynamic therapy for tumours. J. Colloid Interface Sci. 2021, 604, 80–90. [Google Scholar] [CrossRef]
- Yang, C.; Guo, C.; Guo, W.; Zhao, X.; Liu, S.; Han, X. Multifunctional bismuth nanoparticles as theranostic agent for PA/CT imaging and NIR laser-driven photothermal therapy. ACS Appl. Nano Mater. 2018, 1, 820–830. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Faghfouri, L.; Ferreira, M.P.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The versatile biomedical applications of bismuth-based nanoparticles and composites: Therapeutic, diagnostic, biosensing, and regenerative properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef] [PubMed]
- Keogan, D.M.; Griffith, D.M. Current and potential applications of bismuth-based drugs. Molecules 2014, 19, 15258–15297. [Google Scholar] [CrossRef]
- Stewart, C.; Konstantinov, K.; McDonald, M.; Bogusz, K.; Cardillo, D.; Oktaria, S.; Shi, D.; Lerch, M.; Devers, T.; Corde, S. Engineering of bismuth oxide nanoparticles to induce differential biochemical activity in malignant and nonmalignant cells. Part. Part. Syst. Charact. 2014, 31, 960–964. [Google Scholar] [CrossRef]
- Bogusz, K.; Tehei, M.; Cardillo, D.; Lerch, M.; Rosenfeld, A.; Dou, S.X.; Liu, H.-K.; Konstantinov, K. High toxicity of Bi(OH)3 and α-Bi2O3 nanoparticles towards malignant 9L and MCF-7 cells. Mater. Sci. Eng. C 2018, 93, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.; Liu, Y.; Liu, J.; Yuan, Q.; He, Y.; Lu, L. Large-scale synthesis of Bi2S3 nanodots as a contrast agent for in vivo X-ray computed tomography imaging. Adv. Mater. 2011, 23, 4886–4891. [Google Scholar] [CrossRef]
- Akbarzadeh, F.; Khoshgard, K.; Hosseinzadeh, L.; Arkan, E.; Rezazadeh, D. Investigating the cytotoxicity of folate-conjugated bismuth oxide nanoparticles on KB and A549 cell lines. Adv. Pharm. Bull. 2018, 8, 627. [Google Scholar] [CrossRef]
- Shakibaie, M.; Forootanfar, H.; Ameri, A.; Adeli-Sardou, M.; Jafari, M.; Rahimi, H.R. Cytotoxicity of biologically synthesised bismuth nanoparticles against HT-29 cell line. IET Nanobiotechnol. 2018, 12, 653–657. [Google Scholar] [CrossRef]
- Mishra, V.; Baranwal, V.; Mishra, R.K.; Sharma, S.; Paul, B.; Pandey, A.C. Immunotoxicological impact and biodistribution assessment of bismuth selenide (Bi2Se3) nanoparticles following intratracheal instillation in mice. Sci. Rep. 2017, 7, 18032. [Google Scholar] [CrossRef]
- Song, Z.; Chang, Y.; Xie, H.; Yu, X.-F.; Chu, P.K.; Chen, T. Decorated ultrathin bismuth selenide nanosheets as targeted theranostic agents for in vivo imaging guided cancer radiation therapy. NPG Asia Mater. 2017, 9, e439. [Google Scholar] [CrossRef]
- da Luz, J.Z.; Machado, T.N.; Bezerra, A.G.; de Oliveira Ribeiro, C.A.; Neto, F.F. Cytotoxicity of bismuth nanoparticles in the murine macrophage cell line RAW 264.7. J. Mater. Sci. Mater. Med. 2020, 31, 95. [Google Scholar] [CrossRef] [PubMed]
- Alamer, A.; Ali, D.; Alarifi, S.; Alkahtane, A.; Al-Zharani, M.; Abdel-Daim, M.M.; Albasher, G.; Almeer, R.; Al-Sultan, N.K.; Almalik, A. Bismuth oxide nanoparticles induce oxidative stress and apoptosis in human breast cancer cells. Environ. Sci. Pollut. Res. 2021, 28, 7379–7389. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, K.; Cardillo, D.; Tehei, M.; Boutard, T.; Barker, P.J.; Devers, T.; Rosenfeld, A.; Dou, S.X.; Liu, H.K.; Konstantinov, K. Biocompatible Bi(OH)3 nanoparticles with reduced photocatalytic activity as possible ultraviolet filter in sunscreens. Mater. Res. Bull. 2018, 108, 130–141. [Google Scholar] [CrossRef]
- Di, D.-R.; He, Z.-Z.; Sun, Z.-Q.; Liu, J. A new nano-cryosurgical modality for tumor treatment using biodegradable MgO nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, R.S.; Choi, Y.-K.; Singh, V.; Song, H.-J.; Song, K.-G.; Kim, K.J.; Kim, H.J. In vitro cytotoxic evaluation of MgO nanoparticles and their effect on the expression of ROS genes. Int. J. Mol. Sci. 2015, 16, 7551–7564. [Google Scholar] [CrossRef]
- Prucnal, S.; Shalimov, A.; Ozerov, M.; Potzger, K.; Skorupa, W. Magnetic and optical properties of virgin arc furnace grown MgO crystals. J. Cryst. Growth 2012, 339, 70–74. [Google Scholar] [CrossRef]
- Khalid, A.; Norello, R.; Abraham, A.N.; Tetienne, J.-P.; Karle, T.J.; Lui, E.W.C.; Xia, K.; Tran, P.A.; O’Connor, A.J.; Mann, B.G.; et al. Biocompatible and biodegradable magnesium oxide nanoparticles with in vitro photostable near-infrared emission: Short-term fluorescent markers. Nanomaterials 2019, 9, 1360. [Google Scholar] [CrossRef]
- Mahmoud, A.; Ezgi, Ö.; Merve, A.; Özhan, G. In vitro toxicological assessment of magnesium oxide nanoparticle exposure in several mammalian cell types. Int. J. Toxicol. 2016, 35, 429–437. [Google Scholar] [CrossRef]
- Ghorbani, S.; Moshtaghi, H.; Shekarforoush, S.S.; Ghaisari, H.R.; Sedaghati, F.; Abbasvali, M. In vitro toxicological assessment of MgO and Silica Nanoparticle in human colon carcinoma cells. Nanomed. Res. J. 2019, 4, 77–83. [Google Scholar]
- Alfaro, A.; León, A.; Guajardo-Correa, E.; Reuquen, P.; Torres, F.; Mery, M.; Segura, R.; Zapata, P.A.; Orihuela, P.A. MgO nanoparticles coated with polyethylene glycol as carrier for 2-Methoxyestradiol anticancer drug. PLoS ONE 2019, 14, e0214900. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Moon, J.Y.; Hyun, H.B.; Cho, S.K.; Kim, S.-J. Mechanistic investigation on the toxicity of MgO nanoparticles toward cancer cells. J. Mater. Chem. 2012, 22, 24610–24617. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Dey, A.; Neogi, S. An insight into the Mechanism of Antibacterial activity by Magnesium oxide nanoparticles. J. Mater. Chem. B 2021, 9, 5329–5339. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Khalid, A.; Verma, R.; Abraham, A.; Qazi, F.; Dong, X.; Liang, G.; Tomljenovic-Hanic, S. Silk fibroin coated magnesium oxide nanospheres: A biocompatible and biodegradable tool for noninvasive bioimaging applications. Nanomaterials 2021, 11, 695. [Google Scholar] [CrossRef]
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Oraby, H.F.; Al Hamoud, G.A.; Bukhari, S.I.; Moubayed, N.M. Biogenic green synthesis of MgO nanoparticles using Saussurea costus biomasses for a comprehensive detection of their antimicrobial, cytotoxicity against MCF-7 breast cancer cells and photocatalysis potentials. PLoS ONE 2020, 15, e0237567. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef]
- Poon, W.; Zhang, Y.-N.; Ouyang, B.; Kingston, B.R.; Wu, J.L.; Wilhelm, S.; Chan, W.C. Elimination pathways of nanoparticles. ACS Nano 2019, 13, 5785–5798. [Google Scholar] [CrossRef]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef]
- Von Maltzahn, G.; Park, J.-H.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef]
- Van der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.; Hollman, P.C. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, Z.; Wu, Y.; Zhang, W.; Zhang, T.; Yu, B.-Y.; Zheng, X.; Tian, J. Renal-clearable and biodegradable black phosphorus quantum dots for photoacoustic imaging of kidney dysfunction. Anal. Chim. Acta 2022, 1204, 339737. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, M.; Zhou, C.; Yang, S.; Ning, X.; Zheng, J. Passive tumor targeting of renal-clearable luminescent gold nanoparticles: Long tumor retention and fast normal tissue clearance. J. Am. Chem. Soc. 2013, 135, 4978–4981. [Google Scholar] [CrossRef] [PubMed]
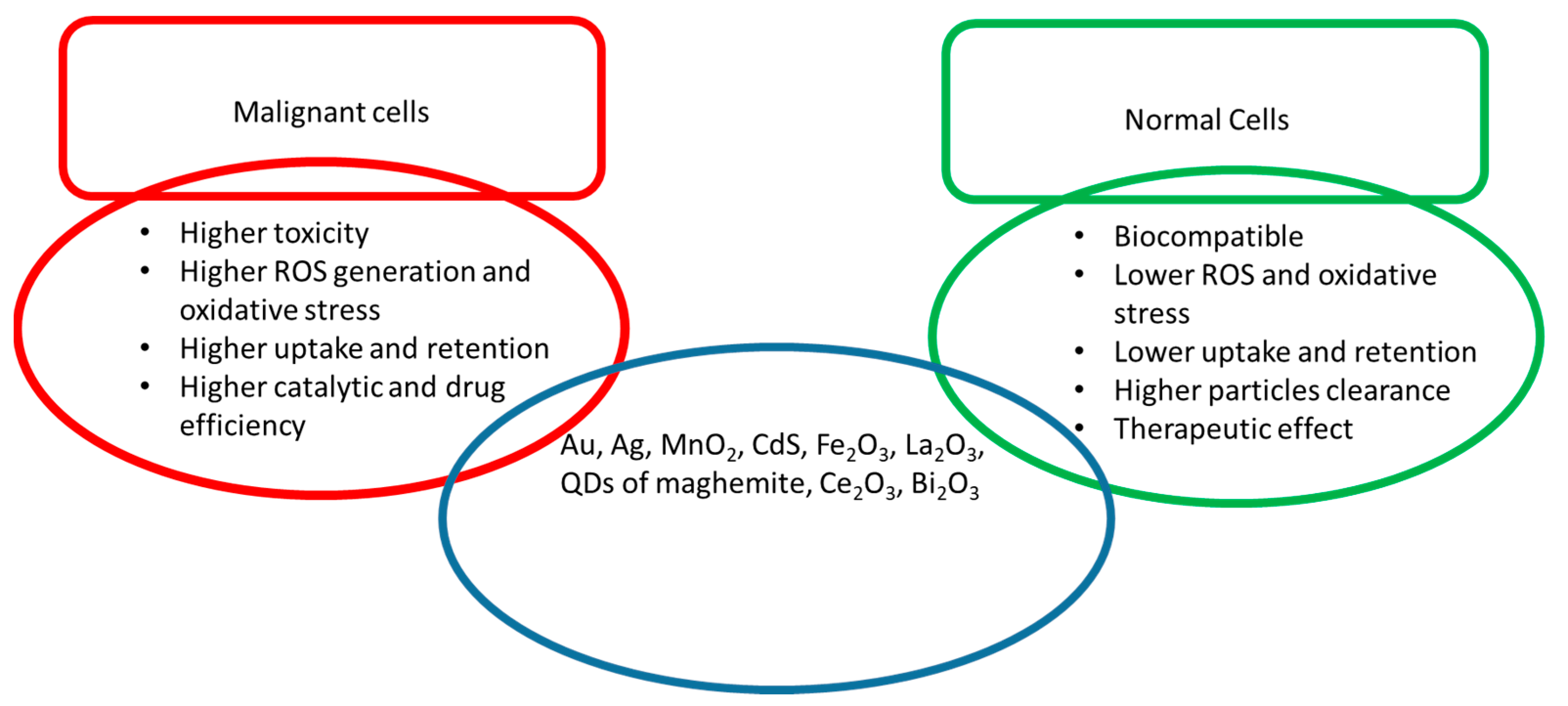
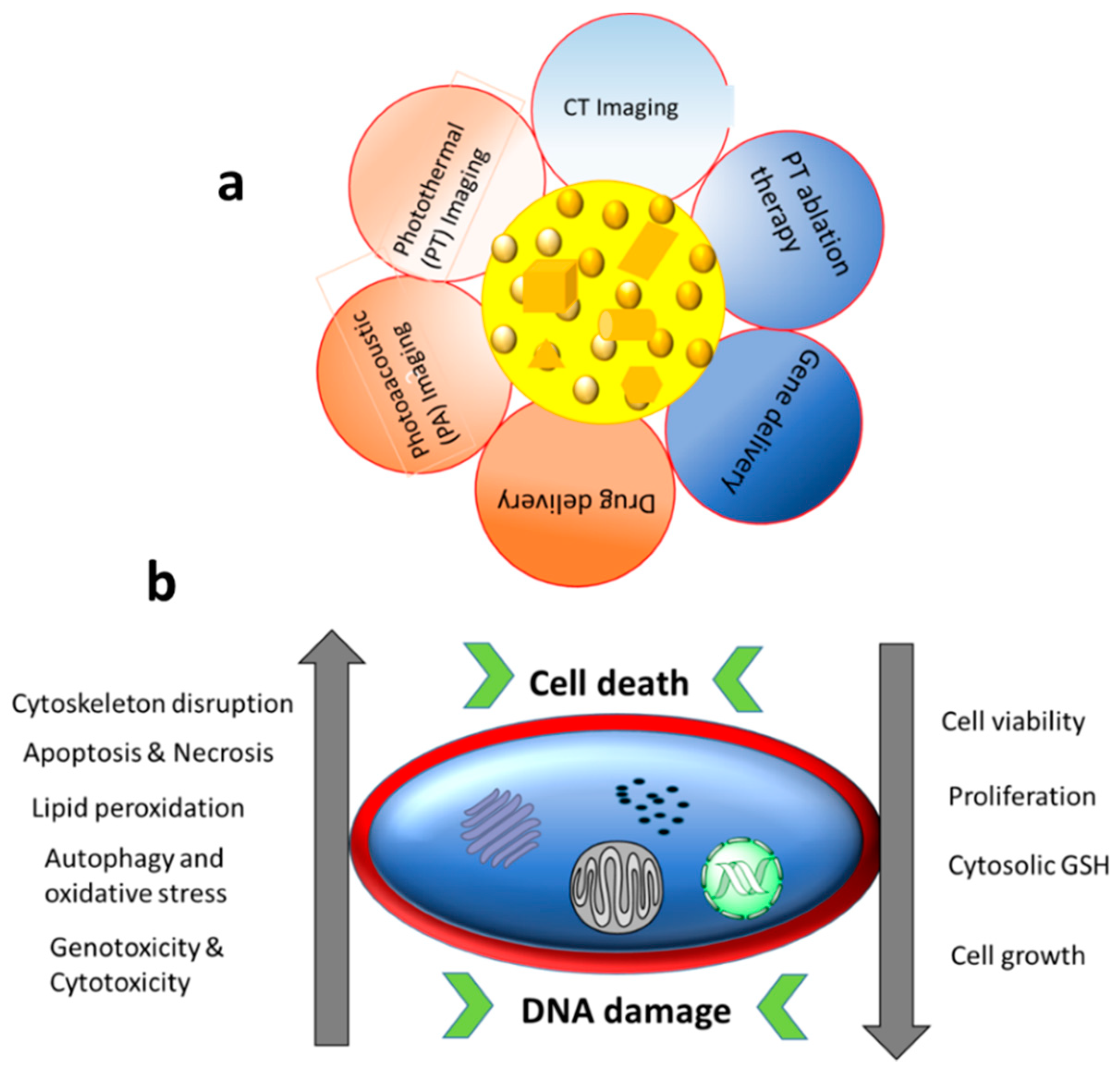

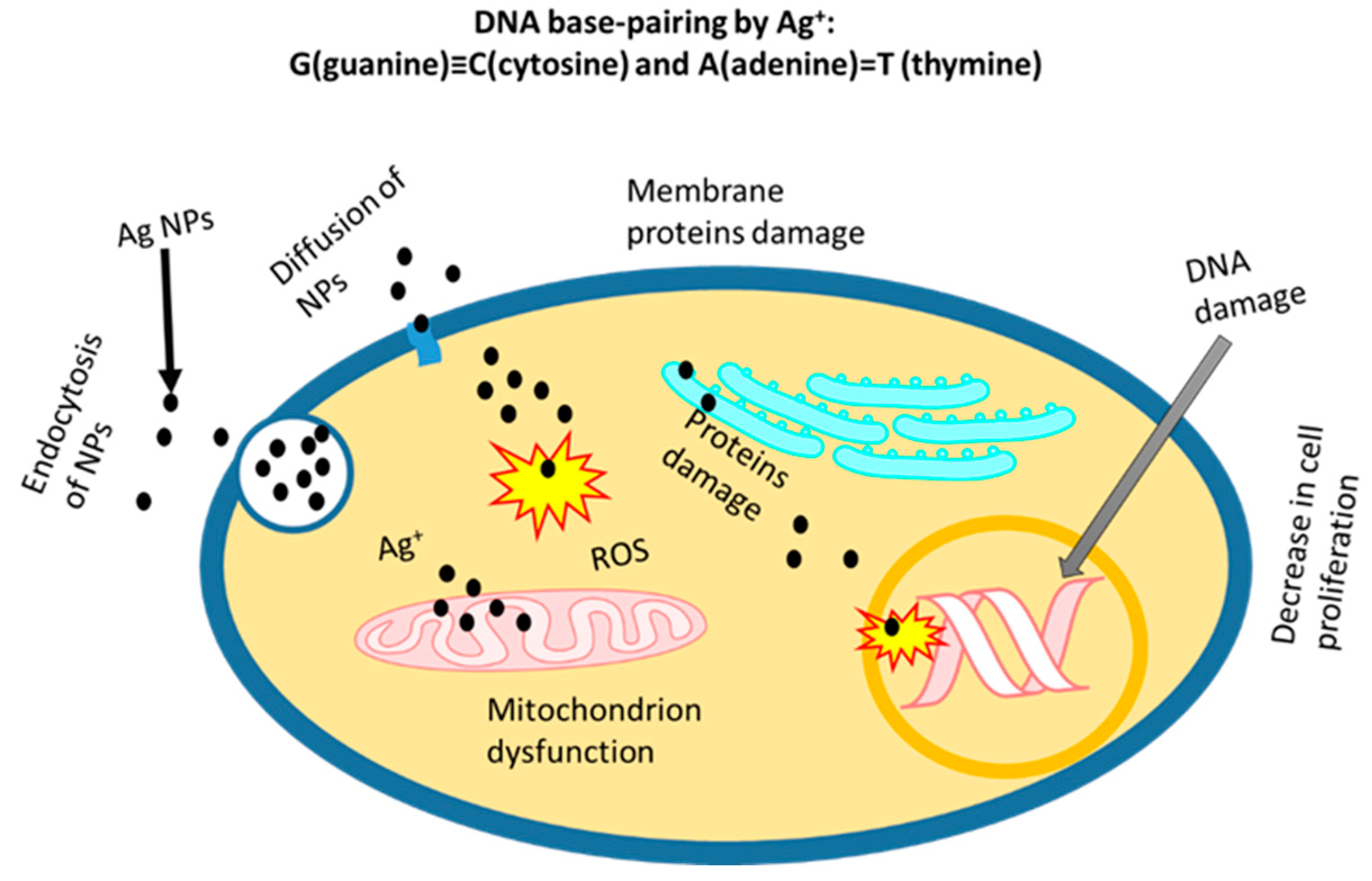


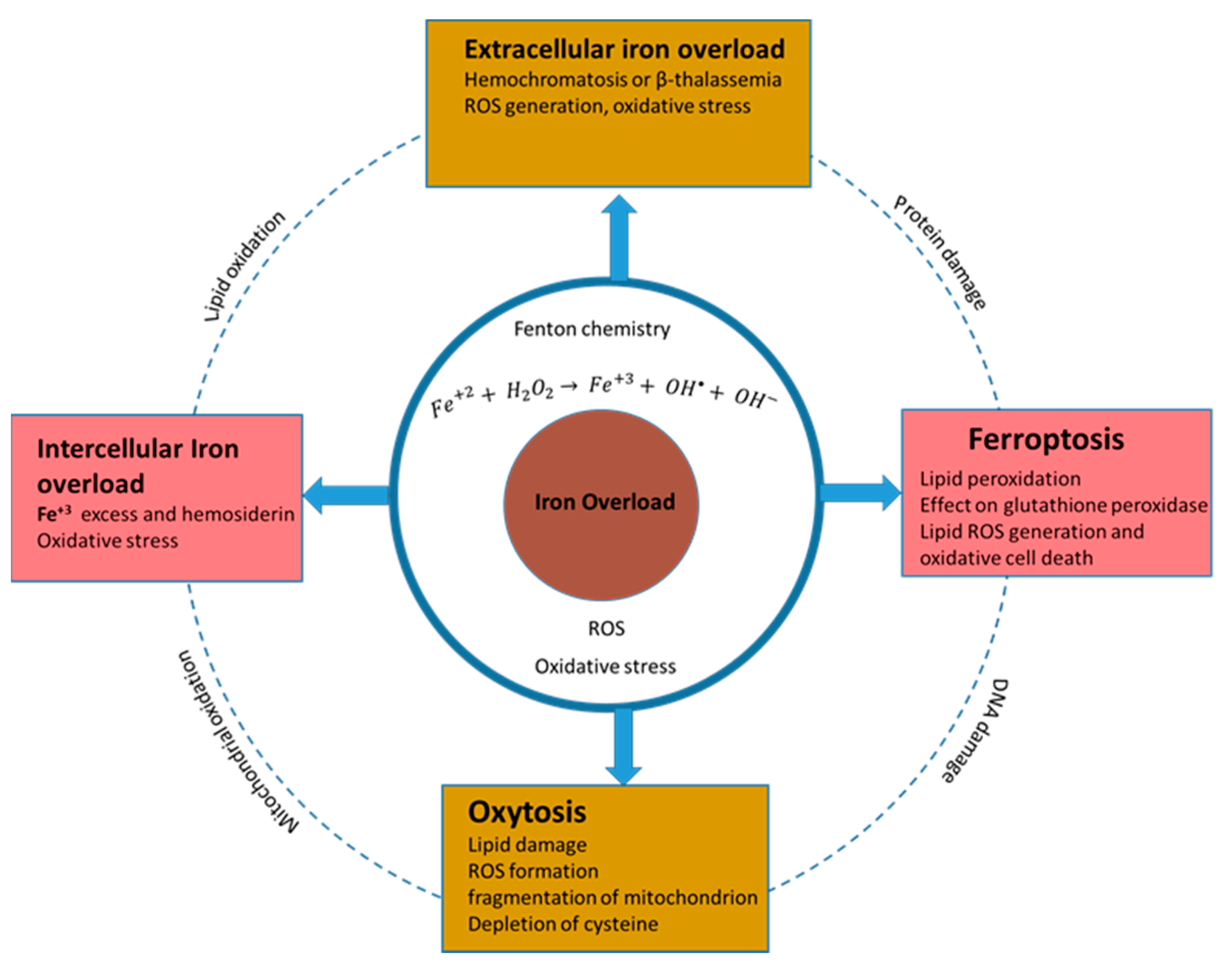

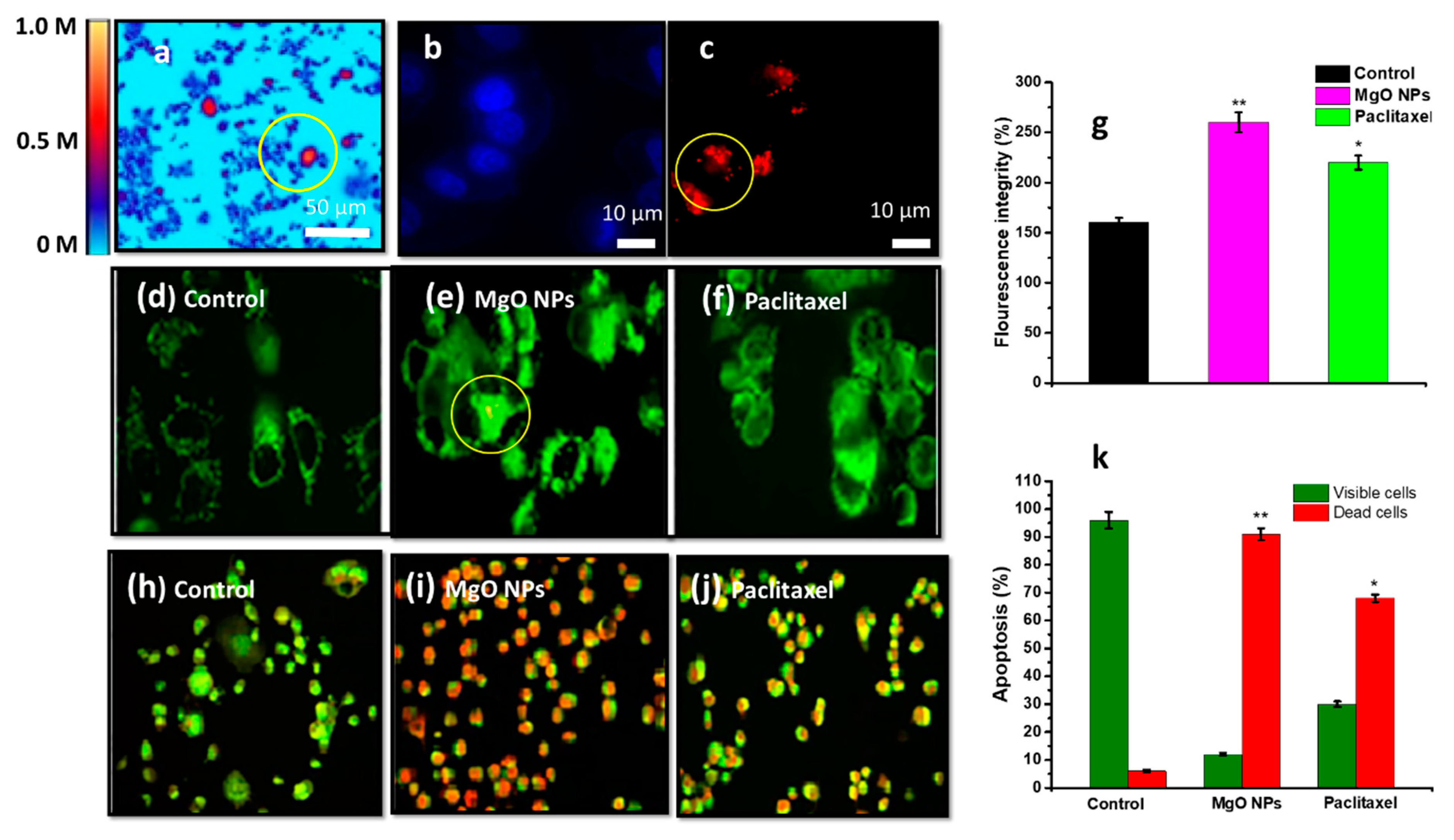
| Cell Line | NP Size and Shape | Synthesis Method | Effect |
|---|---|---|---|
| Human breast cancer (MDA-MB 231) cell line | ITO NPs, irregular (10.78 nm) | Chemical precipitation and calcination | Higher NP uptake, increased ROS generation caused higher oxidative stress, cellular apoptosis evaluated by Annexin-V binding [157]. |
| Breast cancer (MCF-7) cell line | ITO NPs, irregular (10.78 nm) | Chemical precipitation and calcination | ROS induced oxidative stress, cellular apoptosis [157]. |
| Human epithelial (MCF-10A) normal breast cells | ITO NPs, irregular (10.78 nm) | Chemical precipitation and calcination | ROS scavenging, lower cellular apoptosis due to lower oxidative stress [157]. |
| Cell Line | NP Size and Shape | Synthesis Method | Effect |
|---|---|---|---|
| 9L cell line, MCF cell line | α-Bi2O3 (6–10 nm, round and ellipsoidal shapes) | Precipitation method | Higher cellular internalisation, increased ROS generation, induced higher toxicity and, hence, lowered cell viability [205]. |
| MDCK cell line | α-Bi2O3 (6–10 nm, round and ellipsoidal shapes) | Precipitation method | Lower cellular internalisation, decreased ROS generation and, hence, minimum toxicity generation, biocompatible behaviour [205]. |
| HeLa cells | Bi2S3 (2–3 nm, nanodots, coated with PVP) | Hot injection method | Dose-dependent uptake, good biocompatibility even at higher doze (2 mg Bi mL−1, excellent for CT imaging [206]. |
| KB cells, A549 cell lines | Bi2O3 (15–25 nm, hexagonal shape) | Solvothermal synthesis | Higher ROS generation and toxicity of bare Bi2O3 NPs than folic acid-coated NPs, significant increase in oxidative stress by bare Bi2O3 NPs [207]. |
| HT-29 cell line | Bi2O3 (40–120 nm, spherical shape) | Biogenic synthesis | Reduced GSH level, increased MDA level, decreased SOD and CAT activities, induction of cytotoxicity through late apoptosis [208]. |
| Mice lung epithelial and fibroblast cells | Bi2Se3 (70 nm, flake-like shape) | Sonochemical method | Cytotoxic inflammation due to increased expression of IL-1β, MIP-2 and IL-6; increased oxidative stress through neutrophilic and ROS generation [209]. |
| HeLa cells | Bi2S3 decorated with chitosan and RGD peptide (nanosheets, 53.8 nm wide and 6 nm thick) | Solution-based method using poly(vinylpyrrolidone) | Mitochondria-mediated intrinsic cell apoptosis, G0/GI cell cycle arrest, TrxR inhibition, ROS generation, X-rays induced apoptosis, increased radio-sensitisation effect. Excellent photoacoustic imaging [210]. |
| Murine macrophage cell line (RAW 264.7) | Bi NPs (unknown 25–60 nm) | Laser ablation method | ROS generation and increased oxidative stress, DNA and plasma membrane damage, increased phagocytic activity [211]. |
| MCF-7 cell line | Bi2O3 (unknown 14 nm) | Commercial | Increased ROS generation, SOD and CAT activity, and increased GSH concentration, mitochondrion dysfunction, DNA damage and cellular apoptosis [212]. |
| HaCaT and MDCK cell lines | Bi(OH)3 (6 nm, spherical shape) | Precipitation method | Higher cell viability, biocompatible nature of the synthesised NPs. No DNA or mitochondrion damage [213]. |
| Cell Line | NP Size and Shape | Synthesis Method | Effect |
|---|---|---|---|
| Prostate cancer (PC-3) cells | Spherical, commercial (20–40 nm), irregular shape, ball milling (70–230 nm) | Commercial (MTI Corporation, St. Richmond, CA, USA), ball milling | Dose-dependent toxicity at concentration of ≥62.5 µg mL−1. Fluorescent imaging properties [217]. |
| Human liver cancer (HepG2) cell lines | Unknown (20 nm) | Thermal decomposition of metal–oleate complex | No significant toxicity towards oxidative stress genes (GST and catalase), demonstrated high biocompatibility and low toxicity, also recorded high stability in microenvironment [215]. |
| Human lung (A549) cells | Irregular shape (43.9 nm) | Commercial (Sigma-Aldrich, St. Louis, Missouri, USA) | Generation of ROS, oxidative damage and depletion of GSH, mitochondrial apoptosis and DNA damage [218]. |
| Human colon adenocarcinoma (HT-29) | Irregular shape (50 nm) | Precipitation–aging–calcination method | Lipid peroxidation, increased ROS generation oxidative stress, depletion of GSH, overall dose-dependent toxicity [219]. |
| Prostate cancer cell line (LNCap) | Spherical (15–30 nm), PEG-coated (93 nm) | Sol–gel method | Decrease in cell viability, coated MgO NPs used as anticancer drug carriers [220]. |
| (HeLa), human gastric adenocarcinoma and carcinoma (AGS, SNU-A6) cell line | Irregular shape (20 nm) | Precipitation method | Ultrasound induced lipid peroxidation, increased ROS level, ROS induced cellular apoptosis [221]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, Y.; Qutaish, H.; Kim, J.H.; Huang, X.-F.; Alvi, S.; Konstantinov, K. Microenvironmental Behaviour of Nanotheranostic Systems for Controlled Oxidative Stress and Cancer Treatment. Nanomaterials 2022, 12, 2462. https://doi.org/10.3390/nano12142462
Rehman Y, Qutaish H, Kim JH, Huang X-F, Alvi S, Konstantinov K. Microenvironmental Behaviour of Nanotheranostic Systems for Controlled Oxidative Stress and Cancer Treatment. Nanomaterials. 2022; 12(14):2462. https://doi.org/10.3390/nano12142462
Chicago/Turabian StyleRehman, Yaser, Hamzeh Qutaish, Jung Ho Kim, Xu-Feng Huang, Sadia Alvi, and Konstantin Konstantinov. 2022. "Microenvironmental Behaviour of Nanotheranostic Systems for Controlled Oxidative Stress and Cancer Treatment" Nanomaterials 12, no. 14: 2462. https://doi.org/10.3390/nano12142462
APA StyleRehman, Y., Qutaish, H., Kim, J. H., Huang, X.-F., Alvi, S., & Konstantinov, K. (2022). Microenvironmental Behaviour of Nanotheranostic Systems for Controlled Oxidative Stress and Cancer Treatment. Nanomaterials, 12(14), 2462. https://doi.org/10.3390/nano12142462








